Beneficial Effects of Cornelian Cherries on Lipid Profile and NO/ROS Balance in Obese Zucker Rats: Comparison with CoQ10
Abstract
1. Introduction
2. Results
2.1. Cornelian Cherry: Preparation and Characterisation
2.2. Bodyweight, Relative Heart Weight and Blood Pressure
2.3. Plasma Lipid Profile
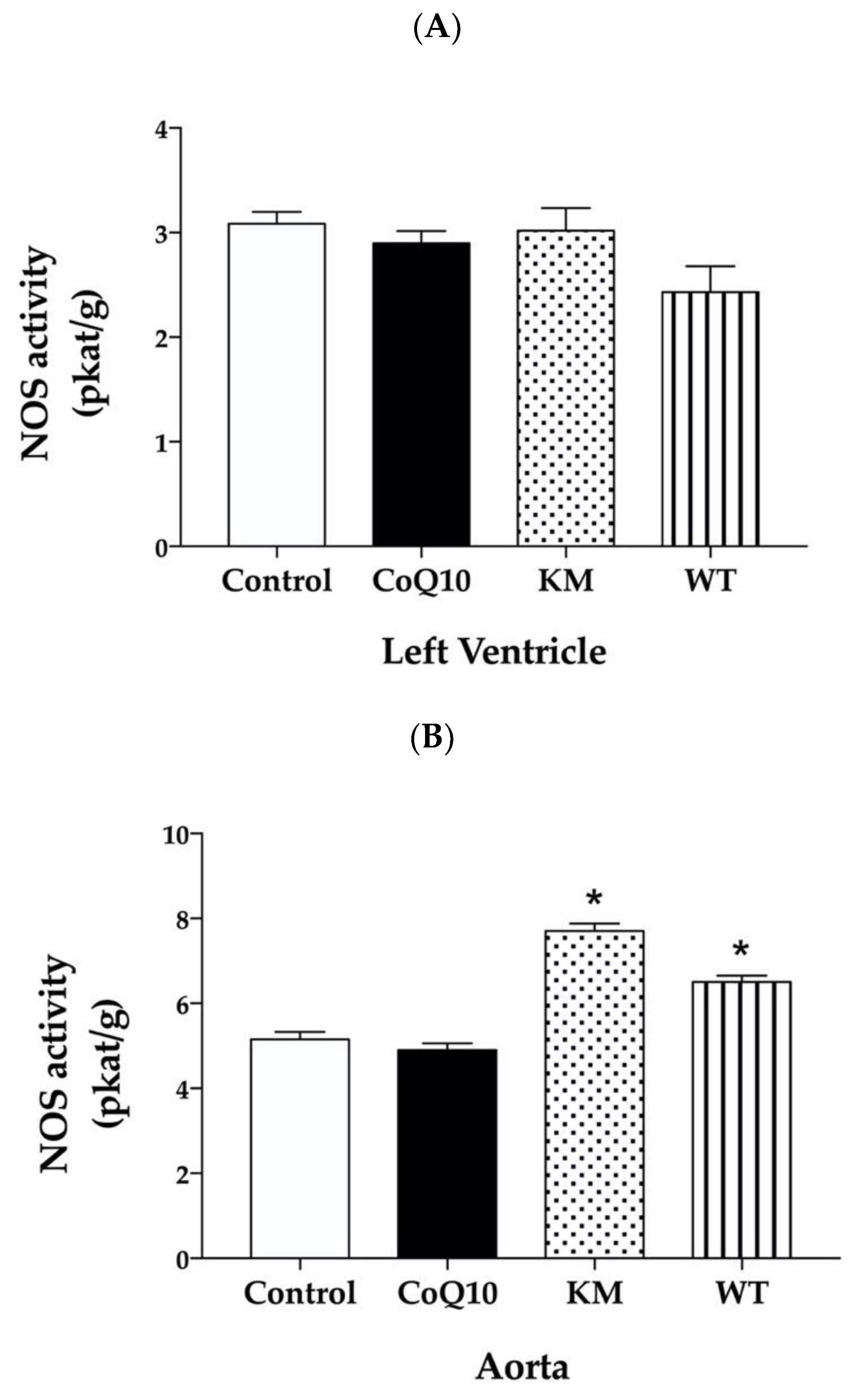
2.4. Total NOS Activity
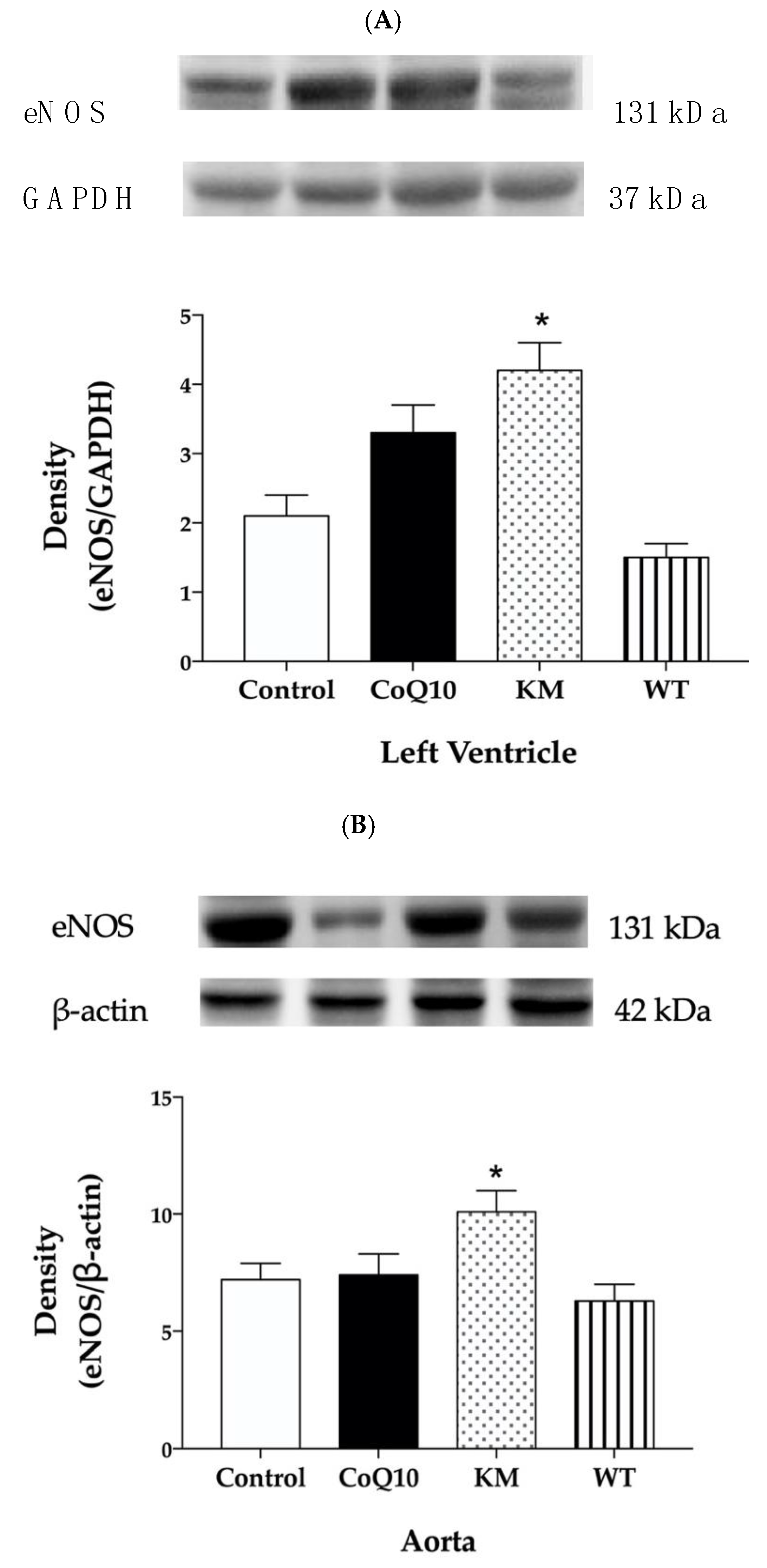
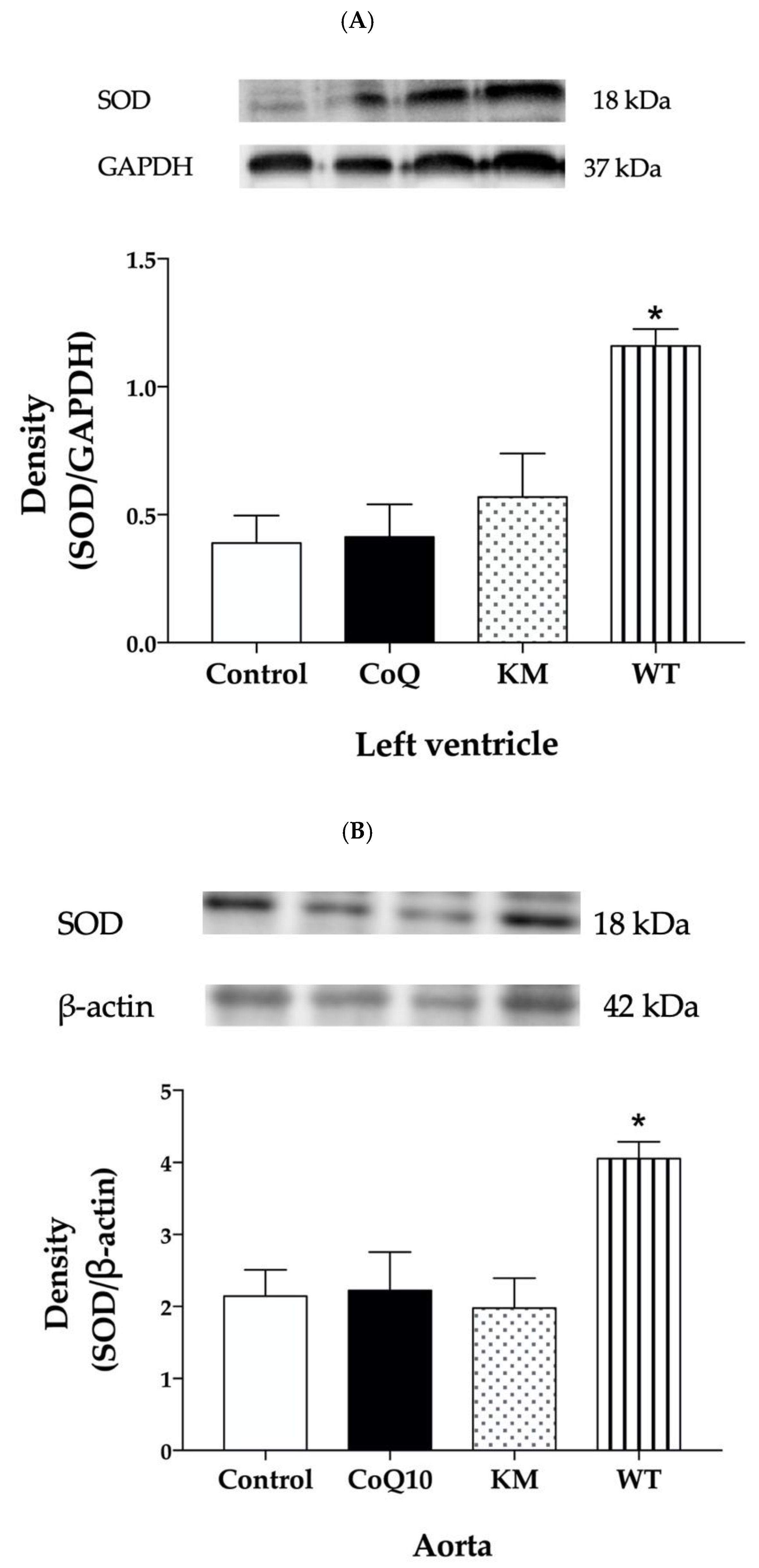
2.5. Protein Expressions of eNOS, NADPH Oxidase, and SOD
2.6. Conjugated Diene Concentration
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cornelian Cherry Preparation and Characterisation
4.3. Animals and Treatment
4.4. Plasma Lipid Profile
4.5. Total NOS Activity
4.6. Protein Expression Analysis by Western Blot
4.7. Conjugated Dienes Determination
4.8. Statistics
5. Conclusions
Sample availability
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, P.L. eNOS, metabolic syndrome and cardiovascular disease. Trends Endocrinol. Metab. 2009, 20, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Chin, K.Y.; Suh, F.H.; Fairus, A.; Ima-Nirwana, S. Animal models of metabolic syndrome: A review. Nutr. Metab. (Lond.) 2016, 13, 65. [Google Scholar] [CrossRef] [PubMed]
- Pechánová, O.; Varga, Z.V.; Cebová, M.; Giricz, Z.; Pacher, P.; Ferdinandy, P. Cardiac NO signalling in the metabolic syndrome. Br. J. Pharmacol. 2015, 172, 1415–1433. [Google Scholar] [CrossRef] [PubMed]
- Münzel, T.; Gori, T.; Bruno, R.M.; Taddei, S. Is oxidative stress a therapeutic target in cardiovascular disease? Eur. Heart J. 2010, 31, 2741–2749. [Google Scholar] [CrossRef] [PubMed]
- Cebova, M.; Rehakova, R.; Kosutova, M.; Pechanova, O. Simvastatin does not affect nitric oxide generation increased by sesame oil in obese Zucker rats. Oxid. Med. Cell. Longev. 2018, 2018, 5413423. [Google Scholar] [CrossRef]
