Synthesis and Evaluation of [18F]FEtLos and [18F]AMBF3Los as Novel 18F-Labelled Losartan Derivatives for Molecular Imaging of Angiotensin II Type 1 Receptors
Abstract
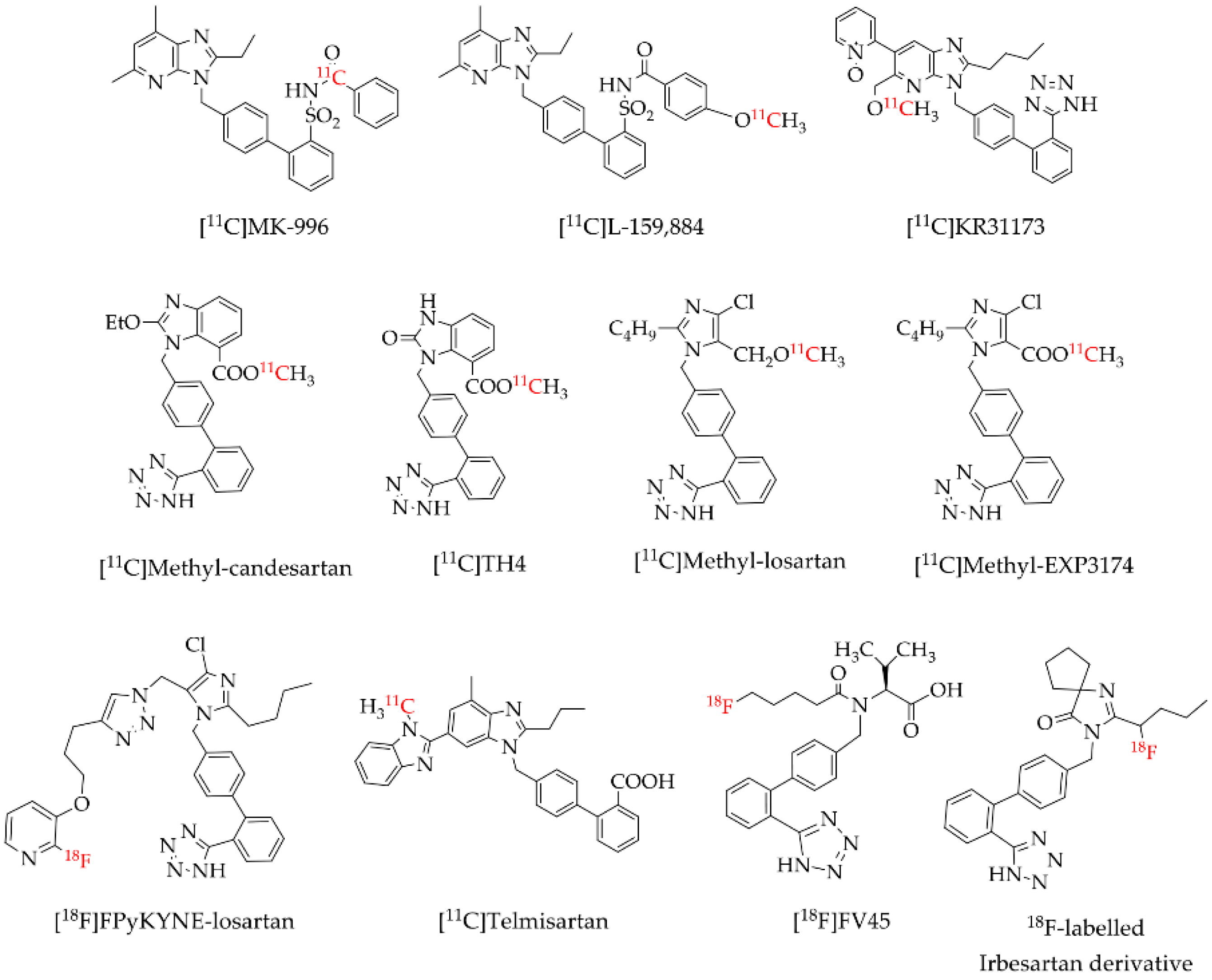
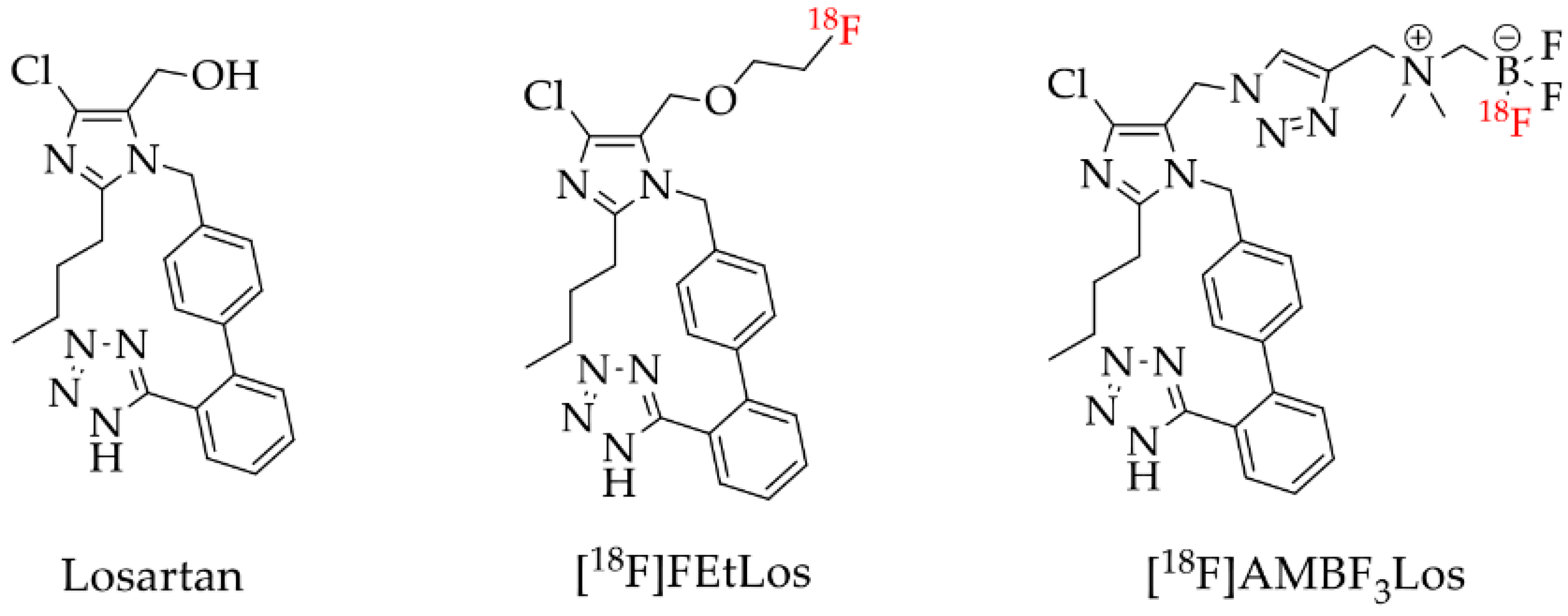
1. Introduction
2. Results
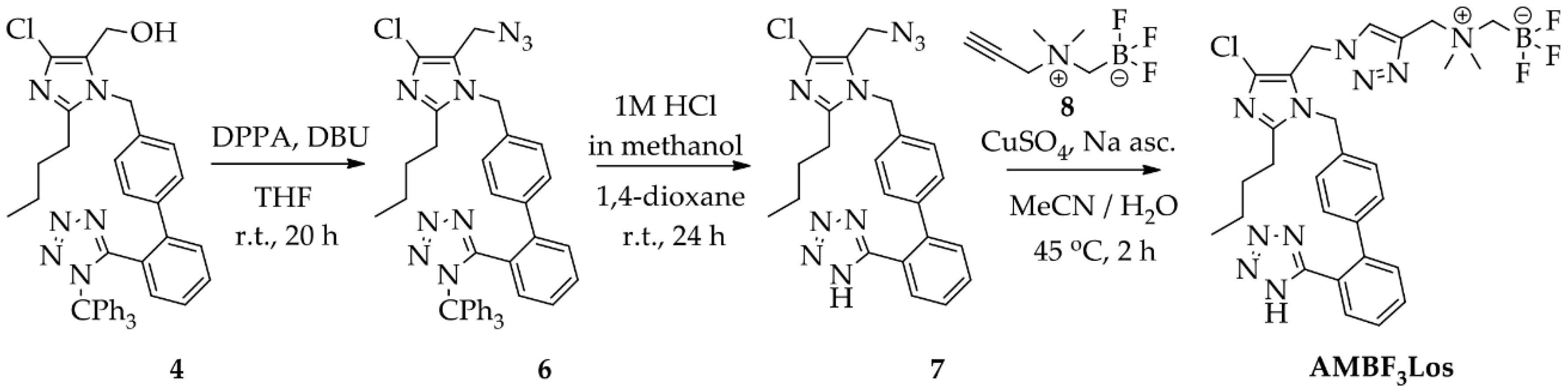
2.1. Chemistry
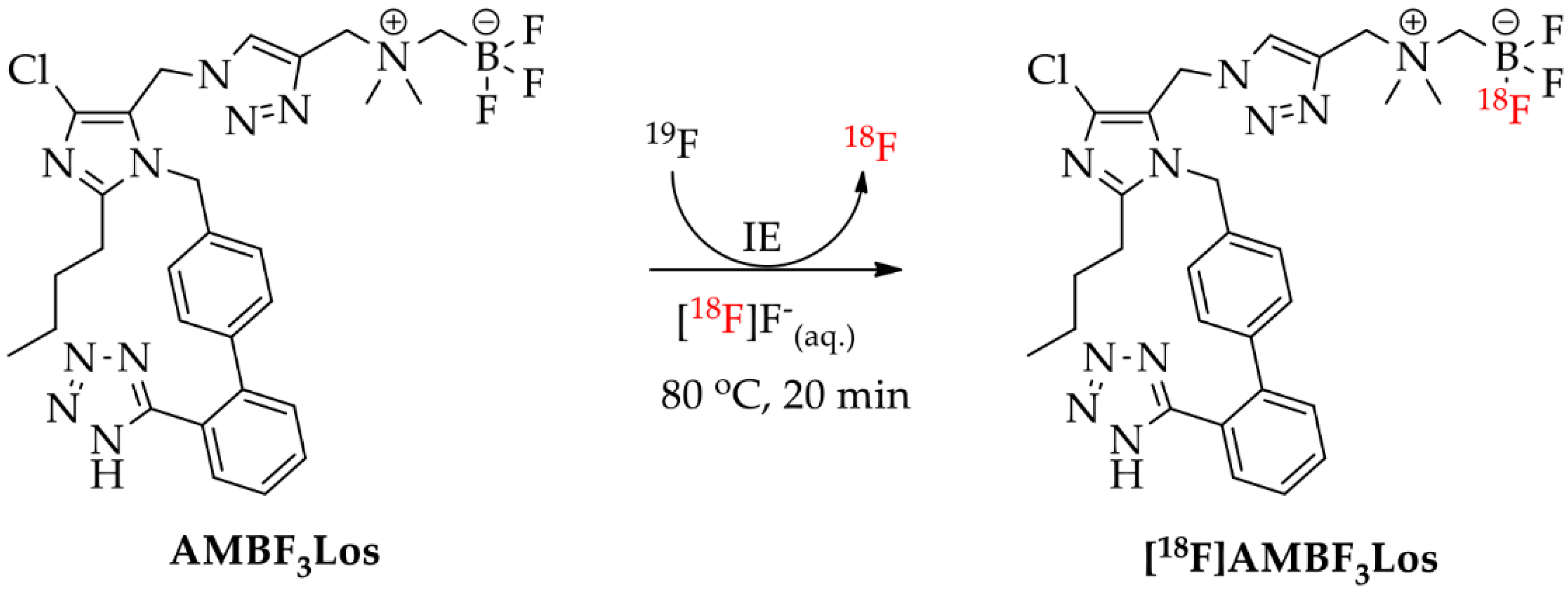
2.2. Radiochemistry
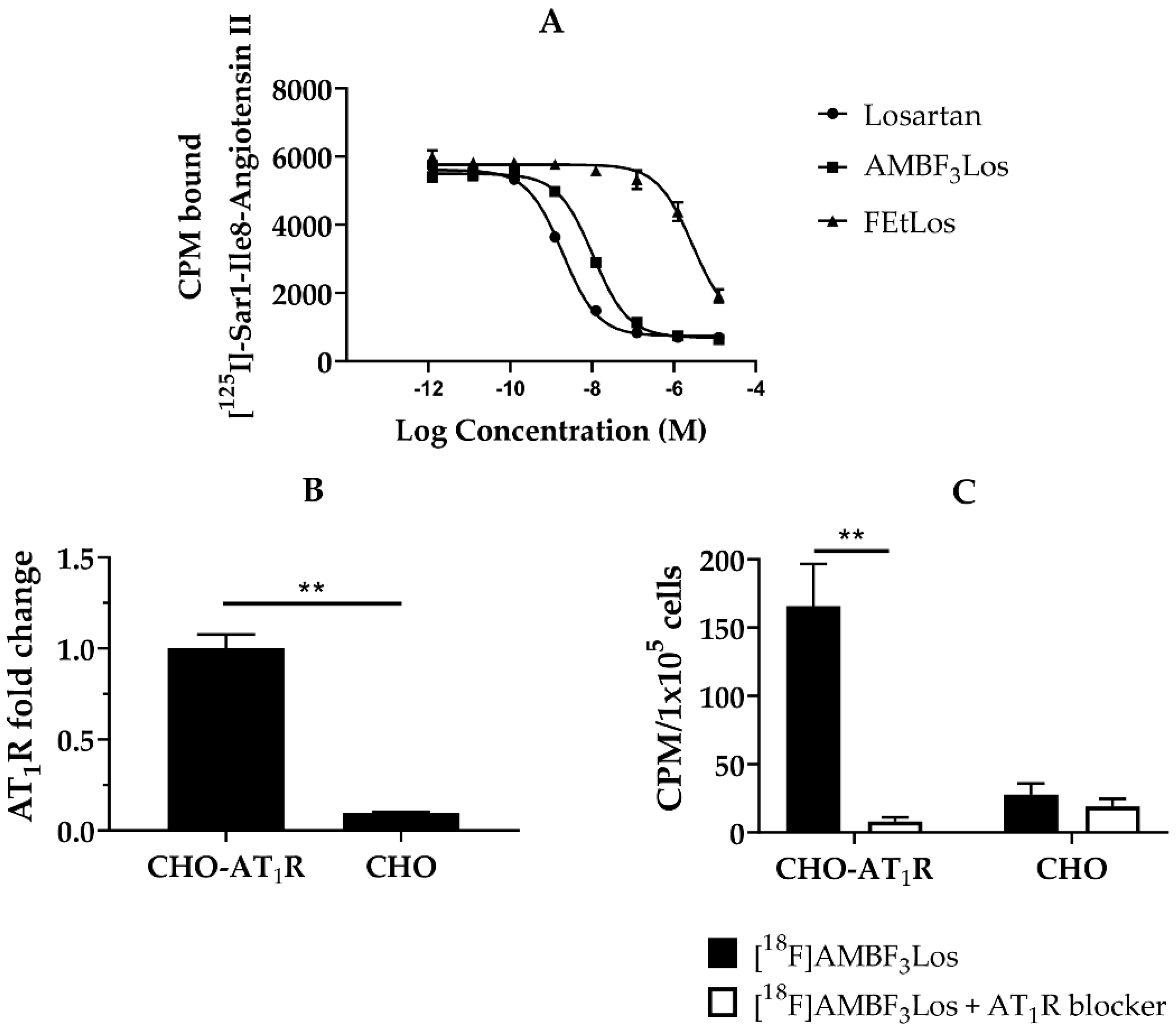
2.3. In Vitro Binding Assays
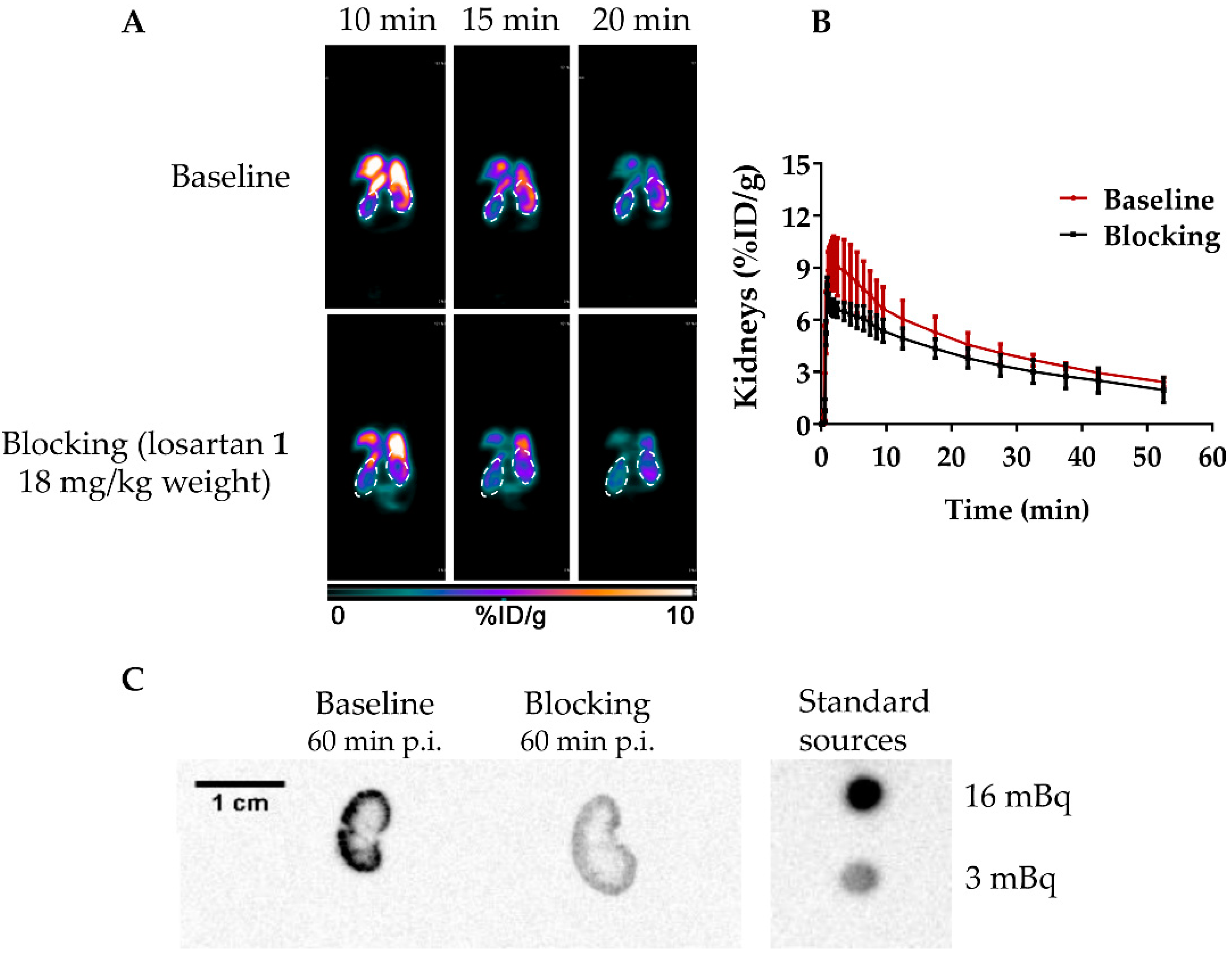
2.4. In Vivo Assays
3. Discussion
4. Materials and Methods
4.1. General Methods
4.2. Chemistry
4.2.1. Synthesis of 2-Butyl-4-chloro-5-(2-fluoroethoxy)methyl-1-[(2′-(1H-(1-(triphenylmethyl)) tetrazol-5-yl) biphenyl-4-yl)methyl]-1H-imidazole (5)
4.2.2. Synthesis of 2-Butyl-4-chloro-5-(2-fluoroethoxy)methyl-1-[(2′-(1H-tetrazol-5-yl)biphenyl-4-yl)methyl]-1H-imidazole (FEtLos)
4.2.3. Synthesis of 2-Butyl-4-chloro-5-(azidomethyl)-1-[(2′-(1H-tetrazol-5-yl)biphenyl-4-yl)methyl]-1H-imidazole (7)
4.2.4. Synthesis of 2-Butyl-4-chloro-5-[((1H-1,2,3-triazol-4-yl)-(N,N-dimethyl-ammoniomethyl-trifluoroborate)methyl)methyl]-1-[(2′-(1H-tetrazol-5-yl)biphenyl-4-yl)methyl]-1H-imidazole (AMBF3Los)
4.3. Radiochemistry
4.3.1. Concentration and [18F]F− Drying
4.3.2. Radiosynthesis of 2-[18F]Fluoroethyl Tosylate (2*)
4.3.3. Radiosynthesis of [18F]FEtLos
4.3.4. Radiosynthesis of [18F]AMBF3Los
Radiosynthesis Using Low Activities (0.7 ± 0.2 GBq) of [18F]F−
Radiosynthesis Using High Activities (61 ± 23 GBq) of [18F]F−
4.3.5. Quality Control
Log D7.4 Measurements
4.4. In Vitro Assays
4.4.1. Competition Binding Assays in Membranes Containing Human AT1R
4.4.2. Cell Culture
4.4.3. Real-Time Polymerase Chain Reaction (RT-PCR)
4.4.4. Cell Binding Assays
4.5. In Vivo Assays
4.5.1. Animals
4.5.2. Imaging and Biodistribution Studies
4.5.3. Autoradiography
4.5.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Crowley, S.D.; Coffman, T.M. Recent advances involving the renin-angiotensin system. Exp. Cell Res. 2012, 318, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Unger, T. The role of the renin-angiotensin system in the development of cardiovascular disease. Am. J. Cardiol. 2002, 89, 3A–9A, discussion 10A. [Google Scholar] [CrossRef]
- Xu, F.; Mao, C.; Hu, Y.; Rui, C.; Xu, Z.; Zhang, L. Cardiovascular effects of losartan and its relevant clinical application. Curr. Med. Chem. 2009, 16, 3841–3857. [Google Scholar] [PubMed]
