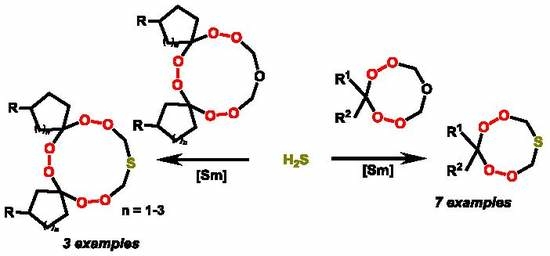
First Example of Catalytic Synthesis of Cyclic S-Containing Di- and Triperoxides
Abstract
1. Introduction
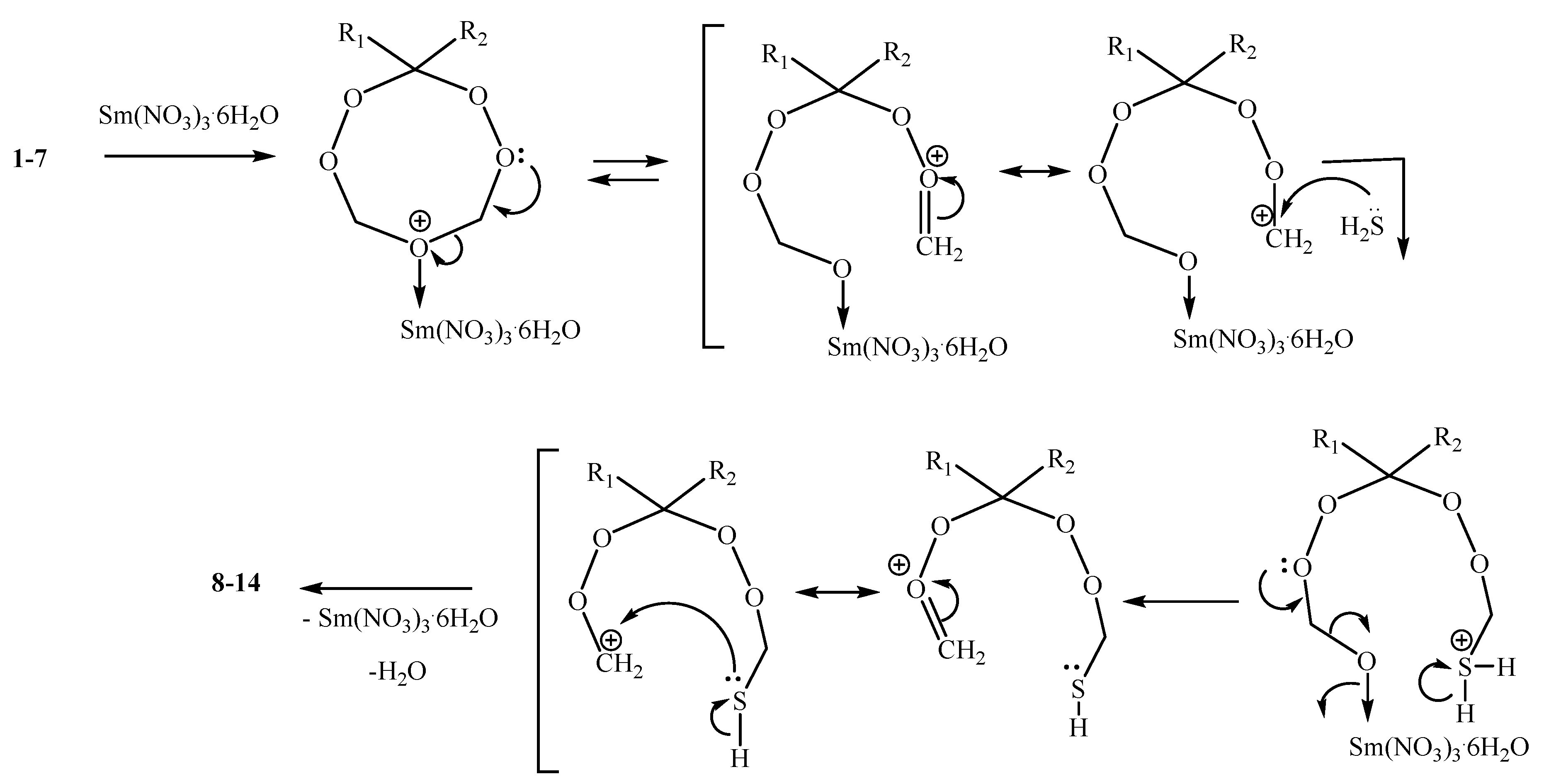
2. Results and Discussion
2.1. Chemistry
2.2. Biological Evaluation
3. Materials and Methods
3.1. Chemistry
3.1.1. Reactions of Pentaoxacanes with Hydrogen Sulfide in the Presence of a Catalyst, Sm(NO3)3·6H2O
3.1.2. Reactions Heptaoxadispiroalkanes with Hydrogen Sulfide in Presence of a Catalyst, Sm(NO3)3·6H2O
3.2. Biology
3.2.1. Cell Culturing
3.2.2. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yamazaki, M.; Suzuki, S.; Miyaki, K. Tremorgenic toxins from Aspergillus fumigatus Fres. Chem. Pharm. Bull. 1971, 19, 1739–1740. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, M.; Fujimoto, H.; Kawasaki, T. Chemistry of tremorogenic metabolites. I. Fumitremorgin A from Aspergillus fumigatus. Chem. Pharm. Bull. 1980, 28, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Cole, R.J.; Kirksey, J.W. Mycotoxin verruculogen. 6-O-Methylindole. J. Agric. Food Chem. 1973, 21, 927–929. [Google Scholar] [CrossRef] [PubMed]
- Fayos, J.; Lokensgard, D.; Clardy, J.; Cole, R.J.; Kirksey, J.W. Structure of verruculogen, a tremor producing peroxide from Penicillium verruculosum. J. Am. Chem. Soc. 1974, 96, 6785–6787. [Google Scholar] [CrossRef] [PubMed]
- Cole, R.J.; Kirksey, J.W.; Cox, R.H.; Clardy, J. Structure of the tremor-producing indole, TR-2. J. Agric. Food Chem. 1975, 23, 1015–1018. [Google Scholar] [CrossRef]
- Yoshizawa, T.; Morooka, N.; Sawada, Y.; Udagawa, S. Host specificity of filamentous, segmented microorganisms adherent to the small bowel epithelium in mice and race. Appl. Environ. Microbiol. 1976, 32, 441–442. [Google Scholar] [CrossRef]
- Liu, D.-Z.; Liu, J.-K. Peroxy natural products. Natural Products and Bioprospecting. Nat. Prod. Bioprospect. 2013, 3, 161–206. [Google Scholar] [CrossRef]
- Cole, R.J.; Kirksey, J.W.; Moore, J.H.; Blankenship, B.R.; Diner, U.L.; Davis, N.D. Tremorgenic Toxin from Penicillium verruculosum. J. Appl. Microbiol. 1972, 24, 248–256. [Google Scholar]
- Schroeder, H.W.; Cole, R.J.; Hein, H.; Kirksey, J.W. Tremorgenic mycotoxins from Aspergillus caespitosus. J. Appl. Microbiol. 1975, 29, 857–858. [Google Scholar] [CrossRef][Green Version]
- Dorner, J.W.; Cole, R.J.; Hill, R.A. Tremorgenic mycotoxins produced by Aspergillus fumigatus and Penicillium crustosum isolated from molded corn implicated in a natural intoxication of cattle. J. Agric. Food. Chem. 1984, 32, 411–413. [Google Scholar] [CrossRef]