- Bagetta, D.; Maruca, A.; Lupia, A.; Mesiti, F.; Catalano, R.; Romeo, I.; Moraca, F.; Ambrosio, F.A.; Costa, G.; Artese, A.; et al. Mediterranean products as promising source of multi-target agents in the treatment of metabolic syndrome. Eur. J. Med. Chem. 2020, 186, 111903. [Google Scholar] [CrossRef]
- Ramkumar, S.; Raghunath, A.; Raghunath, S. Statin therapy: Review of safety and potential side effects. Acta Cardiol. Sin. 2016, 32, 631–639. [Google Scholar]
- Dinda, B.; Kyriakopoulos, A.M.; Dinda, S.; Zoumpouris, V.; Thomaidis, N.S.; Velegraki, A.; Markopoulos, C.; Dinda, M. Cornus mas L. (cornelian cherry), an important European and Asian traditional food and medicine: Ethnomedicine, phytochemistry and pharmacology for Its commercial utilization in drug industry. J. Ethnopharmacol. 2016, 193, 670–690. [Google Scholar] [CrossRef]
- Gholamrezayi, A.; Yaghubi, E.; Ghafouri, A. A review of probable effects of cornelian cherry fruit. J. Biochem. Tech. 2019, 2, 71–74. [Google Scholar]
- Szczepaniak, O.M.; Kobus-Cisowska, J.; Kusek, W.; Przeor, M. Functional properties of Cornelian cherry (Cornus mas L.): A comprehensive review. Eur. Food Res. Technol. 2019, 245, 2071–2087. [Google Scholar] [CrossRef]
- Kazimierski, M.; Regula, J.; Molska, M. Cornelian cherry (Cornus mas L.)—characteristics, nutritional and pro-health properties. Acta Sci. Pol. Technol. Aliment. 2019, 18, 5–12. [Google Scholar] [PubMed]
- Gąstoł, M.; Krośniak, M.; Derwisz, M.; Dobrowolska-Iwanek, J. Cornelian cherry (Cornus mas L.) juice as a potential source of biological compounds. J. Med. Food 2013, 16, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Lietava, J.; Beerova, N.; Klymenko, S.V.; Panghyova, E.; Varga, I.; Pechanova, O. Effects of Cornelian cherry on atherosclerosis and its risk factors. Oxid. Med. Cell. Longev. 