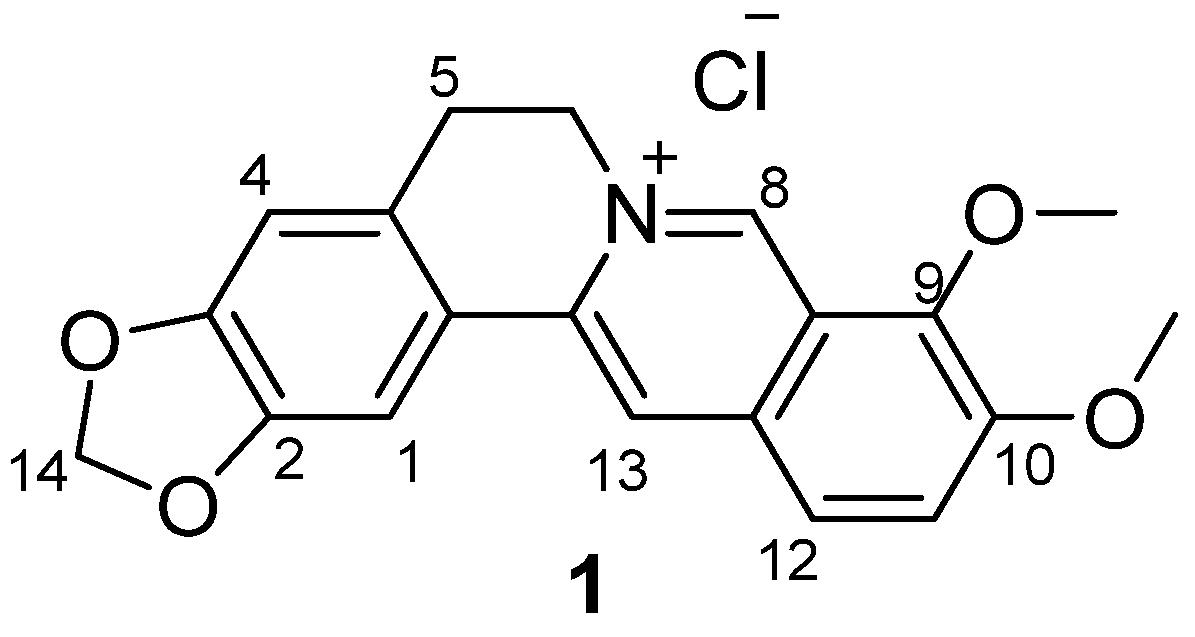
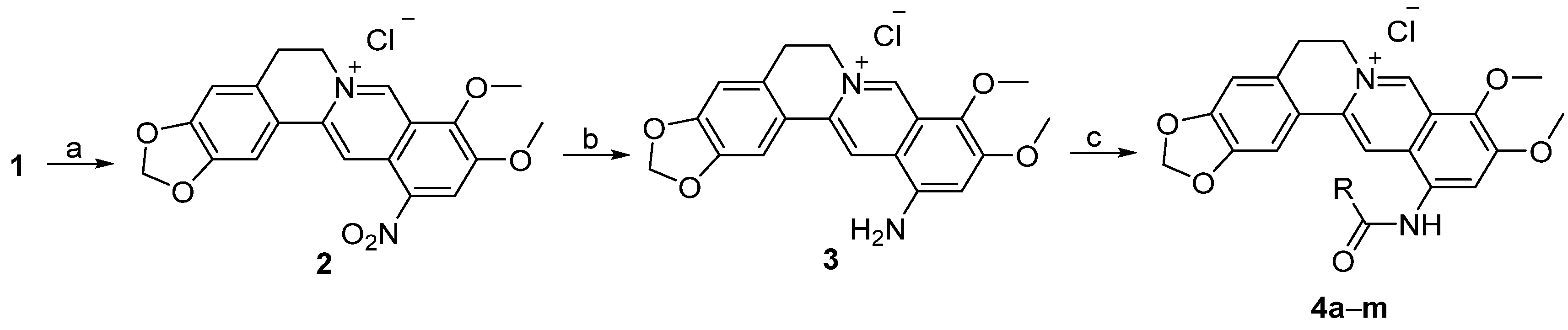
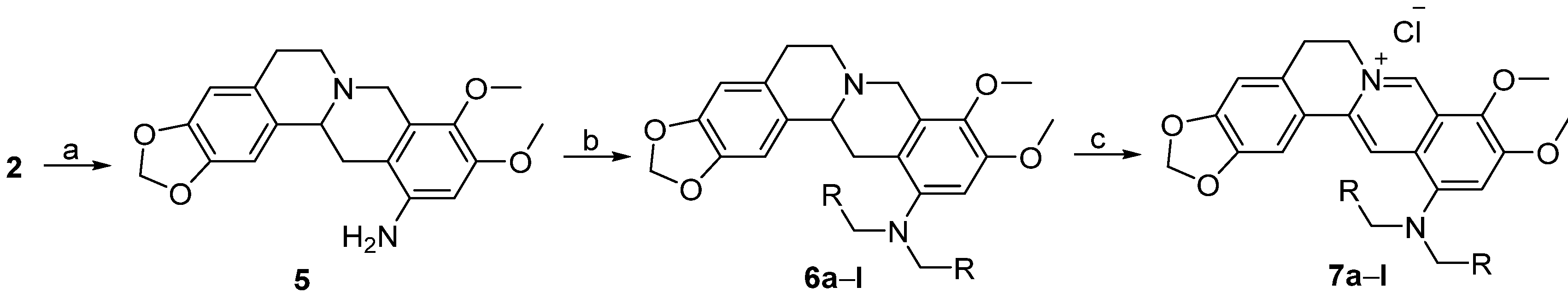
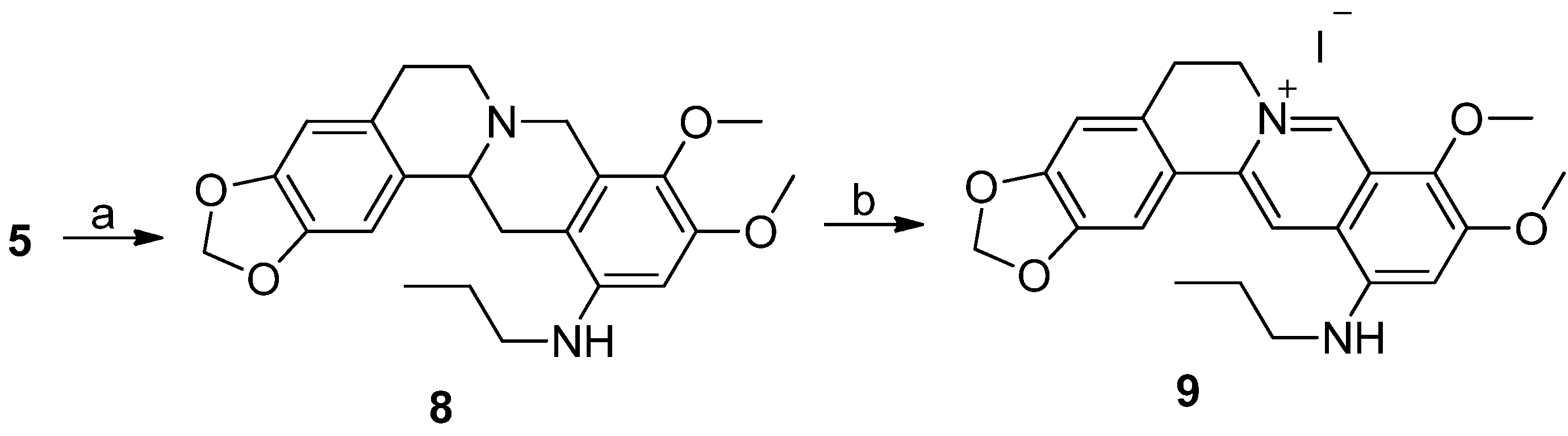
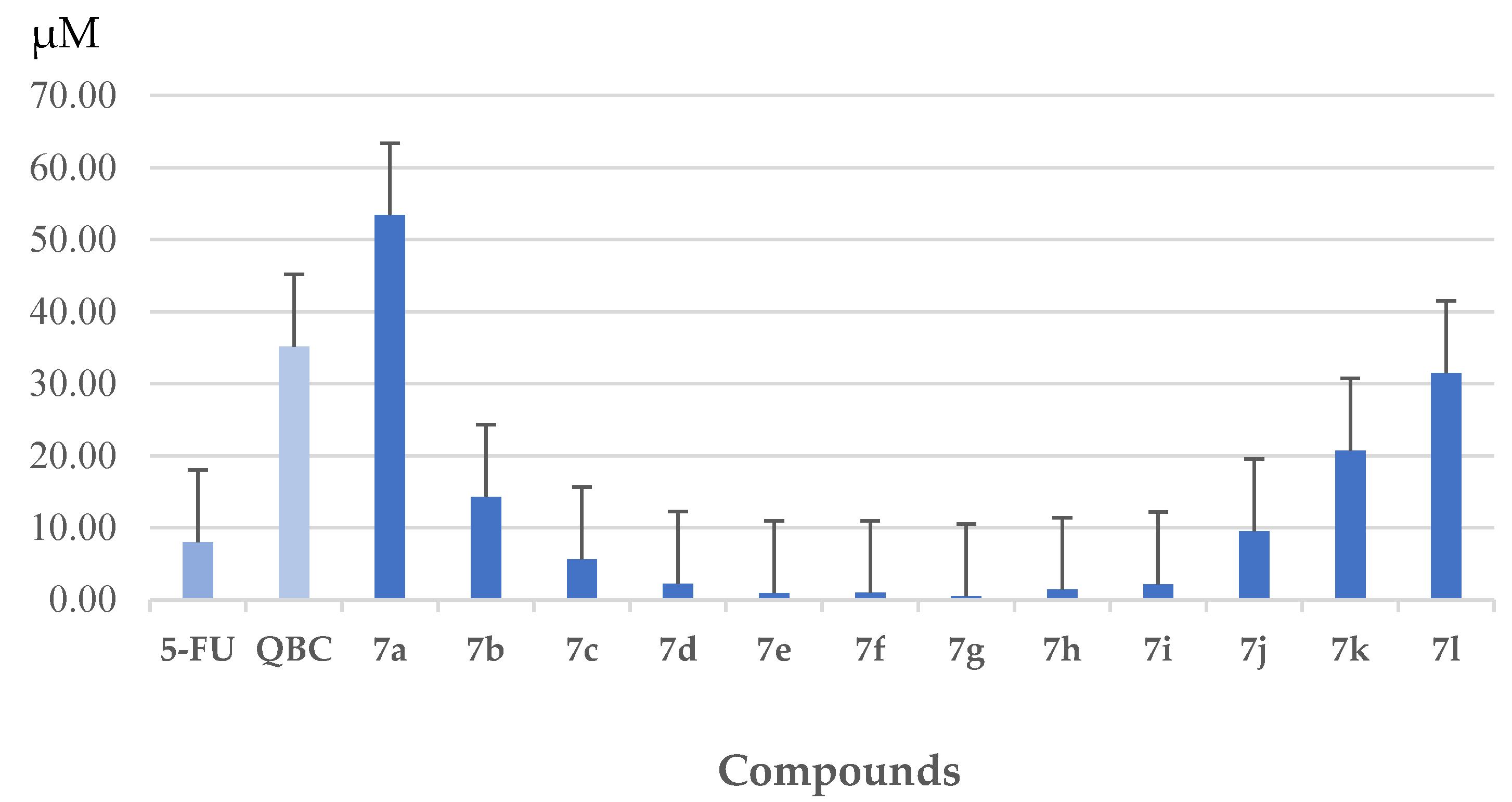
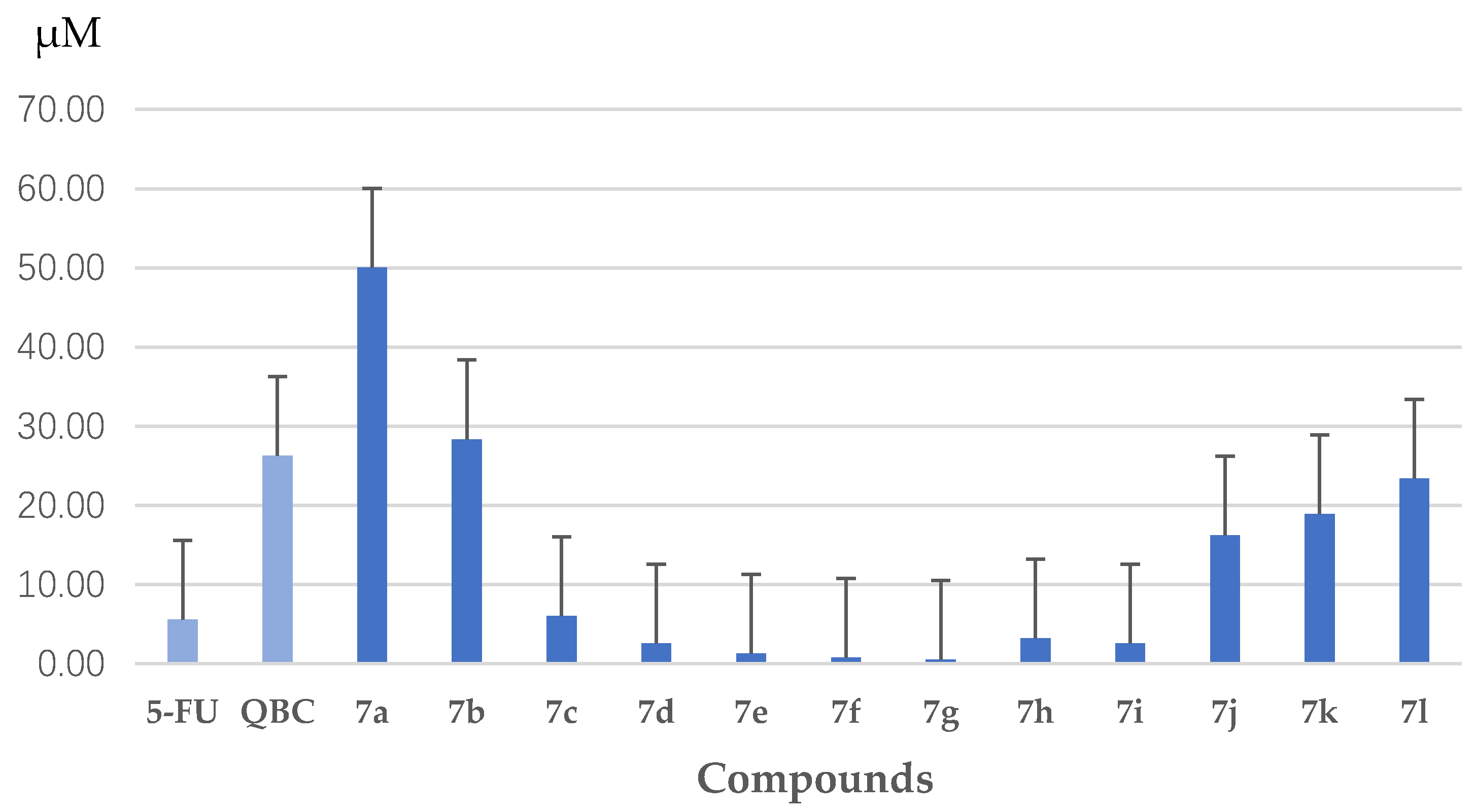
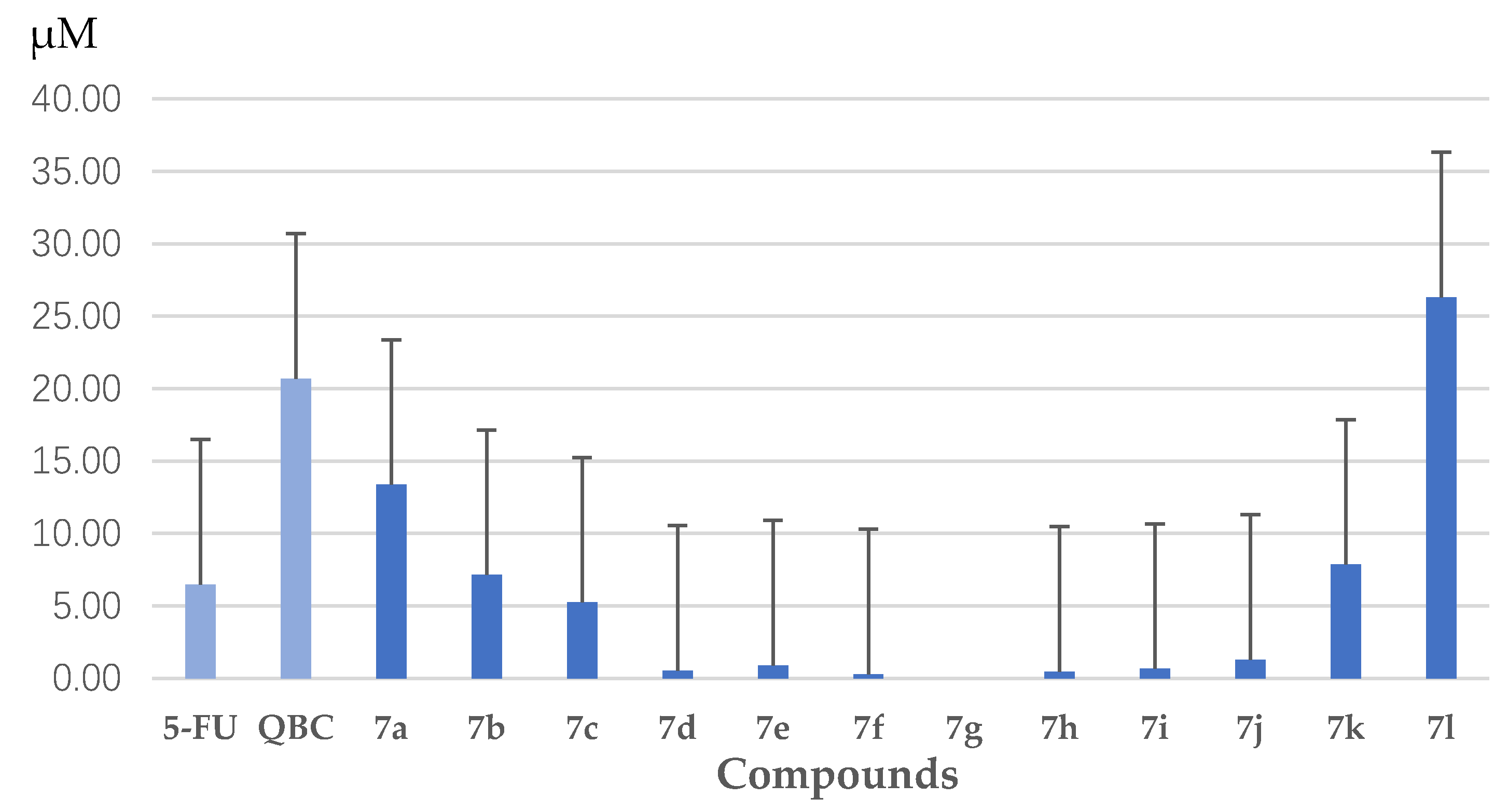
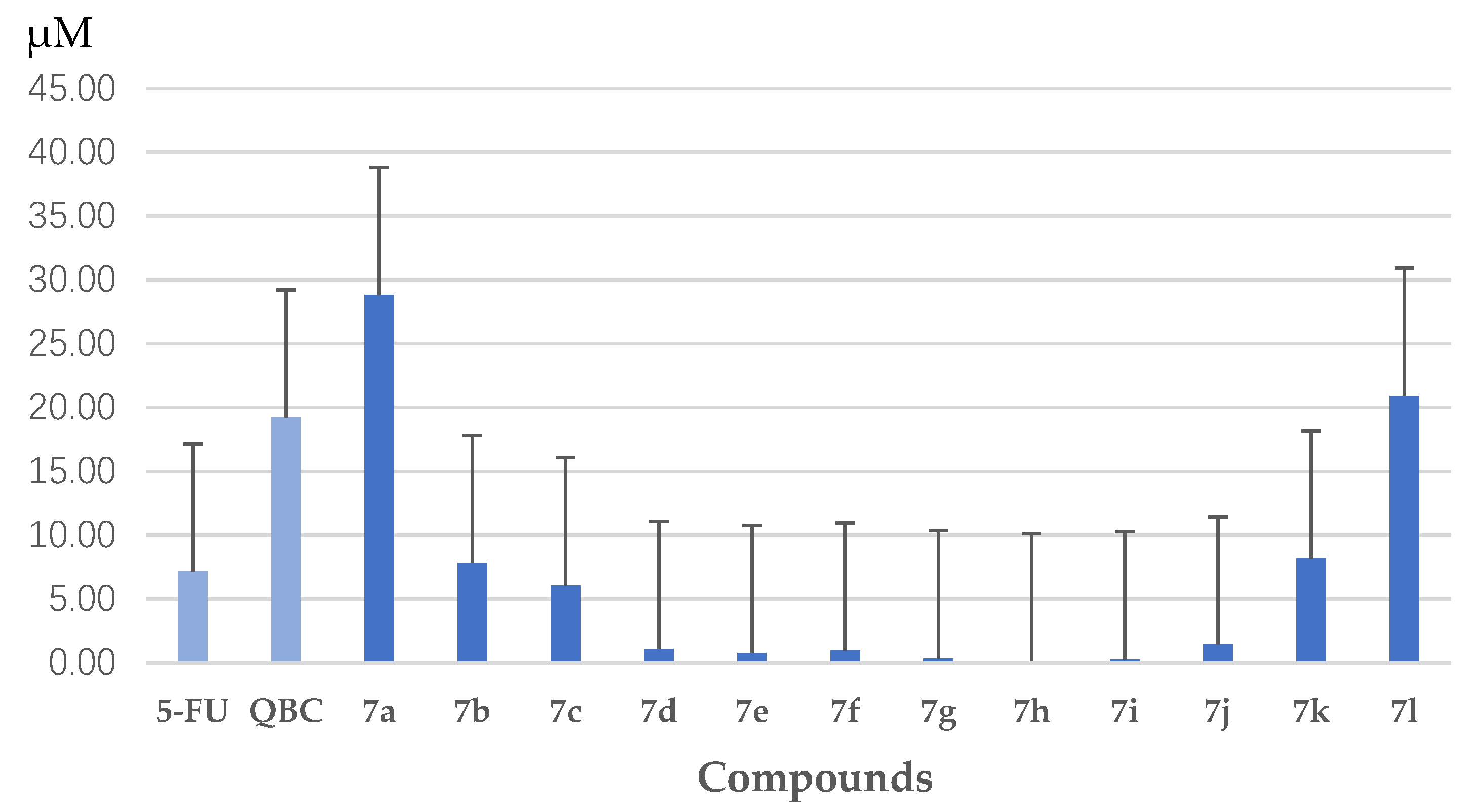
3.1.2. Synthesis
Quaternary 12-nitroberberine chloride (2). NaNO2 (18.6 g, 269.57 mmol) was added into a reaction bottle containing a solution of QBC (20 g, 53.84 mmol) in acetic acid (250 mL) under the condition of stirring at 0 °C batchwise. Then, concentrated HNO3 (30 mL) was added dropwise. The reaction mixture was stirred for 5 min at 0 °C, then heated at 50 °C under stirring for 1h until the raw material was completely reacted. Water (200 mL) was immediately added into the reaction mixture to quench the reaction, and