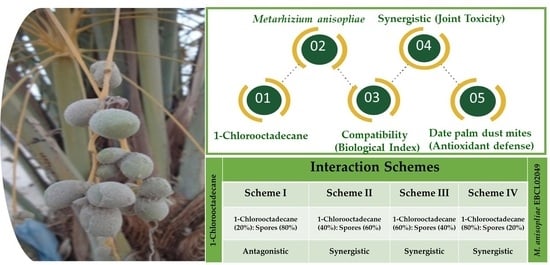
Potential Synergy between Spores of Metarhizium anisopliae and Plant Secondary Metabolite, 1-Chlorooctadecane for Effective Natural Acaricide Development
Abstract
1. Introduction
2. Results
2.1. Compatibility Bioassays
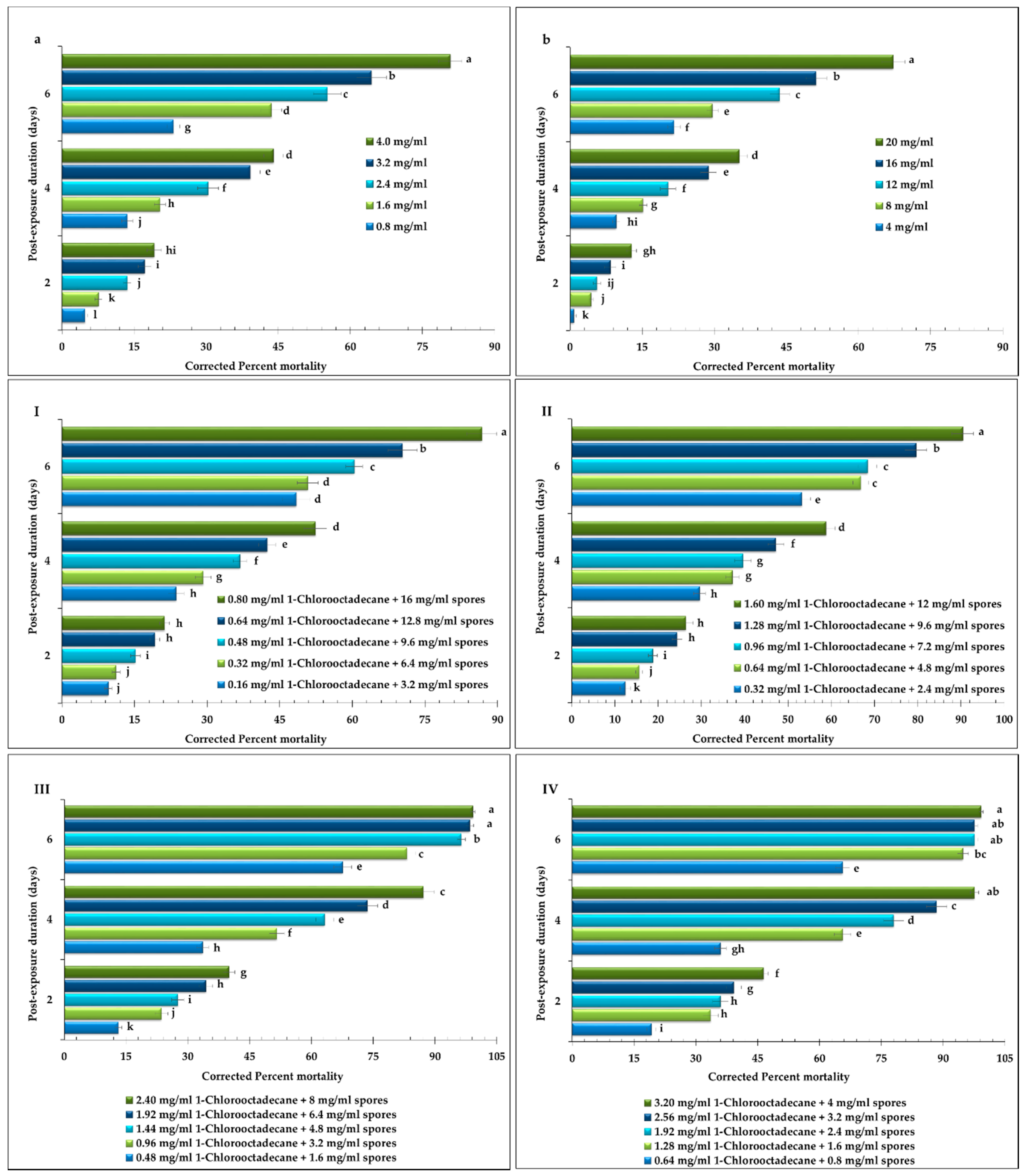
2.2. Screening Biossays
2.3. Host Antioxidant Defense Response
3. Discussion
4. Materials and Methods
4.1. Date Palm Dust Mites
4.2. M. anisopliae
4.3. 1-Chlorooctadecane
4.4. Compatibility of M. anisopliae Spores with 1-Chlorooctadecane
4.5. Laboratory Evaluation of M. anisopliae Spores with 1-Chlorooctadecane in Different Proportions against Date Palm Dust Mites
4.6. Exploration of Host Antioxidant Defense Response
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Diallo, H. The role of date palm in combat desertification. In The Date Palm: From Traditional Resource to Green Wealth; Emirates Centre for Strategic Studies and Research: Abu Dhabi, UAE, 2005; pp. 13–19. [Google Scholar]
- Bashah, M. Date Varieties in the Kingdom of Saudi Arabia. Guidance Booklet: Palms and Dates; King Abdulaziz University Press: Riyadh, Saudi Arabia, 1996. [Google Scholar]
- El-Hadrami, I.; El-Hadrami, A. Breeding Date Palm. In Breeding Plantation Tree Crops: Tropical Species; Springer: New York, NY, USA, 2009; pp. 191–216. [Google Scholar]
- FAOSTAT Food and Agricultural Commodities Production. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 26 February 2020).