- Husain, K.; Hernandez, W.; Ansari, R.A.; Ferder, L. Inflammation, oxidative stress and renin angiotensin system in atherosclerosis. World J. Biol. Chem. 2015, 6, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Brenner, B.M.; Cooper, M.E.; de Zeeuw, D.; Keane, W.F.; Mitch, W.E.; Parving, H.H.; Remuzzi, G.; Snapinn, S.M.; Zhang, Z.; Shahinfar, S.; et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 2001, 345, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Bai, Y.; Ni, Z.; Quiroz, Y.; Pandian, R.; Rodriguez-Iturbe, B. Intra-renal angiotensin II/AT1 receptor, oxidative stress, inflammation, and progressive injury in renal mass reduction. J. Pharmacol. Exp. Ther. 2007, 323, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.M.; Wang, J.Y.; Chen, T.T.; Wu, Y.C.; Wu, Y.L.; Lin, H.T.; Chang, T.J.; Chu, N.F.; Su, S.L.; Chen, J.S.; et al. Angiotensin-converting enzyme inhibitors or angiotensin receptor blocker monotherapy retard deterioration of renal function in Taiwanese chronic kidney disease population. Sci. Rep. 2019, 9, 2694. [Google Scholar]
- Joglar, B.; Rodriguez-Pallares, J.; Rodriguez-Perez, A.I.; Rey, P.; Guerra, M.J.; Labandeira-Garcia, J.L. The inflammatory response in the MPTP model of Parkinson’s disease is mediated by brain angiotensin: relevance to progression of the disease. J. Neurochem. 2009, 109, 656–669. [Google Scholar]
- Li, N.C.; Lee, A.; Whitmer, R.A.; Kivipelto, M.; Lawler, E.; Kazis, L.E.; Wolozin, B. Use of angiotensin receptor blockers and risk of dementia in a predominantly male population: Prospective cohort analysis. BMJ 2010, 340, b5465. [Google Scholar] [CrossRef]
- Labandeira-Garcia, J.L.; Rodríguez-Perez, A.I.; Garrido-Gil, P.; Rodriguez-Pallares, J.; Lanciego, J.L.; Guerra, M.J. Brain Renin-Angiotensin System and Microglial Polarization: Implications for Aging and Neurodegeneration. Front. Aging Neurosci. 2017, 9, 129. [Google Scholar] [CrossRef]
- Jackson, L.; Eldahshan, W.; Fagan, S.C.; Ergul, A. Within the Brain: The Renin Angiotensin System. Int. J. Mol. Sci. 2018, 19, 876. [Google Scholar] [CrossRef] [PubMed]
- George, A.J.; Thomas, W.G.; Hannan, R.D. The renin-angiotensin system and cancer: old dog, new tricks. Nat. Rev. Cancer 2010, 10, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Wegman-Ostrosky, T.; Soto-Reyes, E.; Vidal-Millán, S.; Sánchez-Corona, J. The renin-angiotensin system meets the hallmarks of cancer. J. Renin Angiotensin Aldosterone Syst. 2015, 16, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Munro, M.J.; Wickremesekera, A.C.; Davis, P.F.; Marsh, R.; Tan, S.T.; Itinteang, T. Renin-angiotensin system and cancer: a review. Integr. Cancer Sci. Ther. 2017, 4, 1–6. [Google Scholar]
- Mathews, W.B.; Burns, H.D.; Dannals, R.F.; Ravert, H.T.; Naylor, E.M. Carbon-11 Labeling of a Potent, Nonpeptide, AT1-Selective Angiotensin-I1 Receptor Antagonist: MK-996. J. Label Compd. Radiopharm. 1995, 36, 729–737. [Google Scholar] [CrossRef]
- Hamill, T.G.; Burns, H.D.; Dannals, R.F.; Mathews, W.B.; Musachio, J.L.; Ravert, H.T.; Naylor, E.M. Development of [11C] L-159,884: a radiolabelled, nonpeptide angiotensin II antagonist that is useful for angiotensin II, AT1 receptor imaging. Appl. Radiat. Isot. 1996, 47, 211–218. [Google Scholar] [CrossRef]
