Characterization of Free and Bound Phenolic Acids and Flavonoid Aglycones in Rosa rugosa Thunb. Leaves and Achenes Using LC–ESI–MS/MS–MRM Methods
Abstract
1. Introduction
2. Results and Discussion
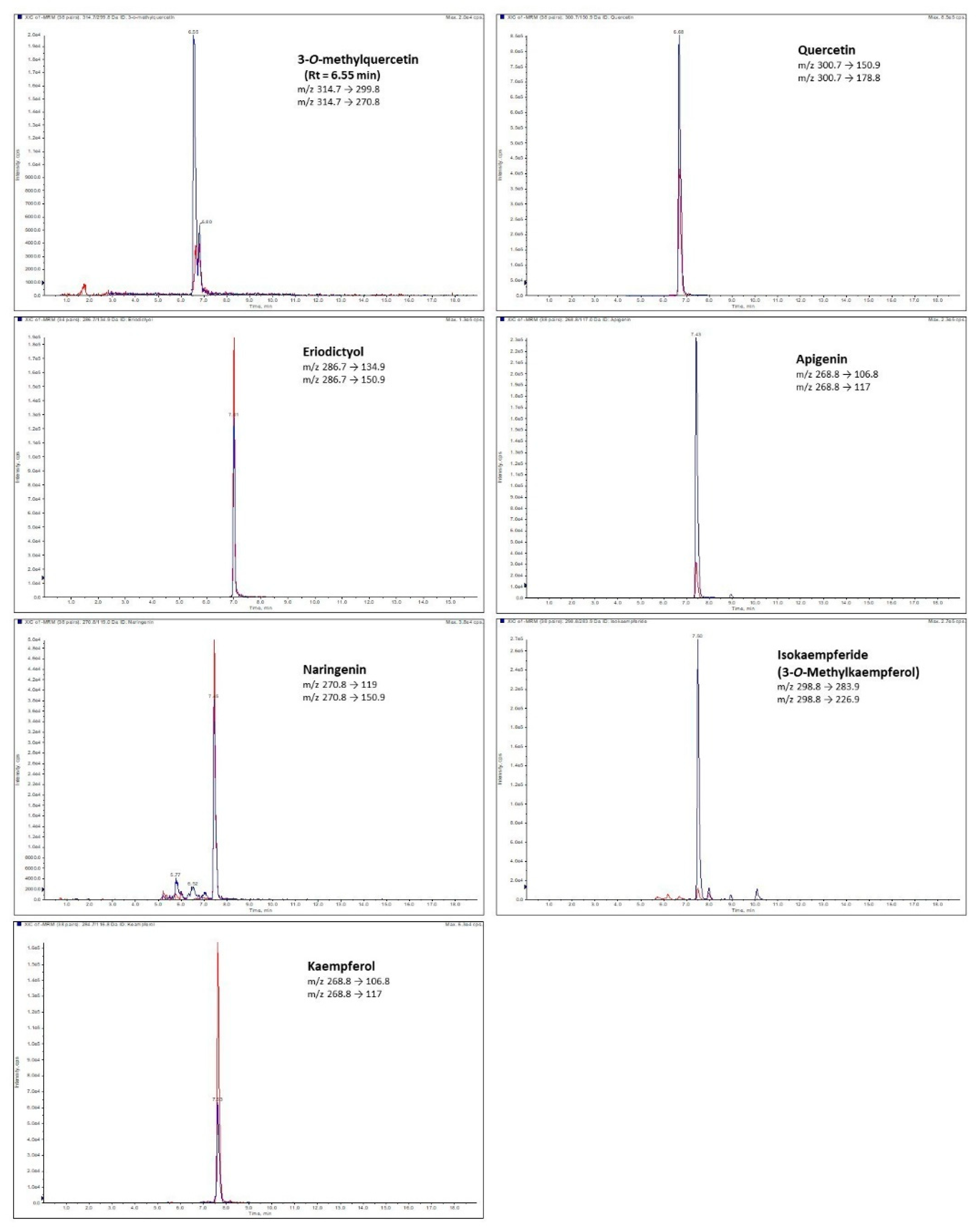
2.1. Conditions of the Methods
2.2. Validation of the Methods
2.3. Identification and Quantification of Polyphenols in R. rugosa Leaves and Achenes
2.3.1. Phenolic Acids
2.3.2. Flavonoid Aglycones
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material
3.3. Sample Preparation
3.4. LC-ESI-MS/MS Analysis of Phenolic Acids and Flavonoid Aglycones
3.4.1. Chromatographic Conditions and Apparatus
3.4.2. Optimization of MS Parameters
3.5. Method Validation
3.5.1. Linearity
3.5.2. Limit of Detection (LOD) and Limit of Quantification (LOQ)
3.5.3. Repeatability, Intra-Day, and Inter-Day Precision
3.5.4. Matrix Effect
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Olech, M.; Nowak, R.; Loś, R.; Rzymowska, J.; Malm, A.; Chrusciel, K. Biological activity and composition of teas and tinctures prepared from Rosa rugosa Thunb. Open Life Sci. 2012, 7, 172–182. [Google Scholar] [CrossRef]
- Nowak, R.; Olech, M.; Nowacka-Jechalke, N. Plant Polyphenols as Chemopreventive Agents. In Polyphenols in Human Health and Disease; Elsevier BV: Amsterdam, The Netherlands, 2014; Volume 2, pp. 1289–1307. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phospho-molybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Lamaison, J.L.; Carnart, A. Teneurs en principaux flavonoides des fleurs et des feuilles de Crataegus mo-nogyna Jacq. et de Crataegus laevigata (Poiret) DC. En fonction de la periode de vegetation. Plantes Med. Phyther. 1991, 25, 12–16. [Google Scholar]
- Nichiforesco, E.; Coucou, V. Sur le dosage des o-dihydrophénols de type acide caféique présents dans les feuilles d’artichaut (Cynara scolymus L.). Ann. Pharm. Fr. 1965, 23, 419–427. [Google Scholar]
- Elessawy, F.M.; Bazghaleh, N.; Vandenberg, A.; Purves, R. Polyphenol profile comparisons of seed coats of five pulse crops using a semi-quantitative liquid chromatography-mass spectrometric method. Phytochem. Anal. 2019, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ciesla, L.M.; Moaddel, R. Comparison of analytical techniques for the identification of bioactive compounds from natural products. Nat. Prod. Rep. 2016, 33, 1131–1145. [Google Scholar] [CrossRef]
- Kumar, B.R. Application of HPLC and ESI-MS techniques in the analysis of phenolic acids and flavonoids from green leafy vegetables (GLVs). J. Pharm. Anal. 2017, 7, 349–364. [Google Scholar] [CrossRef]
- Cendrowski, A.; Ścibisz, I.; Kieliszek, M.; Kolniak-Ostek, J.; Mitek, M. UPLC-PDA-Q/TOF-MS Profile of Polyphenolic Compounds of Liqueurs from Rose Petals (Rosa rugosa). Molecules 2017, 22, 1832. [Google Scholar] [CrossRef]
- Fougère, L.; Da Silva, D.; Destandau, E.; Elfakir, C. TLC-MALDI-TOF-MS-based identification of flavonoid compounds using an inorganic matrix. Phytochem. Anal. 2018, 30, 218–225. [Google Scholar] [CrossRef]