- Chaaban, S.B.; Chermiti, B.; Kreiter, S. Comparative demography of the spider mite, Oligonychus afrasiaticus, on four date palm varieties in Southwestern Tunisia. J. Insect Sci. 2011, 11, Article 136. [Google Scholar] [CrossRef]
- Amer, S.A.A.; Refaat, A.M.; Momen, F.M. Repellent and oviposition-deterring activity of Rosemary and Sweet Marjoram on the spider mites Tetranychus urticae and Eutetranychus orientalis (Acari: Tetranychidae). Acta Phytopathol. Entomol. Hungarica 2001, 36, 155–164. [Google Scholar]
- Reddy, G.; Srinivasa, N.; Muralidhara, M.S. Potentiality of Cinnamomum extracts to two spotted spider mite, Tetranychus urticae Koch and its predator Neoseiulus longispinosus (Evans). J. Biopestic. 2014, 7, 1–11. [Google Scholar]
- Chiasson, H.; Bélanger, A.; Bostanian, N.; Vincent, C.; Poliquin, A. Acaricidal properties of Artemisia absinthium and Tanacetum vulgare (Asteraceae) essential oils obtained by three methods of extraction. J. Econ. Entomol. 2001, 94, 167–171. [Google Scholar] [CrossRef]
- Idrees, A.; Qasim, M.; Ali, H.; Qadir, Z.A.; Idrees, A.; Qing, J. Acaricidal potential of some botanicals against the stored grain mites, Rhizoglyphus tritici. J. Entomol. Zool. Stud. 2016, 4, 611–617. [Google Scholar]
- Elkertati, M.; Blenzar, A.; Jotei, A.B.; Belkoura, I.; Tazi, B. Acaricide effect of some extracts and fractions on Tetranychus urticae Koch (Acari: Tetranychidae). African J. Agric. Res. 2013, 8, 2970–2976. [Google Scholar]
- Bussaman, P.; Sa-uth, C.; Rattanasena, P.; Chandrapatya, A. Effect of crude plant extracts on Mushroom mite, Luciaphorus sp. (Acari: Pygmephoridae). Psyche A J. Entomol. 2012, 2012, Article ID 150958. [Google Scholar] [CrossRef]
- Miresmailli, S.; Bradbury, R.; Isman, M.B. Comparative toxicity of Rosmarinus officinalis L. essential oil and blends of its major constituents against Tetranychus urticae Koch (Acari: Tetranychidae) on two different host plants. Pest Manag. Sci. 2006, 62, 366–371. [Google Scholar] [CrossRef]
- Hussain, A.; Tian, M.Y.; He, Y.R.; Ahmed, S. Entomopathogenic fungi disturbed the larval growth and feeding performance of Ocinara varians (Lepidoptera: Bombycidae) larvae. Insect Sci. 2009, 16, 511–517. [Google Scholar] [CrossRef]
- Hussain, A.; Tian, M.Y.; He, Y.R.; Lei, Y.Y. Differential fluctuation in virulence and VOC profiles among different cultures of entomopathogenic fungi. J. Invertebr. Pathol. 2010, 104, 166–171. [Google Scholar] [CrossRef]
- Hussain, A.; Tian, M.Y.; Wen, S.-Y. Proteomic analysis of Formosan Subterranean Termites during exposure to entomopathogenic fungi. Curr. Proteomics 2018, 15, 229–240. [Google Scholar] [CrossRef]
- Hussain, A.; Rizwan-ul-haq, M.; AlJabr, A.M.; Al-Ayedh, H. Host-pathogen interaction for screening potential of Metarhizium anisopliae isolates against the date-palm dust mite, Oligonychus afrasiaticus (McGregor) (Acari: Tetranychidae). Egypt. J. Biol. Pest Control 2019, 29, 63. [Google Scholar] [CrossRef]
- Kristiani, E.B.E.; Nugroho, L.H.; Moeljopawiro, S.; Widyarini, S. Characterization of volatile compounds of Albertisia papuana Becc root extracts and cytotoxic activity in breast cancer cell line T47D. Trop. J. Pharm. Res. 2016, 15, 959–964. [Google Scholar] [CrossRef][Green Version]
- Swami, S.B.; Thakor, N.S.J.; Patil, M.M.; Haldankar, P.M. Jamun (Syzygium cumini (L.)): A review of its food and medicinal uses. Food Nutr. Sci. 2012, 3, 1100–1117. [Google Scholar]