2019, 2019, 2515270. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, H. Antioxidant capacity and phytochemical properties of cornelian cherry (Cornus mas L.) genotypes in Iran. Sci. Hortic. 2011, 129, 459–463. [Google Scholar] [CrossRef]
- Narimani-Rad, M.; Zendehdel, M.; Abbasi, M.M.; Abdollahi, B.; Lotfi, A. Cornelian cherry (Cornus mas L.) extract affects glycemic status in Wistar rats. Bull. Environ. 2013, 2, 48–50. [Google Scholar]
- Mirbadalzadeh, R.; Shirdel, Z. Antihyperglycemic and antihyperlipidemic effects of Cornus mas extract in diabetic rats compared with glibenclamide. Horm. Signal. 2012, 47, 8969–8972. [Google Scholar]
- Asgary, S.; Rafieian-Kopaei, M.; Shamsi, F.; Najafi, S.; Sahebkar, A. Biochemical and histopathological study of the anti-hyperglycemic and anti-hyperlipidemic effects of cornelian cherry (Cornus mas L.) in alloxan-induced diabetic rats. J. Complement. Integr. Med. 2014, 11, 63–69. [Google Scholar] [CrossRef]
- Hosseinpour-Jaghdani, F.; Shomali, T.; Gholipour-Shahraki, S.; Rahimi-Madiseh, M.; Rafieian-Kopaei, M. Cornus mas: A review on traditional uses and pharmacological properties. J. Complement. Integr. Med. 2017, 14. [Google Scholar] [CrossRef]
- Asgary, S.; Rafieian-Kopaei, M.; Adelnia, A.; Kazemi, S.; Shamsi, F. Comparing the effects of lovastatin and Cornus mas fruit on fibrinogen level in hypercholesterolemic rabbits. ARYA Atheroscler. J. 2010, 6, 1–5. [Google Scholar]
- Jayaprakasam, B.; Olson, L.K.; Schutzki, R.E.; Tai, M.H.; Nair, M.G. Amelioration of obesity and glucose intolerance in high-fat-fed C57BL/6 mice by anthocyanins and ursolic acid in Cornelian cherry (Cornus mas). J. Agric. Food Chem. 2006, 54, 243–248. [Google Scholar] [CrossRef]