- Mathews, W.B.; Yoo, S.E.; Lee, S.H.; Scheffel, U.; Rauseo, P.A.; Zober, T.G.; Gocco, G.; Sandberg, K.; Ravert, H.T.; Dannals, R.F.; et al. A novel radioligand for imaging the AT1 angiotensin receptor with PET. Nucl. Med. Biol. 2004, 31, 571–574. [Google Scholar] [CrossRef]
- Hadizad, T.; Kirkpatrick, S.A.; Mason, S.; Burns, K.; Beanlands, R.S.; DaSilva, J.N. Novel O-[11C]methylated derivatives of candesartan as angiotensin II AT1 receptor imaging ligands: Radiosynthesis and ex vivo evaluation in rats. Bioorg. Med. Chem. 2009, 17, 7971–7977. [Google Scholar] [CrossRef]
- Iimori, H.; Hashizume, Y.; Sasaki, M.; Kajiwara, Y.; Sugimoto, Y.; Sugiyama, Y.; Watanabe, Y.; Senda, M. First automatic radiosynthesis of 11C labeled Telmisartan using a multipurpose synthesizer for clinical research use. Ann. Nucl. Med. 2011, 25, 333–337. [Google Scholar] [CrossRef]
- Hadizad, T.; Collins, J.; E Antoun, R.; S Beanlands, R.; N DaSilva, J. [11C] Methyl-losartan as a potential ligand for PET imaging angiotensin II AT1 receptors. J. Label. Compd. Radiopharm. 2011, 54, 754–757. [Google Scholar] [CrossRef]
- Arksey, N.; Hadizad, T.; Ismail, B.; Hachem, M.; Valdivia, A.C.; Beanlands, R.S.; deKemp, R.A.; DaSilva, J.N. Synthesis and evaluation of the novel 2-[¹⁸F]fluoro-3-propoxy-triazole-pyridine-substituted losartan for imaging AT1 receptors. Bioorg. Med. Chem. 2014, 22, 3931–3937. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hirano, M.; Werner, R.A.; Decker, M.; Higuchi, T. Novel 18 F-Labeled PET Imaging Agent FV45 Targeting the Renin - Angiotensin System. ACS Omega 2018, 3, 10460–10470. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Chen, X.; Hirano, M.; Arimitsu, K.; Kimura, H.; Higuchi, T.; Decker, M. F-Labeled Derivatives of Irbesartan for Angiotensin II Receptor PET Imaging. ChemMedChem 2018, 13, 2546–2557. [Google Scholar] [CrossRef] [PubMed]
- Ismail, B.; Hadizad, T.; Antoun, R.; Lortie, M.; deKemp, R.A.; Beanlands, R.S.; DaSilva, J.N. Evaluation of [(11)C]methyl-losartan and [(11)C]methyl-EXP3174 for PET imaging of renal AT1receptor in rats. Nucl. Med. Biol. 2015, 42, 850–857. [Google Scholar] [CrossRef]
- Conti, M.; Eriksson, L. Physics of pure and non-pure positron emitters for PET: A review and a discussion. EJNMMI Phys. 2016, 3, 8. [Google Scholar] [CrossRef]
- Cole, E.L.; Stewart, M.N.; Littich, R.; Hoareau, R.; Scott, P.J. Radiosyntheses using fluorine-18: The art and science of late stage fluorination. Curr. Top Med. Chem. 2014, 14, 875–900. [Google Scholar] [CrossRef]
- Jacobson, O.; Kiesewetter, D.O.; Chen, X. Fluorine-18 radiochemistry, labeling strategies and synthetic routes. Bioconjug. Chem. 2015, 26, 1–18. [Google Scholar] [CrossRef]