the solution was extracted three times (200 mL/time) using a mixed solution of CHCl3/MeOH (v/v = 10:1) in a separatory funnel. The organic layer was incorporated and concentrated under reduced pressure to remove the solvent. The residue was purified using silica gel CC eluted using a mixed solution of CHCl3/CH3OH (v/v = 20:1) to yield 2 (9.65 g, 43% yield) as a red solid. 1H-NMR (400 MHz, DMSO-d6): δ 3.23 (t, J = 6 Hz, 2H, ArCH2CH2N), 4.16 (s, 3H, OCH3), 4.28 (s, 3H, OCH3), 4.96 (t, J = 6 Hz, 2H, ArCH2CH2N), 6.20 (s, 2H, OCH2O), 7.13 (s, 1H, Ar–H), 7.83 (s, 1H, Ar–H), 8.89 (s, 1H, Ar–H), 9.05 (s, 1H, Ar–H), 10.12 (s, 1H, Ar–H); 13C-NMR: (100 MHz, DMSO-d6) δ 26.02, 55.18, 57.67, 62.56, 102.22, 105.70, 108.41, 115.38, 119.90, 120.66, 124.43, 125.18, 131.71, 138.90, 139.94, 147.19, 147.86, 147.90, 149.65, 150.51; ESI–MS (m/z): 381.2 [M − Cl]+.
