Isolation and Identification of Anthocyanin Component in the Fruits of Acanthopanax Sessiliflorus (Rupr. & Maxim.) Seem. by Means of High Speed Counter Current Chromatography and Evaluation of Its Antioxidant Activity
Abstract
1. Introduction
2. Results and Discussion
2.1. Total Anthocyanin Determination
2.2. Selection of Two-Phase Solvent System for Purification
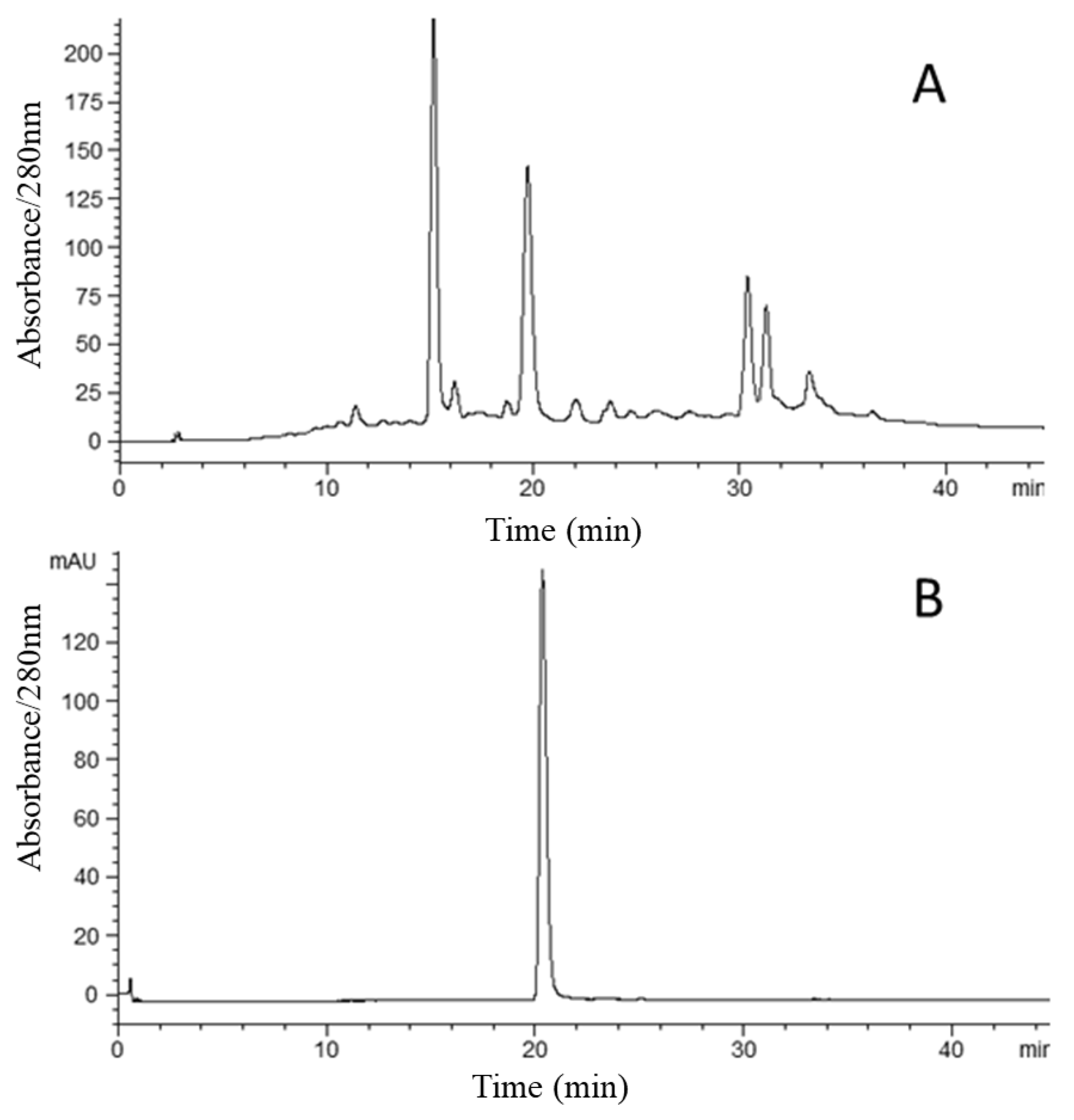
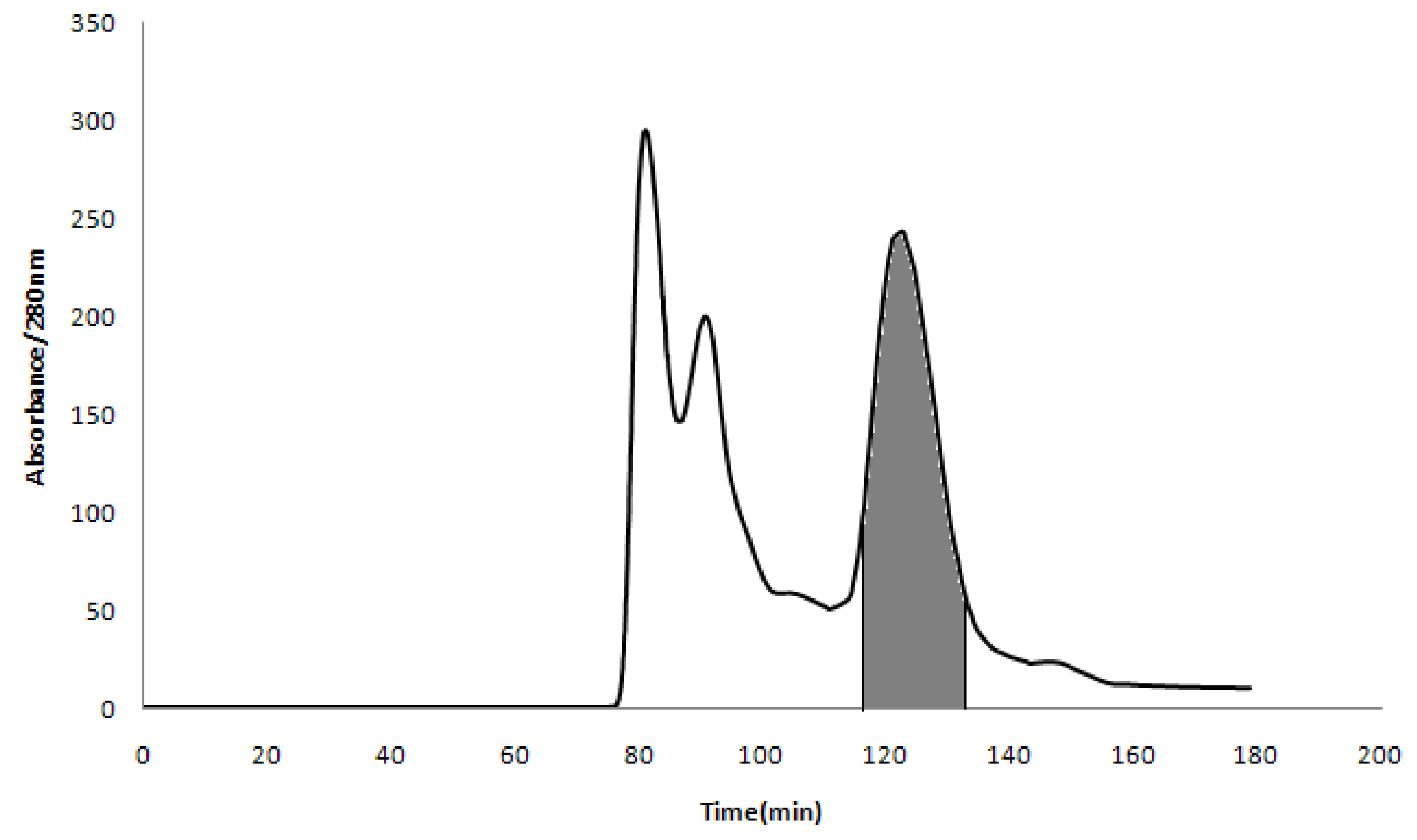
2.3. HSCCC Separation
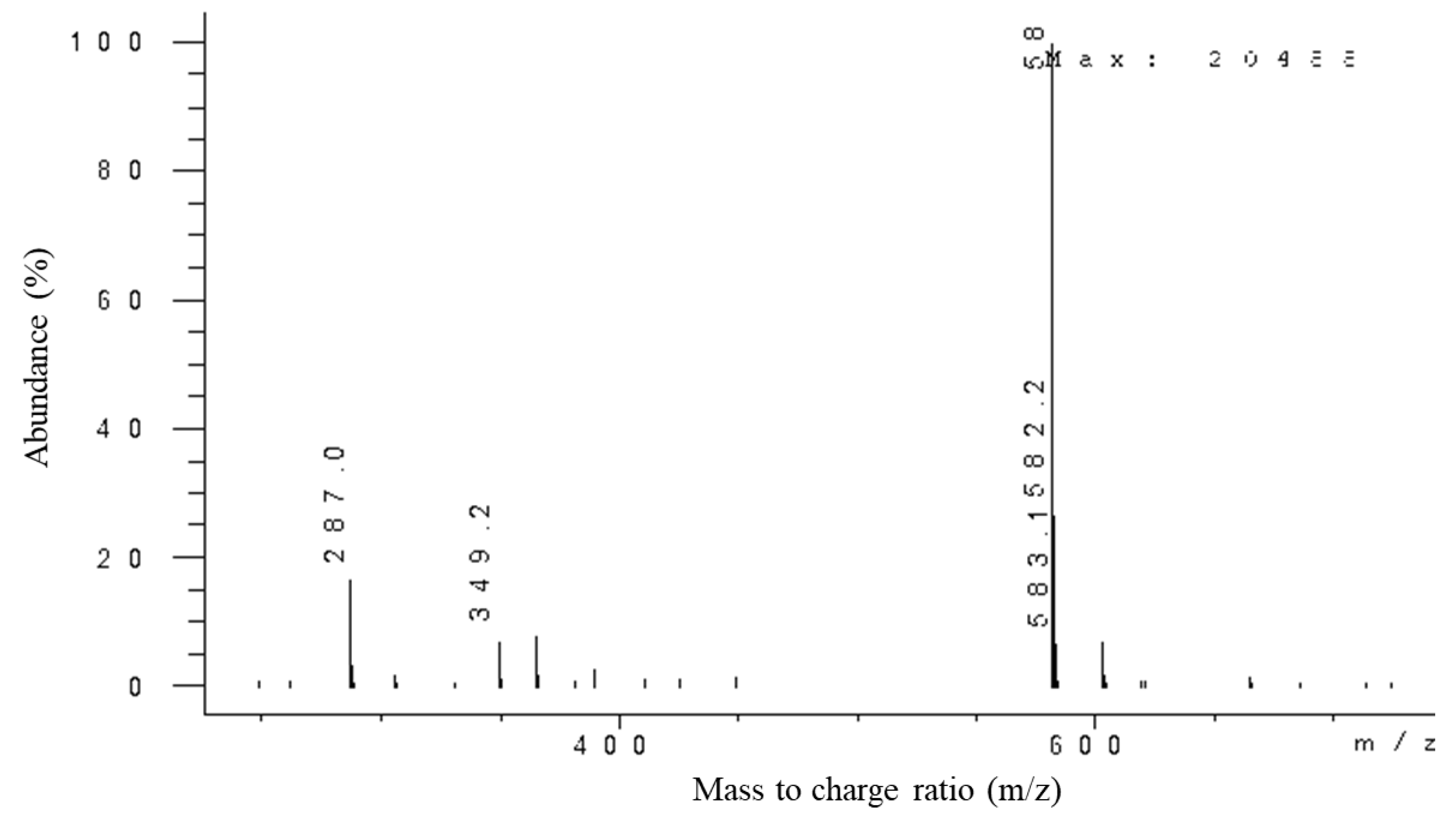
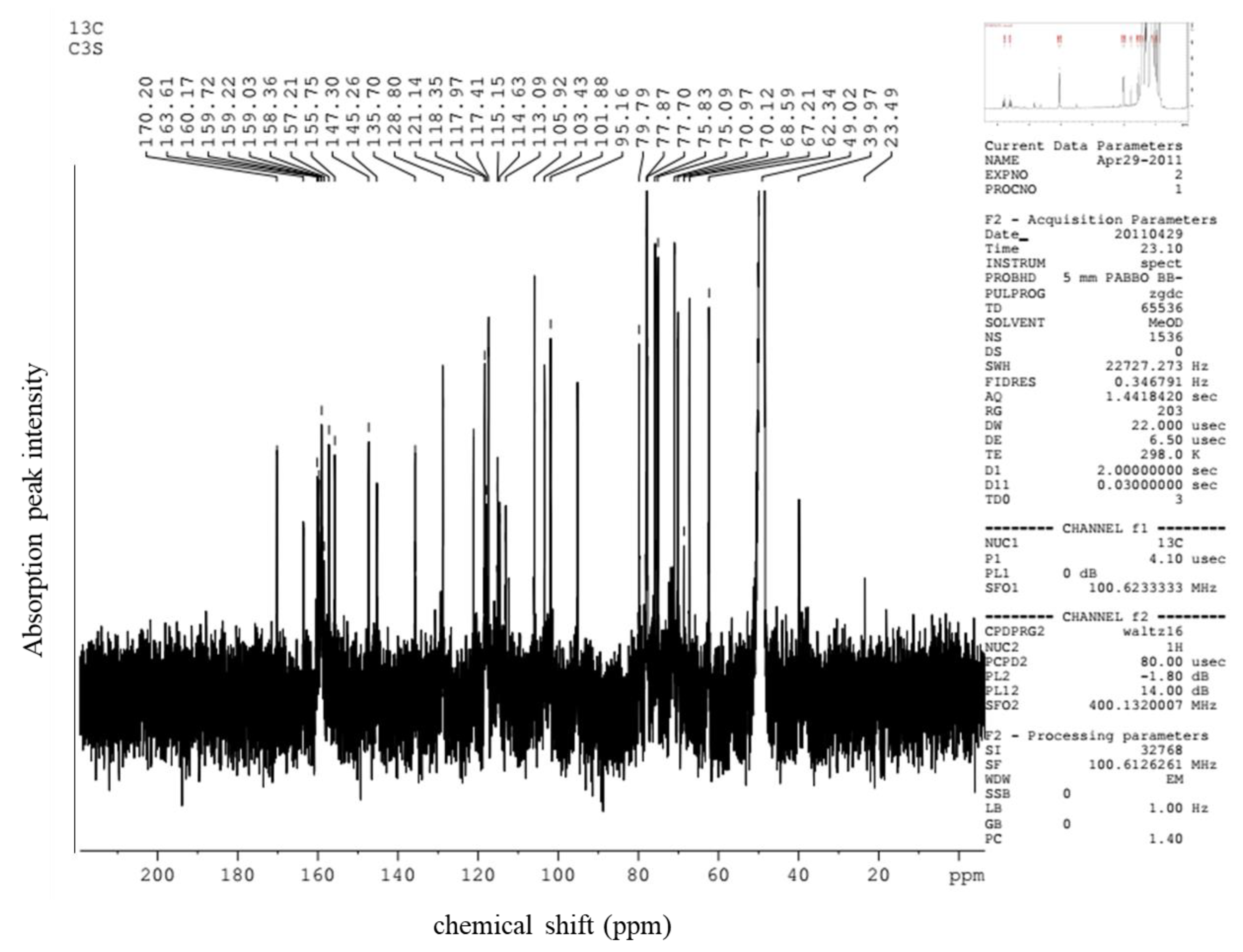
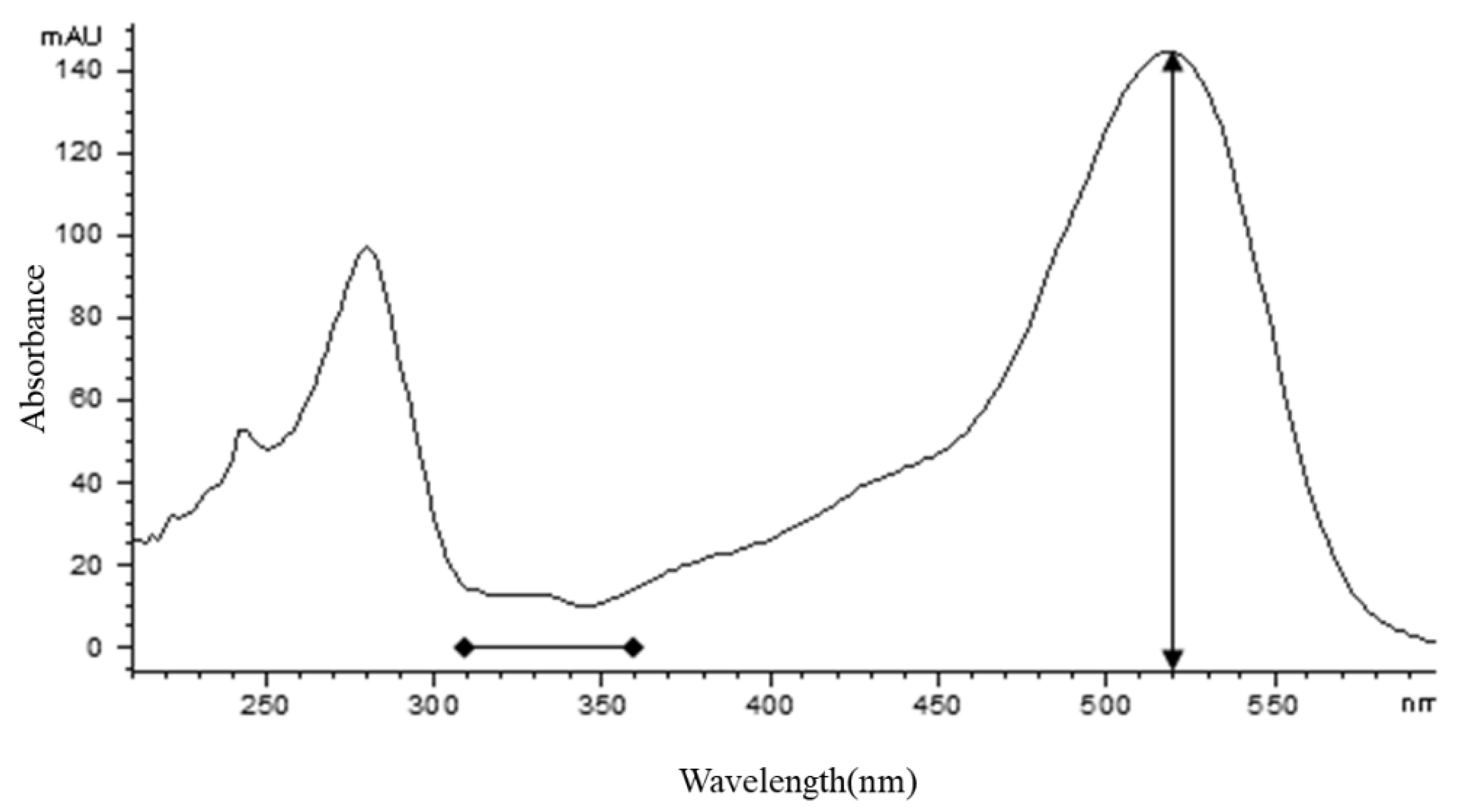
2.4. Confirmation of Anthocyanin Compound
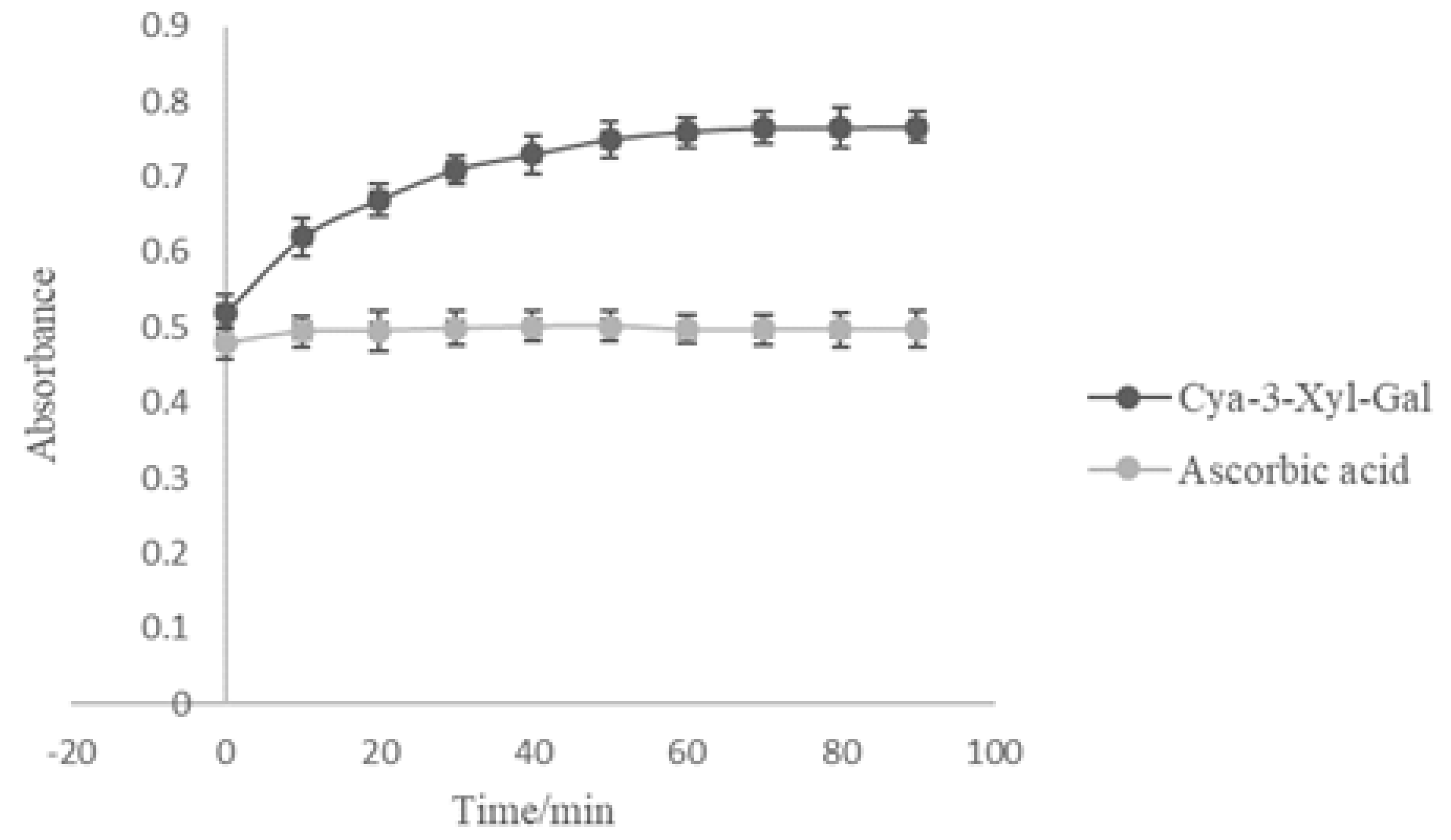
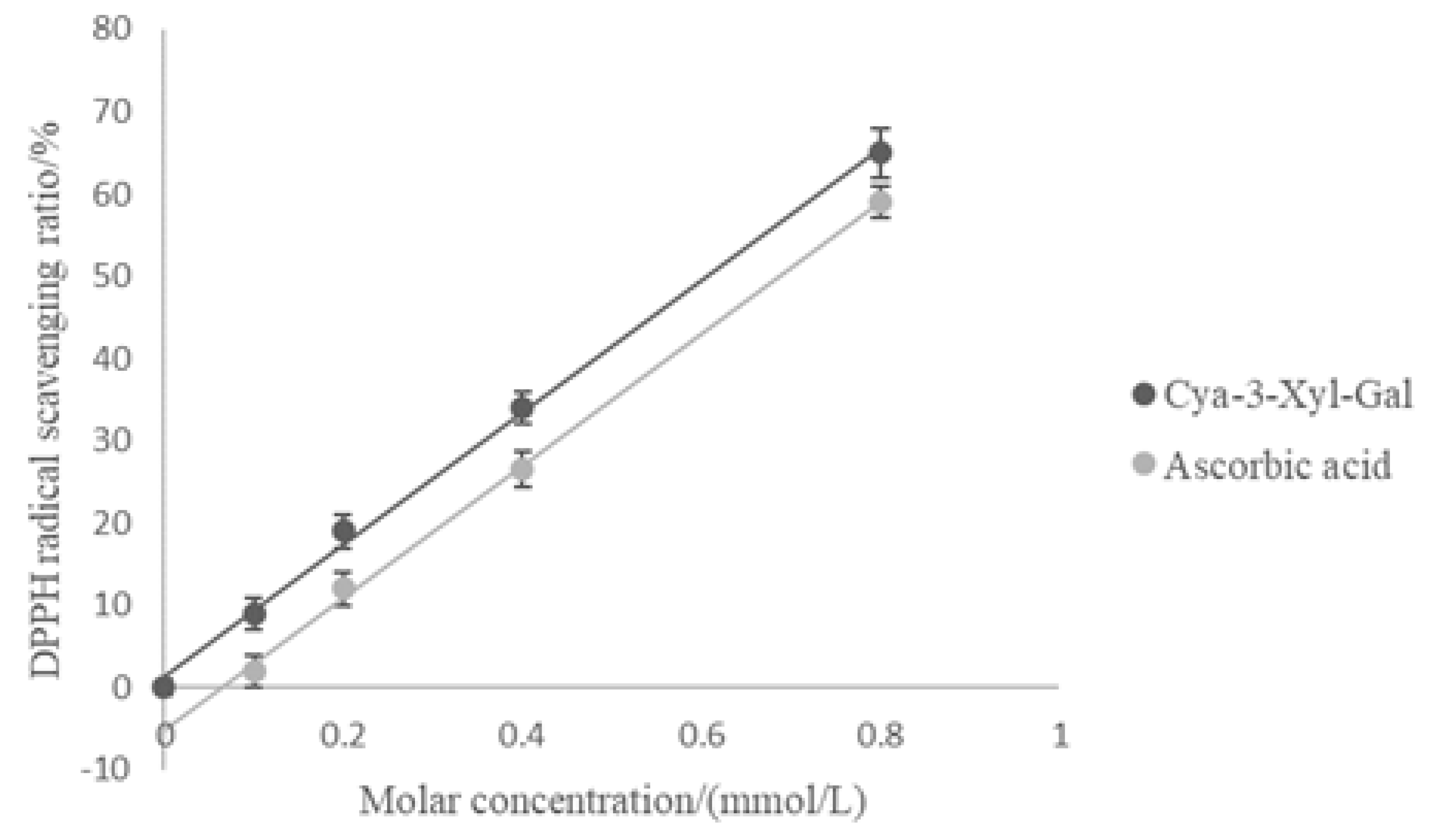
2.5. Antioxidant Activity of Cyanidin 3-Xylosyl-Galactoside
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Instruments and Apparatus
3.3. Total Anthocyanin Determination
3.4. Bulk Anthocyanin Extract of A. sessiliflorus
3.5. Preparation of Two-Phase Solvent Systems and Sample Solutions
3.6. Determination of Partition Coefficients (K)
3.7. HSCCC Separation
3.8. Analytical Controls and Structure Elucidation
3.9. Determination of Molar Absorptivity of Anthocyanin Component
3.10. Evaluation of Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A

References
- Zhou, C.C. Anti-inflammatory action of ethanol extracts from Acanthopanax sessiliflorus. Chin. J. Chin. Mater. Med. 1985, 10, 37–41. [Google Scholar]