- Sozanski, T.; Kucharska, A.Z.; Szumny, A.; Magdalan, J.; Bielska, K.; Merwid-Lad, A.; Wozniak, A.; Dzimira, S.; Piorecki, N.; Trocha, M. The protective effect of the Cornus mas fruits (cornelian cherry) on hypertriglyceridemia and atherosclerosis through PPARα activation in hypercholesterolemic rabbits. Phytomedicine 2014, 21, 1774–1784. [Google Scholar] [CrossRef]
- Potgieter, M.; Pretorius, E.; Pepper, M.S. Primary and secondary coenzyme Q10 deficiency: The role of therapeutic supplementation. Nutr. Rev. 2013, 71, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.L.; Huang, Y.H.; Kao, C.L.; Yang, D.M.; Lee, H.C.; Chou, H.Y.; Chen, Y.C.; Chiou, G.Y.; Chen, L.H.; Yang, Y.P.; et al. A novel mechanism of coenzyme Q10 protects against human endothelial cells from oxidative stress-induced injury by modulating NO-related pathways. J. Nutr. Biochem. 2012, 23, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Perova, I.B.; Zhogova, A.A.; Poliakova, A.V.; Éller, K.I.; Ramenskaia, G.V.; Samylina, I.A. [Biologically active substances of cornelian cherry fruits (Cornus mas L.)]. Vopr. Pitan. 2014, 83, 86–94. [Google Scholar]
- Rasoulian, H.; Shahryar, H.A.; Abbaspour, R.; Lotfi, H. Effects of dietary inclusion of cornelian cherry (Cornus mas L.) fruit on body weight, insulin level and glycemic status of hamsters. Pak. J. Biol. Sci. 2012, 15, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Sozański, T.; Kucharska, A.Z.; Dzimira, S.; Magdalan, J.; Szumny, D.; Matuszewska, A.; Nowak, B.; Piórecki, N.; Szeląg, A.; Trocha, M. Loganic acid and anthocyanins from cornelian cherry (Cornus mas L.) fruits modulate diet-induced atherosclerosis and redox status in rabbits. Adv. Clin. Exp. Med. 2018, 27, 1505–1513. [Google Scholar]
- Alavian, S.M.; Banihabib, N.; Es Haghi, M.; Panahi, F. Protective Effect of Cornus mas Fruits Extract on Serum Biomarkers in CCl4-Induced Hepatotoxicity in Male Rats. Hepat. Mon. 2014, 14, e10330. [Google Scholar]