- Nowacka-Jechalke, N.; Nowak, R.; Drozd, M.; Olech, M.; Loś, R.; Malm, A. Antibacterial, Antiradical Potential and Phenolic Compounds of Thirty-One Polish Mushrooms. PLoS ONE 2015, 10, e0140355. [Google Scholar]
- Li, J.-M.; Liang, H.-Q.; Qiao, P.; Su, K.-M.; Liu, P.-G.; Guo, S.-X.; Chen, J.; Juan, C. Chemical Composition and Antioxidant Activity of Tuber indicum from Different Geographical Regions of China. Chem. Biodivers. 2019, 16, e1800609. [Google Scholar] [CrossRef] [PubMed]
- Olech, M.; Nowak, R.; Załuski, D.; Kapusta, I.; Amarowicz, R.; Oleszek, W. Hyaluronidase, acetylcholinesterase inhibiting potential, antioxidant activity, and LC-ESI-MS/MS analysis of polyphenolics of rose (Rosa rugosa Thunb.) teas and tinctures. Int. J. Food Prop. 2017, 20, S16–S25. [Google Scholar] [CrossRef]
- Nowak, R.; Tuzimski, T. A solid-phase extraction-thin-layer chromatographic-fiber optical scanning densitometric method for determination of flavonol aglycones in extracts of rose leaves. J. Planar Chromatogr. – Mod. TLC 2005, 18, 437–442. [Google Scholar] [CrossRef]
- Baydar, N.G.; Baydar, H. Phenolic compounds, antiradical activity and antioxidant capacity of oil-bearing rose (Rosa damascena Mill.) extracts. Ind. Crop. Prod. 2013, 41, 375–380. [Google Scholar] [CrossRef]
- Cunja, V.; Mikulic-Petkovsek, M.; Stampar, F.; Schmitzer, V. Compound Identification of Selected Rose Species and Cultivars: an Insight to Petal and Leaf Phenolic Profiles. J. Am. Soc. Hortic. Sci. 2014, 139, 157–166. [Google Scholar] [CrossRef]
- Ieri, F.; Innocenti, M.; Possieri, L.; Gallori, S.; Mulinacci, N. Phenolic composition of “bud extracts” of Ribes nigrum L., Rosa canina L. and Tilia tomentosa M. J. Pharm. Biomed. Anal. 2015, 115, 1–9. [Google Scholar] [CrossRef]
- Živković, J.; Stojković, D.; Petrović, J.; Zdunić, G.; Glamočlija, J.; Soković, M. Rosa canina L. – new possibilities for an old medicinal herb. Food Funct. 2015, 6, 3687–3692. [Google Scholar] [CrossRef]
- Bitis, L.; Sen, A.; Ozsoy, N.; Birteksoz-Tan, S.; Kultur, S.; Melikoglu, G. Flavonoids and biological activities of various extracts from Rosa sempervirens leaves. Biotechnol. Biotechnol. Equip. 2017, 31, 299–303. [Google Scholar] [CrossRef]
- Kitahiro, Y.; Ikeda, H.; Im, H.-T.; Kodaira, E.; Shibano, M. Phytochemical characterization of Rosa multiflora Thunb. (Rosaceae) in Japan and South Korea, with a focus on the bioactive flavonol glycoside ‘multiflorin A’. J. Nat. Med. 2019, 73, 555–565. [Google Scholar] [CrossRef]
- Karczmarz, K.; Szmagara, A.; Stefaniak, E.A. Ellagic acid content in selected wild species of fruit roses. Acta Sci. Pol. Hortorum Cultus 2019, 18, 131–140. [Google Scholar] [CrossRef]