- Dézsi, C.A. The different therapeutic choices with ARBs. Which one to give? When? Why? Am. J. Cardiovasc. Drugs 2016, 16, 255–266. [Google Scholar] [CrossRef]
- Schoultz, B.W.; Reed, B.J.; Marton, J.; Willoch, F.; Henriksen, G. A fully automated radiosynthesis of [18F]fluoroethyl-diprenorphine on a single module by use of SPE cartridges for preparation of high quality 2-[18F]fluoroethyl tosylate. Molecules 2013, 18, 7271–7278. [Google Scholar] [CrossRef]
- Kniess, T.; Laube, M.; Brust, P.; Steinbach, J. 2-[18 F] Fluoroethyl tosylate–a versatile tool for building 18 F-based radiotracers for positron emission tomography. MedChemComm 2015, 6, 1714–1754. [Google Scholar] [CrossRef][Green Version]
- Kettenbach, K.; Schieferstein, H.; Ross, T.L. 18F-labeling using click cycloadditions. Biomed. Res. Int. 2014, 2014, 361329. [Google Scholar] [CrossRef] [PubMed]
- Glaser, M.; Robins, E.G. “Click labeling” in PET radiochemistry. J. Label Compd. Radiopharm. 2009, 52, 407–414. [Google Scholar] [CrossRef]
- Pretze, M.; Pietzsch, D.; Mamat, C. Recent trends in bioorthogonal click-radiolabeling reactions using fluorine-18. Molecules 2013, 18, 8618–8665. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Pourghiasian, M.; Radtke, M.A.; Lau, J.; Pan, J.; Dias, G.M.; Yapp, D.; Lin, K.S.; Bénard, F.; Perrin, D.M. An organotrifluoroborate for broadly applicable one-step 18F-labeling. Angew. Chem. Int. Ed. Engl. 2014, 53, 11876–11880. [Google Scholar] [CrossRef]
- Liu, Z.; Radtke, M.A.; Wong, M.Q.; Lin, K.S.; Yapp, D.T.; Perrin, D.M. Dual mode fluorescent (18)F-PET tracers: efficient modular synthesis of rhodamine-[cRGD]2-[(18)F]-organotrifluoroborate, rapid, and high yielding one-step (18)F-labeling at high specific activity, and correlated in vivo PET imaging and ex vivo fluorescence. Bioconjug. Chem. 2014, 25, 1951–1962. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, K.S.; Bénard, F.; Pourghiasian, M.; Kiesewetter, D.O.; Perrin, D.M.; Chen, X. One-step (18)F labeling of biomolecules using organotrifluoroborates. Nat. Protoc. 2015, 10, 1423–1432. [Google Scholar] [CrossRef]
- Bernard-Gauthier, V.; Aliaga, A.; Boudjemeline, M.; Hopewell, R.; Kostikov, A.; Rosa-Neto, P.; Thiel, A.; Schirrmacher, R. Syntheses and evaluation of carbon-11- and fluorine-18-radiolabeled pan-tropomyosin receptor kinase (Trk) inhibitors: exploration of the 4-aza-2-oxindole scaffold as Trk PET imaging agents. ACS Chem. Neurosci. 2015, 6, 260–276. [Google Scholar] [CrossRef]
- Pourghiasian, M.; Liu, Z.; Pan, J.; Zhang, Z.; Colpo, N.; Lin, K.S.; Perrin, D.M.; Bénard, F. (18)F-AmBF3-MJ9: a novel radiofluorinated bombesin derivative for prostate cancer imaging. Bioorg. Med. Chem. 2015, 23, 1500–1506. [Google Scholar] [CrossRef]