- Li, G.; Jiang, Y.; Li, Y.; He, T.; Wang, Y.; Ji, T.; Zhai, W.; Zhao, L.; Zhou, X. Analysis and biological evaluation of Arisaema amuremse Maxim essential oil. Open Chem. 2019, 17, 647–654. [Google Scholar] [CrossRef]
- Siddiquee, S.; Cheong, B.E.; Taslima, K.; Kausar, H.; Hasan, M.M. Separation and identification of volatile compounds from liquid cultures of Trichoderma harzianum by GC-MS using three different Capillary Columns. J. Chromatogr. Sci. 2012, 50, 358–367. [Google Scholar] [CrossRef]
- Fetoh, B.; Al-Shammery, K. Acaricidal ovicial and repellent activities of some plant extracts on the date palm dust mite, Oligonychus afrasiaticus Meg. (Acari: Tetranychidae). Int. J. Environ. Sci. Eng. 2011, 2, 45–52. [Google Scholar]
- Lakhdari, W.; Dehliz, A.; Acheuk, F.; Soud, A.; Hammi, H.; Mlik, R.; Doumandji-Mitiche, B. Acaricidal activity of aqueous extracts against the mite of date palm Oligonychus afrasiaticus Meg (Acari: Tetranychidae). J. Med. Plants 2015, 3, 113–117. [Google Scholar]
- AlJabr, A.; Hussain, A.; Rizwan-ul-haq, M. Toxin-Pathogen synergy reshaping detoxification and antioxidant defense mechanism of Oligonychus afrasiaticus (McGregor). Molecules 2018, 23, 1978. [Google Scholar] [CrossRef]
- Hussain, A.; Rizwan-ul-Haq, M.; AlJabr, A.M.; Al-Ayedh, H. Evaluation of host–pathogen interactions for selection of entomopathogenic fungal isolates against Oligonychus afrasiaticus (McGregor). BioControl 2020, 65, 185–195. [Google Scholar] [CrossRef]
- Shi, W.-B.; Zhang, L.; Feng, M.-G. Time-concentration-mortality responses of carmine spider mite (Acari: Tetranychidae) females to three hypocrealean fungi as biocontrol agents. Biol. Control 2008, 46, 495–501. [Google Scholar] [CrossRef]
- Gonçalves Diniz, A.; Barbosa, L.F.S.; da Silva Santos, A.C.; de Oliveira, N.T.; da Costa, A.F.; Carneiro-Leão, M.P.; Tiago, P.V. Bio-insecticide effect of isolates of Fusarium caatingaense (Sordariomycetes: Hypocreales) combined to botanical extracts against Dactylopius opuntiae (Hemiptera: Dactylopiidae). Biocontrol Sci. Technol. 2020, 30, 1–12. [Google Scholar]
- Wraight, S.P.; Filotas, M.J.; Sanderson, J.P. Comparative efficacy of emulsifiable-oil, wettable-powder, and unformulated-powder preparations of Beauveria bassiana against the melon aphid Aphis gossypii. Biocontrol Sci. Technol. 2016, 26, 894–914. [Google Scholar] [CrossRef]
- Hernández, M.M.; Martínez-Villar, E.; Peace, C.; Pérez-Moreno, I.; Marco, V. Compatibility of the entomopathogenic fungus Beauveria bassiana with flufenoxuron and azadirachtin against Tetranychus urticae. Exp. Appl. Acarol. 2012, 58, 395–405. [Google Scholar] [CrossRef]
- Hussain, A.; Rizwan-ul-Haq, M.; Al-Ayedh, H.; AlJabr, A. Susceptibility and immune defence mechanisms of Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) against entomopathogenic fungal infections. Int. J. Mol. Sci. 2016, 17, 1518. [Google Scholar] [CrossRef]
- Hussain, A.; Ahmed, S.; Shahid, M. Laboratory and field evaluation of Metarhizium anisopliae var. anisopliae for controlling subterranean termites. Neotrop. Entomol. 2011, 40, 244–250. [Google Scholar]
- Hussain, A. Reprogramming the virulence: Insect defense molecules navigating the epigenetic landscape of Metarhizium robertsii. Virulence 2018, 9, 447–449. [Google Scholar] [CrossRef]
- Hussain, A.; Rizwan-ul-Haq, M.; Al-Ayedh, H.; Al-Jabr, A. Mycoinsecticides: Potential and future perspective. Recent Pat. Food. Nutr. Agric. 2014, 6, 45–53. [Google Scholar] [CrossRef]