- Yoshizumi, K.; Hirano, K.; Ando, H.; Hirai, Y.; Ida, Y.; Tsuji, T.; Tanaka, T.; Satouchi, K.; Terao, J. Lupanetype saponins from leaves of Acanthopanax sessiliflorus and their inhibitory activity on pancreatic lipase. J. Agric. Food Chem. 2006, 54, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; An, Q.; Xiong, Z.; Song, Y.; Yu, K.; Li, F. Triterpenes from Acanthopanax sessiliflorus fruits and their antiplatelet aggregation activities. Planta Med. 2009, 75, 656–659. [Google Scholar] [CrossRef] [PubMed]
- Meiyu, G.; Yuqiang, L.; Qian, C.; Yutong, L.; Shenlei, Z. Study on the Anti-fatigue Effect of Acanthopanax Sessiliflorus Fruits. Asia-Pac. Tradit. Med. 2015, 19, 6. [Google Scholar]
- Billingham, M.; Davies, G. Experimental models of arthritis in animals as screening tests for drugs to treat arthritis in man. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 1979; Volume 50, pp. 108–144. [Google Scholar]
- Deyama, T.; Nishibe, S.; Nakazawa, Y. Constituents and pharmacological effects of Eucommia and Siberian ginseng. Acta Pharmacol. Sin. 2001, 22, 1057–1070. [Google Scholar]
- Lee, S.H.; Jung, S.H.; Shin, K.H.; Kang, S.S. Antitumor and immunostimulating activities of Acanthopanax sessiliflorus fruits. Nat. Prod. Sci. 2003, 9, 112–116. [Google Scholar]
- Jeong, S.C.; Jeong, Y.T.; Yang, B.K.; Song, C.H. Chemical characteristics and immuno-stimulating properties of biopolymers extracted from Acanthopanax sessiliflorus. BMB Rep. 2006, 39, 84–90. [Google Scholar] [CrossRef]
- Sumiyoshi, M.; Kimura, Y. Effects of Eleutherococcus sicentosus cortex on recovery from the forced swimming test and fatty acid β-oxidation in the liver and skeletal muscle of mice. Nat. Prod. J. 2016, 6, 49–55. [Google Scholar]
