Volatilome of Chill-Stored European Seabass (Dicentrarchus labrax) Fillets and Atlantic Salmon (Salmo salar) Slices under Modified Atmosphere Packaging
Abstract
:1. Introduction
2. Results and Discussion
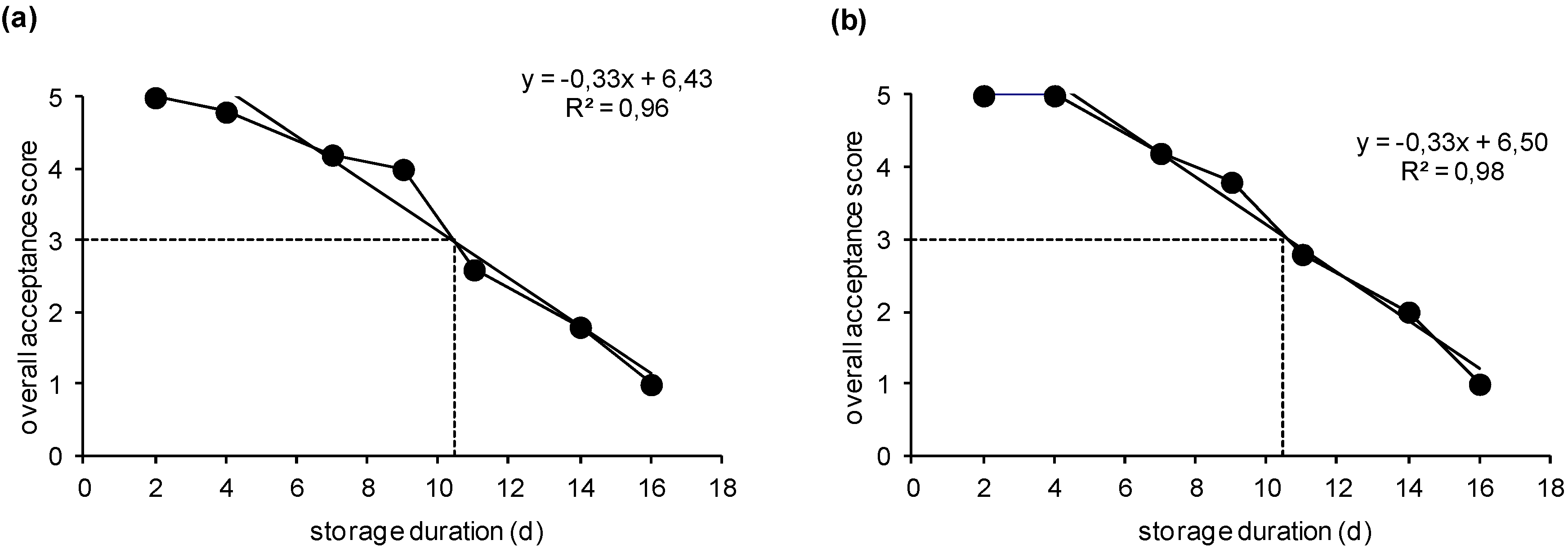
2.1. Sensory Acceptance Evaluation and Shelf-Life Determination
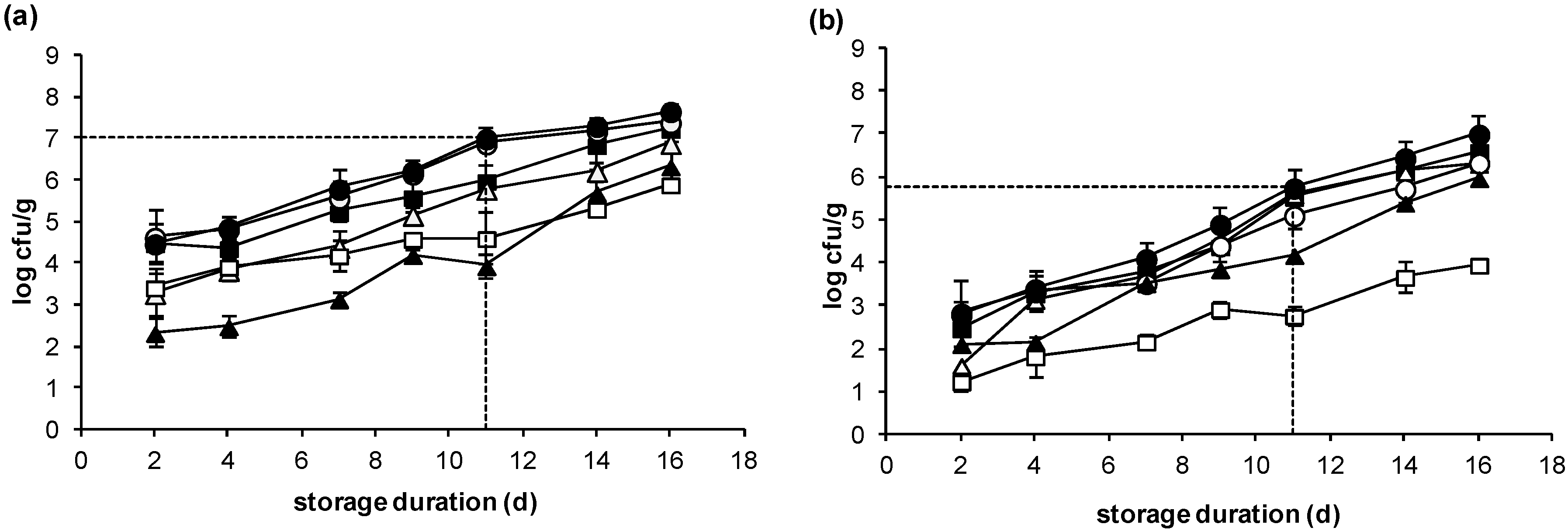
2.2. Microbiological Changes
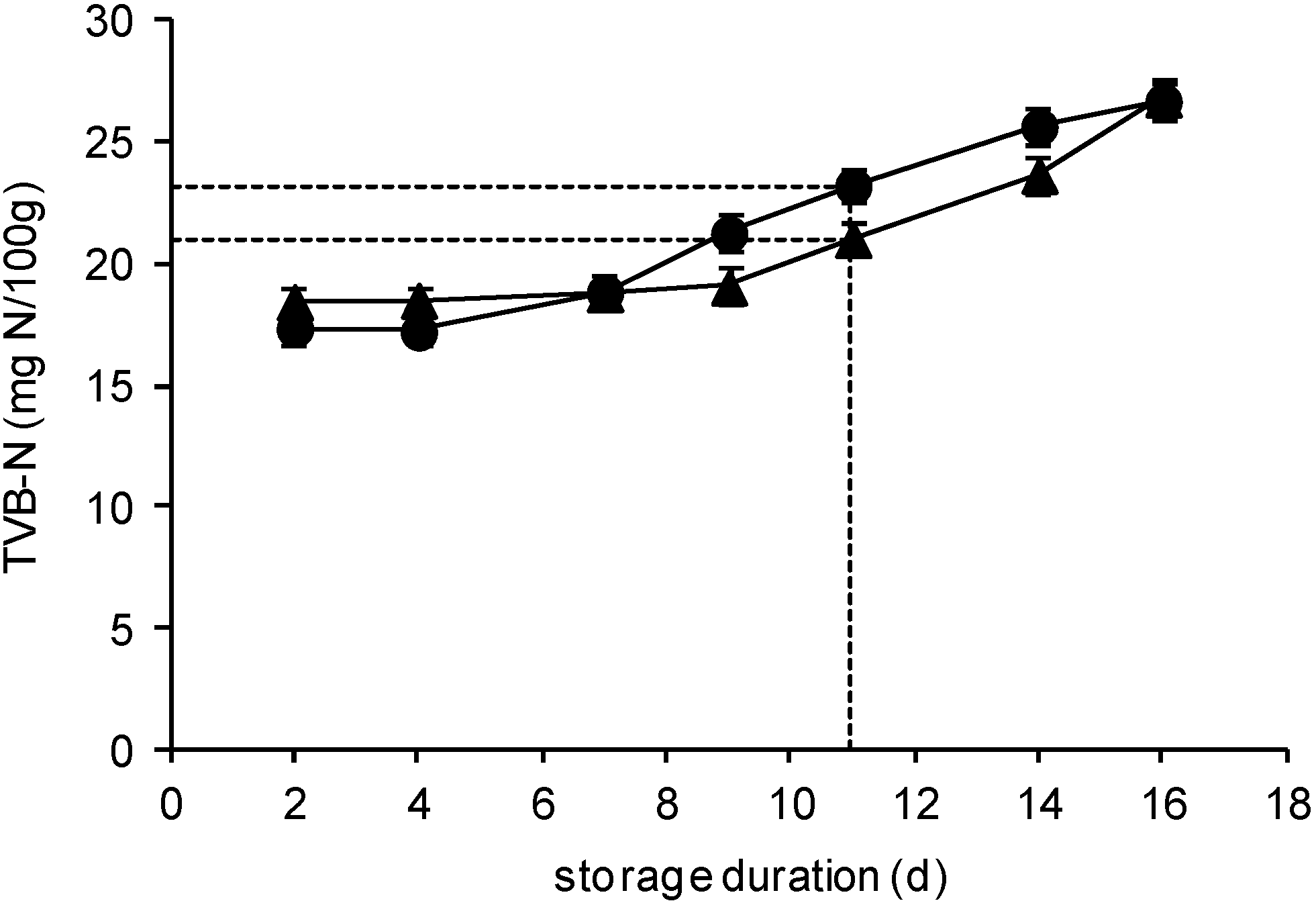
2.3. TVB-N Determination
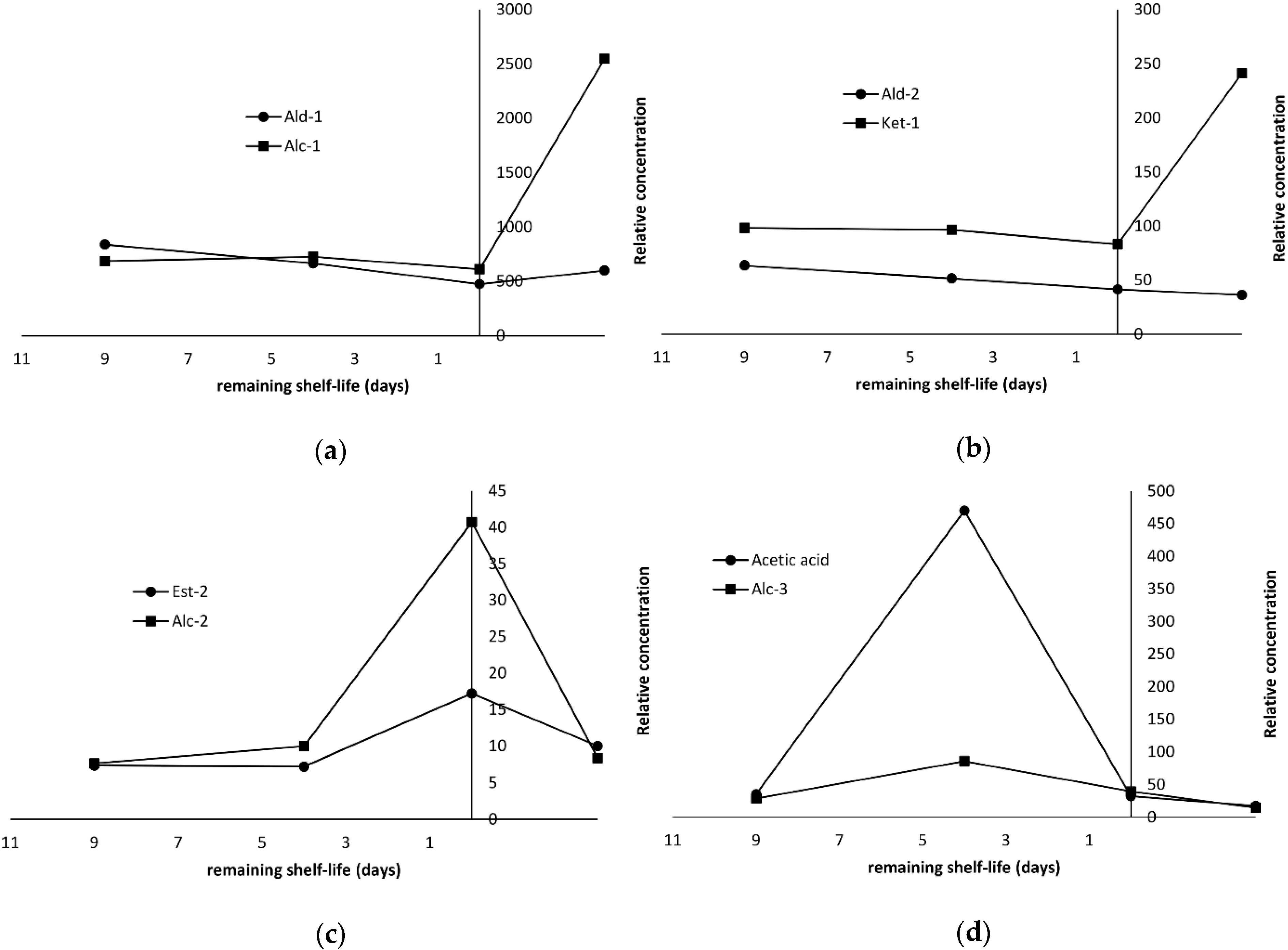
2.4. Production of Volatile Compounds During Fish Storage
3. Materials and Methods
3.1. Fish Provision, Handling and Storage
3.2. Sensory Acceptance Evaluation
3.3. Microbiological Analysis
3.4. Determination of TVB-N
3.5. VOCs Determination by Headspace SPME-GC/MS
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ashie, I.N.A.; Smith, J.P.; Simpson, B.K.; Haard, D.N.F. Spoilage and shelf-life extension of fresh fish and shellfish. Crit. Rev. Food Sci. Nutr. 2009, 36, 87–121. [Google Scholar] [CrossRef]
- Gram, L.; Huss, H.H. Microbiological spoilage of fish and fish products. Int. J. Food Microbiol. 1996, 33, 121–137. [Google Scholar] [CrossRef]
- Olafsdóttir, G.; Martinsdóttir, E.; Oehlenschläger, J.; Dalgaard, P.; Jensen, B.; Undeland, I.; Mackie, I.M.; Henehan, G.; Nielsen, J.; Nilsen, H. Methods to evaluate fish freshness in research and industry. Trends Food Sci. Technol. 1997, 8, 258–265. [Google Scholar] [CrossRef]
- Dalgaard, P. FISH | Spoilage of Seafood. In Encyclopedia of Food Sciences and Nutrition; Caballero, B., Trugo, L., Finglas, P.M., Eds.; Academic Press: Cambridge, UK, 2003; pp. 2462–2471. ISBN 978-0-12-227055-0. [Google Scholar]
- DeWitt, C.A.M.; Oliveira, A.C.M. Modified Atmosphere Systems and Shelf Life Extension of Fish and Fishery Products. Foods 2016, 5, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oehlenschläger, J. Seafood Quality Assessment. In Seafood Processing: Technology, Quality and Safety; John Wiley & Sons, Ltd.: Chichester, UK, 2013; pp. 359–386. ISBN 978-1-118-34617-4. [Google Scholar]