- Fukushima, K.; Bravo, P.E.; Higuchi, T.; Schuleri, K.H.; Lin, X.; Abraham, M.R.; Xia, J.; Mathews, W.B.; Dannals, R.F.; Lardo, A.C.; et al. Molecular hybrid positron emission tomography/computed tomography imaging of cardiac angiotensin II type 1 receptors. J. Am. Coll. Cardiol. 2012, 60, 2527–2534. [Google Scholar] [CrossRef]
- Valenta, I.; Szabo, Z.; Mathews, W.; Dannals, R.; Pomper, M.; Abraham, T.; Schindler, T. Abnormal regional increase of myocardial angiotensin II type 1 receptors in hypertrophic obstructive cardiomyopathy patients as determined with 11C-KR31173 and PET/CT. J. Nucl. Med. 2017, 58, 439. [Google Scholar]
- Szabo, Z.; Speth, R.C.; Brown, P.R.; Kerenyi, L.; Kao, P.F.; Mathews, W.B.; Ravert, H.T.; Hilton, J.; Rauseo, P.; Dannals, R.F.; et al. Use of positron emission tomography to study AT1 receptor regulation in vivo. J. Am. Soc. Nephrol. 2001, 12, 1350–1358. [Google Scholar] [PubMed]
- Zober, T.G.; Fabucci, M.E.; Zheng, W.; Brown, P.R.; Seckin, E.; Mathews, W.B.; Sandberg, K.; Szabo, Z. Chronic ACE inhibitor treatment increases angiotensin type 1 receptor binding in vivo in the dog kidney. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Lortie, M.; DaSilva, J.N.; Kirkpatrick, S.A.; Hadizad, T.; Ismail, B.A.; Beanlands, R.S.; deKemp, R.A. Analysis of [11C]methyl-candesartan kinetics in the rat kidney for the assessment of angiotensin II type 1 receptor density in vivo with PET. Nucl. Med. Biol. 2013, 40, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Hachem, M.; Tiberi, M.; Ismail, B.; Hunter, C.R.; Arksey, N.; Hadizad, T.; Beanlands, R.S.; deKemp, R.A.; DaSilva, J.N. Characterization of 18F-FPyKYNE-Losartan for Imaging AT1 Receptors. J. Nucl. Med. 2016, 57, 1612–1617. [Google Scholar] [CrossRef] [PubMed]
- Noda, A.; Fushiki, H.; Murakami, Y.; Sasaki, H.; Miyoshi, S.; Kakuta, H.; Nishimura, S. Brain penetration of telmisartan, a unique centrally acting angiotensin II type 1 receptor blocker, studied by PET in conscious rhesus macaques. Nucl. Med. Biol. 2012, 39, 1232–1235. [Google Scholar] [CrossRef] [PubMed]
- Verjans, J.W.; Lovhaug, D.; Narula, N.; Petrov, A.D.; Indrevoll, B.; Bjurgert, E.; Krasieva, T.B.; Petersen, L.B.; Kindberg, G.M.; Solbakken, M.; et al. Noninvasive imaging of angiotensin receptors after myocardial infarction. JACC Cardiovasc. Imaging 2008, 1, 354–362. [Google Scholar] [CrossRef]
- Breschi, M.C.; Calderone, V.; Digiacomo, M.; Martelli, A.; Martinotti, E.; Minutolo, F.; Rapposelli, S.; Balsamo, A. NO-sartans: A new class of pharmacodynamic hybrids as cardiovascular drugs. J. Med. Chem. 2004, 47, 5597–5600. [Google Scholar] [CrossRef]