- Bian, X.; Liu, X.; Liu, J.; Zhao, Y.; Li, H.; Zhang, L.; Li, P.; Gao, Y. Hepatoprotective effect of chiisanoside from Acanthopanax sessiliflorus against LPS/D-GalN-induced acute liver injury by inhibiting NF-κB and activating Nrf2/HO-1 signaling pathways. J. Sci. Food Agr. 2019, 99, 3283–3290. [Google Scholar] [CrossRef]
- Lee, S.; Kim, B.K.; Cho, S.H.; Shin, K.H. Phytochemical constituents from the fruits of Acanthopanax sessiliflorus. Arch. Pharm. Res. 2002, 25, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, Y.; Cui, L.; Pei, K.; Li, F. Determination of the contents of seopoletin and isofraxidin in fruit of Acanthopanax sessiliflorus (Rupr. et Maxim.) Seem. by HPLC. J. Shenyang Pharm. Univ. 2006, 9, 6. [Google Scholar]
- He, F.; Han, Y.; Liu, M.; Liu, R.; Li, F. Determination of the contents of total coumarin and 6, 7-dimethoxycoumarin in fruit of Acanthopanax sessiliflorum (Rupr. et Maxim.) Seem. J. Shenyang Pharm. Univ. 2006, 1, 5. [Google Scholar]
- Yang, C.; An, Q.; Song, Y.; Xiong, Z.; Li, F. Isolation and identification of chemical constituents of fruits of Acanthopanax sessiliflorus. China J. Chin. Mater. Med. 2009, 34, 715. [Google Scholar]
- Yang, C.; Yu, K.; Song, Y.; Qin, F.; Li, F. Determination and pharmacokinetic study of chiisanogenin in rat plasma by ultra-performance liquid chromatography-tandem mass spectrometry. Phytochem. Anal. 2011, 22, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Jun, W.J.; Seong, H.S.; Chun, H.; Lim, E.J.; Kim, K.I.; Cho, H.Y. Determination of antioxidative potentials of Acanthopanax sessiliflorus (Rupr. & Maxim.) Seem. in differentiated HL-60 cells. Food Chem. 2007, 105, 1557–1563. [Google Scholar]
- Tsuda, T. Dietary anthocyanin-rich plants: Biochemical basis and recent progress in health benefits studies. Mol. Nutr. Food Res. 2012, 56, 159–170. [Google Scholar] [CrossRef]