- Huss, H.H. Quality and Quality Changes in Fresh Fish; FAO Fisheries Technical Paper - 348; Food and Agriculture Organization of the United Nations: Rome, Italy, 1995. [Google Scholar]
- Gram, L.; Dalgaard, P. Fish spoilage bacteria – problems and solutions. Curr. Opin. Biotechnol. 2002, 13, 262–266. [Google Scholar] [CrossRef]
- Boziaris, I.S.; Parlapani, F.F. Chapter 3 - Specific Spoilage Organisms (SSOs) in Fish. In The Microbiological Quality of Food; Bevilacqua, A., Corbo, M.R., Sinigaglia, M., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Duxford, UK, 2017; pp. 61–98. ISBN 978-0-08-100502-6. [Google Scholar]
- Parlapani, F.F.; Mallouchos, A.; Haroutounian, S.A.; Boziaris, I.S. Microbiological spoilage and investigation of volatile profile during storage of sea bream fillets under various conditions. Int. J. Food Microbiol. 2014, 189, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Leduc, F.; Tournayre, P.; Kondjoyan, N.; Mercier, F.; Malle, P.; Kol, O.; Berdagué, J.L.; Duflos, G. Evolution of volatile odorous compounds during the storage of European seabass (Dicentrarchus labrax). Food Chem. 2012, 131, 1304–1311. [Google Scholar] [CrossRef]
- Wierda, R.L.; Fletcher, G.; Xu, L.; Dufour, J.-P. Analysis of Volatile Compounds as Spoilage Indicators in Fresh King Salmon (Oncorhynchus tshawytscha) During Storage Using SPME−GC−MS. J. Agric. Food Chem. 2006, 54, 8480–8490. [Google Scholar] [CrossRef]
- Soncin, S.; Chiesa, L.M.; Panseri, S.; Biondi, P.; Cantoni, C. Determination of volatile compounds of precooked prawn (Penaeus vannamei) and cultured gilthead sea bream (Sparus aurata) stored in ice as possible spoilage markers using solid phase microextraction and gas chromatography/mass spectrometry. J. Sci. Food Agric. 2009, 89, 436–442. [Google Scholar] [CrossRef]
- Edirisinghe, R.K.B.; Graffham, A.J.; Taylor, S.J. Characterisation of the volatiles of yellowfin tuna (Thunnus albacares) during storage by solid phase microextraction and GC–MS and their relationship to fish quality parameters. Int. J. Food Sci. Technol. 2007, 42, 1139–1147. [Google Scholar] [CrossRef]