- Hussain, A.; Tian, M.Y. Germination pattern and inoculum transfer of entomopathogenic fungi and their role in disease resistance among Coptotermes formosanus (Isoptera: Rhinotermitidae). Int. J. Agric. Biol. 2013, 15, 319–324. [Google Scholar]
- Wink, M. Plant secondary metabolites modulate insect behavior-steps toward addiction? Front. Physiol. 2018, 9, 364. [Google Scholar] [CrossRef]
- Wink, M. Interference of alkaloids with neuroreceptors and ion channels. Stud. Nat. Prod. Chem. 2000, 21, 3–122. [Google Scholar]
- AlJabr, A.M.; Hussain, A.; Rizwan-ul-Haq, M.; Al-Ayedh, H. Toxicity of Plant Secondary Metabolites Modulating Detoxification Genes Expression for Natural Red Palm Weevil Pesticide Development. Molecules 2017, 22, 169. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Rizwan-ul-haq, M.; AlJabr, A.M.; Al-Ayedh, H. Lethality of Sesquiterpenes Reprogramming Red Palm Weevil Detoxification Mechanism for Natural Novel Biopesticide Development. Molecules 2019, 24, 1648. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Rizwan-ul-Haq, M.; Al-Ayedh, H.; Aljabr, A.M. Toxicity and detoxification mechanism of black pepper and its major constituent in controlling Rhynchophorus ferrugineus Olivier (Curculionidae: Coleoptera). Neotrop. Entomol. 2017, 46, 685–693. [Google Scholar] [CrossRef]
- Sáenz-de-Cabezón Irigaray, F.J.; Marco-Mancebón, V.; Pérez-Moreno, I. The entomopathogenic fungus Beauveria bassiana and its compatibility with triflumuron: Effects on the twospotted spider mite Tetranychus urticae. Biol. Control 2003, 26, 168–173. [Google Scholar] [CrossRef]
- Zou, C.; Li, L.; Dong, T.; Zhang, B.; Hu, Q. Joint action of the entomopathogenic fungus Isaria fumosorosea and four chemical insecticides against the whitefly Bemisia tabaci. Biocontrol Sci. Technol. 2014, 24, 315–324. [Google Scholar] [CrossRef]
- Akbar, W.; Lord, J.C.; Nechols, J.R.; Loughin, T.M. Efficacy of Beauveria bassiana for red flour beetle when applied with plant essential oils or in mineral oil and organosilicone carriers. J. Econ. Entomol. 2005, 98, 683–688. [Google Scholar] [CrossRef]
- Lu, F.; Liang, X.; Lu, H.; Li, Q.; Chen, Q.; Zhang, P.; Li, K.; Liu, G.; Yan, W.; Song, J.; et al. Overproduction of superoxide dismutase and catalase confers cassava resistance to Tetranychus cinnabarinus. Sci. Rep. 2017, 7, 40179. [Google Scholar] [CrossRef]
- Deisseroth, A.; Dounce, A.L. Catalase: Physical and chemical properties, mechanism of catalysis, and physiological role. Physiol. Rev. 1970, 50, 319–375. [Google Scholar] [CrossRef]
- Hussain, A.; Tian, M.Y.; He, Y.R.; Ruan, L.; Ahmed, S. In vitro and in vivo culturing impacts on the virulence characteristics of serially passed entomopathogenic fungi. J. Food Agric. Environ. 2010, 8, 481–487. [Google Scholar]
- Hussain, A.; Tian, M.-Y.; Wen, S.-Y. Exploring the caste-specific multi-layer defense mechanism of Formosan Subterranean Termites, Coptotermes formosanus Shiraki. Int. J. Mol. Sci. 2017, 18, 2694. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, V.; Poehling, H.-M. In vitro effect of pesticides on the germination, vegetative growth, and conidial production of two strains of Metarhizium anisopliae. Fungal Biol. 2012, 116, 121–132. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- StatsDirect StatsDirect Statistical Software. England. StatsDirect Ltd., 2013. Available online: http://www.statsdirect.com (accessed on 25 February 2020).