Quaternary 12-aminoberberine chloride (3). SnCl2·2H2O (1173 mg, 5.2 mmol) and concentrated HCl (1.3 mL) were added, in turn, into a solution containing compound 2 (540 mg, 1.3 mol) and absolute ethanol (20 mL) in a reaction bottle under stirring. The reaction mixture was refluxed for 0.5 h under stirring until the raw material was completely reacted according to thin-layer chromatography (TLC) test. The solution was concentrated under reduced pressure to remove the solvent. Aqueous 5% NaOH solution was added into the residue dropwise to make the solution alkaline (pH = 10). The solution was extracted using n-butanol three times (20 mL/time) in a separatory funnel. The organic layer was integrated and concentrated under reduced pressure to yield a residue, which was purified using silica gel CC eluted using a mixed solvent of CHCl3/CH3OH (v/v = 15:1) to yield 3 (200 mg, 40% yield) as a red solid. 1H-NMR (400 MHz, DMSO-d6): δ 3.18 (t, J = 6 Hz, 2H, ArCH2CH2N), 3.89 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 4.87 (t, J = 6 Hz, 2H, ArCH2CH2N), 6.16 (s, 2H, OCH2O), 6.62 (s, 2H, Ar–NH2), 7.06 (s, 1H, Ar–H), 7.14 (s, 1H, Ar–H), 7.88 (s, 1H, Ar–H), 8.85 (s, 1H, Ar–H), 9.61 (s, 1H, Ar–H); 13C-NMR: (100 MHz, DMSO-d6) δ 26.28, 54.82, 56.17, 61.74, 101.88, 104.10, 105.12, 108.31, 116.10, 120.09, 120.89, 121.44, 129.82, 133.21, 134.72, 143.19, 144.50, 147.49, 149.18, 152.32; ESI–MS (m/z): 351.5 [M − Cl]+.
Quaternary berberine-12-N-p-trifluoromethylphenylcarbonylamine chloride (4a). Pyridine (63 μL, 0.78 mmol) was added into a solution containing compound 3 (150 mg, 0.39 mmol) in anhydrous CH2Cl2 (6 mL) in a reaction bottle under stirring. The reaction mixture was stirred at 0°C for 10 min. Then, p-trifluoromethylbenzoyl chloride (63 μL, 0.429 mmol) was added into the reaction mixture, and the reaction solution was stirred at room temperature for 8 h until the raw material was completely reacted according to TLC analysis. After adding a small amount of water (10 mL), the solution was extracted three times (20 mL/time) using n-butanol in a separatory funnel. The organic layer was integrated, dried using anhydrous MgSO4, and filtered. The filtrate was concentrated under reduced pressure to remove the solvent. The residue was purified using silica gel CC eluted using a mixed solvent of CHCl3/CH3OH (v/v = 20:1) to yield 4a (60 mg, 28% yield) as a yellow amorphous solid. 1H-NMR (400 MHz, DMSO-d6): δ 3.21 (t, J = 6 Hz, 2H, ArCH2CH2N), 4.07 (s, 3H, OCH3), 4.11 (s, 3H, OCH3), 4.95 (t, J = 6 Hz, 2H, ArCH2CH2N), 6.15 (s, 2H, OCH2O), 7.10 (s, 1H, Ar–H), 7.96 (s, 1H, Ar–H), 8.01 (d, J = 8 Hz, 2H, Ar–H), 8.39 (d, J = 8 Hz, 2H, Ar–H), 8.41 (s, 1H, Ar–H), 8.78 (s, 1H, Ar–H), 9.95 (s, 1H, Ar–H), 11.17 (s, 1H, ArNHCO); ESI–MS (m/z): 523.2 [M − Cl]+.
Quaternary berberine-12-N-methylcarbonylamine chloride (4b). Target compound 4b was obtained (51 mg, 51% yield) as a yellow amorphous solid from compound 3 (90 mg, 0.233 mmol), pyridine (75 μL, 0.932 mmol), and acetyl chloride (36 μL, 0.513 mmol) using a procedure similar to that for synthesizing compound 4a. 1H-NMR (400 MHz, DMSO-d6): δ 2.31 (s, 3H, NHCOCH3), 3.21 (t, J = 6 Hz, 2H, ArCH2CH2N), 4.03 (s, 3H, OCH3), 4.06 (s, 3H, OCH3), 4.93 (t, J = 6 Hz, 2H, ArCH2CH2N), 6.18 (s, 2H, OCH2O), 7.10 (s, 1H, Ar–H), 8.01 (s, 1H, Ar–H), 8.47 (s, 1H, Ar–H), 8.88 (s, 1H, Ar–H), 9.87 (s, 1H, Ar–H), 10.54 (s, 1H, ArNHCO); 13C-NMR: (100 MHz, DMSO-d6) δ 23.99, 26.31, 55.14, 56.93, 62.03, 102.14, 105.90, 108.49, 116.00, 119.30, 120.62, 121.22, 126.03, 130.78, 130.82, 136.98, 140.42, 145.62, 147.68, 149.86, 150.09, 169.59; ESI–MS (m/z): 393.3 [M − Cl]+.
Quaternary berberine-12-N-isopropylcarbonylamine chloride (4c). Target compound 4c was obtained (31 mg, 59.6% yield) as a yellow amorphous solid from compound 3 (44 mg, 0.114 mmol), pyridine (37 μL, 0.456 mmol), and isobutyryl chloride (26 μL, 0.251 mmol) using a procedure similar to that for synthesizing 4a. 1H-NMR (400 MHz, DMSO-d6): δ 1.22 (d, J = 6.8 Hz, 6H, NHCOCHC2H6), 3.08 (septet, 1H, J = 6.8 Hz, NHCOCHC2H6), 3.20 (t, J = 6 Hz, 2H, ArCH2CH2N), 4.04 (s, 3H, OCH3), 4.06 (s, 3H, OCH3), 4.93 (t, J = 6 Hz, 2H, ArCH2CH2N), 6.18 (s, 2H, OCH2O), 7.10 (s, 1H, Ar–H), 8.02 (s, 1H, Ar–H), 8.51 (s, 1H, Ar–H), 8.91 (s, 1H, Ar–H), 9.88 (s, 1H, Ar–H), 10.58 (s, 1H, ArNHCO); 13C-NMR: (100 MHz, DMSO-d6) δ 19.62 (2 × C), 26.31, 34.53, 55.11, 56.92, 62.03, 102.12, 105.85, 108.46, 116.05, 119.10, 120.67, 121.22, 125.92, 130.80, 130.87, 136.88, 140.29, 145.56, 147.68, 149.85, 150.07, 176.48; ESI–MS (m/z): 421.3 [M − Cl]+.
Quaternary berberine-12-N-p-bromophenylcarbonylamine chloride (4d). Target compound 4d was obtained (84 mg, 56.9% yield) as a yellow amorphous solid from compound 3 (100 mg, 0.259 mmol), pyridine (83 μL, 1.036 mmol), and p-bromobenzoyl chloride (125μL, 0.57 mmol) using a procedure similar to that for 4a. 1H-NMR (400 MHz, DMSO-d6): δ 3.21 (t, J = 6 Hz, 2H, ArCH2CH2N), 4.06 (s, 3H, OCH3), 4.11 (s, 3H, OCH3), 4.95 (t, J = 6 Hz, 2H, ArCH2CH2N), 6.15 (s, 2H, OCH2O), 7.10 (s, 1H, Ar–H), 7.83 (d, J = 8 Hz, 2H, Ar–H), 7.93 (s, 1H, Ar–H), 8.13 (d, J = 8 Hz, 2H, Ar–H), 8.39 (s, 1H, Ar–H), 8.75 (s, 1H, Ar–H), 9.93 (s, 1H, Ar–H), 10.94 (s, 1H, ArNHCO); 13C-NMR: (100 MHz, DMSO-d6) δ 26.32, 55.20, 57.11, 62.08, 102.06, 106.09, 108.41, 116.89, 120.60, 121.26, 122.39, 125.85, 127.66, 130.44 (2 × C), 130.64, 130.83, 131.44 (2 × C), 133.22, 136.93, 141.64, 145.82, 147.60, 149.81, 149.99,165.84; ESI–MS (m/z): 535.0 [M − Cl]+.
Quaternary berberine-12-N-phenylcarbonylamine chloride (4e). Target compound 4e was obtained (74 mg, 58.3% yield) as a yellow amorphous solid from compound 3 (100 mg, 0.259 mmol), pyridine (83 μL, 1.036 mmol), and benzoyl chloride (66 μL, 0.57 mmol) using a procedure similar to that for 4a. 1H-NMR (400 MHz, DMSO-d6): δ 3.22 (t, J = 6 Hz, 2H, ArCH2CH2N), 4.07 (s, 3H, OCH3), 4.11 (s, 3H, OCH3), 4.95 (t, J = 6 Hz, 2H, ArCH2CH2N), 6.15 (s, 2H, OCH2O), 7.10 (s, 1H, Ar–H), 7.62 (t, J = 7.6 Hz, 2H, Ar–H), 7.66 (t, J = 7.2 Hz, 1H, Ar–H) 7.91 (s, 1H, Ar–H), 8.17 (m, 2H, Ar–H), 8.43 (s, 1H, Ar–H), 8.74 (s, 1H, Ar–H), 9.93 (s, 1H, Ar–H), 10.80 (s, 1H, ArNHCO); 13C-NMR: (100 MHz, DMSO-d6) δ 26.33, 55.19, 57.09, 62.09, 102.06, 106.02, 108.42, 116.89, 120.63, 121.27, 122.09, 127.53, 128.32 (2 × C), 128.46(2 × C), 130.83, 130.89, 132.07, 134.11, 136.87, 141.47, 145.78, 147.61, 149.80, 150.02, 166.75; ESI-MS (m/z): 455.2 [M − Cl]+.