- Orhan, D.D.; Hartevioglu, A.; Kupeli, E.; Yesilada, E. In vivo anti-inflammatory and antinociceptive activity of the crude extract and fractions from Rosa canina L. fruits. J. Ethnopharmacol. 2007, 112, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.-J.; Sa, J.-H.; Song, Y.S.; Shim, T.-H.; Park, E.-H.; Lim, C.-J. Anti-inflammatory, anti-angiogenic, and anti-nociceptive activities of the chloroform fraction of a methanol extract from Rosa davurica Pall. leaves in experimental animal models. Immunopharmacol. Immunotoxicol. 2010, 33, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Nowak, R.; Zgórka, G. Phenolic acids in fruits and leaves of Ribes nigrum L. and Ribes grossularia L. Acta Pol. Pharm.-Drug Res. 1997, 54, 155–160. [Google Scholar]
- Pietrzak, W.; Nowak, R.; Gawlik-Dziki, U.; Lemieszek, M.K.; Rzeski, W. LC-ESI-MS/MS Identification of Biologically Active Phenolic Compounds in Mistletoe Berry Extracts from Different Host Trees. Molecules 2017, 22, 624. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.P.; Midha, K.K.; Dighe, S.; McGilveray, I.J.; Skelly, J.P.; Yacobi, A.; Layloff, T.; Viswanathan, C.T.; Cook, C.E.; McDowall, R.D.; et al. Analytical Methods Validation: Bioavailability, Bioequivalence, and Pharmacokinetic Studies. J. Pharm. Sci. 1992, 81, 309–312. [Google Scholar] [CrossRef]
- Robbins, K.S.; Gong, Y.; Wells, M.L.; Greenspan, P.; Pegg, R.B. Investigation of the antioxidant capacity and phenolic constituents of U.S. pecans. J. Funct. Foods 2015, 15, 11–22. [Google Scholar] [CrossRef]
- Nowak, R.; Gawlik-Dziki, U. Polyphenols of Rosa L. leaves extracts and their radical scavenging activity. Zeitschrift für Naturforschung C 2007, 62, 32–38. [Google Scholar] [CrossRef]
- Hashidoko, Y. The phytochemistry of Rosa rugosa. Phytochemistry 1996, 43, 535–549. [Google Scholar] [CrossRef]
- Krzaczek, W.; Krzaczek, T. Phenolic acids of native species of the Rosa L. genus in Poland. Acta Soc. Bot. Pol. 2015, 48, 327–336. [Google Scholar] [CrossRef]
- Nowak, R.; Olech, M.; Pecio, Ł.; Oleszek, W.; Los, R.; Malm, A.; Rzymowska, J. Cytotoxic, antioxidant, antimicrobial properties and chemical composition of rose petals. J. Sci. Food Agric. 2013, 94, 560–567. [Google Scholar] [CrossRef]
- Olech, M.; Nowak, R.; Nowacka-Jechalke, N.; Pecio, Ł.; Oleszek, W.; Los, R.; Malm, A.; Rzymowska, J. Evaluation of rose roots, a post-harvest plantation residue as a source of phytochemicals with radical scavenging, cytotoxic, and antimicrobial activity. Ind. Crop. Prod. 2015, 69, 129–136. [Google Scholar] [CrossRef]
- Olech, M.; Nowak, R.; Pecio, Ł.; Łoś, R.; Malm, A.; Rzymowska, J.; Oleszek, W. Multidirectional characterisation of chemical composition and health-promoting potential of Rosa rugosa hips. Nat. Prod. Res. 2016, 31, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ouerghemmi, S.; Sebei, H.; Siracusa, L.; Ruberto, G.; Saija, A.; Cimino, F.; Cristani, M. Comparative study of phenolic composition and antioxidant activity of leaf extracts from three wild Rosa species grown in different Tunisia regions: Rosa canina L., Rosa moschata Herrm. and Rosa sempervirens L. Ind. Crop. Prod. 2016, 94, 167–177. [Google Scholar] [CrossRef]