- Statistix Statistix 8.1; Analytical Software: Tallahassee, FL, USA, 2003.
- Sun, Y.-P.; Johnson, E.R. Analysis of joint action of insecticides against House flies. J. Econ. Entomol. 1960, 53, 887–892. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- SAS Institute SAS User’s Guide: Statistics; SAS Institute: Cary, NC, USA, 2000.
Sample Availability: Samples of the compound, 1-Chlorooctadecane and Metarhizium anisopliae EBCL 02049 are available from the authors. |
| Treatments | Vegetative Growth (mm) 1 | Germination (%) 1 | Sporulation (×106 Spores/mL) 1 | Biological Index | Classification 2 |
|---|---|---|---|---|---|
| Control | 85.70 ± 2.25a | 98.70 ± 0.79a | 7.80 ± 0.63a | - | - |
| 0.8 mg/mL | 84.40 ± 2.28ab | 98.20 ± 0.99a | 7.30 ± 0.60ab | 96.48 | Compatible |
| 1.6 mg/mL | 84.20 ± 21.69ab | 97.90 ± 1.03a | 6.40 ± 0.54abc | 91.38 | Compatible |
| 2.4 mg/mL | 79.30 ± 1.82bc | 97.30 ± 0.71a | 6.20 ± 0.61bc | 87.53 | Compatible |
| 3.2 mg/mL | 78.10 ± 2.11c | 96.70 ± 0.93a | 5.60 ± 0.40c | 83.50 | Compatible |
| 4.0 mg/mL | 77.70 ± 1.54c | 97.20 ± 0.81a | 5.50 ± 0.34c | 82.78 | Compatible |
| Combinations | LC50 (mg/mL) | Joint Toxicity | Interaction 1 |
|---|---|---|---|
| Scheme I: 20% 1-Chlorooctadecane: 80% Spores | |||
| 0.16 mg/mL 1-Chlorooctadecane + 3.2 mg/mL spores | 8.75 (7.10–10.75) | 47 | Antagonistic |
| 0.32 mg/mL 1-Chlorooctadecane + 6.4 mg/mL spores | |||
| 0.48 mg/mL 1-Chlorooctadecane + 9.6 mg/mL spores | |||
| 0.64 mg/mL 1-Chlorooctadecane + 12.8 mg/mL spores | |||
| 0.80 mg/mL 1-Chlorooctadecane + 16.0 mg/mL spores | |||
| Scheme II: 40% 1-Chlorooctadecane: 60% Spores | |||
| 0.32 mg/mL 1-Chlorooctadecane + 2.4 mg/mL spores | 4.62 (3.41–5.65) | 112 | Synergistic |
| 0.64 mg/mL 1-Chlorooctadecane + 4.8 mg/mL spores | |||
| 0.96 mg/mL 1-Chlorooctadecane + 7.2 mg/mL spores | |||
| 1.28 mg/mL 1-Chlorooctadecane + 9.6 mg/mL spores | |||
| 1.60 mg/mL 1-Chlorooctadecane + 12 mg/mL spores | |||
| Scheme III: 60% 1-Chlorooctadecane: 40% Spores | |||
| 0.48 mg/mL 1-Chlorooctadecane + 1.6 mg/mL spores | 2.39 (2.00–2.72) | 289 | Synergistic |
| 0.96 mg/mL 1-Chlorooctadecane + 3.2 mg/mL spores | |||
| 1.44 mg/mL 1-Chlorooctadecane + 4.8 mg/mL spores | |||
| 1.92 mg/mL 1-Chlorooctadecane + 6.4 mg/mL spores | |||
| 2.40 mg/mL 1-Chlorooctadecane + 8.0 mg/mL spores | |||
| Scheme IV: 80% 1-Chlorooctadecane: 20% Spores | |||
| 0.64 mg/mL 1-Chlorooctadecane + 0.8 mg/mL spores | 1.47 (1.24–1.67) | 713 | Synergistic |
| 1.28 mg/mL 1-Chlorooctadecane + 1.6 mg/mL spores | |||
| 1.92 mg/mL 1-Chlorooctadecane + 2.4 mg/mL spores | |||
| 2.56 mg/mL 1-Chlorooctadecane + 3.2 mg/mL spores | |||
| 3.20 mg/mL 1-Chlorooctadecane + 4.0 mg/mL spores | |||