- Duflos, G.; Coin, V.M.; Cornu, M.; Antinelli, J.-F.; Malle, P. Determination of volatile compounds to characterize fish spoilage using headspace/mass spectrometry and solid-phase microextraction/gas chromatography/mass spectrometry. J. Sci. Food Agric. 2006, 86, 600–611. [Google Scholar] [CrossRef]
- Noseda, B.; Vermeulen, A.; Ragaert, P.; Devlieghere, F. Packaging of Fish and Fishery Products. In Seafood Processing; John Wiley & Sons, Ltd.: Chichester, UK, 2013; pp. 237–261. ISBN 978-1-118-34617-4. [Google Scholar]
- Sivertsvik, M.; Jeksrud, W.K.; Rosnes, J.T. A review of modified atmosphere packaging of fish and fishery products – significance of microbial growth, activities and safety. Int. J. Food Sci. Technol. 2002, 37, 107–127. [Google Scholar] [CrossRef]
- Parlapani, F.F.; Haroutounian, S.A.; Nychas, G.-J.E.; Boziaris, I.S. Microbiological spoilage and volatiles production of gutted European sea bass stored under air and commercial modified atmosphere package at 2 °C. Food Microbiol. 2015, 50, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Kostaki, M.; Giatrakou, V.; Savvaidis, I.N.; Kontominas, M.G. Combined effect of MAP and thyme essential oil on the microbiological, chemical and sensory attributes of organically aquacultured sea bass (Dicentrarchus labrax) fillets. Food Microbiol. 2009, 26, 475–482. [Google Scholar] [CrossRef]
- Poli, B.M.; Messini, A.; Parisi, G.; Scappini, F.; Vigiani, V.; Giorgi, G.; Vincenzini, M. Sensory, physical, chemical and microbiological changes in European sea bass (Dicentrarchus labrax) fillets packed under modified atmosphere/air or prepared from whole fish stored in ice. Int. J. Food Sci. Technol. 2006, 41, 444–454. [Google Scholar] [CrossRef]
- Emborg, J.; Laursen, B.G.; Rathjen, T.; Dalgaard, P. Microbial spoilage and formation of biogenic amines in fresh and thawed modified atmosphere-packed salmon (Salmo salar) at 2 °C. J. Appl. Microbiol. 2002, 92, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Macé, S.; Cornet, J.; Chevalier, F.; Cardinal, M.; Pilet, M.-F.; Dousset, X.; Joffraud, J.-J. Characterisation of the spoilage microbiota in raw salmon (Salmo salar) steaks stored under vacuum or modified atmosphere packaging combining conventional methods and PCR–TTGE. Food Microbiol. 2012, 30, 164–172. [Google Scholar] [CrossRef] [Green Version]