- Carini, D.J.; Duncia, J.J.V.; Aldrich, P.E.; Chiu, A.T.; Johnson, A.L.; Pierce, M.E.; Price, W.A.; Santella, J.B.; Wells, G.J.; Wexler, R.R.; et al. Nonpeptide angiotensin II receptor antagonists: the discovery of a series of N-(biphenylylmethyl) imidazoles as potent, orally active antihypertensives. J. Med. Chem. 1991, 34, 2525–2547. [Google Scholar] [CrossRef]
- Vauquelin, G.; Fierens, F.L.; Gáborik, Z.; Le Minh, T.; De Backer, J.P.; Hunyady, L.; Vanderheyden, P.M. Role of basic amino acids of the human angiotensin type 1 receptor in the binding of the non-peptide antagonist candesartan. J. Renin Angiotensin Aldosterone Syst. 2001, 2, S32–S36. [Google Scholar] [CrossRef]
- Zhang, H.; Unal, H.; Gati, C.; Han, G.W.; Liu, W.; Zatsepin, N.A.; James, D.; Wang, D.; Nelson, G.; Weierstall, U.; et al. Structure of the Angiotensin receptor revealed by serial femtosecond crystallography. Cell 2015, 161, 833–844. [Google Scholar] [CrossRef]
- Neochoritis, C.G.; Zhao, T.; Dömling, A. Tetrazoles via Multicomponent Reactions. Chem. Rev. 2019, 119, 1970–2042. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.; Liu, Z.; Lin, K.S.; Pan, J.; Zhang, Z.; Vullo, D.; Supuran, C.T.; Perrin, D.M.; Bénard, F. Trimeric Radiofluorinated Sulfonamide Derivatives to Achieve In Vivo Selectivity for Carbonic Anhydrase IX-Targeted PET Imaging. J. Nucl. Med. 2015, 56, 1434–1440. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Pourghiasian, M.; Bénard, F.; Pan, J.; Lin, K.S.; Perrin, D.M. Preclinical evaluation of a high-affinity 18F-trifluoroborate octreotate derivative for somatostatin receptor imaging. J. Nucl. Med. 2014, 55, 1499–1505. [Google Scholar] [CrossRef]
- Gonçalves Nunes, P.S.; Zhang, Z.; Kuo, H.T.; Zhang, C.; Rousseau, J.R.; Rousseau, E.; Lau, J.; Kwon, D.; Carvalho, I.; Bénard, F.; et al. Synthesis and evaluation of an 18F-labeled trifluoroborate derivative of 2-nitroimidazole for imaging tumor hypoxia with positron emission tomography. J. Label Compd. Radiopharm. 2018, 61, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-S.; Benard, F.; Perrin, D.M. Organoboronates: Captors of 18F-fluoride for one-step radiofluorination of biomolecules. In Fluorine in Life Sciences: Pharmaceuticals, Medicinal Diagnostics, and Agrochemicals; Inc., E., Haufe, G., Leroux, F.R., Eds.; Academic Press: London, UK, 2019; pp. 519–549. [Google Scholar]
- Kalgutkar, A.S.; Daniels, J.S. Carboxylic acids and their bioisosteres. In Metabolism, Pharmacokinetics and Toxicity of Functional Groups: Impact of Chemical Buildins Blocks on ADMET; Smith, D.A., Ed.; Royal Society of Chemistry: Cambridge, UK, 2010; pp. 99–167. [Google Scholar]
- Watanabe, Y.; Shibata, K.; Kikkawa, F.; Kajiyama, H.; Ino, K.; Hattori, A.; Tsujimoto, M.; Mizutani, S. Adipocyte-derived leucine aminopeptidase suppresses angiogenesis in human endometrial carcinoma via renin-angiotensin system. Clin. Cancer Res. 2003, 9, 6497–6503. [Google Scholar]