- Hashidoko, Y.; Tahara, S.; Mizutani, J. 2-Phenoxychromones and a structurally related flavone from leaves of Rosa rugosa. Phytochemistry 1991, 30, 3837–3838. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Z.; Huang, Y.; Wen, X.; Wu, Y.; Zhao, Y.; Ni, Y. Extraction, Purification, and Hydrolysis Behavior of Apigenin-7-O-Glucoside from Chrysanthemum Morifolium Tea. Molecules 2018, 23, 2933. [Google Scholar] [CrossRef]
- Salminen, J.-P.; Karonen, M.; Lempa, K.; Liimatainen, J.; Sinkkonen, J.; Lukkarinen, M.; Pihlaja, K. Characterisation of proanthocyanidin aglycones and glycosides from rose hips by high-performance liquid chromatography-mass spectrometry, and their rapid quantification together with vitamin C. J. Chromatogr. A 2005, 1077, 170–180. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, J.-L.; Zhou, Q.; Wang, L.; Huang, W.; Wang, R. Effect of ultrasonic and ball-milling treatment on cell wall, nutrients, and antioxidant capacity of rose (Rosa rugosa) bee pollen, and identification of bioactive components. J. Sci. Food Agric. 2019, 99, 5350–5357. [Google Scholar] [CrossRef]
- Kawakami, S.; Matsunami, K.; Otsuka, H.; Kawahata, M.; Yamaguchi, K. Chemical constituents of imported Rosae fructus. J. Nat. Med. 2008, 63, 46–51. [Google Scholar] [CrossRef]
- Ibrahim, R.; Towers, G. The identification, by chromatography, of plant phenolic acids. Arch. Biochem. Biophys. 1960, 87, 125–128. [Google Scholar] [CrossRef]
- Bioanalytical Method Validation, Guidance for Industry, U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CMV), Biopharmaceutics Federal register, 2018. Available online: https://www.fda.gov/downloads/drugs/guidances/ucm070107.pdf (accessed on 12 April 2020).
- Bonfiglio, R.; King, R.C.; Olah, T.V.; Merkle, K. The effects of sample preparation methods on the varia-bility of the electrospray ionization response for model drug compounds. Rapid Commun Mass Spectrom. 1999, 13, 1175–1185. [Google Scholar] [CrossRef]
Sample Availability: Samples of the extracts are not available. |
| Analyte | Nominal Concentration (ng/mL) | Repeatability (% CVa) (n = 10) | Precision | |||
|---|---|---|---|---|---|---|
| Inter-day | Intra-day | |||||
| Measured Concentration (ng/mL) | CVa (%) | Measured Concentration (ng/mL) | CVa (%) | |||
| Gallic acid | 167 | 4.7 | 152.5 | 4.9 | 156.6 | 2.1 |
| 666 | 2.9 | 651.2 | 2.0 | 645 | 1.6 | |
| 3300 | 1.5 | 2899 | 0.9 | 2912 | 0.5 | |
| 5-O-caffeoylqunic acid | 17.65 | 2.2 | 15.0 | 2.4 | 14.9 | 3.3 |
| 176.5 | 4.0 | 151.0 | 3.8 | 151. | 5.3 | |
| 3530 | 1.2 | 3052 | 2.1 | 3077 | 2.1 | |
| Homogentisic acid | 167 | 2.2 | 156.4 | 2.2 | 158.4 | 0.1 |
| 1665 | 1.1 | 1477 | 0.5 | 1481 | 0.1 | |
| 11,100 | 2.4 | 10475 | 0.0 | 10,475 | 0.0 | |
| α-Resorcylic acid | 347 | 2.9 | 337.8 | 4.7 | 339.1 | 6.5 |
| 1735 | 5.0 | 1669 | 1.5 | 1663.7 | 2.0 | |
| 3470 | 4.2 | 3462 | 1.1 | 3443.3 | 0.8 | |