Quaternary berberine-12-N-p-fluorophenylcarbonylamine chloride (4f). Target compound 4f was obtained (46 mg, 35% yield) as a yellow amorphous solid from compound 3 (100 mg, 0.259 mmol), pyridine (83 μL, 1.036 mmol), and p-fluorobenzoyl chloride (68 μL, 0.57 mmol) using a procedure similar to that for 4a. 1H-NMR (400 MHz, DMSO-d6): δ 3.21 (t, J = 6 Hz, 2H, ArCH2CH2N), 4.06 (s, 3H, OCH3), 4.11 (s, 3H, OCH3), 4.95 (t, J = 6 Hz, 2H, ArCH2CH2N), 6.15 (s, 2H, OCH2O), 7.09 (s, 1H, Ar–H), 7.46 (t, J = 8.8 Hz, 2H, Ar–H), 7.93 (s, 1H, Ar–H), 8.26 (m, 2H, Ar–H), 8.40 (s, 1H, Ar–H), 8.76 (s, 1H, Ar–H), 9.93 (s, 1H, Ar–H), 10.88 (s, 1H, ArNHCO); 13C-NMR: (150 MHz, DMSO-d6) δ 26.33, 55.17, 57.09, 62.07, 102.02, 106.12, 108.36, 115.32 (d, J=21 Hz, 2C), 117.08, 120.63, 121.24, 122.39, 127.64, 130.48, 130.50, 130.78, 131.17 (d, J=9 Hz, 2C), 136.80, 141.52, 145.70, 147.58, 149.76, 149.96, 164.37 (d, J = 249 Hz, 1C), 165.62; ESI–MS (m/z): 473.2 [M − Cl]+.
Quaternary berberine-12-N-p-methylphenylcarbonylamine chloride (4g). Target compound 4g was obtained (54 mg, 41.3% yield) as a yellow amorphous solid from compound 3 (100 mg, 0.259 mmol), pyridine (83 μL, 1.036 mmol), and p-methylbenzoyl chloride (76 μL, 0.57 mmol) using a procedure similar to that for 4a. 1H-NMR (400 MHz, DMSO-d6): δ 2.44 (s, 3H, NHCOArCH3), 3.21 (t, J = 6 Hz, 2H, ArCH2CH2N), 4.06 (s, 3H, OCH3), 4.11 (s, 3H, OCH3), 4.95 (t, J = 6 Hz, 2H, ArCH2CH2N), 6.15 (s, 2H, OCH2O), 7.10 (s, 1H, Ar–H), 7.42 (d, J = 8 Hz, 2H, Ar–H), 7.88 (s, 1H, Ar–H), 8.06 (d, J = 8 Hz, 2H, Ar–H), 8.41 (s, 1H, Ar–H), 8.71 (s, 1H, Ar–H), 9.92 (s, 1H, Ar–H), 10.70 (s, 1H, ArNHCO); 13C-NMR: (150 MHz, DMSO-d6) δ 21.08, 26.30, 55.14, 57.05, 62.04, 102.01, 105.93, 108.37, 116.91, 120.59, 121.22, 122.05, 127.48, 128.32 (2 × C), 128.90 (2 × C) 130.77, 130.94, 131.20, 136.74, 141.34, 142.09, 145.68, 147.57, 149.75, 149.98, 166.53; ESI–MS (m/z): 469.1 [M − Cl]+.
Quaternary berberine-12-N-m-methylphenylcarbonylamine chloride (4h). Target compound 4h was obtained (90 mg, 68.8% yield) as a yellow amorphous solid from compound 3 (100 mg, 0.259 mmol), pyridine (83 μL, 1.036 mmol), and m-methylbenzoyl chloride (75 μL, 0.57 mmol) using a procedure similar to that for 4a. 1H-NMR (400 MHz, DMSO-d6): δ 2.45 (s, 3H, NHCOArCH3), 3.21 (t, J = 6 Hz, 2H, ArCH2CH2N), 4.06 (s, 3H, OCH3), 4.11 (s, 3H, OCH3), 4.95 (t, J = 6 Hz, 2H, ArCH2CH2N), 6.15 (s, 2H, OCH2O), 7.09 (s, 1H, Ar–H), 7.49 (m, 2H, Ar–H), 7.91 (s, 1H, Ar–H), 7.98 (m, 2H, Ar–H), 8.39 (s, 1H, Ar–H), 8.74 (s, 1H, Ar–H), 9.93 (s, 1H, Ar–H), 10.82 (s, 1H, ArNHCO); 13C-NMR: (100 MHz, DMSO-d6) δ 21.02, 26.33, 55.18, 57.08, 62.08, 102.05, 106.00, 108.41, 116.96, 120.63, 121.27, 122.15, 125.47, 127.57, 128.35, 128.80, 130.82, 130.95, 132.61, 134.09, 136.83, 137.74, 141.47, 145.78, 147.61, 149.80, 150.02, 166.86; ESI–MS (m/z): 469.2 [M − Cl]+.
Quaternary berberine-12-N-ethylcarbonylamine chloride (4i). Target compound 4i was obtained (46 mg, 40.1% yield) as a yellow amorphous solid from compound 3 (100 mg, 0.259 mmol), pyridine (83 μL, 1.036 mmol), and propionyl chloride (50 μL, 0.57 mmol) using a procedure similar to that for 4a. 1H-NMR (400 MHz, DMSO-d6): δ 1.18 (t, J = 7.6 Hz, 3H, NHCOCH2CH3), 2.67 (q, J = 7.6 Hz, 2H, NHCOCH2CH3), 3.21 (t, J = 6 Hz, 2H, ArCH2CH2N), 4.03 (s, 3H, OCH3), 4.06 (s, 3H, OCH3), 4.93 (t, J = 6 Hz, 2H, ArCH2CH2N), 6.18 (s, 2H, OCH2O), 7.09 (s, 1H, Ar-H), 8.02 (s, 1H, Ar-H), 8.51 (s, 1H, Ar–H), 8.91 (s, 1H, Ar–H), 9.87 (s, 1H, Ar–H), 10.53 (s, 1H, ArNHCO); 13C-NMR: (150 MHz, DMSO-d6) δ 9.48, 26.30, 29.22, 55.10, 56.88, 62.00, 102.06, 106.09, 108.37, 116.24, 118.93, 120.67, 121.18, 125.85, 130.69, 130.95, 136.85, 140.13, 145.44, 147.63, 149.77, 150.04, 173.27; ESI–MS (m/z): 407.2 [M − Cl]+.
Quaternary berberine-12-N-n-butylcarbonylamine chloride (4j). Target compound 4j was obtained (60 mg, 49.2% yield) as a yellow amorphous solid from compound 3 (100 mg, 0.259 mmol), pyridine (83 μL, 1.036 mmol), and n-pentanoyl chloride (68 μL, 0.57 mmol) using a procedure similar to that for 4a. 1H-NMR (400 MHz, DMSO-d6): δ 0.95 (t, J = 7.6 Hz, 3H, NHCOCH2CH2CH2CH3), 1.42 (m, 2H, NHCOCH2CH2CH2CH3), 1.68 (m, 2H, NHCOCH2CH2CH2CH3), 2.65 (t, J = 7.6 Hz, 2H, NHCOCH2CH2CH2CH3), 3.21 (t, J = 6 Hz, 2H, ArCH2CH2N), 4.03 (s, 3H, OCH3), 4.06 (s, 3H, OCH3), 4.93 (t, J = 6 Hz, 2H, ArCH2CH2N), 6.18 (s, 2H, OCH2O), 7.10 (s, 1H, Ar–H), 8.02 (s, 1H, Ar–H), 8.50 (s, 1H, Ar–H), 8.90 (s, 1H, Ar–H), 9.87 (s, 1H, Ar–H), 10.60 (s, 1H, ArNHCO); 13C-NMR: (100 MHz, DMSO-d6) δ 13.86, 21.89, 26.30, 27.09, 35.73, 55.11, 56.91, 62.02, 102.12, 105.89, 108.45, 116.09, 119.11, 120.65, 121.21, 125.93, 130.77, 130.85, 136.90, 140.29, 145.55, 147.67, 149.83, 150.08, 172.53; ESI–MS (m/z): 435.3 [M − Cl]+.
Quaternary berberine-12-N-p-methoxyphenylcarbonylamine chloride (4k). Target compound 4k was obtained (60 mg, 44.4% yield) as a yellow amorphous solid from compound 3 (100 mg, 0.259 mmol), pyridine (83 μL, 1.036 mmol), and p-methoxybenzoyl chloride (97 mg, 0.57 mmol) using a procedure similar to that for 4a. 1H-NMR (400 MHz, DMSO-d6): δ 3.21 (t, J = 6 Hz, 2H, ArCH2CH2N), 3.88 (s, 3H, NHCOArOCH3), 4.06 (s, 3H, OCH3), 4.10 (s, 3H, OCH3), 4.95 (t, J = 6 Hz, 2H, ArCH2CH2N), 6.15 (s, 2H, OCH2O), 7.09 (s, 1H, Ar–H), 7.14 (d, J = 8.8 Hz, 2H, Ar–H), 7.92 (s, 1H, Ar–H), 8.19 (d, J = 8.8 Hz, 2H, Ar–H), 8.40 (s, 1H, Ar–H), 8.75 (s, 1H, Ar–H), 9.92 (s, 1H, Ar–H), 10.77 (s, 1H, ArNHCO); 13C-NMR: (100 MHz, DMSO-d6) δ 26.35, 55.17, 55.54, 57.06, 62.07, 102.05, 106.00, 108.42, 113.67 (2 × C), 117.00, 120.66, 121.26, 121.96, 126.12, 127.49, 130.34 (2 × C), 130.81, 131.13, 136.73, 141.27, 145.71, 147.62, 149.78, 150.04, 162.33, 166.08; ESI–MS (m/z): 485.2 [M − Cl]+.
Quaternary berberine-12-N-p-nitrophenylcarbonylamine chloride (4l). Target compound 4l was obtained (36 mg, 26% yield) as a yellow amorphous solid from compound 3 (100 mg, 0.259 mmol), pyridine (83 μL, 1.036 mmol), and p-nitrobenzoyl chloride (106 mg, 0.57 mmol) using a procedure similar to that for 4a. 1H-NMR (400 MHz, DMSO-d6): δ 3.22 (t, J = 6 Hz, 2H, ArCH2CH2N), 4.07 (s, 3H, OCH3), 4.12 (s, 3H, OCH3), 4.95 (t, J = 6 Hz, 2H, ArCH2CH2N), 6.15 (s, 2H, OCH2O), 7.09 (s, 1H, Ar–H), 7.93 (s, 1H, Ar–H), 8.39-8.47 (m, 5H, Ar–H), 8.77 (s, 1H, Ar–H), 9.94 (s, 1H, Ar–H), 11.17 (s, 1H, ArNHCO); 13C-NMR: (100 MHz, DMSO-d6) δ 26.32, 55.23, 57.16, 62.11, 102.07, 106.11, 108.42, 116.82, 120.56, 121.27, 122.69, 123.55 (2 × C), 127.77, 129.85 (2 × C), 130.28, 130.86, 137.07, 139.87, 141.93, 145.92, 147.60, 149.42, 149.84, 149.98, 165.22; ESI–MS (m/z): 500.2 [M − Cl]+.
Quaternary berberine-12-N-t-butylcarbonylamine chloride (4m). Target compound 4m was obtained (30 mg, 24.6% yield) as a yellow amorphous solid from compound 3 (100 mg, 0.259 mmol), pyridine (83 μL, 1.036 mmol), and pivaloyl chloride (70 μL, 0.57 mmol) using a procedure similar to that for 4a. 1H-NMR (400 MHz, DMSO-d6): δ 1.39 (s, 9H, NHCOC(CH3)3), 3.21 (t, J = 6 Hz, 2H, ArCH2CH2N), 4.05 (s, 3H, OCH3), 4.09 (s, 3H, OCH3), 4.94 (t, J = 6 Hz, 2H, ArCH2CH2N), 6.18 (s, 2H, OCH2O), 7.11 (s, 1H, Ar–H), 7.68 (s, 1H, Ar–H), 8.11 (s, 1H, Ar–H), 8.38 (s, 1H, Ar–H), 9.91 (s, 1H, Ar–H), 10.02 (s, 1H, ArNHCO); 13C-NMR: (100 MHz, DMSO-d6) δ 26.33, 27.30 (3 × C), 55.08, 57.13, 62.06, 102.14, 105.12, 108.53, 116.54, 120.54, 121.22, 123.06, 127.87, 130.77, 130.96, 136.58, 141.50, 145.85, 147.77, 149.91, 150.03, 177.67; ESI–MS (m/z): 435.3 [M − Cl]+.