- Liang, H.; Li, C.; Yuan, Q.; Vriesekoop, F. Application of high-speed countercurrent chromatography for the isolation of sulforaphane from broccoli seed meal. J. Agric. Food Chem. 2008, 56, 7746–7749. [Google Scholar] [CrossRef]
- Oka, F.; Oka, H.; Ito, Y. Systematic search for suitable two-phase solvent systems for high-speed counter-current chromatography. J. Chromatogr. A. 1991, 538, 99–108. [Google Scholar] [CrossRef]
- Ito, Y. Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. J. Chromatogr. A. 2005, 1065, 145–168. [Google Scholar] [CrossRef]
- Marston, A.; Hostettmann, K. Developments in the application of counter-current chromatography to plant analysis. J. Chromatogr. A. 2006, 1112, 181–194. [Google Scholar] [CrossRef]
- Friesen, J.B.; Pauli, G.F. Rational development of solvent system families in counter-current chromatography. J. Chromatogr. A. 2007, 1151, 51–59. [Google Scholar] [CrossRef]
- Kan, X.; Yan, Y.; Ran, L.; Lu, L.; Mi, J.; Zhang, Z.; Li, X.; Zeng, X.; Cao, Y. Ultrasonic-assisted extraction and high-speed counter-current chromatography purification of zeaxanthin dipalmitate from the fruits of Lycium barbarum L. Food Chem. 2020, 310, 125854. [Google Scholar] [CrossRef]
- Feger, W.; Brandauer, H.; Gabris, P.; Ziegler, H. Nonvolatiles of commercial lime and grapefruit oils separated by high-speed countercurrent chromatography. J. Agric. Food Chem. 2006, 54, 224–252. [Google Scholar] [CrossRef]
- Chen, L.; Xin, X.; Yuan, Q.; Su, D.; Liu, W. Phytochemical properties and antioxidant capacities of various colored berries. J. Sci. Food Agr. 2014, 94, 180–188. [Google Scholar] [CrossRef]