| Protocatechuic acid | 347 | 3.6 | 299 | 2.5 | 301.3 | 3.3 |
| 1700 | 2.1 | 1723 | 1.8 | 1716 | 2.4 | |
| 17,200 | 2.5 | 14,820 | 1.2 | 14,850 | 1.7 | |
| trans-Caffeic acid | 350 | 2.7 | 314.7 | 4.7 | 322.9 | 1.8 |
| 700 | 1.7 | 597.4 | 0.4 | 597.6 | 0.5 | |
| 3500 | 1.6 | 3015 | 2.8 | 3017 | 4.0 | |
| Syringic acid | 666 | 4.9 | 589.6 | 0.8 | 592 | 0.7 |
| 3330 | 2.8 | 2852 | 1.8 | 2855 | 2.5 | |
| 11,100 | 1.7 | 9510 | 1.3 | 9546 | 1.6 | |
| 4-Hydroxybenzoic acid | 347 | 3.5 | 283.0 | 4.7 | 275.8 | 2.6 |
| 694 | 4.2 | 618.7 | 0.3 | 619.65 | 0.3 | |
| 3470 | 2.6 | 2975 | 0.5 | 2983 | 0.3 | |
| Vanillic acid | 333 | 4.7 | 293.2 | 4.2 | 295.15 | 5.7 |
| 1650 | 4.8 | 1439 | 1.3 | 1450 | 0.3 | |
| 33,000 | 2.0 | 30694 | 1.7 | 30,400 | 0.3 | |
| Gentisic acid | 333 | 5.0 | 306.5 | 0.9 | 307.9 | 0.6 |
| 1670 | 2.5 | 1914 | 2.5 | 1941 | 0.6 | |
| 3300 | 2.6 | 3653 | 1.9 | 3692 | 0.4 | |
| γ-Resorcylic acid | 194 | 3.6 | 186.7 | 1.0 | 185.65 | 0.5 |
| 1935 | 3.4 | 1873 | 1.3 | 1881 | 1.6 | |
| 7740 | 2.0 | 6648 | 1.3 | 6679 | 1.4 | |
| 3-Hydroxybenzoic acid | 667 | 2.3 | 590 | 1.6 | 593.4 | 1.9 |
| 1334 | 2.6 | 1289 | 1.3 | 1298 | 0.1 | |
| 6670 | 1.6 | 6462 | 1.4 | 6487 | 1.7 | |
| β-Resorcylic acid | 1970 | 3.4 | 1760 | 2.1 | 1781 | 0.7 |
| 7860 | 0.5 | 6871 | 0.4 | 6885 | 0.1 | |
| 19,700 | 2.1 | 18180 | 0.4 | 18135 | 0.2 | |
| trans-Sinapic acid | 69.4 | 4.3 | 78.3 | 1.0 | 78.8 | 0.0 |
| 694 | 3.1 | 669 | 3.1 | 681.6 | 0.1 | |
| 3470 | 0.8 | 3026 | 2.7 | 3060 | 2.5 | |
| 4-Hydroxycinnamic acid (p-coumaric) | 373 | 3.8 | 322 | 2.8 | 319.1 | 2.9 |
| 746 | 3.3 | 638 | 0.8 | 635.1 | 0.3 | |
| 3730 | 1.9 | 3476 | 2.3 | 3523 | 0.2 | |
| Ferulic acid | 347 | 2.9 | 319.2 | 4.9 | 324.5 | 5.6 |
| 1735 | 3.0 | 1663 | 1.0 | 1660 | 1.3 | |
| 11,600 | 2.1 | 10,508 | 1.2 | 10437 | 0.5 | |
| Rosmarinic acid | 357 | 2.2 | 377.9 | 3.4 | 371.2 | 2.2 |
| 1790 | 1.4 | 1685 | 1.3 | 1677 | 1.5 | |
| 7140 | 1.3 | 6982 | 0.8 | 7005 | 0.9 | |
| Isoferulic acid | 343 | 4.9 | 299.8 | 4.8 | 299 | 6.8 |
| 3430 | 1.8 | 3749 | 1.0 | 3738 | 1.3 | |
| 11,400 | 0.9 | 11627 | 0.2 | 11,625 | 0.3 | |
| 3-Hydroxycinnamic acid (m-coumaric) | 1740 | 2.0 | 1613 | 0.5 | 1617 | 0.4 |
| 6940 | 3.2 | 6406 | 0.5 | 6387 | 0.1 | |
| 17,000 | 0.9 | 16,233 | 0.9 | 16,150 | 0.4 | |
| Veratric acid | 3140 | 3.0 | 2931 | 2.4 | 2961 | 2.2 |
| 15,700 | 1.5 | 15,403 | 0.5 | 15,375 | 0.5 | |
| 31,400 | 1.0 | 30547 | 0.3 | 30,487 | 0.1 | |
| 3.4.5-Trimethoxyphenyl acetic acid | 33.3 | 3.5 | 29.6 | 3.1 | 30 | 2.4 |
| 333 | 1.9 | 362.5 | 4.0 | 361.9 | 5.6 | |
| 3330 | 0.6 | 3304 | 2.1 | 3341 | 1.3 | |
| 2-Hydroxybenzoic acid (o-coumaric) | 182 | 4.7 | 178.2 | 4.4 | 182.5 | 1.9 |
| 1820 | 1.9 | 1859 | 3.7 | 1898 | 0.3 | |
| 3630 | 2.5 | 3728 | 0.5 | 3740 | 0.0 | |
| 3.4-Dimethoxycinnamic acid | 167 | 2.8 | 158.7 | 0.5 | 159 | 0.4 |
| 1665 | 1.8 | 1444 | 1.7 | 1450 | 2.2 | |
| 11,100 | 0.7 | 10,738 | 0.8 | 10,687 | 0.2 | |