- Sivertsvik, M.; Rosnes, J.T.; Kleiberg, G.H. Effect of Modified Atmosphere Packaging and Superchilled Storage on the Microbial and Sensory Quality of Atlantic Salmon (Salmo salar) Fillets. J. Food Sci. 2003, 68, 1467–1472. [Google Scholar] [CrossRef]
- Koutsoumanis, K.P.; Taoukis, P.S.; Drosinos, E.H.; Nychas, G.-J.E. Applicability of an Arrhenius Model for the Combined Effect of Temperature and CO2 Packaging on the Spoilage Microflora of Fish. Appl. Environ. Microbiol. 2000, 66, 3528–3534. [Google Scholar] [CrossRef] [Green Version]
- Parlapani, F.F.; Kormas, K.A.; Boziaris, I.S. Microbiological changes, shelf life and identification of initial and spoilage microbiota of sea bream fillets stored under various conditions using 16S rRNA gene analysis. J. Sci. Food Agric. 2015, 95, 2386–2394. [Google Scholar] [CrossRef]
- Powell, S.M.; Tamplin, M.L. Microbial communities on Australian modified atmosphere packaged Atlantic salmon. Food Microbiol. 2012, 30, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Cakli, S.; Kilinc, B.; Cadun, A.; Dincer, T.; Tolasa, S. Quality differences of whole ungutted sea bream (Sparus aurata) and sea bass (Dicentrarchus labrax) while stored in ice. Food Control. 2007, 18, 391–397. [Google Scholar] [CrossRef]
- Castro, P.; Padrón, J.C.P.; Cansino, M.J.C.; Velázquez, E.S.; Larriva, R.M.D. Total volatile base nitrogen and its use to assess freshness in European sea bass stored in ice. Food Control. 2006, 17, 245–248. [Google Scholar] [CrossRef]
- Macé, S.; Joffraud, J.-J.; Cardinal, M.; Malcheva, M.; Cornet, J.; Lalanne, V.; Chevalier, F.; Sérot, T.; Pilet, M.-F.; Dousset, X. Evaluation of the spoilage potential of bacteria isolated from spoiled raw salmon (Salmo salar) fillets stored under modified atmosphere packaging. Int. J. Food Microbiol. 2013, 160, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Commission Regulation (EC) No 2074/2005. Off. J. Eur. Union 2005, L 338, 27–59.