- Chen, L.; Xin, X.; Zhang, H.; Yuan, Q. Phytochemical properties and antioxidant capacities of commercial raspberry varieties. J. Func. Foods. 2013, 5, 508–515. [Google Scholar] [CrossRef]
- Victor, H.; Ronald, E.W. Use of HPLC separation/photodiode array detection for characterization of anthocyanins. J. Agric. Food Chem. 1990, 38, 708–715. [Google Scholar]
- Harborne, J. Spectral methods of characterizing anthocyanins. Biochem. J. 1958, 70, 22–28. [Google Scholar] [CrossRef]
- Kim, D.M.; Bae, J.S.; Lee, D.S.; Lee, H.; Joo, M.H.; Yoo, S.H. Positive effects of glycosylated anthocyanin isolated from an edible berry fruit (Acanthopanax sessiliflorum) on its antioxidant activity and color stability. Food Res. Int. 2011, 44, 2258–2263. [Google Scholar] [CrossRef]
- Degenhardt, A.; Knapp, H.; Winterhalter, P. Separation and purification of anthocyanins by high-speed countercurrent chromatography and screening for antioxidant activity. J. Agric. Food Chem. 2000, 48, 338–343. [Google Scholar] [CrossRef]
- Du, Q.; Jerz, G.; Winterhalter, P. Isolation of two anthocyanin sambubiosides from bilberry (Vaccinium myrtillus) by high-speed counter-current chromatography. J. Chromatogr. A. 2004, 1045, 59–63. [Google Scholar] [CrossRef]
- Chen, L.; Xin, X.; Lan, R.; Yuan, Q.; Wang, X.; Li, Y. Isolation of cyanidin 3-glucoside from blue honeysuckle fruits by high-speed counter-current chromatography. Food Chem. 2014, 152, 386–390. [Google Scholar] [CrossRef]
- Mullen, W.; Lean, M.E.; Crozier, A. Rapid characterization of anthocyanins in red raspberry fruit by high-performance liquid chromatography coupled to single quadrupole mass spectrometry. J. Chromatogr. A. 2002, 966, 63–70. [Google Scholar] [CrossRef]