- Patterson, D.S.P.; Shreeve, B.J.; Roberts, B.A.; MacDonald, S.M. Verruculogen Produced by soil fungi in England and Wales. Appl. Environ. Microbiol. 1981, 42, 916–917. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, R.T.; Latch, G.C.M. Production of the Tremorgenic Mycotoxins Verruculogen and Fumitremorgin B by Penicillium piscarium Westling. Appl. Environ. Microbiol. 1977, 33, 730–731. [Google Scholar] [CrossRef] [PubMed]
- Cockrum, P.A.; Culvenor, C.C.J.; Edgar, J.A.; Payne, A.L. Chemically Different Tremorgenic Mycotoxins in Isolates of Penicillium paxilli From Australia and North America. J. Nat. Prod. 1979, 42, 534–536. [Google Scholar] [CrossRef]
- Day, J.B.; Mantle, P.G.; Show, B.I. Production of verruculogen by Penicillium estinogenum in stirred fermenters. J. Gen. Microbiol. 1980, 117, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Day, J.B.; Mantle, P.G. Biosynthesis of radiolabeled verruculogen by Penicillium simplicissimum. Appl. Environ. Microbiol. 1982, 43, 514–516. [Google Scholar] [CrossRef] [PubMed]
- Nelsen, P.V.; Beuchat, L.R.; Frisvad, J.C. Growth of and fumitremorgin production by Neosartorya fischeri as affected by temperature, light, and water activity. Appl. Environ. Microbiol. 1988, 54, 1504–1510. [Google Scholar] [CrossRef]
- Feng, Y.; Holte, D.; Zoller, J.; Umemiya, S.; Simke, L.R.; Baran, P.S. Total Synthesis of Verruculogen and Fumitremorgin A Enabled by Ligand-Controlled C–H Borylation. J. Am. Chem. Soc. 2015, 137, 10160–10163. [Google Scholar] [CrossRef] [PubMed]
- Rabindran, S.K.; Ross, D.D.; Doyle, L.A.; Yang, W.; Greenberger, L.M. Fumitremorgin C reverses multidrug resistance in cells transfected with the breast cancer resistance protein. Cancer Res. 2000, 60, 47–50. [Google Scholar]
- Allen, J.D.; van Loevezijn, A.; Lakhai, J.M.; van der Valk, M.; van Tellingen, O.; Reid, G.; Schellens, J.H.M.; Koomen, G.-J.; Schinkel, A.H. Potent and Specific Inhibition of the Breast Cancer Resistance Protein Multidrug Transporter in Vitro and in Mouse Intestine by a Novel Analogue of Fumitremorgin C 1. Mol. Cancer Ther. 1 2002, 417–425. [Google Scholar]
- Wang, X.; Furukawa, T.; Nitanda, T.; Okamoto, M.; Sugimoto, Y.; Akiyama, S.-I.; Baba, M. Breast Cancer Resistance Protein (BCRP/ABCG2) Induces Cellular Resistance to HIV-1 Nucleoside Reverse Transcriptase Inhibitors. Mol. Pharmacol. 2003, 63, 65–72. [Google Scholar] [CrossRef]