| Salicylic acid | 330 | 4.6 | 362.2 | 4.4 | 370.5 | 2.5 |
| 1650 | 0.5 | 1893 | 2.2 | 1914 | 1.5 | |
| 3300 | 2.6 | 3730 | 2.2 | 3773 | 1.3 | |
| 3.5-Dimethoxybenzoic acid | 1770 | 3.2 | 1693 | 1.3 | 1700 | 1.7 |
| 7060 | 1.9 | 6416 | 0.4 | 6403 | 0.3 | |
| 35,300 | 3.9 | 30,072 | 2.2 | 30,075 | 3.2 | |
| Analyte | Nominal Concentration (ng/mL) | Repeatability (% CV) (n = 10) | Precision | |||
|---|---|---|---|---|---|---|
| Inter-day | Intra-day | |||||
| Measured Concentration (ng/mL) | CV (%) | Measured Concentration (ng/mL) | CV (%) | |||
| Taxifolin | 50 | 2.7 | 52 | 4.0 | 51.5 | 3.0 |
| 1000 | 2.0 | 1003 | 0.3 | 997 | 0.3 | |
| 5000 | 2.3 | 4870 | 2.6 | 4950 | 1.0 | |
| Myricetin | 11 | 2.0 | 10.59 | 3.7 | 10.81 | 1.7 |
| 1000 | 1.0 | 953 | 4.7 | 1004 | 0.4 | |
| 3600 | 3.2 | 3450 | 4.2 | 3488 | 3.1 | |
| Morin | 10 | 2.2 | 10.5 | 5.0 | 10.2 | 2.0 |
| 1000 | 4.7 | 1042 | 4.2 | 1022 | 2.2 | |
| 5000 | 4.0 | 4862 | 2.8 | 4750 | 5.0 | |
| Eriodictyol | 15 | 3.0 | 14.3 | 4.7 | 15.1 | 0.7 |
| 1000 | 3.2 | 974 | 2.6 | 982 | 1.8 | |
| 5000 | 1.8 | 4766 | 4.7 | 5012 | 0.2 | |
| Luteolin | 40 | 2.5 | 38 | 5.0 | 39.2 | 2.0 |
| 1000 | 4.8 | 988 | 1.2 | 989 | 1.1 | |
| 4000 | 4.1 | 3850 | 3.8 | 3886 | 2.9 | |
| Quercetin | 30 | 2.8 | 29.8 | 0.7 | 28.9 | 3.7 |
| 500 | 2.7 | 489 | 2.2 | 475 | 5.0 | |
| 3000 | 2.2 | 2876 | 4.1 | 2995 | 0.2 | |
| 3-O-Methylquercetin | 15 | 1.9 | 14.8 | 1.3 | 15.2 | 1.3 |
| 500 | 1.7 | 496 | 0.8 | 485 | 3.0 | |
| 3700 | 1.7 | 3665 | 0.9 | 3710 | 0.3 | |
| Apigenin | 12 | 3.9 | 12 | 0.0 | 11.9 | 0.8 |
| 1000 | 3.7 | 954 | 4.6 | 996 | 0.4 | |
| 6000 | 1.2 | 5800 | 3.3 | 5870 | 2.2 | |
| Naringenin | 33 | 0.5 | 32.2 | 2.4 | 33.5 | 1.5 |
| 500 | 2.2 | 489 | 2.2 | 515 | 3.0 | |
| 3000 | 3.5 | 3100 | 3.3 | 3150 | 5.0 | |
| Kaempferol | 33 | 1.9 | 33.5 | 1.5 | 33.6 | 1.8 |
| 3000 | 0.1 | 2850 | 5.0 | 3100 | 3.3 | |
| 20,000 | 2.0 | 19,950 | 0.3 | 19000 | 5.0 | |
| Isorhamnetin | 40 | 1.7 | 39.2 | 2.0 | 40.5 | 1.3 |
| 3000 | 4.5 | 2890 | 3.7 | 2900 | 3.3 | |
| 60,000 | 2.0 | 57,000 | 5.0 | 61,000 | 1.7 | |
| Isokaempferide(3-O-Methylkaempferol) | 250 | 4.6 | 240 | 4.0 | 242 | 3.2 |
| 3000 | 1.2 | 3050 | 1.7 | 3100 | 3.3 | |
| 10,000 | 1.0 | 9800 | 2.0 | 9560 | 4.4 | |
| Rhamnetin | 5 | 1.3 | 4.9 | 2.0 | 5.1 | 2.0 |
| 200 | 2.1 | 198 | 1.0 | 205 | 2.5 | |
| 625 | 1.9 | 620 | 0.8 | 630 | 0.8 | |
| Chrysin | 42 | 3.4 | 41 | 2.4 | 44 | 4.8 |
| 500 | 2.7 | 498 | 0.4 | 480 | 4.0 | |
| 2500 | 2.1 | 2420 | 3.2 | 2600 | 4.0 | |
| Sakuranetin | 70 | 4.3 | 68 | 2.9 | 69 | 1.4 |
| 1000 | 4.1 | 1050 | 5.0 | 1020 | 2.0 | |
| 7000 | 0.9 | 6800 | 2.9 | 6700 | 4.3 | |
| Prunetin | 200 | 3.8 | 195 | 2.5 | 205 | 2.5 |
| 3000 | 4.1 | 2900 | 3.3 | 3050 | 1.7 | |
| 20,000 | 4.8 | 19,000 | 5.0 | 20,500 | 2.5 | |
| Rhamnazin | 70 | 0.2 | 69 | 1.4 | 72 | 2.9 |
| 3000 | 3.5 | 2850 | 5.0 | 3050 | 1.7 | |
| 7000 | 0.3 | 6800 | 2.9 | 7100 | 1.4 | |
| Phenolic Acid | RT (min) | LF-Free | LF-AcH | LF-AlkH | FRU-Free | FRU-AcH | FRU-AlkH |