- Ui, S.; Masuda, T.; Masuda, H.; Muraki, H. Mechanism for the formation of 2,3-butanediol stereoisomers in Bacillus polymyxa. J. Ferment. Technol. 1986, 64, 481–486. [Google Scholar] [CrossRef]
- Jónsdóttir, R.; Ólafsdóttir, G.; Chanie, E.; Haugen, J.-E. Volatile compounds suitable for rapid detection as quality indicators of cold smoked salmon (Salmo salar). Food Chem. 2008, 109, 184–195. [Google Scholar] [CrossRef]
- Olafsdottir, G.; Jonsdottir, R.; Lauzon, H.L.; Luten, J.; Kristbergsson, K. Characterization of Volatile Compounds in Chilled Cod (Gadus morhua) Fillets by Gas Chromatography and Detection of Quality Indicators by an Electronic Nose. J. Agric. Food Chem. 2005, 53, 10140–10147. [Google Scholar] [CrossRef]
- Macé, S.; Cardinal, M.; Jaffrès, E.; Cornet, J.; Lalanne, V.; Chevalier, F.; Sérot, T.; Pilet, M.-F.; Dousset, X.; Joffraud, J.-J. Evaluation of the spoilage potential of bacteria isolated from spoiled cooked whole tropical shrimp (Penaeus vannamei) stored under modified atmosphere packaging. Food Microbiol. 2014, 40, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Ercolini, D.; Casaburi, A.; Nasi, A.; Ferrocino, I.; Di Monaco, R.; Ferranti, P.; Mauriello, G.; Villani, F. Different molecular types of Pseudomonas fragi have the same overall behaviour as meat spoilers. Int. J. Food Microbiol. 2010, 142, 120–131. [Google Scholar] [CrossRef]
- Joffraud, J.J.; Leroi, F.; Roy, C.; Berdagué, J.L. Characterisation of volatile compounds produced by bacteria isolated from the spoilage flora of cold-smoked salmon. Int. J. Food Microbiol. 2001, 66, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Laursen, B.G.; Leisner, J.J.; Dalgaard, P. Carnobacterium Species: Effect of Metabolic Activity and Interaction with Brochothrix thermosphacta on Sensory Characteristics of Modified Atmosphere Packed Shrimp. J. Agric. Food Chem. 2006, 54, 3604–3611. [Google Scholar] [CrossRef] [PubMed]
- Tsigarida, E.; Boziaris, I.S.; Nychas, G.-J.E. Bacterial Synergism or Antagonism in a Gel Cassette System. Appl. Environ. Microbiol. 2003, 69, 7204–7209. [Google Scholar] [CrossRef] [Green Version]
- Parlapani, F.F.; Mallouchos, A.; Haroutounian, S.A.; Boziaris, I.S. Volatile organic compounds of microbial and non-microbial origin produced on model fish substrate un-inoculated and inoculated with gilt-head sea bream spoilage bacteria. LWT 2017, 78, 54–62. [Google Scholar] [CrossRef]
- Fratini, G.; Lois, S.; Pazos, M.; Parisi, G.; Medina, I. Volatile profile of Atlantic shellfish species by HS-SPME GC/MS. Food Res. Int. 2012, 48, 856–865. [Google Scholar] [CrossRef]
- Noseda, B.; Islam, M.T.; Eriksson, M.; Heyndrickx, M.; De Reu, K.; Van Langenhove, H.; Devlieghere, F. Microbiological spoilage of vacuum and modified atmosphere packaged Vietnamese Pangasius hypophthalmus fillets. Food Microbiol. 2012, 30, 408–419. [Google Scholar] [CrossRef]
