Stability Evaluation of DMT and Harmala Alkaloids in Ayahuasca Tea Samples
Abstract
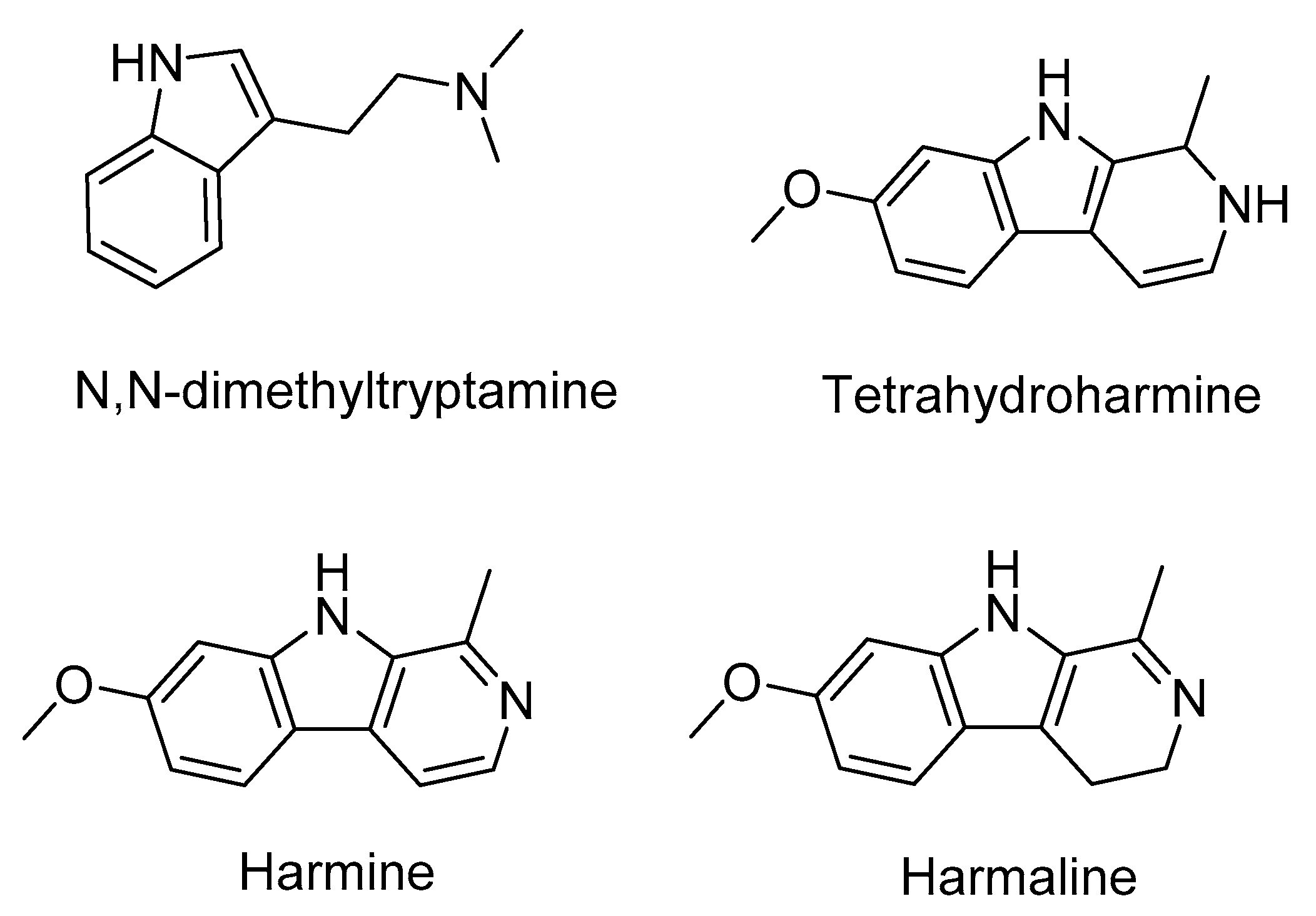
1. Introduction
2. Results
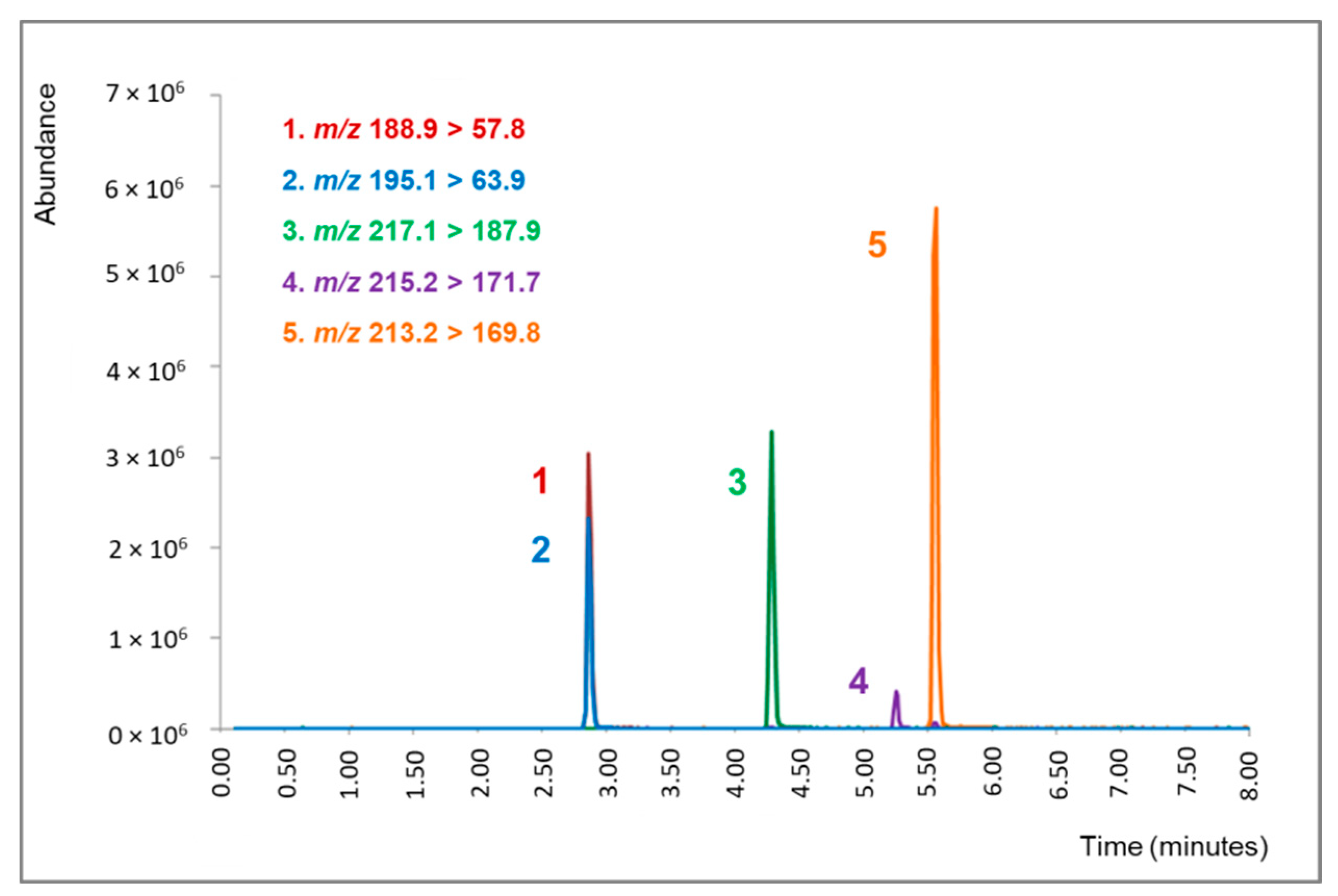
2.1. LC-ESI-MS/MS Analysis
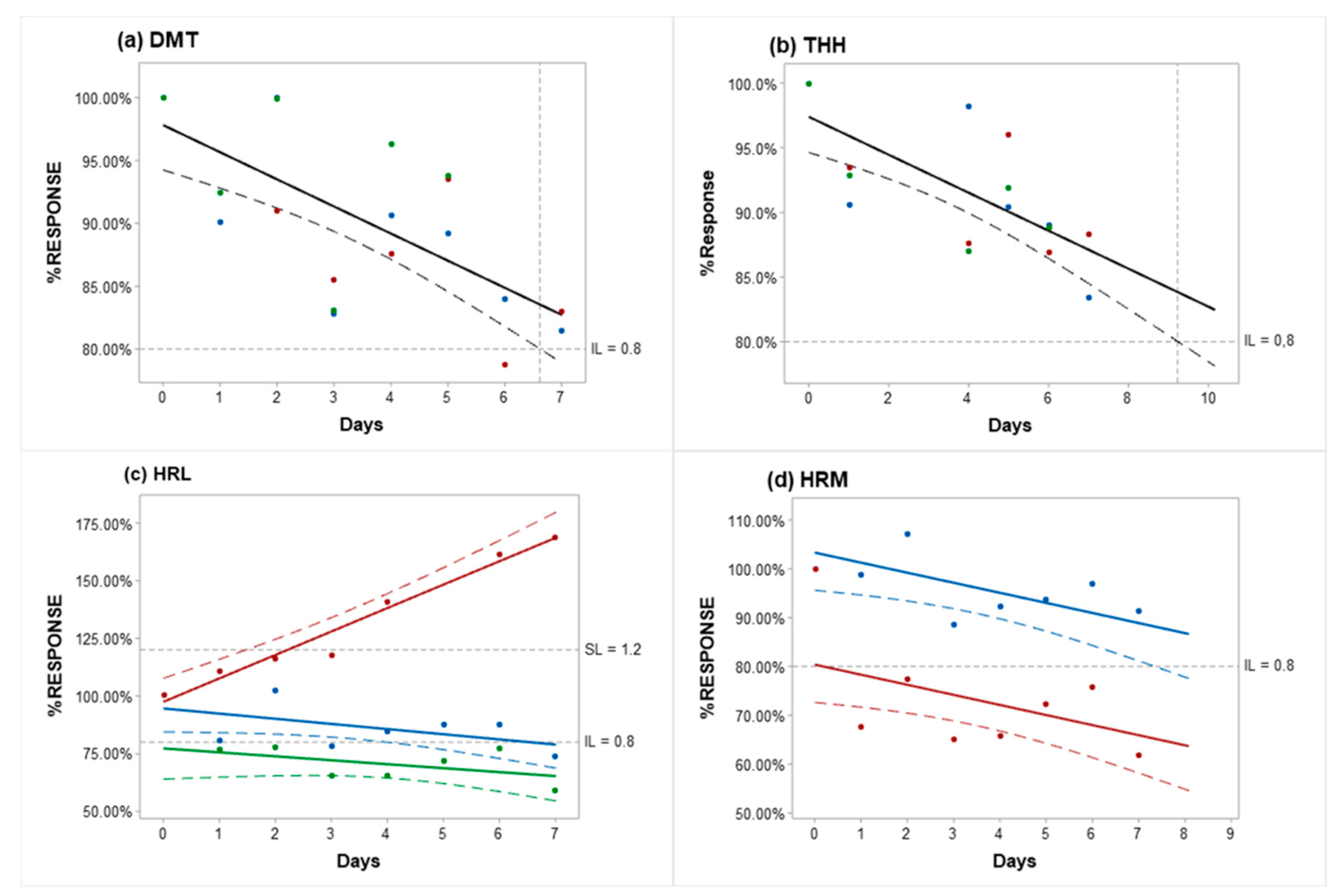
2.2. Long-Term Stability
2.3. Transportation Stability
2.4. Freeze-Thaw Cycles
3. Discussion
4. Materials and Methods
4.1. Standards and Reagents
4.2. Plant Material and Sample Preparation
4.3. Instrumental Analysis
4.4. Stability Design and Data Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mckenna, D.J. Clinical investigations of the therapeutic potential of ayahuasca: Rationale and regulatory challenges. Pharmacol. Ther. 2004, 102, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, P.C.R.; Mizumoto, S.; Bogenschutz, M.P.; Strassman, R.J. Health status of ayahuasca users. Drug Test. Anal. 2012, 4, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Bouso, J.C.; González, D.; Fondevila, S.; Cutchet, M.; Fernández, X.; Barbosa, P.C.R.; Riba, J. Personality, psychopathology, life attitudes and neuropsychological performance among ritual users of ayahuasca: A longitudinal study. PLoS ONE 2012, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.J.; Albarracin-Jordan, J.; Moore, C.; Capriles, J.M. Chemical evidence for the use of multiple psychotropic plants in a 1,000-year-old ritual bundle from South America. Proc. Natl. Acad. Sci. USA 2019, 116, 11207–11212. [Google Scholar] [CrossRef]