12-Aminotetrahydroberberine (5). NiCl2·6H2O (855.7 mg, 3.6 mmol) was added into a solution containing compound 2 (300 mg, 0.72 mmol) in a mixed solution of THF/MeOH (12 mL, v/v = 1:1). Then, NaBH4 (272 mg, 7.2 mmol) was added batchwise. The reaction was performed at 66 °C for 20 min under stirring until the raw material was completely reacted. The reaction mixture was filtered. The filtrate was evaporated under reduced pressure. The residue was dissolved in CH2Cl2 solvent. The solution was washed in a separatory funnel, first, three times using water, then one time using saturated aqueous NaCl solution. The organic layer was dried using anhydrous MgSO4, and then filtered. The filtrate was concentrated under reduced pressure to yield compound 5 (213 mg, 83.5% yield) as a hazel amorphous solid. 1H-NMR (400 MHz, DMSO-d6): δ 2.07–3.34 (m, 8H), 3.59 (s, 3H, OCH3), 3.69 (s, 3H, OCH3), 3.96 (d, J = 15.2 Hz, 1H), 4.68 (s, 2H, ArNH2), 5.94, 5.96 (2 × br s, 2H, OCH2O), 6.24 (s, 1H, Ar-H), 6.66 (s, 1H, Ar-H), 6.96 (s, 1H, Ar-H); 13C-NMR: (100 MHz, DMSO-d6) δ 28.96, 31.63, 50.95, 53.67, 55.29, 59.09, 59.72, 97.39, 100.52, 105.90, 108.00, 110.63, 127.40, 128.07, 131.40, 135.26, 142.24, 145.36, 145.64, 150.08; ESI–MS (m/z): 355.3 [M + H]+.
Tetrahydroberberine-12-N,N-dimethylamine (6a). To a stirred solution of compound 5 (200 mg, 0.564 mmol) in CH2Cl2 (8 mL), we added aqueous 37% formaldehyde (186 μL, 2.48 mmol), sodium triacetoxyborohydride (597 mg, 2.82 mmol) and HOAc (16 drops). The reaction mixture was stirred for 2h at room temperature until the raw material was completely reacted. A small amount of saturated aqueous NaHCO3 solution was added into the reaction mixture dropwise to make the solution alkaline (pH = 8). The solution was stirred for 2h at room temperature, and then extracted using CH2Cl2 three times in a separatory funnel. The organic layer was washed, first, three times using water, then, one time using saturated aqueous NaCl solution. Then, the organic layer was dried with anhydrous MgSO4 and filtered. The filtrate was concentrated under reduced pressure. The residue was purified using silica gel CC eluted using a mixed solvent of CHCl3/CH3OH (v/v = 80:1) to yield 6a (205 mg, 95% yield) as a hazel solid. 1H-NMR (400 MHz, DMSO-d6): δ 2.32–3.41 (m, 8H), 2.59 (s, 6H, N(CH3)2), 3.68 (s, 3H, OCH3), 3.79 (s, 3H, OCH3), 4.04 (d, J = 16 Hz, 1H), 5.94, 5.96 (2 × br s, 2H, OCH2O), 6.65 (s, 1H, Ar–H), 6.68 (s, 1H, Ar–H), 6.86 (s, 1H, Ar–H); 13C-NMR (100 MHz, DMSO-d6): δ 29.08, 33.45, 44.08 (2 × C), 50.54, 53.80, 55.67, 58.96, 59.51, 100.57, 102.32, 105.77, 108.11, 121.61, 127.63, 128.89, 131.05, 140.06, 145.46, 145.70, 148.09, 149.84; ESI–MS (m/z): 383.4 [M + H]+.
Tetrahydroberberine-12-N,N-diethylamine (6b). Target compound 6b was obtained (277 mg, 96% yield) as a hazel solid from compound 5 (250 mg, 0.705 mmol), aqueous 40% acetaldehyde (313 μL, 3.1 mmol), sodium triacetoxyborohydride (747 mg, 3.525 mmol), and HOAc (16 drops) using a procedure similar to that for 6a. 1H-NMR (400 MHz, CDCl3): δ 0.98 (t, J = 7.2 Hz, 6H, N(CH2CH3)2), 2.47-2.68 (m, 3H), 2.94 (t, J = 7.2 Hz, 4H, N(CH2CH3)2), 3.11-3.21 (m, 2H), 3.41–3.56 (m, 3H), 3.82 (s, 3H, OCH3), 3.84 (s, 3H, OCH3), 4.23 (d, J = 16 Hz,1H), 5.93 (s, 2H, OCH2O), 6.60 (s, 1H, Ar–H), 6.61 (s, 1H, Ar–H), 6.82 (s, 1H, Ar–H); 13C-NMR: (100 MHz, CDCl3) δ 12.76 (2 × C), 29.71, 33.76, 47.89 (2 × C), 51.60, 54.53, 56.09, 59.91, 60.33, 100.89, 105.68, 105.97, 108.50, 125.29, 127.93, 129.02, 131.41, 141.47, 145.51, 145.99, 146.23, 150.29; ESI–MS (m/z): 411.3 [M + H]+.
Tetrahydroberberine-12-N,N-di-n-propylamine (6c). Target compound 6c was obtained (200 mg, 54% yield) as a hazel solid from compound 5 (300 mg, 0.846 mmol), propionaldehyde (147.4 μL, 2.03 mmol), sodium triacetoxyborohydride (537.8 mg, 2.538 mmol), and HOAc (12 drops) using a procedure similar to that for 6a. 1H-NMR (400 MHz, CDCl3): δ 0.83 (t, J = 7.2 Hz, 6H, N(CH2CH2CH3)2), 1.43 (m, 4H), 2.51 (m, 1H), 2.61 (m, 1H), 2.67 (ov, 1H), 2.82 (m, 4H), 3.13 (ov, 1H), 3.19 (ov, 1H), 3.40 (d, J = 10.0 Hz, 1H), 3.49 (m, 1H), 3.53 (d, J = 16.0 Hz), 3.82 (s, 3H, 10-OCH3), 3.83 (s, 3H, 9-OCH3), 4.23 (d, J = 16.0 Hz, 1H), 5.92, 5.93 (2 × br s, 2H, OCH2O), 6.60 (s, 1H, Ar–H), 6.62 (s, 1H, Ar–H), 6.78 (s, 1H, Ar–H); 13C-NMR: (100 MHz, CDCl3) δ 11.92 (2 × C), 20.53 (2 × C), 29.73, 33.79, 51.64, 54.57, 56.11 (3 × C), 59.93, 60.32, 100.88, 105.71, 105.86, 108.51, 124.97, 127.96, 128.98, 131.39, 141.38, 145.98, 146.26 (2 × C), 150.28; ESI–MS (m/z): 439.3 [M + H]+.
Tetrahydroberberine-12-N,N-di-n-butylamine (6d). Target compound 6d was obtained (287 mg, 87.2% yield) as a hazel solid from compound 5 (250 mg, 0.705 mmol), n-butyraldehyde (152 μL, 1.69 mmol), sodium triacetoxyborohydride (448 mg, 2.115 mmol), and HOAc (10 drops) using a procedure similar to that for 6a. 1H-NMR (400 MHz, DMSO-d6): δ 0.83 (t, J = 7.2 Hz, 6H, N(CH2CH2CH2CH3)2), 1.21–1.40 (m, 8H), 2.24–3.39 (m, 8H), 2.83 (t, J = 6.8 Hz, 4H, N(CH2CH2CH2CH3)2), 3.70 (s, 3H, OCH3), 3.76 (s, 3H, OCH3), 4.04 (d, J = 16 Hz, 1H), 5.96 (s, 2H, OCH2O), 6.68 (s, 1H, Ar-H), 6.73 (br s, 2H, Ar–H); 13C-NMR: (100 MHz, DMSO-d6) δ 13.92 (2 × C), 19.93 (2 × C), 28.98, 29.12 (2 × C), 33.34, 50.63, 53.36 (2 × C), 53.71, 55.73, 58.97, 59.51, 100.62, 105.17, 105.55, 108.20, 124.41, 127.63, 128.58, 131.10, 140.65, 145.47, 145.55, 145.73, 149.85; ESI–MS (m/z): 467.3 [M + H]+.
Tetrahydroberberine-12-N,N-di-n-pentylamine (6e). Target compound 6e was obtained (320 mg, 91.8% yield) as a hazel oil from compound 5 (250 mg, 0.705 mmol), n-valeraldehyde (180 μL, 1.69 mmol), sodium triacetoxyborohydride (448 mg, 2.115 mmol), and HOAc (10 drops) using a procedure similar to that for 6a. 1H-NMR (400 MHz, CDCl3): δ 0.85 (t, J = 6.8 Hz, 6H, N(CH2CH2CH2CH2CH3)2), 1.24 (m, 8H), 1.40 (m, 4H), 2.46–2.68 (m, 3H), 2.85 (t, J = 7.2 Hz, 4H, N(CH2CH2CH2CH2CH3)2), 3.11–3.20 (m, 2H), 3.39-3.55 (m, 3H), 3.83 (br s, 6H, OCH3), 4.23 (d, J = 15.6 Hz,1H), 5.92 (s, 2H, OCH2O), 6.60 (s, 1H, Ar–H), 6.62 (s, 1H, Ar–H), 6.79 (s, 1H, Ar-H); 13C-NMR: (100 MHz, CDCl3) δ 14.30 (2 × C), 22.74 (2 × C), 27.06 (2 × C), 29.72, 29.79 (2 × C), 33.79, 51.67, 54.19 (2 × C), 54.55, 56.09, 59.91, 60.33, 100.89, 105.65, 105.88, 108.50, 124.94, 127.93, 128.95, 131.41, 141.33, 145.98, 146.29 (2 × C), 150.26; ESI–MS (m/z): 495.4 [M + H]+.
Tetrahydroberberine-12-N,N-di-n-hexylamine (6f). Target compound 6f was obtained (230 mg, 78% yield) as a hazel oil from compound 5 (200 mg, 0.564 mmol), n-hexanal (164 μL, 1.354 mmol), sodium triacetoxyborohydride (358 mg, 1.692 mmol), and HOAc (10 drops) using a procedure similar to that for 6a. 1H-NMR (400 MHz, CDCl3): δ 0.85 (t, J = 6.8 Hz, 6H, N(CH2CH2CH2CH2CH2CH3)2), 1.23 (m, 12H), 1.40 (m, 4H), 2.46-2.68 (m, 3H), 2.85 (t, J = 7.2 Hz, 4H, N(CH2CH2CH2CH2CH2CH3)2)), 3.12–3.20 (m, 2H), 3.39–3.56 (m, 3H), 3.82 (s, 3H, OCH3), 3.83 (s, 3H, OCH3), 4.23 (d, J = 15.6 Hz,1H), 5.92 (s, 2H, OCH2O), 6.60 (s, 1H, Ar-H), 6.62 (s, 1H, Ar-H), 6.79 (s, 1H, Ar-H); 13C-NMR: (100 MHz, CDCl3) δ 14.17 (2 × C), 22.84 (2 × C), 27.22 (2 × C), 27.33 (2 × C), 29.69, 31.88 (2 × C), 33.77, 51.63, 54.21 (2 × C), 54.52, 56.08, 59.89, 60.32, 100.88, 105.64, 105.87, 108.48, 124.88, 127.89, 128.92, 131.38, 141.31, 145.99, 146.29 (2 × C), 150.25; ESI–MS (m/z): 523.4 [M + H]+.