| Treatments | Post-Exposure Duration | ||
|---|---|---|---|
| 24 h 1 | 72 h 1 | 120 h 1 | |
| 1-Chlorooctadecane | (%) | (%) | (%) |
| 0.8 mg/mL | 13.35 ± 0.55p | 32.18 ± 0.34m | 46.03 ± 0.80g |
| 1.6 mg/mL | 20.32 ± 1.02lm | 35.56 ± 0.49l | 39.59 ± 0.88i |
| 2.4 mg/mL | 21.17 ± 1.03kl | 40.09 ± 0.69ij | 23.27 ± 0.44l |
| 3.2 mg/mL | 24.38 ± 1.18hi | 46.16 ± 0.97g | 17.13 ± 0.34op |
| 4.0 mg/mL | 27.14 ± 1.32fg | 52.86 ± 0.92d | 15.14 ± 0.25q |
| Scheme I: 20% 1-Chlorooctadecane: 80% Spores | |||
| 0.16 mg/mL 1-Chlorooctadecane + 3.2 mg/mL spores | 15.04 ± 0.21op | 31.52 ± 0.37m | 60.78 ± 0.94b |
| 0.32 mg/mL 1-Chlorooctadecane + 6.4 mg/mL spores | 16.38 ± 0.24no | 39.64 ± 0.59j | 53.88 ± 0.98e |
| 0.48 mg/mL 1-Chlorooctadecane + 9.6 mg/mL spores | 20.22 ± 0.54lm | 44.17 ± 0.72gh | 56.42 ± 0.93d |
| 0.64 mg/mL 1-Chlorooctadecane + 12.8 mg/mL spores | 21.19 ± 0.78kl | 50.11 ± 0.95ef | 52.78 ± 0.95e |
| 0.80 mg/mL 1-Chlorooctadecane + 16.0 mg/mL spores | 25.15 ± 0.92ghi | 43.11 ± 0.84h | 41.46 ± 0.76h |
| Scheme II: 40% 1-Chlorooctadecane: 60% Spores | |||
| 0.32 mg/mL 1-Chlorooctadecane + 2.4 mg/mL spores | 20.32 ± 0.31lm | 35.30 ± 0.55l | 40.03 ± 0.64hi |
| 0.64 mg/mL 1-Chlorooctadecane + 4.8 mg/mL spores | 22.06 ± 0.70jkl | 36.50 ± 0.57l | 29.74 ± 0.55j |
| 0.96 mg/mL 1-Chlorooctadecane + 7.2 mg/mL spores | 27.15 ± 0.72fg | 39.68 ± 0.78j | 24.09 ± 0.50l |
| 1.28 mg/mL 1-Chlorooctadecane + 9.6 mg/mL spores | 30.01 ± 0.90de | 48.45 ± 0.91f | 20.10 ± 0.24m |
| 1.60 mg/mL 1-Chlorooctadecane + 12 mg/mL spores | 34.18 ± 0.92bc | 52.93 ± 0.93d | 15.20 ± 0.20q |
| Scheme III: 60% 1-Chlorooctadecane: 40% Spores | |||
| 0.48 mg/mL 1-Chlorooctadecane + 1.6 mg/mL spores | 20.10 ± 0.24lm | 35.69 ± 0.46l | 26.54 ± 0.72k |
| 0.96 mg/mL 1-Chlorooctadecane + 3.2 mg/mL spores | 24.19 ± 0.60hij | 37.43 ± 0.56kl | 24.19 ± 0.60l |
| 1.44 mg/mL 1-Chlorooctadecane + 4.8 mg/mL spores | 29.12 ± 0.78ef | 42.02 ± 0.70hi | 20.29 ± 0.39m |
| 1.92 mg/mL 1-Chlorooctadecane + 6.4 mg/mL spores | 32.22 ± 0.87cd | 55.90 ± 0.76c | 17.44 ± 0.32no |
| 2.40 mg/mL 1-Chlorooctadecane + 8.0 mg/mL spores | 33.22 ± 0.90bc | 63.85 ± 0.93b | 09.79 ± 0.26r |
| Scheme IV: 80% 1-Chlorooctadecane: 20% Spores | |||
| 0.64 mg/mL 1-Chlorooctadecane + 0.8 mg/mL spores | 23.08 ± 0.57ijk | 38.92 ± 0.57jk | 15.58 ± 0.28pq |
| 1.28 mg/mL 1-Chlorooctadecane + 1.6 mg/mL spores | 29.37 ± 0.64ef | 43.35 ± 0.69h | 09.67 ± 0.25r |
| 1.92 mg/mL 1-Chlorooctadecane + 2.4 mg/mL spores | 33.02 ± 0.81bc | 50.16 ± 0.79ef | 03.58 ± 0.24s |
| 2.56 mg/mL 1-Chlorooctadecane + 3.2 mg/mL spores | 35.12 ± 0.93b | 63.96 ± 0.91b | 01.79 ± 0.15t |