- Suganuma, T.; Ino, K.; Shibata, K.; Kajiyama, H.; Nagasaka, T.; Mizutani, S.; Kikkawa, F. Functional expression of the angiotensin II type1 receptor in human ovarian carcinoma cells and its blockade therapy resulting in suppression of tumor invasion, angiogenesis, and peritoneal dissemination. Clin Cancer Res. 2005, 11, 2686–2694. [Google Scholar] [CrossRef]
- Uemura, H.; Hasumi, H.; Ishiguro, H.; Teranishi, J.; Miyoshi, Y.; Kubota, Y. Renin-angiotensin system is an important factor in hormone refractory prostate cancer. Prostate 2006, 66, 822–830. [Google Scholar] [CrossRef]
- Tanaka, N.; Miyajima, A.; Kosaka, T.; Shirotake, S.; Hasegawa, M.; Kikuchi, E.; Oya, M. Cis-dichlorodiammineplatinum upregulates angiotensin II type 1 receptors through reactive oxygen species generation and enhances VEGF production in bladder cancer. Mol. Cancer Ther. 2010, 9, 2982–2992. [Google Scholar] [CrossRef]
- Du, N.; Feng, J.; Hu, L.J.; Sun, X.; Sun, H.B.; Zhao, Y.; Yang, Y.P.; Ren, H. Angiotensin II receptor type 1 blockers suppress the cell proliferation effects of angiotensin II in breast cancer cells by inhibiting AT1R signaling. Oncol. Rep. 2012, 27, 1893–1903. [Google Scholar]
- Huang, M.M.; Guo, A.B.; Sun, J.F.; Chen, X.L.; Yin, Z.Y. Angiotensin II promotes the progression of human gastric cancer. Mol. Med. Rep. 2014, 9, 1056–1060. [Google Scholar] [CrossRef][Green Version]
- Coulson, R.; Liew, S.H.; Connelly, A.A.; Yee, N.S.; Deb, S.; Kumar, B.; Vargas, A.C.; O’Toole, S.A.; Parslow, A.C.; Poh, A.; et al. The angiotensin receptor blocker, Losartan, inhibits mammary tumor development and progression to invasive carcinoma. Oncotarget 2017, 8, 18640–18656. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Cao, J.; Melamed, A.; Worley, M.; Gockley, A.; Jones, D.; Nia, H.T.; Zhang, Y.; Stylianopoulos, T.; Kumar, A.S.; et al. Losartan treatment enhances chemotherapy efficacy and reduces ascites in ovarian cancer models by normalizing the tumor stroma. Proc. Natl. Acad. Sci. USA 2019, 116, 2210–2219. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.S.; Pan, J.; Amouroux, G.; Turashvili, G.; Mesak, F.; Hundal-Jabal, N.; Pourghiasian, M.; Lau, J.; Jenni, S.; Aparicio, S.; et al. In vivo radioimaging of bradykinin receptor b1, a widely overexpressed molecule in human cancer. Cancer Res. 2015, 75, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Oroujeni, M.; Abouzayed, A.; Lundmark, F.; Mitran, B.; Orlova, A.; Tolmachev, V.; Rosenström, U. Evaluation of Tumor-Targeting Properties of an Antagonistic Bombesin Analogue RM26 Conjugated with a Non-Residualizing Radioiodine Label Comparison with a Radiometal-Labelled Counterpart. Pharmaceutics 2019, 11, 380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, Z.; Lin, K.-S.; Pan, J.; Dude, I.; Hundal-Jabal, N.; Colpo, N.; Bénard, F. Preclinical melanoma imaging with 68Ga-labeled α-melanocyte-stimulating hormone derivatives using PET. Theranostics 2017, 7, 805–8013. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds FEtLos and AMBF3Los are available from the authors. |