- Tian, Q.; Giusti, M.M.; Stoner, G.D.; Schwartz, S.J. Screening for anthocyanins using high-performance liquid chromatography coupled to electrospray ionization tandem mass spectrometry with precursor-ion analysis, product-ion analysis, common-neutral-loss analysis, and selected reaction monitoring. J. Chromatogr. A. 2005, 1091, 72–82. [Google Scholar] [CrossRef]
- Sakamoto, K.; Iida, K.; Sawamura, K.; Hajiro, K.; Asada, Y.; Yoshikawa, T.; Furuya, T. Effects of nutrients on anthocyanin production in cultured cells of Aralia cordata. Phytochemistry 1993, 33, 357–360. [Google Scholar] [CrossRef]
- Asada, Y.; Sakamoto, K.; Furuya, T. A minor anthocyanin from cultured cells of Aralia cordata. Phytochemistry 1994, 35, 1471–1473. [Google Scholar] [CrossRef]
- Schwarz, M.; Wray, V.; Winterhalter, P. Isolation and identification of novel pyranoanthocyanins from black carrot (Daucus carota L.) juice. J. Agric. Food Chem. 2004, 52, 5095–5101. [Google Scholar] [CrossRef]
- Sato, K.; Goda, Y.; Yoshihira, K.; Noguchi, H. Structure and contents of main coloring constituents in the calyces of Hibiscus sabdariffa and commercial roselle color. Food Hyg. Soc. Jpn. 1991, 32, 301–307. [Google Scholar] [CrossRef]
- Kawano, K.; Sakamura, S. .Cyanidin 3-[O-β-D-Xylopyranosyl (1→2)-β-D-galactopyranoside] from Aralia elata and Aralia cordata. Agric. Biol. Chem. 1972, 36, 27–32. [Google Scholar] [CrossRef][Green Version]
- Agcam, E.; Akyıldız, A.; Balasubramaniam, V.M. Optimization of anthocyanins extraction from black carrot pomace with thermosonication. Food Chem. 2017, 237, 461–470. [Google Scholar] [CrossRef]