- Vennerstrom, J.L. Amine peroxides as potential antimalarials. J. Med. Chem. 1989, 32, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Yadav, L.; Tiwari, M.K.; Shyamlal, B.R.K.; Mathur, M.; Swami, A.K.; Puri, S.K.; Naikade, N.K.; Chaudhary, S. Synthesis and antimalarial activity of novel bicyclic and tricyclic aza-peroxides. RSC Adv. 2016, 6, 23718–23725. [Google Scholar] [CrossRef]
- Sundar, N.; Jacob, V.T.; Bhat, S.V.; Valecha, N.; Biswas, S. Antimalarial t-butylperoxyamines. Bioorg. Med. Chem. Lett. 2001, 11, 2269–2272. [Google Scholar] [CrossRef]
- Crespo-Ortiz, M.P.; Wei, M.Q. Antitumor Activity of Artemisinin and Its Derivatives: From a Well-Known Antimalarial Agent to a Potential Anticancer Drug. J. Biomed. Biotechnol. 2012, 257597. [Google Scholar] [CrossRef]
- Ellis, G.L.; Amewu, R.; Sabbani, S.; Stocks, P.A.; Shone, A.; Stanford, D.; Gibbons, P.; Davies, J.; Vivas, L.; Charnand, S.; et al. Two-Step Synthesis of Achiral Dispiro-1,2,4,5-tetraoxanes with Outstanding Antimalarial Activity, Low Toxicity, and High-Stability Profiles. J. Med. Chem. 2008, 51, 2170–2177. [Google Scholar] [CrossRef]
- Opsenica, I.; Opsenica, D.; Lanteri, C.A.; Anova, L.; Milhous, W.K.; Smith, K.S.; Solaja, B.A. New Chimeric Antimalarials with 4-Aminoquinoline Moiety Linked to a Tetraoxane Skeleton. J. Med. Chem. 2008, 51, 6216–6219. [Google Scholar] [CrossRef]
- Rode, A.B.; Chung, K.; Kim, Y.W.; Hong, I.S. Synthesis and cetane-improving performance of 1,2,4,5-tetraoxane and 1,2,4,5,7,8-hexaoxonane derivatives. Energy Fuels 2010, 24, 1636–1639. [Google Scholar] [CrossRef]
- Coghi, P.; Yaremenko, I.A.; Prommana, P.; Radulov, P.S.; Syroeshkin, M.A.; Wu, Y.J.; Gao, J.Y.; Gordillo-Martinez, F.M.; Mok, S.; Kam-Wai Wong, V.; et al. Novel Peroxides as Promising Anticancer Agents with Unexpected Depressed Antimalarial Activity. Chem. Med. Chem. 2018, 13, 902–908. [Google Scholar] [CrossRef]
- Vil’, V.A.; Yaremenko, I.A.; Ilovaisky, A.I.; Terent’ev, A.O. Peroxides with Anthelmintic, Antiprotozoal, Fungicidal and Antiviral Bioactivity: Properties, Synthesis and Reactions. Molecules 2017, 22, 1881. [Google Scholar] [CrossRef]
- Vil’, V.A.; Yaremenko, I.A.; Ilovaisky, A.I.; Terent’ev, A.O. Synthetic Strategies for Peroxide Ring Construction in Artemisinin. Molecules 2017, 22, 117. [Google Scholar] [CrossRef]