Tetrahydroberberine-12-N,N-di-n-heptylamine (6g). Target compound 6g was obtained (272 mg, 87.6% yield) as a hazel oil from compound 5 (200 mg, 0.564 mmol), n-heptanal (189 μL, 1.354 mmol), sodium triacetoxyborohydride (358 mg, 1.692 mmol), and HOAc (10 drops) using a procedure similar to that for 6a. 1H-NMR (400 MHz, CDCl3): δ 0.85 (t, J = 6.8 Hz, 6H, N(CH2CH2(CH2)4CH3)2)), 1.23 (m, 16H), 1.40 (m, 4H), 2.46–2.68 (m, 3H), 2.85 (t, J = 7.2 Hz, 4H, N(CH2CH2(CH2)4CH3)2)), 3.12–3.21 (m, 2H), 3.39-3.55 (m, 3H), 3.82 (s, 3H, OCH3), 3.83 (s, 3H, OCH3), 4.23 (d, J = 16 Hz,1H), 5.92 (s, 2H, OCH2O), 6.60 (s, 1H, Ar–H), 6.62 (s, 1H, Ar–H), 6.79 (s, 1H, Ar–H); 13C-NMR: (100 MHz, CDCl3) δ 14.20 (2 × C), 22.75 (2 × C), 27.39 (2 × C), 27.52 (2 × C), 29.36 (2 × C), 29.68, 32.05 (2 × C), 33.74, 51.63, 54.21 (2 × C), 54.51, 56.08, 59.89, 60.32, 100.88, 105.65, 105.87, 108.48, 124.90, 127.88, 128.89, 131.36, 141.32, 146.01, 146.30 (2 × C), 150.26; ESI–MS (m/z): 551.4 [M + H]+.
Tetrahydroberberine-12-N,N-di-n-octylamine (6h). Target compound 6h was obtained (290 mg, 88.8% yield) as a hazel oil from compound 5 (200 mg, 0.564 mmol), n-octanal (300 μL, 1.92 mmol), sodium triacetoxyborohydride (478 mg, 2.256 mmol), and HOAc (13 drops) using a procedure similar to that for 6a. 1H-NMR (400 MHz, CDCl3): δ 0.85 (t, J = 6.8 Hz, 6H, N(CH2CH2(CH2)5CH3)2), 1.23 (m, 20H), 1.40 (m, 4H), 2.46-2.68 (m, 3H), 2.85 (t, J = 7.2 Hz, 4H, N(CH2CH2(CH2)5CH3)2), 3.12-3.21 (m, 2H), 3.39–3.55 (m, 3H), 3.82 (s, 3H, OCH3), 3.83 (s, 3H, OCH3), 4.23 (d, J = 16 Hz, 1H), 5.92 (s, 2H, OCH2O), 6.60 (s, 1H, Ar–H), 6.61 (s, 1H, Ar–H), 6.78 (s, 1H, Ar–H); 13C-NMR: (150 MHz, CDCl3) δ 14.23 (2 × C), 22.78 (2 × C), 27.39 (2 × C), 27.57 (2 × C), 29.51 (2 × C), 29.66 (3 × C), 31.98 (2 × C), 33.76, 51.65, 54.23 (2 × C), 54.53, 56.10, 59.91, 60.34, 100.89, 105.68, 105.88, 108.49, 124.92, 127.89, 128.93, 131.39, 141.33, 146.01, 146.31 (2 × C), 150.27; ESI–MS (m/z): 579.4 [M + H]+.
Tetrahydroberberine-12-N,N-di-n-nonylamine (6i). Target compound 6i was obtained (303 mg, 88.5% yield) as a hazel oil from compound 5 (200 mg, 0.564 mmol), n-nonanal (330 μL, 1.92 mmol), sodium triacetoxyborohydride (478 mg, 2.256 mmol), and HOAc (13 drops) using a procedure similar to that for 6a. 1H-NMR (400 MHz, CDCl3): δ 0.86 (t, J = 6.8 Hz, 6H, N(CH2CH2(CH2)6CH3)2), 1.23 (m, 24H), 1.39 (m, 4H), 2.46-2.68 (m, 3H), 2.85 (t, J = 7.2 Hz, 4H, N(CH2CH2(CH2)6CH3)2), 3.12–3.20 (m, 2H), 3.39–3.55 (m, 3H), 3.82 (s, 3H, OCH3), 3.83 (s, 3H, OCH3), 4.23 (d, J = 15.6 Hz, 1H), 5.92 (s, 2H, OCH2O), 6.60 (s, 1H, Ar-H), 6.61 (s, 1H, Ar-H), 6.79 (s, 1H, Ar-H); 13C NMR: (100 MHz, CDCl3) δ 14.25 (2 × C), 22.80 (2 × C), 27.38 (2 × C), 27.57 (2 × C), 29.43 (2 × C), 29.71 (3 × C), 29.81 (2 × C), 32.00 (2 × C), 33.75, 51.65, 54.22 (2 × C), 54.53, 56.08, 59.90, 60.32, 100.87, 105.65, 105.88, 108.48, 124.91, 127.89, 128.91, 131.38, 141.32, 146.00, 146.29, 146.30, 150.25; ESI-MS (m/z): 607.5 [M + H]+.
Tetrahydroberberine-12-N,N-di-n-decylamine (6j). Target compound 6j was obtained (323 mg, 90% yield) as a hazel oil from compound 5 (200 mg, 0.564 mmol), n-decanal (362 μL, 1.92 mmol), sodium triacetoxyborohydride (478 mg, 2.256 mmol), and HOAc (13 drops) using a procedure similar to that for 6a. 1H-NMR (400 MHz, CDCl3): δ 0.87 (t, J = 6.8 Hz, 6H, N(CH2CH2(CH2)7CH3)2), 1.23 (m, 28H), 1.39 (m, 4H), 2.46-2.68 (m, 3H), 2.85 (t, J = 7.2 Hz, 4H, N(CH2CH2(CH2)7CH3)2), 3.12-3.20 (m, 2H), 3.39-3.55 (m, 3H), 3.82 (s, 3H, OCH3), 3.83 (s, 3H, OCH3), 4.23 (d, J = 16 Hz, 1H), 5.92 (s, 2H, OCH2O), 6.60 (s, 1H, Ar–H), 6.61 (s, 1H, Ar–H), 6.79 (s, 1H, Ar–H); 13C-NMR: (100 MHz, CDCl3) δ 14.25 (2 × C), 22.82 (2 × C), 27.38 (2 × C), 27.58 (2 × C), 29.46 (2 × C), 29.72 (3 × C), 29.73 (2 × C), 29.87 (2 × C), 32.04 (2 × C), 33.77, 51.66, 54.21 (2 × C), 54.55, 56.08, 59.91, 60.32, 100.87, 105.65, 105.88, 108.49, 124.92, 127.90, 128.93, 131.40, 141.32, 145.99, 146.30 (2 × C), 150.25; ESI–MS (m/z): 635.5 [M + H]+.
Tetrahydroberberine-12-N,N-di-n-undecylamine (6k). Target compound 6k was obtained (344 mg, 92% yield) as a hazel oil from compound 5 (200 mg, 0.564 mmol), n-undecaldehyde (393 μL, 1.92 mmol), sodium triacetoxyborohydride (478 mg, 2.256 mmol), and HOAc (13 drops) using a procedure similar to that for 6a. 1H-NMR (400 MHz, CDCl3): δ 0.87 (t, J = 6.8 Hz, 6H, N(CH2CH2(CH2)8CH3)2), 1.23 (m, 32H), 1.39 (m, 4H), 2.45-2.68 (m, 3H), 2.85 (t, J = 7.2 Hz, 4H, N(CH2CH2(CH2)8CH3)2), 3.12–3.20 (m, 2H), 3.38–3.55 (m, 3H), 3.82 (s, 3H, OCH3), 3.83 (s, 3H, OCH3), 4.23 (d, J = 16 Hz, 1H), 5.92 (s, 2H, OCH2O), 6.60 (s, 1H, Ar-H), 6.61 (s, 1H, Ar-H), 6.78 (s, 1H, Ar-H); 13C–NMR: (100 MHz, CDCl3) δ 14.27 (2 × C), 22.83 (2 × C), 27.39 (2 × C), 27.59 (2 × C), 29.49 (2 × C), 29.73 (3 × C) 29.76 (2 × C), 29.79 (2 × C), 29.87 (2 × C), 32.06 (2 × C), 33.77, 51.67, 54.22 (2 × C), 54.55, 56.09, 59.92, 60.34, 100.88, 105.66, 105.89, 108.50, 124.93, 127.90, 128.95, 131.41, 141.33, 145.99, 146.30 (2 × C), 150.27; ESI–MS (m/z): 663.6 [M + H]+.
Tetrahydroberberine-12-N,N-di-n-dodecylamine (6l). Target compound 6l was obtained (330 mg, 84.7% yield) as a hazel oil from compound 5 (200 mg, 0.564 mmol), n-dodecaldehyde (300 μL, 1.35 mmol), sodium triacetoxyborohydride (358 mg, 1.69 mmol), and HOAc (10 drops) using a procedure similar to that for 6a. 1H NMR (400 MHz, CDCl3): δ 0.87 (t, J = 6.8 Hz, 6H, N(CH2CH2(CH2)9CH3)2), 1.23 (m, 36H), 1.40 (m, 4H), 2.45–2.68 (m, 3H), 2.85 (t, J = 7.2 Hz, 4H, N(CH2CH2(CH2)9CH3)2), 3.11–3.21 (m, 2H), 3.39–3.55 (m, 3H), 3.82 (s, 3H, OCH3), 3.83 (s, 3H, OCH3), 4.23 (d, J = 15.6 Hz,1H), 5.92 (s, 2H, OCH2O), 6.60 (s, 1H, Ar–H), 6.61 (s, 1H, Ar–H), 6.78 (s, 1H, Ar–H); 13C-NMR: (100 MHz, CDCl3) δ 14.27 (2 × C), 22.83 (2 × C), 27.39 (2 × C), 27.59 (2 × C), 29.51 (2 × C), 29.72 (3 × C), 29.79 (6 × C), 29.88 (2 × C), 32.06 (2 × C), 33.73, 51.63, 54.22 (2 × C), 54.52, 56.09, 59.90, 60.33, 100.88, 105.66, 105.89, 108.50, 124.91, 127.89, 128.90, 131.37, 141.28, 146.00, 146.30 (2 × C), 150.26; ESI–MS (m/z): 691.6 [M + H]+.