| Tissues | Baseline | AT1R Blocking |
|---|---|---|
| Blood | 0.05 ± 0.03 | 0.08 ± 0.08 |
| Fat | 0.011 ± 0.004 | 0.008 ± 0.002 |
| Testis | 0.03 ± 0.01 | 0.029 ± 0.008 |
| Intestines | 63 ± 9 | 62 ± 3 |
| Stomach | 0.06 ± 0.05 | 2 ± 2 |
| Spleen | 0.2 ± 0.1 | 0.08 ± 0.03 |
| Liver | 0.5 ± 0.5 | 0.6 ± 0.3 |
| Pancreas | 0.05 ± 0.03 | 0.07 ± 0.06 |
| Adrenal glands | 0.1 ± 0.1 | 0.11 ± 0.06 |
| Kidneys | 3.5 ± 1.2 | 1.9 ± 1.3 |
| Lungs | 0.06 ± 0.03 | 0.08 ± 0.04 |
| Heart | 0.03 ± 0.01 | 0.04 ± 0.03 |
| Muscle | 0.02 ± 0.01 | 0.05 ± 0.04 |
| Bone | 0.11 ± 0.06 | 0.11 ± 0.03 |
| Brain | 0.006 ± 0.005 | 0.005 ± 0.004 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahylí Ortega Pijeira, M.; Sérgio Gonçalves Nunes, P.; Nascimento dos Santos, S.; Zhang, Z.; Pérez Nario, A.; Araujo Perini, E.; Miguel Turato, W.; Rodríguez Riera, Z.; Chammas, R.; H. Elsinga, P.; et al. Synthesis and Evaluation of [18F]FEtLos and [18F]AMBF3Los as Novel 18F-Labelled Losartan Derivatives for Molecular Imaging of Angiotensin II Type 1 Receptors. Molecules 2020, 25, 1872. https://doi.org/10.3390/molecules25081872
Sahylí Ortega Pijeira M, Sérgio Gonçalves Nunes P, Nascimento dos Santos S, Zhang Z, Pérez Nario A, Araujo Perini E, Miguel Turato W, Rodríguez Riera Z, Chammas R, H. Elsinga P, et al. Synthesis and Evaluation of [18F]FEtLos and [18F]AMBF3Los as Novel 18F-Labelled Losartan Derivatives for Molecular Imaging of Angiotensin II Type 1 Receptors. Molecules. 2020; 25(8):1872. https://doi.org/10.3390/molecules25081872
Chicago/Turabian StyleSahylí Ortega Pijeira, Martha, Paulo Sérgio Gonçalves Nunes, Sofia Nascimento dos Santos, Zhengxing Zhang, Arian Pérez Nario, Efrain Araujo Perini, Walter Miguel Turato, Zalua Rodríguez Riera, Roger Chammas, Philip H. Elsinga, and et al. 2020. "Synthesis and Evaluation of [18F]FEtLos and [18F]AMBF3Los as Novel 18F-Labelled Losartan Derivatives for Molecular Imaging of Angiotensin II Type 1 Receptors" Molecules 25, no. 8: 1872. https://doi.org/10.3390/molecules25081872
APA StyleSahylí Ortega Pijeira, M., Sérgio Gonçalves Nunes, P., Nascimento dos Santos, S., Zhang, Z., Pérez Nario, A., Araujo Perini, E., Miguel Turato, W., Rodríguez Riera, Z., Chammas, R., H. Elsinga, P., Lin, K.-S., Carvalho, I., & Soares Bernardes, E. (2020). Synthesis and Evaluation of [18F]FEtLos and [18F]AMBF3Los as Novel 18F-Labelled Losartan Derivatives for Molecular Imaging of Angiotensin II Type 1 Receptors. Molecules, 25(8), 1872. https://doi.org/10.3390/molecules25081872