- Yaremenko, I.A.; Syroeshkin, M.A.; Levitsky, D.O.; Fleury, F.; Terent’ev, A.O. Cyclic peroxides as promising anticancer agents: In vitro cytotoxicity study of synthetic ozonides and tetraoxanes on human prostate cancer cell lines. Med. Chem. Res. 2017, 26, 170–179. [Google Scholar] [CrossRef]
- Zheng, W.; Wojtas, L.; Antilla, J.C. Chiral Phosphoric Acid Catalyzed Peroxidation of Imines. Angew. Chem., Int. Ed. 2010, 49, 6589–6591. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, H.; Liebscher, J. Isoquinoline- and piperazinedione-derived α-acylamino peroxide moieties in asymmetric oxidation of sulphides and epoxidation of naphthoquinones (09-3919NP). Arkivoc 2009, 11, 204–220. [Google Scholar]
- Kienle, M.; Argyrakis, W.; Baro, A.; Laschat, S. Diketopiperazine-derived hydroperoxide for chemoselective oxidations of sulfides and enantioselective Weitz–Scheffer epoxidations. Tetrahedron Lett. 2008, 49, 1971–1974. [Google Scholar] [CrossRef]
- Rebek, J.; McCready, R. Olefin epoxidation with.alpha.-substituted hydroperoxides. J. Am. Chem. Soc. 1980, 102, 5602–5605. [Google Scholar] [CrossRef]
- Rebek, J. Progress in the Development of New Epoxidation Reagents. Heterocycles 1981, 15, 517–545. [Google Scholar] [CrossRef]
- Schmidt, U.; Hausler, J. Trialkylallenes from 1,1-disubstituted propargylic acetates. Angew. Chem., Int. Ed. Engl. 1976, 15, 497–498. [Google Scholar] [CrossRef]
- Casteel, D.A. Peroxy natural products. Nat. Prod. Rep. 1999, 16, 55–73. [Google Scholar] [CrossRef]
- Chung, L.W.; Hayashi, S.; Lundberg, M.; Nakatsu, T.; Kato, H.; Morokuma, K. Mechanism of efficient firefly bioluminescence via adiabatic transition state and seam of sloped conical intersection. J. Am. Chem. Soc. 2008, 130, 12880–12881. [Google Scholar] [CrossRef]
- Gollnick, K.; Griesbeck, A. Thiaozonide formation by singlet oxygen cycloaddition to 2,5-dimethylthiophene. Tetrahedron Lett. 1984, 25, 4921–4924. [Google Scholar] [CrossRef]
- Skold, C.N.; Schlessinger, R.H. The reaction of singlet oxygen with a simple thiophene. Tetrahedron Lett. 1970, 10, 791–794. [Google Scholar] [CrossRef]
- Hoffman, J.M., Jr.; Schlessinger, R.M. Thioozonides: A new class of reactive organosulfur compounds. Tetrahedron Lett. 1970, 10, 797–804. [Google Scholar] [CrossRef]
- Adam, W.; Eggelte, H.J. 2,3-Dioxa-7-thiabicyclo[2.2.1]heptane: A New Heterobicyclic System Possessing the Thiaozonide Structure. Angew. Chern. Znt. Ed. Engl. 1978. [Google Scholar] [CrossRef]