Quaternary berberine-12-N,N-dimethylamine chloride (7a). DDQ (220 mg) was weighed and dissolved in 8 mL of CH2Cl2. The DDQ solution was added dropwise into a solution containing compound 6a (185 mg, 0.484 mmol) in CH2Cl2 (4 mL) under stirring. The reaction solution was stirred for 2h at room temperature until the raw material was completely reacted. The reaction mixture was concentrated to remove the solvent under reduced pressure, then aqueous 10% HCl solution (8 mL) was added into the residue. After stirring the mixture for 2h at room temperature, aqueous 1 N NaOH solution was added to make the solution alkaline. Then, the mixture was stirred for 0.5 h at room temperature and extracted using a mixed solution of CHCl3/CH3OH (v/v = 10:1) in a separatory funnel. The organic layer was washed, first, three times using water, then, one time using saturated aqueous NaCl solution. The organic layer was dried using anhydrous MgSO4 and filtered. The filtrate was concentrated under reduced pressure to remove the solvent. The residue was purified using silica gel CC eluted using a mixed solution of CHCl3/MeOH (v/v = 25:1) to yield 7a (38 mg, 19% yield) as a red solid. 1H-NMR (400 MHz, DMSO-d6): δ 2.95 (s, 6H, N(CH3)2), 3.20 (t, J = 6 Hz, 2H, ArCH2CH2N), 4.00 (s, 3H, OCH3), 4.09 (s, 3H, OCH3), 4.92 (t, J = 6 Hz, 2H, ArCH2CH2N), 6.17 (s, 2H, OCH2O), 7.09 (s, 1H, Ar-H), 7.56 (s, 1H, Ar–H), 7.87 (s, 1H, Ar–H), 8.59 (s, 1H, Ar–H), 9.81 (s, 1H, Ar–H); 13C-NMR (100 MHz, DMSO-d6): δ 26.40, 44.83 (2 × C), 55.10, 56.95, 61.90, 102.05, 105.82, 108.40, 114.15, 116.65, 120.56, 122.14, 127.61, 130.71, 136.75, 138.24, 145.49, 147.57, 147.76, 149.77, 150.89; ESI–MS (m/z): 379.3 [M − Cl]+.
Quaternary berberine-12-N,N-diethylamine chloride (7b). Target compound 7b was obtained (32 mg, 11.6% yield) as a red solid from compound 6b (257 mg, 0.626 mmol) and DDQ (285 mg DDQ in 8 mL CH2Cl2) using a procedure similar to that for 7a. 1H-NMR (400 MHz, DMSO-d6): δ 1.02 (t, J = 7.2 Hz, 6H, N(CH2CH3)2), 3.20 (t, J = 6 Hz, 2H, ArCH2CH2N), 3.27 (q, J = 7.2 Hz, 4H, N(CH2CH3)2), 4.04 (s, 3H, OCH3), 4.07 (s, 3H, OCH3), 4.92 (t, J = 6 Hz, 2H, ArCH2CH2N), 6.18 (s, 2H, OCH2O), 7.10 (s, 1H, Ar–H), 7.72 (s, 1H, Ar–H), 7.76 (s, 1H, Ar–H), 8.60 (s, 1H, Ar–H), 9.84 (s, 1H, Ar–H); 13C-NMR: (100 MHz, DMSO-d6) δ 12.04 (2 × C), 26.39, 47.68 (2 × C), 55.08, 57.15, 61.93, 102.09, 105.51, 108.46, 116.25, 118.78, 120.56, 122.02, 130.41, 130.85, 137.02, 139.37, 144.48, 145.59, 147.79, 149.82, 150.79; ESI–MS (m/z): 407.3 [M − Cl]+.
Quaternary berberine-12-N,N-di-n-propylamine chloride (7c). Target compound 7c was obtained (50 mg, 46.6% yield) as a red solid from compound 6c (100 mg, 0.228 mmol) and DDQ (103 mg DDQ in 6 mL CH2Cl2) using a procedure similar to that for 7a. 1H-NMR (400 MHz, DMSO-d6): δ 0.85 (t, J = 7.2 Hz, 6H, N(CH2CH2CH3)2), 1.47 (m, 4H, N(CH2CH2CH3)2), 3.19 (m, 6H, ArCH2CH2N, N(CH2CH2CH3)2), 4.03 (s, 3H, OCH3), 4.07 (s, 3H, OCH3), 4.92 (t, J = 6 Hz, 2H, ArCH2CH2N), 6.18 (s, 2H, OCH2O), 7.10 (s, 1H, Ar–H), 7.65 (s, 1H, Ar–H), 7.78 (s, 1H, Ar–H), 8.61 (s, 1H, Ar–H), 9.84 (s, 1H, Ar–H); 13C-NMR: (150 MHz, DMSO-d6) δ 11.62 (2 × C), 19.83 (2 × C), 26.38, 55.06, 55.75 (2 × C), 57.17, 61.93, 102.12, 105.11, 108.52, 115.94, 119.14, 120.50, 121.97, 130.25, 130.87, 136.97, 139.53, 145.08, 145.71, 147.78, 149.85, 150.85; ESI–MS (m/z): 435.3 [M − Cl]+.
Quaternary berberine-12-N,N-di-n-butylamine chloride (7d). Target compound 7d was obtained (100 mg, 46.7% yield) as a red solid from compound 6d (200 mg, 0.429 mmol) and DDQ (195 mg DDQ in 8 mL CH2Cl2) using a procedure similar to that for 7a. 1H-NMR (400 MHz, DMSO-d6): δ 0.84 (t, J = 7.2 Hz, 6H, N(CH2CH2CH2CH3)2), 1.28(m, 4H, N(CH2CH2CH2CH3)2), 1.45 (m, 4H, N(CH2CH2CH2CH3)2), 3.21 (m, 6H, ArCH2CH2N, N(CH2CH2CH2CH3)2), 4.03 (s, 3H, OCH3), 4.06 (s, 3H, OCH3), 4.92 (t, J = 6 Hz, 2H, ArCH2CH2N), 6.19 (s, 2H, OCH2O), 7.10 (s, 1H, Ar–H), 7.63 (s, 1H, Ar–H), 7.76 (s, 1H, Ar–H), 8.57 (s, 1H, Ar–H), 9.84 (s, 1H, Ar–H); 13C-NMR: (100 MHz, DMSO-d6) δ 13.86 (2 × C), 19.93 (2 × C), 26.37, 28.74 (2 × C), 53.65 (2 × C), 55.06, 57.18, 61.95, 102.14, 105.09, 108.55, 115.89, 118.86, 120.50, 121.99, 130.14, 130.88, 136.95, 139.43, 145.15, 145.76, 147.79, 149.86, 150.84; ESI–MS (m/z): 463.3 [M − Cl]+.
Quaternary berberine-12-N,N-di-n-pentylamine chloride (7e). Target compound 7e was obtained (70 mg, 11.4% yield) as a red solid from compound 6e (273 mg, 0.552 mmol) and DDQ (251 mg DDQ in 10 mL CH2Cl2) using a procedure similar to that for 7a. 1H-NMR (400 MHz, DMSO-d6): δ 0.82 (t, J = 6.4 Hz, 6H, N(CH2CH2CH2CH2CH3)2), 1.26(m, 8H, N(CH2CH2CH2CH2CH3)2), 1.47 (m, 4H, N(CH2CH2CH2CH2CH3)2), 3.20 (m, 6H, ArCH2CH2N, N(CH2CH2CH2CH2CH3)2), 4.04 (s, 3H, OCH3), 4.06 (s, 3H, OCH3), 4.92 (t, J = 6 Hz, 2H, ArCH2CH2N), 6.18 (s, 2H, OCH2O), 7.11 (s, 1H, Ar–H), 7.60 (s, 1H, Ar–H), 7.78 (s, 1H, Ar–H), 8.59 (s, 1H, Ar–H), 9.84 (s, 1H, Ar–H); 13C-NMR: (100 MHz, DMSO-d6) δ 13.99 (2 × C), 21.96 (2 × C), 26.31 (2 × C), 26.37, 29.03 (2 × C), 53.85 (2 × C), 55.04, 57.19, 61.95, 102.16, 104.96, 108.58, 115.87, 118.95, 120.50, 121.99, 130.19, 130.88, 136.94, 139.49, 145.22, 145.77, 147.80, 149.87, 150.86; ESI–MS (m/z): 491.3 [M − Cl]+.
Quaternary berberine-12-N,N-di-n-hexylamine chloride (7f). Target compound 7f was obtained (52 mg, 29.4% yield) as a red solid from compound 6f (167 mg, 0.32 mmol) and DDQ (145 mg DDQ in 6 mL CH2Cl2) using a procedure similar to that for 7a. 1H-NMR (400 MHz, DMSO-d6): δ 0.81 (t, J = 6.4 Hz, 6H, N(CH2CH2CH2CH2CH2CH3)2), 1.22(m, 12H, N(CH2CH2CH2CH2CH2CH3)2), 1.47 (m, 4H, N(CH2CH2CH2CH2CH2CH3)2), 3.20 (m, 6H, ArCH2CH2N, N(CH2CH2CH2CH2CH2CH3)2), 4.04 (s, 3H, OCH3), 4.06 (s, 3H, OCH3), 4.92 (t, J = 6 Hz, 2H, ArCH2CH2N), 6.18 (s, 2H, OCH2O), 7.11 (s, 1H, Ar–H), 7.59 (s, 1H, Ar–H), 7.78 (s, 1H, Ar–H), 8.58 (s, 1H, Ar–H), 9.85 (s, 1H, Ar–H); 13C-NMR: (100 MHz, DMSO-d6) δ 13.84 (2 × C), 22.13 (2 × C), 26.37, 26.43 (2 × C), 26.60 (2 × C), 31.05 (2 × C), 53.85 (2 × C), 55.04, 57.18, 61.94, 102.17, 104.93, 108.58, 115.87, 118.97, 120.49, 121.98, 130.21, 130.88, 136.93, 139.50, 145.27, 145.76, 147.80, 149.86, 150.87; ESI–MS (m/z): 519.4 [M − Cl]+.
Quaternary berberine-12-N,N-di-n-heptylamine chloride (7g). Target compound 7g was obtained (50 mg, 22.7% yield) as a red solid from compound 6g (208 mg, 0.378 mmol) and DDQ (172 mg DDQ in 8 mL CH2Cl2) using a procedure similar to that for 7a. 1H-NMR (400 MHz, DMSO-d6): δ 0.80 (m, 6H, N(CH2CH2(CH2)4CH3)2), 1.20 (m, 16H, ArN(CH2CH2(CH2)4CH3)2), 1.47 (m, 4H, N(CH2CH2(CH2)4CH3)2), 3.19 (m, 6H, ArCH2CH2N, N(CH2CH2(CH2)4CH3)2), 4.04 (s, 3H, OCH3), 4.07 (s, 3H, OCH3), 4.93 (t, J = 6 Hz, 2H, ArCH2CH2N), 6.18 (s, 2H, OCH2O), 7.12 (s, 1H, Ar–H), 7.58 (s, 1H, Ar–H), 7.79 (s, 1H, Ar-H), 8.59 (s, 1H, Ar-H), 9.87 (s, 1H, Ar-H); 13C-NMR: (100 MHz, DMSO-d6) δ 13.88(2 × C), 22.02 (2 × C), 26.38, 26.65 (2 × C), 26.73 (2 × C), 28.49 (2 × C), 31.32 (2 × C), 53.83 (2 × C), 55.04, 57.18, 61.95, 102.16, 104.87, 108.59, 115.84, 119.09, 120.49, 121.96, 130.26, 130.89, 136.91, 139.57, 145.28, 145.80, 147.80, 149.86, 150.89; ESI–MS (m/z): 547.4 [M − Cl]+.