- Farr, J.E.; Sigurdson, G.T.; Giusti, M.M. Influence of cyanidin glycosylation patterns on carboxypyranoanthocyanin formation. Food Chem. 2018, 259, 261–269. [Google Scholar] [CrossRef]
- Katalinic, V.; Milos, M.; Kulisic, T.; Jukic, M. Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem. 2006, 94, 550–557. [Google Scholar] [CrossRef]
- Dudonne, S.; Vitrac, X.; Coutiere, P.; Woillez, M.; Mérillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Zhou, H.; Lv, J.; Yuan, Q. Preparative isolation and purification of punicalin from pomegranate husk by high-speed countercurrent chromatography. Sep. Purif. Technol. 2010, 72, 225–228. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Brand, W.W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
Sample Availability: Due to the current epidemic situation, samples are temporarily unavailable. |

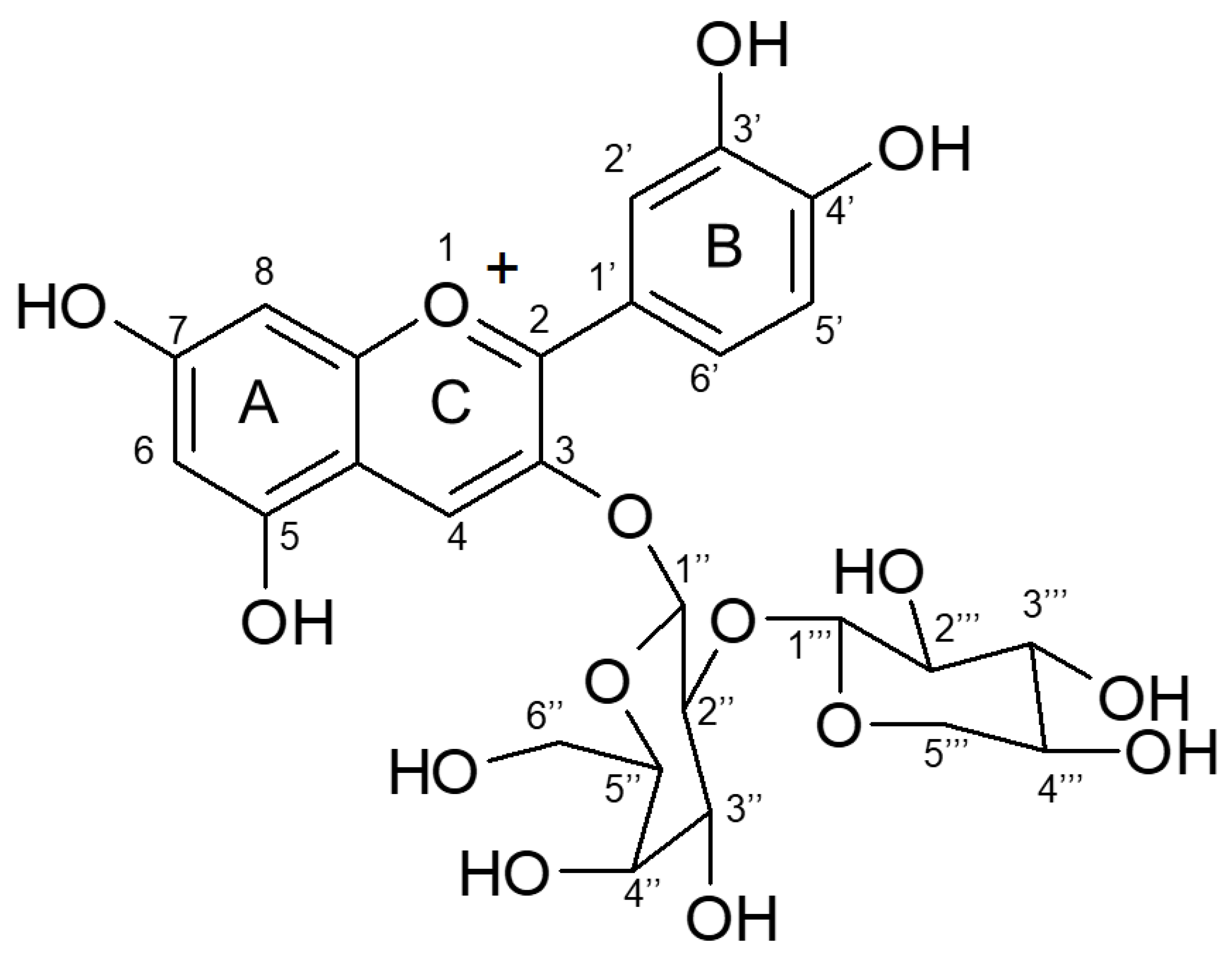
| Cyanidin 3-Xylosyl-Galactoside | 13C (ppm) Also Figure A2 | 1H (ppm) Also Figure A3 |
|---|---|---|
| aglycone | ||
| 2 | 164.08 | |
| 3 | 145.33 | |
| 4 | 136.12 | 8.88 s |
| 5 | 159.18 | |
| 6 | 103.41 | 6.63 d2.0 |
| 7 | 170.34 | |
| 8 | 95.00 | 6.95 d9.5 |
| 9 | 157.47 | |
| 10 | 113.19 | |
| 1′ | 121.25 | |
| 2′ | 118.58 | 7.96 d2.5 |
| 3′ | 147.29 | |
| 4′ | 155.78 | |
| 5′ | 117.39 | 6.86 d1.0 |
| 6′ | 128.59 | 8.23 dd2.5,9.0 |
| galactose | ||
| 1″ | 102.10 | 5.39 d7.5 |
| 2″ | 80.02 | 4.26 t9.0 |
| 3″ | 75.10 | 3.91 dd3.5,9.5 |
| 4″ | 70.06 | 3.98 d3.0 |
| 5″ | 77.70 | 3.84 d9.0 |
| 6A″ | 62.33 | 3.81 d3.5 |
| 6B″ | 3.79 t5.5 | |
| xylose | ||
| 1‴ | 106.01 | 4.71 d7.5 |
| 2‴ | 75.81 | 3.17 dd8.0,9.0 |