- Tabuchi, T.; Nojima, M.; Kusabayashi, S. Reaction of thioketones with carbonyl oxides and 3,3-dimethyl-1,2-dioxirane. [3 + 2] Cycloaddition vs. oxygen atom transfer. J Chem. Soc. Perkin Trans. 1 1991, 3043–3046. [Google Scholar] [CrossRef]
- Yur’ev, Y.K. Catalytic transformations of heterocyclic compounds. I. Transformations of furan into pyrrole and thiophene. Ber. Dtsch. Chem. Ges. [Abteilung] B: Abhandlungen 1936, 69B, 440–443. [Google Scholar]
- Makhmudiyarova, N.N.; Khatmullina, G.M.; Rakhimov, R.S.; Meshcheryakova, E.S.; Ibragimov, A.G.; Dzhemilev, U.M. The first example of catalytic synthesis of N-aryl-substituted tetraoxazaspiroalkanes. Tetrahedron 2016, 72, 3277–3281. [Google Scholar] [CrossRef]
- Tyumkina, T.V.; Makhmudiyarova, N.N.; Kiyamutdinova, G.M.; Meshcheryakova, E.S.; Bikmukhametov, K.S.; Abdullin, M.F.; Khalilov, L.M.; Ibragimov, A.G.; Dzhemilev, U.M. Synthesis, molecular structure, conformation and biological activity of Ad-substituted N-aryl-tetraoxaspiroalkanes. Tetrahedron 2018, 74, 1749–1758. [Google Scholar] [CrossRef]
- Makhmudiyarova, N.N.; Ishmukhametova, I.R.; Tyumkina, T.V.; Ibragimov, A.G.; Dzhemilev, U.M. Synthesis of N-aryl-hexaoxazadispiroalkanes using lanthanide catalysts. Tetrahedron Lett. 2018, 59, 3161–3164. [Google Scholar] [CrossRef]
- Makhmudiyarova, N.N.; Ishmukhametova, I.R.; Dzhemileva, L.U.; Tyumkina, T.V.; D’yakonov, V.A.; Ibragimov, A.G.; Dzhemilev, U.M. Synthesis and anticancer activity novel dimeric azatriperoxides. RSC Adv. 2019, 9, 18923–18929. [Google Scholar] [CrossRef]
- Makhmudiyarova, N.N.; Rakhimov, R.S.; Tyumkina, T.V.; Meshcheryakova, E.S.; Ibragimov, A.G.; Dzhemilev, U.M. Sm-Catalyzed Synthesis and Biological Activity of Acyclic and Cyclic Azadiperoxides. Russ. J. Org. Chem. 2019, 5, 620–632. [Google Scholar] [CrossRef]
- Makhmudiyarova, N.N.; Khatmullina, G.M.; Rakhimov, R.S.; Ibragimov, A.G.; Dzhemilev, U.M. Synthesis of pentaoxaspiroalkanes and pentaoxocanes catalyzed by lanthanide compounds. Arkivoc 2016, 5, 427–433. [Google Scholar] [CrossRef]
- Kukushkin, Y.N. The Reactivity of Coordination Compounds; Khimiya: Leningrad, Russia, 1987; p. 228. [Google Scholar]
- Pearson, R.G. Hard and soft acids and bases. Russ. Chem. Rev. 1971, 40, 1259–1282. [Google Scholar]
- Denekamp, C.; Gottlieb, L.; Tamiri, T.; Tsoglin, A.; Shilav, R.; Karon, M. Two Separable Conformers of TATP and Analogues Exist at Room Temperature. Org. Lett. 2005, 7, 2461–2464. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of all compounds are available from the authors. |