- Callaway, J.C.; McKenna, D.J.; Grob, G.S.; Raymon, L.P.; Poland, R.E.; Andrade, E.N.; Andrade, O.; Mash, D.C. Pharmacokinetics of Hoasca alkaloids in healthy humans. J. Ethnopharmacol. 1999, 65, 243–256. [Google Scholar] [CrossRef]
- Oliveira, C.D.; Okai, G.G.; da Costa, J.L.; de Almeida, R.M.; Oliveira-Silva, D.; Yonamine, M. Determination of dimethyltryptamine and beta-carbolines (ayahuasca alkaloids) in plasma samples by LC-MS/MS. Bioanalysis 2012, 4, 1731–1738. [Google Scholar] [CrossRef]
- Tupper, K.W. The globalization of ayahuasca: Harm reduction or benefit maximization? Int. J. Drug Policy 2008, 19, 297–303. [Google Scholar] [CrossRef]
- Riba, J.; McIlhenny, E.H.; Valle, M.; Bouso, J.C.; Barker, S.A. Metabolism and disposition of N,N-dimethyltryptamine and harmala alkaloids after oral administration of ayahuasca. Drug Test. Anal. 2012, 4, 610–616. [Google Scholar] [CrossRef]
- Cata-Preta, E.G.; Serra, Y.A.; Moreira-Junior, E.D.C.; Reis, H.S.; Kisaki, N.D.; Libarino-Santos, M.; Silva, R.R.; Barros-Santos, T.; Santos, L.C.; Barbosa, P.C.; et al. Ayahuasca and Its DMT- and β-carbolines-Containing ingredients block the expression of ethanol-induced conditioned place preference in mice: Role of the treatment environment. Front. Pharmacol. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Cruz, J.I.; Nappo, S.A. Is Ayahuasca an Option for the Treatment of Crack Cocaine Dependence? J. Psychoact. Drugs 2018, 50, 247–255. [Google Scholar] [CrossRef]
- Frecska, E.; Bokor, P.; Winkelman, M. The Therapeutic Potentials of Ayahuasca: Possible Effects against Various Diseases of Civilization. Front. Pharmacol. 2016, 7, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Brierley, D.I.; Davidson, C. Developments in harmine pharmacology - Implications for ayahuasca use and drug-dependence treatment. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 39, 263–272. [Google Scholar] [CrossRef]
- Cameron, L.P.; Benson, C.J.; Dunlap, L.E.; Olson, D.E. Effects of N,N-Dimethyltryptamine on Rat Behaviors Relevant to Anxiety and Depression. ACS Chem. Neurosci. 2018, 9, 1582–1590. [Google Scholar] [CrossRef]
- Domínguez-Clavé, E.E.; Soler, J.; Elices, M.; Pascual, J.C.; Álvarez, E.; de la Fuente Revenga, M.; Friedlander, P.; Feilding, A.; Riba, J. Ayahuasca: Pharmacology, neuroscience and therapeutic potential. Brain Res. Bull. 2016, 126, 89–101. [Google Scholar] [CrossRef]