- Varlet, V.; Prost, C.; Serot, T. Volatile aldehydes in smoked fish: Analysis methods, occurence and mechanisms of formation. Food Chem. 2007, 105, 1536–1556. [Google Scholar] [CrossRef]
- Parlapani, F.F.; Verdos, G.I.; Haroutounian, S.A.; Boziaris, I.S. The dynamics of Pseudomonas and volatilome during the spoilage of gutted sea bream stored at 2 °C. Food Control. 2015, 55, 257–265. [Google Scholar] [CrossRef]
- Casaburi, A.; Piombino, P.; Nychas, G.-J.; Villani, F.; Ercolini, D. Bacterial populations and the volatilome associated to meat spoilage. Food Microbiol. 2015, 45, 83–102. [Google Scholar] [CrossRef]
- Jørgensen, L.V.; Huss, H.H.; Dalgaard, P. Significance of Volatile Compounds Produced by Spoilage Bacteria in Vacuum-Packed Cold-Smoked Salmon (Salmo salar) Analyzed by GC-MS and Multivariate Regression. J. Agric. Food Chem. 2001, 49, 2376–2381. [Google Scholar] [CrossRef]
- Vyncke, W.; Luten, J.; Brünner, K.; Moermans, R. Determination of total volatile bases in fish: A collaborative study by the West European Fish Technologists’ Association (WEFTA). Z. Für Lebensm.-Unters. Forsch. 1987, 184, 110–114. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
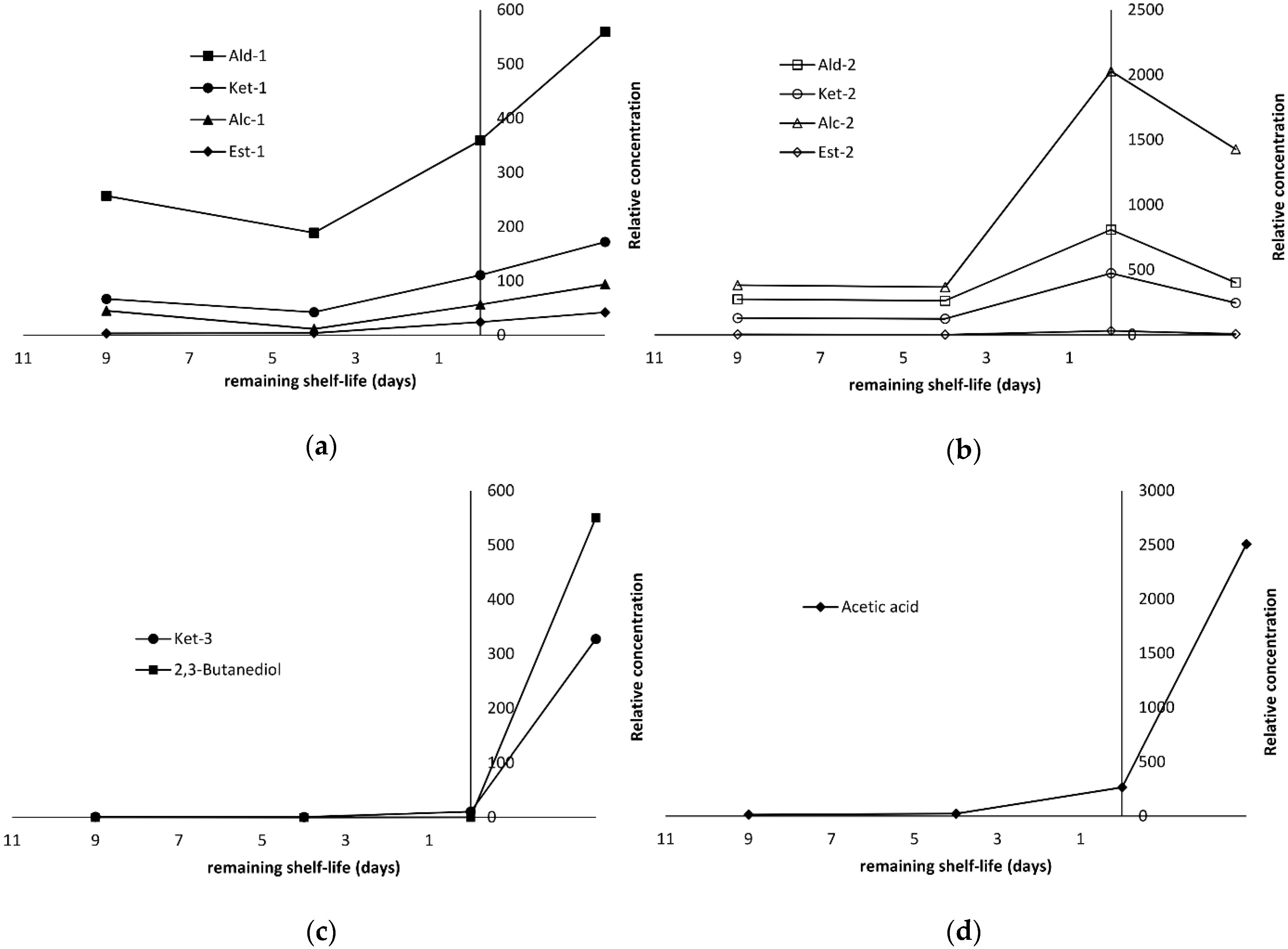
| Compound | Sea Bass Fillets | Salmon Slices | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D2 | D7 | D11 | D14 | Group b | D2 | D7 | D11 | D14 | Group | Identification c | RI d | m/ze | |
| Aldehydes | |||||||||||||
| Acetaldehyde | 12.0 | 11.5 | 43.0 | nd f | Ald-2 | 128 | 62.5 | 77.8 | 61.5 | MS, RI, ref | 459 | 29 | |
| 3-Methylbutanal | nd | nd | 3.1 | nd | Ald-2 | 5.2 | 9.3 | 5.1 | 6.9 | MS, RI, ref | 647 | 58 | |
| 2-Methylbutanal | nd | nd | 2.9 | nd | Ald-2 | 4.7 | 4.9 | 4.0 | 3.1 | MS, RI, ref | 657 | 57 | |
| Pentanal | 10.4 | 9.3 | 33.1 | 12.0 | Ald-2 | 21.9 | 24.4 | 9.0 | 23.1 | Ald-1 | MS, RI | 697 | 58 |
| Hexanal | 132 | 152 | 357 | 164 | Ald-2 | 251 | 157 | 120 | 159 | Ald-1 | MS, RI, ref | 802 | 56 |
| Heptanal | 19.6 | 29.3 | 45.9 | 42.9 | Ald-2 | 53.9 | 33.7 | 31.4 | 36.1 | Ald-1 | MS, RI | 902 | 70 |
| Octanal | 31.6 | 19.5 | 46.3 | 43.2 | Ald-2 | 28.7 | 20.5 | 18.5 | 23.1 | Ald-1 | MS, RI, ref | 1003 | 43 |
| Nonanal | 124 | 115 | 160 | 214 | Ald-1 | 216 | 198 | 145 | 150 | Ald-1 | MS, RI | 1105 | 57 |
| Decanal | 35.6 | 14.0 | 42.7 | 61.1 | Ald-1 | 31.9 | 26.6 | 24.4 | 29.5 | Ald-1 | MS, RI, ref | 1206 | 57 |
| Undecanal | 4.5 | 3.7 | 8.6 | 12.1 | Ald-1 | 5.8 | 7.6 | 4.2 | 5.3 | MS, RI | 1308 | 57 | |
| (E)-2-Pentenal | 6.8 | 3.9 | 25.4 | 13.9 | Ald-2 | 10.7 | 15.9 | 3.9 | 7.4 | Ald-1 | MS, RI | 748 | 55 |
| (E)-2-Hexenal | 3.4 | 1.4 | 13.8 | 9.4 | Ald-2 | 5.5 | 12.4 | 2.8 | 5.8 | Ald-1 | MS, RI | 853 | 83 |
| 4-Heptenal | 12.5 | 6.9 | 51.4 | 20.7 | Ald-2 | 8.8 | 14.6 | 4.0 | 33.1 | Ald-1 | MS, RI | 901 | 84 |
| 2-Heptenal | 4.2 | 2.7 | 10.3 | 8.6 | Ald-2 | 8.3 | 6.4 | 4.6 | 4.5 | Ald-2 | MS, RI | 954 | 83 |