Quaternary berberine-12-N,N-di-n-octylamine chloride (7h). Target compound 7h was obtained (70 mg, 30% yield) as a red solid from compound 6h (220 mg, 0.380 mmol) and DDQ (173 mg DDQ in 8 mL CH2Cl2) using a procedure similar to that for 7a. 1H-NMR (400 MHz, DMSO-d6): δ 0.80 (t, J = 6.4 Hz, 6H, N(CH2CH2(CH2)5CH3)2), 1.20 (m, 20H, N(CH2CH2(CH2)5CH3)2), 1.46 (m, 4H, N(CH2CH2(CH2)5CH3)2), 3.19 (m, 6H, ArCH2CH2N, N(CH2CH2(CH2)5CH3)2), 4.03 (s, 3H, OCH3), 4.06 (s, 3H, OCH3), 4.92 (t, J = 6 Hz, 2H, ArCH2CH2N), 6.17 (s, 2H, OCH2O), 7.11 (s, 1H, Ar–H), 7.58 (s, 1H, Ar–H), 7.78 (s, 1H, Ar–H), 8.58 (s, 1H, Ar–H), 9.84 (s, 1H, Ar–H); 13C-NMR: (100 MHz, DMSO-d6) δ 13.87 (2 × C), 22.04 (2 × C), 26.38, 26.62 (2 × C), 26.76 (2 × C), 28.74 (2 × C), 28.77 (2 × C), 31.19 (2 × C), 53.79(2 × C), 55.05, 57.17, 61.94, 102.16, 104.87, 108.59, 115.84, 119.06, 120.49, 121.97, 130.24, 130.88, 136.91, 139.55, 145.29, 145.80, 147.81, 149.86, 150.89; ESI–MS (m/z): 575.5 [M − Cl]+.
Quaternary berberine-12-N,N-di-n-nonylamine chloride (7i). Target compound 7i was obtained (87 mg, 34.4% yield) as a red solid from compound 6i (240 mg, 0.396 mmol) and DDQ (180 mg DDQ in 6 mL CH2Cl2) using a procedure similar to that for 7a. 1H-NMR (400 MHz, DMSO-d6): δ 0.81 (t, J = 6.4 Hz, 6H, N(CH2CH2(CH2)6CH3)2), 1.18 (m, 24H, N(CH2CH2(CH2)6CH3)2), 1.46 (m, 4H, N(CH2CH2(CH2)6CH3)2), 3.18 (m, 6H, ArCH2CH2N, N(CH2CH2(CH2)6CH3)2), 4.04 (s, 3H, OCH3), 4.06 (s, 3H, OCH3), 4.92 (t, J = 5.6 Hz, 2H, ArCH2CH2N), 6.17 (s, 2H, OCH2O), 7.11 (s, 1H, Ar–H), 7.57 (s, 1H, Ar–H), 7.79 (s, 1H, Ar–H), 8.58 (s, 1H, Ar–H), 9.85 (s, 1H, Ar–H); 13C-NMR: (100 MHz, DMSO-d6) δ 13.91 (2 × C), 22.03 (2 × C), 26.38, 26.62 (2 × C), 26.75 (2 × C), 28.61 (2 × C), 28.81 (2 × C), 29.03 (2 × C), 31.23 (2 × C), 53.78 (2 × C), 55.05, 57.17, 61.94, 102.15, 104.85, 108.60, 115.82, 119.09, 120.48, 121.97, 130.24, 130.88, 136.90, 139.57, 145.30, 145.81, 147.81, 149.86, 150.89; ESI–MS (m/z): 603.5 [M − Cl]+.
Quaternary berberine-12-N,N-di-n-decylamine chloride (7j). Target compound 7j was obtained (47 mg, 14.2% yield) as a red solid from compound 6j (315 mg, 0.496 mmol) and DDQ (225 mg DDQ in 8 mL CH2Cl2) using a procedure similar to that for 7a. 1H-NMR (400 MHz, DMSO-d6): δ 0.82 (t, J = 6.4 Hz, 6H, N(CH2CH2(CH2)7CH3)2), 1.18 (m, 28H, N(CH2CH2(CH2)7CH3)2), 1.46 (m, 4H, N(CH2CH2(CH2)7CH3)2), 3.18 (m, 6H, ArCH2CH2N, N(CH2CH2(CH2)7CH3)2), 4.04 (s, 3H, OCH3), 4.06 (s, 3H, OCH3), 4.92 (t, J = 6 Hz, 2H, ArCH2CH2N), 6.17 (s, 2H, OCH2O), 7.11 (s, 1H, Ar-H), 7.57 (s, 1H, Ar–H), 7.79 (s, 1H, Ar–H), 8.58 (s, 1H, Ar–H), 9.85 (s, 1H, Ar–H); 13C-NMR: (100 MHz, DMSO-d6) δ 13.92 (2 × C), 22.07 (2 × C), 26.37, 26.61 (2 × C), 26.74 (2 × C), 28.66 (2 × C), 28.79 (2 × C), 28.91 (2 × C), 29.08 (2 × C), 31.23 (2 × C), 53.76 (2 × C), 55.05, 57.16, 61.93, 102.14, 104.84, 108.60, 115.81, 119.08, 120.48, 121.96, 130.23, 130.87, 136.89, 139.55, 145.30, 145.80, 147.80, 149.85, 150.88; ESI–MS (m/z): 631.5 [M − Cl]+.
Quaternary berberine-12-N,N-di-n-undecylamine chloride (7k). Target compound 7k was obtained (55 mg, 16% yield) as a red solid from compound 6k (328 mg, 0.495 mmol) and DDQ (225 mg DDQ in 8 mL CH2Cl2) using a procedure similar to that for 7a. 1H-NMR (400 MHz, DMSO-d6): δ 0.83 (t, J = 6.4 Hz, 6H, N(CH2CH2(CH2)8CH3)2), 1.18 (m, 32H, N(CH2CH2(CH2)8CH3)2), 1.46 (m, 4H, N(CH2CH2(CH2)8CH3)2), 3.18 (m, 6H, ArCH2CH2N, N(CH2CH2(CH2)8CH3)2), 4.04 (s, 3H, OCH3), 4.06 (s, 3H, OCH3), 4.92 (t, J = 5.6 Hz, 2H, ArCH2CH2N), 6.17 (s, 2H, OCH2O), 7.11 (s, 1H, Ar–H), 7.57 (s, 1H, Ar–H), 7.79 (s, 1H, Ar–H), 8.58 (s, 1H, Ar–H), 9.85 (s, 1H, Ar–H); 13C-NMR: (100 MHz, DMSO-d6) δ 13.93 (2 × C), 22.07 (2 × C), 26.37, 26.60 (2 × C), 26.73 (2 × C), 28.66 (2 × C), 28.79 (2 × C), 28.96 (4×C), 29.07 (2 × C), 31.27 (2 × C), 53.74 (2 × C), 55.05, 57.16, 61.93, 102.14, 104.83, 108.60, 115.80, 119.08, 120.47, 121.96, 130.23, 130.87, 136.89, 139.56, 145.30, 145.81, 147.80, 149.85, 150.88; ESI–MS (m/z): 659.6 [M − Cl]+.
Quaternary berberine-12-N,N-di-n-dodecylamine chloride (7l). Target compound 7l was obtained (100 mg, 38.3% yield) as a red solid from compound 6l (250 mg, 0.362 mmol) and DDQ (165 mg DDQ in 8 mL CH2Cl2) using a procedure similar to that for 7a. 1H-NMR (400 MHz, DMSO-d6): δ 0.84 (t, J = 6.4 Hz, 6H, N(CH2CH2(CH2)9CH3)2), 1.18 (m, 36H, N(CH2CH2(CH2)9CH3)2), 1.47 (m, 4H, N(CH2CH2(CH2)9CH3)2), 3.18 (m, 6H, ArCH2CH2N, N(CH2CH2(CH2)9CH3)2), 4.04 (s, 3H, OCH3), 4.06 (s, 3H, OCH3), 4.92 (t, J = 6 Hz, 2H, ArCH2CH2N), 6.17 (s, 2H, OCH2O), 7.11 (s, 1H, Ar–H), 7.56 (s, 1H, Ar–H), 7.79 (s, 1H, Ar–H), 8.58 (s, 1H, Ar–H), 9.84 (s, 1H, Ar–H); 13C-NMR: (100 MHz, DMSO-d6) δ 13.94 (2 × C), 22.09 (2 × C), 26.37, 26.60 (2 × C), 26.73 (2 × C), 28.70 (2 × C), 28.78 (2 × C), 28.96 (4×C), 29.01 (2 × C), 29.07 (2 × C), 31.28 (2 × C), 53.74 (2 × C), 55.05, 57.17, 61.94, 102.14, 104.81, 108.61, 115.79, 119.10, 120.47, 121.97, 130.22, 130.88, 136.89, 139.59, 145.31, 145.83, 147.80, 149.86, 150.89; ESI–MS (m/z): 687.6 [M − Cl]+.
Tetrahydroberberine-12-N-n-propylamine derivatives (8). To a stirred solution of compound 5 (200 mg, 0.564 mmol) in CH2Cl2 (4 mL), we added propionaldehyde (41 μL, 0.564 mmol), sodium sodium triacetoxyborohydride (143 mg, 0.677 mmol), and acetic acid (10 drops), respectively. The reaction solution was stirred for 2h at room temperature until the raw material was completely reacted. Then, aqueous saturated NaHCO3 solution was added dropwise to make the solution alkaline (pH = 8). The solution was extracted three times (20mL/time) using CH2Cl2 in a separatory funnel. The organic layer was washed, first, three times using water, then one time using aqueous saturated NaCl solution. The organic layer was dried using anhydrous MgSO4 and filtered. The filtrate was concentrated under reduced pressure to remove the solvent. The residue was purified using silica gel CC eluted using a mixed solution of CH2Cl2/MeOH (v/v = 80:1) to yield 8 (140 mg, 62.8% yield) as a light grey solid. 1H-NMR (400 MHz, CDCl3): δ 1.02 (t, J = 7.6 Hz, 3H, NHCH2CH2CH3), 1.69 (m, 2H), 2.34–3.58 (m, 10H), 3.77 (s, 3H, OCH3), 3.86 (s, 3H, OCH3), 4.20 (d, J = 15.2 Hz,1H), 5.93 (s, 2H, OCH2O), 6.17 (s, 1H, Ar–H), 6.60 (s, 1H, Ar–H), 6.77 (s, 1H, Ar–H); ESI–MS (m/z): 397.3 [M + H]+.
Quaternary berberine-12-N-n-propylamine iodide (9). To a stirred solution of compound 8 (130 mg, 0.328 mmol) in anhydrous ethanol (8 mL), we added iodine (250 mg, 0.984 mmol). After the reaction solution was refluxed for 10 h, a second batch of iodine (125 mg, 0.492 mmol) was added to the solution. The reaction solution was refluxed for another 10 h and a third batch of iodine (83 mg, 0.328 mmol) was added. After the reaction was refluxed for another 10 h, aqueous saturated sodium thiosulfate solution (2 mL) was added to quench the reaction. The reaction mixture was filtered. The filter cake was dissolved in a mixed solution of CHCl3/MeOH (v/v = 10:1). The organic layer was washed, first, three times using water, then, one time using aqueous saturated NaCl solution in a separatory funnel. Then, the organic layer was dried by anhydrous MgSO4 and filtered. The filtrate was concentrated under reduced pressure to remove the solvent. The residue was purified by silica gel CC eluted using a mixed solution of CH2Cl2/MeOH (v/v = 200:1) to yield 9 (35 mg, 15% yield) as a reddish brown solid. 1H-NMR (400 MHz, DMSO-d6): δ 1.04 (t, J = 7.2 Hz, 3H, NHCH2CH2CH3), 1.38 (m, 2H, NHCH2CH2CH3), 1.77 (m, 2H, NHCH2CH2CH3), 3.19 (t, J = 6 Hz, 2H, Ar-CH2CH2N), 3.90 (s, 3H, OCH3), 4.04 (s, 3H, OCH3), 4.88 (t, J = 6 Hz, 2H, Ar–CH2CH2N), 6.18 (s, 2H, OCH2O), 6.84 (s, 1H, Ar–H), 6.97 (t, J = 5.6 Hz, 1H, NHCH2CH2CH3), 7.08 (s, 1H, Ar–H), 7.88 (s, 1H, Ar–H), 8.87 (s, 1H, Ar–H), 9.61 (s, 1H, Ar–H); ESI–MS (m/z): 393.3 [M − I]+.