- Galvão, A.C.D.M.; de Almeida, R.N.; Silva, E.A.; Freire, F.A.; Palhano-Fontes, F.; Onias, H.; Arcoverde, E.; Maia-de-Oliveira, J.P.; de Araújo, D.B.; Lobão-Soares, B.; et al. Cortisol modulation by ayahuasca in patients with treatment resistant depression and healthy controls. Front. Psychiatry 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Palhano-Fontes, F.; Barreto, D.; Onias, H.; Andrade, K.C.; Novaes, M.M.; Pessoa, J.A.; Mota-Rolim, S.A.; Osório, F.L.; Sanches, R.; dos Santos, R.G.; et al. Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: A randomised placebo-controlled trial. Psychol. Med. 2018, 49, 655–663. [Google Scholar] [CrossRef] [PubMed]
- González, D.; Carvalho, M.; Cantillo, J.; Aixalá, M.; Farré, M. Potential Use of Ayahuasca in Grief Therapy. OMEGA-J. Death Dying 2017, 79, 260–285. [Google Scholar] [CrossRef]
- Lafrance, A.; Loizaga-Velder, A.; Fletcher, J.; Renelli, M.; Files, N.; Tupper, K.W. Nourishing the Spirit: Exploratory Research on Ayahuasca Experiences along the Continuum of Recovery from Eating Disorders. J. Psychoact. Drugs 2017, 49, 427–435. [Google Scholar] [CrossRef]
- Gambelunghe, C.; Aroni, K.; Rossi, R.; Moretti, L.; Bacci, M. Identification of N,N-dimethyltryptamine and β-carbolines in psychotropic ayahuasca beverage. Biomed. Chromatogr. 2008, 22, 1056–1059. [Google Scholar] [CrossRef]