| (E)-2-Octenal | 8.6 | 5.5 | 12.0 | 20.0 | Ald-1 | 12.5 | 8.6 | 7.6 | 6.0 | Ald-2 | MS, RI | 1060 | 70 |
| (E)-2-Decenal | 10.5 | 7.0 | 12.6 | 28.6 | Ald-1 | 15.8 | 14.0 | 11.8 | 9.9 | Ald-2 | MS, RI | 1262 | 55 |
| (E)-2-Undecenal | 10.6 | 7.0 | 14.0 | 29.7 | Ald-1 | 15.2 | 14.2 | 10.5 | 9.3 | Ald-2 | MS, RI | 1365 | 70 |
| 2,4-Heptadienal (isomer) | 17.7 | 11.6 | 81.6 | 33.8 | Ald-2 | 28.5 | 40.6 | 5.4 | 21.5 | MS | 998 | 81 | |
| (E,E)-2,4-Heptadienal | 23.4 | 15.0 | 94.2 | 53.9 | Ald-2 | 27.9 | 28.8 | 13.4 | 28.0 | MS, RI | 1011 | 81 | |
| 2,4-Decadienal | 8.7 | 5.6 | 15.5 | 24.8 | Ald-1 | 11.7 | 8.3 | 6.9 | 6.7 | Ald-2 | MS, RI | 1315 | 81 |
| Phenylacetaldehyde | 8.9 | 5.4 | 18.1 | 41.0 | Ald-1 | 13.4 | 26.7 | 13.4 | 17.9 | MS, RI | 1043 | 91 | |
| Benzaldehyde | 46.2 | 25.4 | 75.6 | 127.6 | Ald-1 | 47.0 | 49.4 | 31.5 | 53.5 | MS, RI, ref | 955 | 106 | |
| Ketones | |||||||||||||
| 2,3-Butanedione | 1.0 | 0.6 | 5.7 | 69.2 | Ket-3 | 5.6 | 5.0 | 6.8 | 5.0 | MS, RI, ref | 594 | 86 | |
| 2-Butanone | 12.0 | 7.6 | 26.1 | 28.7 | Ket-1 | 19.8 | 17.1 | 21.1 | 32.7 | Ket-1 | MS, RI | 601 | 72 |
| 2-Pentanone | 28.3 | 17.0 | 35.3 | 29.0 | Ket-2 | 24.8 | 16.5 | 22.5 | 66.7 | Ket-1 | MS, RI | 686 | 43 |
| 2,3-Pentanedione | 20.5 | 17.1 | 65.6 | 38.0 | Ket-2 | 63.9 | 78.6 | 34.4 | 50.7 | MS, RI, ref | 696 | 100 | |
| 3-Hydroxy-2-butanone | nd | nd | 4.8 | 258 | Ket-3 | nd | nd | nd | nd | MS, RI, ref | 715 | 45 | |
| 2-Heptanone | 9.9 | 5.4 | 20.3 | 25.7 | Ket-1 | 5.2 | 4.8 | 8.0 | 13.2 | Ket-1 | MS, RI | 888 | 43 |
| 2,3-Octanedione | 28.7 | 44.9 | 107 | 47.0 | Ket-2 | 17.2 | 15.6 | 14.8 | 42.7 | Ket-1 | MS, RI | 987 | 43 |
| 6-Methyl-5-hepten-2-one | 9.5 | 7.4 | 12.5 | 15.8 | Ket-1 | 7.8 | 8.7 | 7.4 | 5.4 | MS, RI | 989 | 108 | |
| (E,E)-3,5-Octadien-2-one | 42.3 | 36.9 | 216 | 106 | Ket-2 | 28.2 | 37.7 | 7.5 | 50.6 | Ket-1 | MS, RI | 1072 | 95 |
| 2-Nonanone | 10.8 | 4.9 | 18.9 | 30.3 | Ket-1 | 3.3 | 4.9 | 5.1 | 15.4 | Ket-1 | MS, RI | 1094 | 58 |
| 3,5-Octadien-2-one (isomer) | 8.4 | 7.4 | 49.7 | 26.1 | Ket-2 | nd | nd | 4.1 | 19.7 | Ket-1 | MS, RI | 1094 | 95 |
| Acetophenone | 24.8 | 17.2 | 32.9 | 71.4 | Ket-1 | 13.9 | 19.1 | 10.7 | 17.1 | MS, RI, ref | 1065 | 105 | |
| Alcohols | |||||||||||||
| Ethanol | 478 | 111 | 1336 | 567 | Alc-2 | 1294 | 482 | 1151 | 505 | MS, RI, ref | 477 | 45 | |
| Propanol | 12.4 | 10.8 | 54.1 | 8.4 | Alc-2 | 14.8 | 62.4 | 28.7 | 9.7 | Alc-3 | MS, RI, ref | 555 | 31 |
| 3-Methyl-1-butanol | nd | nd | 1.8 | nd | Alc-2 | nd | nd | 6.3 | nd | Alc-2 | MS, RI, ref | 725 | 55 |
| Pentanol | 9.5 | 8.5 | 30.1 | 23.4 | Alc-2 | 11.8 | 11.3 | 13.0 | 11.4 | MS, RI, ref | 759 | 42 | |
| Hexanol | 21.7 | 24.8 | 88.9 | 48.6 | Alc-2 | 7.7 | 10.0 | 34.4 | 8.4 | Alc-2 | MS, RI, ref | 870 | 56 |
| Heptanol | 6.3 | 5.8 | 22.0 | 15.3 | Alc-2 | 7.9 | 4.9 | 6.8 | 6.1 | MS, RI, ref | 973 | 70 | |
| 2-Ethyl-1-hexanol | 40.2 | 7.7 | 49.4 | 81.8 | Alc-1 | 15.7 | 14.2 | 19.4 | 45.4 | Alc-1 | MS, RI, ref | 1030 | 57 |