- Es Haghi, M.; Dehghan, G.; Banihabib, N.; Zare, S.; Mikaili, P.; Panahi, F. Protective effects of Cornus mas fruit extract on carbon tetrachloride induced nephrotoxicity in rats. Indian J. Nephrol. 2014, 24, 291–296. [Google Scholar]
- Sozański, T.; Kucharska, A.Z.; Wiśniewski, J.; Fleszar, M.G.; Rapak, A.; Gomułkiewicz, A.; Dzięgiel, P.; Magdalan, J.; Nowak, B.; Szumny, D.; et al. The iridoid loganic acid and anthocyanins from the cornelian cherry (Cornus mas L.) fruit increase the plasma l-arginine/ADMA ratio and decrease levels of ADMA in rabbits fed a high-cholesterol diet. Phytomedicine 2019, 52, 1–11. [Google Scholar] [CrossRef]
- Gowd, V.; Jia, Y.; Chen, W. Anthocyanins as promising molecules and dietary bioactive components against diabetes. A review of recent advances. Trends Food Sci. Technol. 2017, 68, 1–13. [Google Scholar] [CrossRef]
- Pechánová, O.; Bernátová, I.; Babál, P.; Martínez, M.C.; Kyselá, S.; Stvrtina, S.; Andriantsitohaina, R. Red wine polyphenols prevent cardiovascular alterations in L-NAME-induced hypertension. J. Hypertens. 2004, 22, 1551–1559. [Google Scholar] [CrossRef] [PubMed]
- Mazza, G.; Cacace, J.E.; Kay, C.D. Methods of analysis for anthocyanins in plants and biological fluids. AOAC Int. 2004, 87, 129–145. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Polyphenols and flavonoids: Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu Reagent. Methods Enzym. 1999, 299, 152–178. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berse, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


| Type | Total Polyphenols [mg/100 g] | Total Anthocyanidins [mg/100 g] | EC50 [g/L] |
|---|---|---|---|
| Koralovij Marka | 151.2 ± 18.1 | 41.9 ± 5.0 | 0.54 |
| Wild type | 408.4 ± 49.0 | 50.3 ± 6.0 | 0.98 |