| Entry | [M] | Solvent | Yield * of 8% |
|---|---|---|---|
| 1 | Sm(NO3)3·6H2O | THF | 98 |
| 2 | Sm(NO3)3·6H2O | CH2Cl2 | 85 |
| 3 | Sm(NO3)3·6H2O | Et2O | 79 |
| 4 | Sm(NO3)3·6H2O | C6H12 | 15 |
| 5 | Sm(NO3)3·6H2O | EtOAc | 10 |
| 6 | Sm(NO3)3·6H2O | C2H5OH | 7 |
| 7 | Ho(NO3)3 5H2O | THF | 84 |
| 8 | TbCl3 6H2O | THF | 72 |
| 9 | DyCl3 6H2O | THF | 67 |
| 10 | NdCl3 6H2O | THF | 61 |
| 11 | La(NO3)3·6H2O | THF | 58 |
| Compound | Jurkat (IC50, µM) | K562 (IC50, µM) | HL60 (IC50, µM) | U937 (IC50, µM) | Fibroblasts (IC50, µM) |
|---|---|---|---|---|---|
| 8 | 5.26 ± 0.57 | 7.15 ± 0.64 | 4.59 ± 0.38 | 24.13 ± 1.87 | 118.61 ± 8.74 |
| 9 | 4.91 ± 0.43 | 6.83 ± 0.59 | 4.14 ± 0.34 | 21.17 ± 2.11 | 97.88 ± 6.81 |
| 10 | 3.52 ± 0.31 | 5.77 ± 0.46 | 2.67 ± 0.21 | 15.24 ± 1.26 | 81.42 ± 5.12 |
| 12 | 4.45 ± 0.49 | 6.29 ± 0.57 | 3.91 ± 0.33 | 19.89 ± 1.57 | 85.93 ± 5.47 |
| 13 | 10.21 ± 0.87 | 14.37 ± 0.96 | 8.56 ± 0.69 | 35.24 ± 2.65 | 142.17 ± 9.76 |
| 14 | 9.61 ± 0.79 | 11.97 ± 0.91 | 8.22 ± 0.74 | 32.81 ± 2.89 | 129.23 ± 8.92 |
| 18 | 17.11 ± 1.24 | 21.75 ± 1.59 | 14.96 ± 0.97 | 46.67 ± 3.76 | 188.36 ± 12.91 |
| 19 | 2.81 ± 0.37 | 4.37 ± 0.31 | 2.24 ± 0.29 | 11.79 ± 0.99 | 79.17 ± 5.41 |
| 20 | 23.94 ± 1.67 | 28.26 ± 1.48 | 19.61 ± 1.12 | 65.81 ± 4.84 | 195.87 ± 14.67 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makhmudiyarova, N.; Ishmukhametova, I.; Dzhemileva, L.; D’yakonov, V.; Ibragimov, A.; Dzhemilev, U. First Example of Catalytic Synthesis of Cyclic S-Containing Di- and Triperoxides. Molecules 2020, 25, 1874. https://doi.org/10.3390/molecules25081874
Makhmudiyarova N, Ishmukhametova I, Dzhemileva L, D’yakonov V, Ibragimov A, Dzhemilev U. First Example of Catalytic Synthesis of Cyclic S-Containing Di- and Triperoxides. Molecules. 2020; 25(8):1874. https://doi.org/10.3390/molecules25081874
Chicago/Turabian StyleMakhmudiyarova, Nataliya, Irina Ishmukhametova, Lilya Dzhemileva, Vladimir D’yakonov, Askhat Ibragimov, and Usein Dzhemilev. 2020. "First Example of Catalytic Synthesis of Cyclic S-Containing Di- and Triperoxides" Molecules 25, no. 8: 1874. https://doi.org/10.3390/molecules25081874
APA StyleMakhmudiyarova, N., Ishmukhametova, I., Dzhemileva, L., D’yakonov, V., Ibragimov, A., & Dzhemilev, U. (2020). First Example of Catalytic Synthesis of Cyclic S-Containing Di- and Triperoxides. Molecules, 25(8), 1874. https://doi.org/10.3390/molecules25081874