| Dodecanol | 4.9 | 3.7 | 7.2 | 11.9 | Alc-1 | 13.7 | 23.5 | 10.8 | 4.9 | Alc-3 | MS, RI | 1476 | 55 |
| 1-Penten-3-ol | 145 | 148 | 746 | 600 | Alc-2 | 552 | 548 | 474 | 1468 | Alc-1 | MS, RI, ref | 675 | 57 |
| (Z)-2-Penten-1-ol | 29.3 | 27.5 | 160 | 148 | Alc-2 | 70.0 | 108 | 32.7 | 108 | MS, RI | 766 | 57 | |
| 1-Octen-3-ol | 52.1 | 53.3 | 374 | 196 | Alc-2 | 23.7 | 37.0 | 33.7 | 212 | Alc-1 | MS, RI, ref | 980 | 57 |
| 2,3-Butanediol | nd | nd | nd | 550 | Alc-3 | nd | nd | nd | nd | MS, RI | 794 | 45 | |
| (5Z)-Octa-1,5-dien-3-ol | 106 | 88.9 | 551 | 389 | Alc-2 | 93.1 | 124 | 83.4 | 821 | Alc-1 | MS, RI | 975 | 57 |
| Esters | |||||||||||||
| Ethyl acetate | 3.4 | 4.1 | 24.3 | 42.1 | Est-1 | 13.2 | 7.3 | 17.0 | 5.4 | MS, RI, ref | 613 | 61 | |
| Ethyl butanoate | nd | nd | 4.5 | nd | Est-2 | 17.4 | 5.0 | 15.6 | 6.9 | MS, RI, ref | 805 | 88 | |
| Ethyl lactate | nd | nd | nd | nd | 27.0 | 5.6 | 21.2 | 4.7 | MS, RI, ref | 815 | 45 | ||
| Ethyl hexanoate | 1.8 | 0.8 | 19.4 | 3.0 | Est-2 | 7.3 | 7.2 | 12.8 | 6.8 | Est-1 | MS, RI, ref | 1001 | 88 |
| Ethyl octanoate | 1.1 | nd | 7.2 | 3.3 | Est-2 | nd | nd | 3.1 | 2.4 | Est-1 | MS, RI, ref | 1198 | 88 |
| Ethyl decanoate | nd | nd | 1.8 | nd | Est-2 | nd | nd | 1.3 | 0.8 | Est-1 | MS, RI, ref | 1396 | 88 |
| Acids | |||||||||||||
| Acetic acid | 13.5 | 22.3 | 266 | 2507 | 35.4 | 470 | 32.2 | 17.4 | MS, RI, ref | 624 | 60 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kritikos, A.; Aska, I.; Ekonomou, S.; Mallouchos, A.; Parlapani, F.F.; Haroutounian, S.A.; Boziaris, I.S. Volatilome of Chill-Stored European Seabass (Dicentrarchus labrax) Fillets and Atlantic Salmon (Salmo salar) Slices under Modified Atmosphere Packaging. Molecules 2020, 25, 1981. https://doi.org/10.3390/molecules25081981
Kritikos A, Aska I, Ekonomou S, Mallouchos A, Parlapani FF, Haroutounian SA, Boziaris IS. Volatilome of Chill-Stored European Seabass (Dicentrarchus labrax) Fillets and Atlantic Salmon (Salmo salar) Slices under Modified Atmosphere Packaging. Molecules. 2020; 25(8):1981. https://doi.org/10.3390/molecules25081981
Chicago/Turabian StyleKritikos, Athanasios, Ioanna Aska, Sotirios Ekonomou, Athanasios Mallouchos, Foteini F. Parlapani, Serkos A. Haroutounian, and Ioannis S. Boziaris. 2020. "Volatilome of Chill-Stored European Seabass (Dicentrarchus labrax) Fillets and Atlantic Salmon (Salmo salar) Slices under Modified Atmosphere Packaging" Molecules 25, no. 8: 1981. https://doi.org/10.3390/molecules25081981
APA StyleKritikos, A., Aska, I., Ekonomou, S., Mallouchos, A., Parlapani, F. F., Haroutounian, S. A., & Boziaris, I. S. (2020). Volatilome of Chill-Stored European Seabass (Dicentrarchus labrax) Fillets and Atlantic Salmon (Salmo salar) Slices under Modified Atmosphere Packaging. Molecules, 25(8), 1981. https://doi.org/10.3390/molecules25081981