| BW (g) | HW (g) | HW/TL (×10−2) | BP (mm-Hg) | |
|---|---|---|---|---|
| Control | 698.5 ± 20.4 | 1.33 ± 0.05 | 3.3 ± 0.1 | 147 ± 2.5 |
| CoQ10 | 639.8 ± 42.3 | 1.29 ± 0.05 | 3.1 ± 0.1 | 142 ± 2.3 |
| KM | 611.5 ± 15.0 ** | 1.29 ± 0.02 | 3.2 ± 0.07 | 143 ± 5.4 |
| WT | 664 ± 10.4 | 1.15 ± 0.02 * | 2.8 ± 0.03 ** | 137 ± 3.9 |
| TG (mmol/L) | CHOL (mmol/L) | HDL (mmol/L) | LDL (mmol/L) | |
|---|---|---|---|---|
| Control | 2.87 ± 0.21 | 7.65 ± 0.18 | 147.3 ± 10.1 | 70.9 ± 2.7 |
| CoQ10 | 2.91 ± 0.48 | 6.23 ± 0.52 | 143.2 ± 6.3 | 49.6 ± 4.1 |
| KM | 2.88 ± 0.42 | 6.17 ± 0.40 | 125.7 ± 3.9 | 46.9 ± 3.2 |
| WT | 2.77 ± 0.17 | 4.10 ± 0.33 * | 128.3 ± 4.4 | 27.0 ± 1.3 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dayar, E.; Cebova, M.; Lietava, J.; Panghyova, E.; Pechanova, O. Beneficial Effects of Cornelian Cherries on Lipid Profile and NO/ROS Balance in Obese Zucker Rats: Comparison with CoQ10. Molecules 2020, 25, 1922. https://doi.org/10.3390/molecules25081922
Dayar E, Cebova M, Lietava J, Panghyova E, Pechanova O. Beneficial Effects of Cornelian Cherries on Lipid Profile and NO/ROS Balance in Obese Zucker Rats: Comparison with CoQ10. Molecules. 2020; 25(8):1922. https://doi.org/10.3390/molecules25081922
Chicago/Turabian StyleDayar, Ezgi, Martina Cebova, Jan Lietava, Elena Panghyova, and Olga Pechanova. 2020. "Beneficial Effects of Cornelian Cherries on Lipid Profile and NO/ROS Balance in Obese Zucker Rats: Comparison with CoQ10" Molecules 25, no. 8: 1922. https://doi.org/10.3390/molecules25081922
APA StyleDayar, E., Cebova, M., Lietava, J., Panghyova, E., & Pechanova, O. (2020). Beneficial Effects of Cornelian Cherries on Lipid Profile and NO/ROS Balance in Obese Zucker Rats: Comparison with CoQ10. Molecules, 25(8), 1922. https://doi.org/10.3390/molecules25081922