| 3‴ | 77.87 | 3.35 m |
| 4‴ | 70.97 | 3.64 dd5.0,11.50 |
| 5‴ | 67.16 | 3.73 d2.0 |
| 6‴ | 3.03 t11.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Xin, X.; Feng, H.; Li, S.; Cao, Q.; Wang, X.; Vriesekoop, F. Isolation and Identification of Anthocyanin Component in the Fruits of Acanthopanax Sessiliflorus (Rupr. & Maxim.) Seem. by Means of High Speed Counter Current Chromatography and Evaluation of Its Antioxidant Activity. Molecules 2020, 25, 1781. https://doi.org/10.3390/molecules25081781
Chen L, Xin X, Feng H, Li S, Cao Q, Wang X, Vriesekoop F. Isolation and Identification of Anthocyanin Component in the Fruits of Acanthopanax Sessiliflorus (Rupr. & Maxim.) Seem. by Means of High Speed Counter Current Chromatography and Evaluation of Its Antioxidant Activity. Molecules. 2020; 25(8):1781. https://doi.org/10.3390/molecules25081781
Chicago/Turabian StyleChen, Liang, Xiulan Xin, Hui Feng, Shuangshi Li, Qiguang Cao, Xinying Wang, and Frank Vriesekoop. 2020. "Isolation and Identification of Anthocyanin Component in the Fruits of Acanthopanax Sessiliflorus (Rupr. & Maxim.) Seem. by Means of High Speed Counter Current Chromatography and Evaluation of Its Antioxidant Activity" Molecules 25, no. 8: 1781. https://doi.org/10.3390/molecules25081781
APA StyleChen, L., Xin, X., Feng, H., Li, S., Cao, Q., Wang, X., & Vriesekoop, F. (2020). Isolation and Identification of Anthocyanin Component in the Fruits of Acanthopanax Sessiliflorus (Rupr. & Maxim.) Seem. by Means of High Speed Counter Current Chromatography and Evaluation of Its Antioxidant Activity. Molecules, 25(8), 1781. https://doi.org/10.3390/molecules25081781