|---|---|---|---|---|---|---|---|
| Average ± SD | Average ± SD | Average ± SD | Average ± SD | Average ± SD | Average ± SD | ||
| Gallic | 5.14 | 2.08 a ± 0.03 | 151.31 b ± 1.10 | 115.65 c ± 0.98 | 0.27 d ± 0.01 | 0.07 d ± 0.00 | 0.020 d ± 0.00 |
| Protocatechuic | 5.92 | 4.00 a ± 0.26 | 91.97 b ± 0.74 | 8.17 c ± 0.17 | 0.83 d ± 0.01 | 0.45 d ± 0.01 | 0.20 d ± 0.00 |
| trans-Caffeic | 6.93 | 7.09 a ± 0.05 | 62.97 b ± 1.74 | 17.23 c ± 0.11 | 0.01 d ± 0.00 | 0.30 d ± 0.01 | BQL |
| cis-Caffeic | 7.10 | 0.06 ± 0.00 | 0 | 0 | 0 | 0 | 0 |
| Syringic acid | 7.20 | 0.15 a ± 0.00 | BQL | 0 | 0.56 a ± 0.01 | 1.14 a ± 0.01 | 0.09 a ± 0.00 |
| 4-Hydroxy-benzoic | 7.33 | 1.40 a ± 0.08 | 23.6 b ± 0.26 | BQL | 0.77 a ± 0.02 | 1.37 a ± 0.01 | 0.14 a ± 0.00 |
| Vanillic | 7.45 | BQL | 0 | 0 | 5.22 a ± 0.10 | 11.03 b ± 0.19 | 0.31 c ± 0.00 |
| Gentisic | 7.79 | 0.40 a ± 0.00 | 65.77 b ± 0.90 | 3.06 c ± 0.04 | 0.01 a ± 0.00 | 0.04 a ± 0.00 | BQL |
| trans-Sinapic | 9.23 | 0 | 0 | 0 | 0.05 a ± 0.00 | 0.07 a ± 0.00 | 0.02 a ± 0.00 |
| cis-Sinapic | 9.78 | 0 | 0 | 0 | 0.03 a ± 0.00 | 0.14 a ± 0.00 | 0.03 a ± 0.00 |
| p-Coumaric | 9.33 | 21.38 a ± 0.24 | 118.50 b ± 2.12 | 38.50 c ± 0.56 | 1.77 d ± 0.04 | 5.73 e ± 0.26 | 1.73 d ± 0.05 |
| Ferulic | 9.89 | 2.20ac ± 0.05 | 15.23 b ± 0.25 | 3.83 a ± 0.08 | 0.61 c ± 0.01 | 2.50ad ± 0.01 | 0.40 c ± 0.01 |
| Rosmarinic | 10.23 | 0 | 0 | 0 | 0 | 0.01 a ± 0.00 | 0.01 a ± 0.00 |
| Isoferulic | 10.56 | 1.83 a ± 0.04 | BQL | BQL | 1.36 a ± 0.03 | 14.86 b ± 0.21 | 1.52 a ± 0.02 |
| 3,4-Dimethoxycinnamic | 13.18 | 0 | 0 | 0 | 0.05 a ± 0.00 | 0.39 a ± 0.00 | 0.21 a ± 0.00 |
| Salicylic | 14.19 | 5.17 a ± 0.18 | 24.10 b ± 0.36 | 0.78 c ± 0.02 | 0.02 c ± 0.00 | 0.03 c ± 0.00 | 0.01 c ± 0.00 |
| SUM | 45.76 | 553.45 | 187.23 | 11.58 | 38.15 | 4.69 |
| LF-Free | LF-AcH | FRU-Free | FRU-AcH | |
|---|---|---|---|---|
| µg/g Dry Plant Material | µg/g Dry Plant Material | |||
| Taxifolin | - | - | <BQL | - |
| Eriodictyol | 0.07 a ± 0.00 | - | 0.03 a ± 0.00 | <BQL |
| Quercetin | 6.3 9a,* ± 0.15 | 1.94 b,* ± 0.02 | 0.13 b,* ± 0.00 | 0.11 b,* ± 0.01 |
| 3-O-Methylquercetin | 0.04 ± 0.00 | - | - | - |
| Apigenin | 1.27 ± 0.01 | - | - | - |
| Naringenin | 0.31 ± 0.00 | - | < BQL | - |
| Kaempferol | 2.09 a ± 0.03 | 0.51 a,b ± 0.01 | < BQL | 0.03 b ± 0.00 |
| Isorhamnetin | - | 0.03 a ± 0 | < BQL | 0.05 a ± 0.00 |
| 3-O-Methylkaempferol | 1.81 ± 0.01 | - | - | - |
| Compound | LOD (ng/mL) | LOQ (ng/mL) | R2 | Linearity Range (ng/mL) |
|---|---|---|---|---|
| Gallic acid | 33.3 | 95 | 0.9982 | 167–3300 |
| 5-O-caffeoylqunic acid | 1.7 | 3.5 | 0.9993 | 3.5–3530 |
| Homogentisic acid | 33.3 | 68 | 0.9998 | 68–11100 |
| α-Resorcylic acid | 174 | 347 | 0.9990 | 347–3470 |
| Protocatechuic acid | 68 | 174 | 0.9996 | 174–17200 |
| trans-Caffeic acid | 60 | 160 | 0.9990 | 175–3500 |
| Syringic acid | 167 | 666 | 0.9993 | 666–11100 |
| 4-OH-benzoic acid | 17.4 | 34.7 | 0.9993 | 69.4–3470 |