| 3.20 mg/mL 1-Chlorooctadecane + 4.0 mg/mL spores | 39.32 ± 1.22a | 71.64 ± 1.05a | 01.24 ± 0.13t |
| M. anisopliae EBCL02049 spores | |||
| 4 mg/mL | 15.34 ± 0.43op | 49.13 ± 0.81f | 58.19 ± 0.81c |
| 8 mg/mL | 18.27 ± 0.56mn | 57.16 ± 0.98c | 63.83 ± 0.77a |
| 12 mg/mL | 21.08 ± 0.78kl | 51.35 ± 0.97de | 48.38 ± 0.97f |
| 16 mg/mL | 23.26 ± 0.88ijk | 43.67 ± 0.93h | 28.07 ± 0.62jk |
| 20 mg/mL | 26.17 ± 0.91gh | 36.26 ± 0.68l | 19.13 ± 0.60mn |
| Treatments | Post-Exposure Duration | ||
|---|---|---|---|
| 24 h 1 | 72 h 1 | 120 h 1 | |
| 1-Chlorooctadecane | (%) | (%) | (%) |
| 0.8 mg/mL | 12.89 ± 0.53kl | 30.38 ± 0.85q | 66.27 ± 1.19bc |
| 1.6 mg/mL | 20.42 ± 0.78h | 36.15 ± 0.90op | 57.13 ± 1.21d |
| 2.4 mg/mL | 21.20 ± 0.87h | 43.85 ± 0.93l | 37.31 ± 0.65h |
| 3.2 mg/mL | 26.87 ± 1.05d | 54.04 ± 1.27g | 31.36 ± 0.45ij |
| 4.0 mg/mL | 29.65 ± 1.29c | 66.10 ± 1.35c | 19.77 ± 0.36lm |
| Scheme I: 20% 1-Chlorooctadecane: 80% Spores | |||
| 0.16 mg/mL 1-Chlorooctadecane + 3.2 mg/mL spores | 11.17 ± 0.27l | 53.13 ± 0.97gh | 76.13 ± 1.01a |
| 0.32 mg/mL 1-Chlorooctadecane + 6.4 mg/mL spores | 13.33 ± 0.42k | 61.18 ± 0.99e | 68.07 ± 0.98b |
| 0.48 mg/mL 1-Chlorooctadecane + 9.6 mg/mL spores | 18.13 ± 0.50j | 52.54 ± 1.04gh | 57.30 ± 1.11d |
| 0.64 mg/mL 1-Chlorooctadecane + 12.8 mg/mL spores | 20.11 ± 0.76hi | 46.57 ± 0.95j | 40.43 ± 0.69g |
| 0.80 mg/mL 1-Chlorooctadecane + 16.0 mg/mL spores | 21.32 ± 0.85gh | 29.46 ± 0.50q | 25.30 ± 0.58k |
| Scheme II: 40% 1-Chlorooctadecane: 60% Spores | |||
| 0.32 mg/mL 1-Chlorooctadecane + 2.4 mg/mL spores | 14.17 ± 0.61k | 37.27 ± 0.61o | 43.08 ± 0.82f |
| 0.64 mg/mL 1-Chlorooctadecane + 4.8 mg/mL spores | 21.20 ± 0.63h | 49.65 ± 0.81i | 44.12 ± 0.96f |
| 0.96 mg/mL 1-Chlorooctadecane + 7.2 mg/mL spores | 25.42 ± 0.91de | 58.40 ± 1.18f | 39.80 ± 0.88g |
| 1.28 mg/mL 1-Chlorooctadecane + 9.6 mg/mL spores | 27.07 ± 0.93d | 61.44 ± 1.20e | 25.46 ± 0.51k |
| 1.60 mg/mL 1-Chlorooctadecane + 12.0 mg/mL spores | 30.11 ± 1.02c | 64.55 ± 1.25cd | 21.42 ± 0.50l |
| Scheme III: 60% 1-Chlorooctadecane: 40% Spores | |||
| 0.48 mg/mL 1-Chlorooctadecane + 1.6 mg/mL spores | 17.21 ± 0.39j | 41.14 ± 0.77mn | 36.26 ± 0.68h |
| 0.96 mg/mL 1-Chlorooctadecane + 3.2 mg/mL spores | 23.10 ± 0.65fg | 44.39 ± 0.85kl | 29.32 ± 0.63j |
| 1.44 mg/mL 1-Chlorooctadecane + 4.8 mg/mL spores | 24.27 ± 0.70ef | 54.25 ± 1.03g | 25.37 ± 0.53k |
| 1.92 mg/mL 1-Chlorooctadecane + 6.4 mg/mL spores | 29.22 ± 0.95c | 63.76 ± 1.24d | 20.54 ± 0.43l |
| 2.40 mg/mL 1-Chlorooctadecane + 8.0 mg/mL spores | 34.20 ± 1.02a | 72.26 ± 1.25b | 16.28 ± 0.25n |
| Scheme IV: 80% 1-Chlorooctadecane: 20% Spores | |||
| 0.64 mg/mL 1-Chlorooctadecane + 0.8 mg/mL spores | 23.38 ± 0.52f | 43.15 ± 0.81lm | 32.41 ± 0.52i |
| 1.28 mg/mL 1-Chlorooctadecane + 1.6 mg/mL spores | 25.32 ± 0.70de | 53.19 ± 0.91gh | 26.42 ± 0.43k |
| 1.92 mg/mL 1-Chlorooctadecane + 2.4 mg/mL spores | 26.19 ± 0.98d | 60.45 ± 0.99ef | 21.24 ± 0.37l |
| 2.56 mg/mL 1-Chlorooctadecane + 3.2 mg/mL spores | 32.34 ± 1.12b | 66.23 ± 1.10c | 18.03 ± 0.52mn |
| 3.20 mg/mL 1-Chlorooctadecane + 4.0 mg/mL spores | 35.41 ± 1.18a | 81.28 ± 1.26a | 11.80 ± 0.37o |
| M. anisopliae EBCL02049 spores | |||
| 4 mg/mL | 09.28 ± 0.34m | 34.20 ± 0.79p | 56.53 ± 0.93d |
| 8 mg/mL | 17.11 ± 0.48j | 40.14 ± 0.88n | 65.87 ± 1.03c |
| 12 mg/mL | 18.41 ± 0.58ij | 46.43 ± 0.92jk | 54.14 ± 0.97e |
| 16 mg/mL | 20.13 ± 0.66hi | 51.24 ± 0.98hi | 43.75 ± 0.99f |
| 20 mg/mL | 23.16 ± 0.89fg | 60.24 ± 1.14ef | 25.46 ± 0.72k |
| 1-Chlorooctadecane (mg/mL) | Interaction Schemes | M. anisopliae (mg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Scheme I | Scheme II | Scheme III | Scheme IV | ||||||
| 1-Chlorooctadecane (20%): Spores (80%) | 1-Chlorooctadecane (40%): Spores (60%) | 1-Chlorooctadecane (60%): Spores (40%) | 1-Chlorooctadecane (80%): Spores (20%) | ||||||
| Toxin mg/mL | Spores mg/mL | Toxin mg/mL | Spores mg/mL | Toxin mg/mL | Spores mg/mL | Toxin mg/mL | Spores mg/mL | ||
| 0.8 | 0.16 | 3.2 | 0.32 | 2.4 | 0.48 | 1.6 | 0.64 | 0.8 | 4 |
| 1.6 | 0.32 | 6.4 | 0.64 | 4.8 | 0.96 | 3.2 | 1.28 | 1.6 | 8 |
| 2.4 | 0.48 | 9.6 | 0.96 | 7.2 | 1.44 | 4.8 | 1.92 | 2.4 | 12 |
| 3.2 | 0.64 | 12.8 | 1.28 | 9.6 | 1.92 | 6.4 | 2.56 | 3.2 | 16 |
| 4 | 0.8 | 16 | 1.6 | 12 | 2.4 | 8 | 3.2 | 4 | 20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, A.; AlJabr, A.M. Potential Synergy between Spores of Metarhizium anisopliae and Plant Secondary Metabolite, 1-Chlorooctadecane for Effective Natural Acaricide Development. Molecules 2020, 25, 1900. https://doi.org/10.3390/molecules25081900
Hussain A, AlJabr AM. Potential Synergy between Spores of Metarhizium anisopliae and Plant Secondary Metabolite, 1-Chlorooctadecane for Effective Natural Acaricide Development. Molecules. 2020; 25(8):1900. https://doi.org/10.3390/molecules25081900
Chicago/Turabian StyleHussain, Abid, and Ahmed Mohammed AlJabr. 2020. "Potential Synergy between Spores of Metarhizium anisopliae and Plant Secondary Metabolite, 1-Chlorooctadecane for Effective Natural Acaricide Development" Molecules 25, no. 8: 1900. https://doi.org/10.3390/molecules25081900
APA StyleHussain, A., & AlJabr, A. M. (2020). Potential Synergy between Spores of Metarhizium anisopliae and Plant Secondary Metabolite, 1-Chlorooctadecane for Effective Natural Acaricide Development. Molecules, 25(8), 1900. https://doi.org/10.3390/molecules25081900