| Vanillic acid | 100 | 250 | 0.9997 | 330–33000 |
| Gentisic acid | 16.7 | 67 | 0.9991 | 67–1670 |
| γ- Resorcylic acid | 38.7 | 194 | 0.9978 | 194–7740 |
| 3-Hydroxybenzoic acid | 33.3 | 334 | 0.9994 | 334–6670 |
| β- Resorcylic acid | 39.3 | 78.6 | 0.9992 | 197–19700 |
| trans-Sinapic acid | 17.4 | 69.4 | 0.9999 | 69.4–3470 |
| trans-p-Coumaric acid | 18.7 | 74.6 | 0.9996 | 187–3730 |
| trans-Ferulic acid | 17.4 | 34.7 | 0.9994 | 69.4–11600 |
| Rosmarinic acid | 7.1 | 17.9 | 0.9994 | 19.9–7140 |
| trans-Isoferulic acid | 17.2 | 686 | 0.9997 | 343–11400 |
| trans-m-Coumaric acid | 17 | 68 | 0.9993 | 174–17000 |
| Veratric acid | 1570 | 3140 | 0.9980 | 3140–31400 |
| 3,4,5-Trimethoxyphenylacetic acid | 3.3 | 9.5 | 1.0000 | 16.7–3330 |
| trans-o-Coumaric acid | 7.3 | 18.1 | 0.9996 | 18.1–1820 |
| 3,4-Dimethoxycinnamic acid | 3.3 | 33.3 | 0.9994 | 66.7–11100 |
| Salicylic acid | 3.3 | 16.5 | 0.9989 | 16.5–1650 |
| 3,5-Dimethoxybenzoic acid | 176.5 | 470.7 | 0.9996 | 706–35300 |
| Compound | LOD (ng/mL) | LOQ (ng/mL) | R2 | Linearity Range (ng/mL) |
|---|---|---|---|---|
| Taxifolin | 20 | 50 | 0.9986 | 50–5000 |
| Myricetin | 3 | 5 | 0.9991 | 11–3600 |
| Morin | 2 | 4 | 0.9985 | 10–5000 |
| Eriodictyol | 5 | 15 | 0.9980 | 15–5000 |
| Luteolin | 25 | 40 | 0.9970 | 40–4000 |
| Quercetin | 2 | 3 | 0.9975 | 30–3000 |
| 3-O-Methylquercetin | 1 | 2 | 0.9989 | 15–3700 |
| Apigenin | 3 | 4 | 0.9987 | 12–6000 |
| Naringenin | 25 | 33 | 0.9990 | 33–3000 |
| Kaempferol | 20 | 33 | 0.9986 | 33–20000 |
| Isorhamnetin | 12 | 24 | 0.9975 | 40–60000 |
| Isokaempferide | 1 | 2 | 0.9993 | 250–10000 |
| Rhamnetin | 2 | 5 | 0.9984 | 5–625 |
| Chrysin | 25 | 40 | 0.9959 | 42–2500 |
| Sakuranetin | 34 | 46 | 0.9990 | 70–7000 |
| Prunetin | 50 | 75 | 0.9985 | 200–20000 |
| Rhamnazin | 50 | 70 | 0.9991 | 70–7000 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olech, M.; Pietrzak, W.; Nowak, R. Characterization of Free and Bound Phenolic Acids and Flavonoid Aglycones in Rosa rugosa Thunb. Leaves and Achenes Using LC–ESI–MS/MS–MRM Methods. Molecules 2020, 25, 1804. https://doi.org/10.3390/molecules25081804
Olech M, Pietrzak W, Nowak R. Characterization of Free and Bound Phenolic Acids and Flavonoid Aglycones in Rosa rugosa Thunb. Leaves and Achenes Using LC–ESI–MS/MS–MRM Methods. Molecules. 2020; 25(8):1804. https://doi.org/10.3390/molecules25081804
Chicago/Turabian StyleOlech, Marta, Wioleta Pietrzak, and Renata Nowak. 2020. "Characterization of Free and Bound Phenolic Acids and Flavonoid Aglycones in Rosa rugosa Thunb. Leaves and Achenes Using LC–ESI–MS/MS–MRM Methods" Molecules 25, no. 8: 1804. https://doi.org/10.3390/molecules25081804
APA StyleOlech, M., Pietrzak, W., & Nowak, R. (2020). Characterization of Free and Bound Phenolic Acids and Flavonoid Aglycones in Rosa rugosa Thunb. Leaves and Achenes Using LC–ESI–MS/MS–MRM Methods. Molecules, 25(8), 1804. https://doi.org/10.3390/molecules25081804