- McIlhenny, E.H.; Pipkin, K.E.; Standish, L.J.; Wechkin, H.A.; Strassman, R.; Barker, S.A. Direct analysis of psychoactive tryptamine and harmala alkaloids in the Amazonian botanical medicine ayahuasca by liquid chromatography–electrospray ionization-tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 8960–8968. [Google Scholar] [CrossRef]
- Salum Pires, A.P.; De Oliveira, R.; Dizioli, C.; Moura, S.; Doerr, F.A.; Silva, W.A.E.; Yonamine, M. Gas Chromatographic Analysis of Dimethyltryptamine and beta-Carboline Alkaloids in Ayahuasca, an Amazonian Psychoactive Plant Beverage. Phytochem. Anal. 2009, 20, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Gaujac, A.; Dempster, N.; Navickiene, S.; Brandt, S.D.; de Andrade, J.B. Determination of N,N-dimethyltryptamine in beverages consumed in religious practices by headspace solid-phase microextraction followed by gas chromatography ion trap mass spectrometry. Talanta 2013, 106, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Lanaro, R.; Calemi, D.B.D.A.; Togni, L.R.; Costa, J.L.; Yonamine, M.; Cazenave, S.D.O.S.; Linardi, A. Ritualistic Use of Ayahuasca versus Street Use of Similar Substances Seized by the Police: A Key Factor Involved in the Potential for Intoxications and Overdose? J. Psychoact. Drugs 2015, 47, 132–139. [Google Scholar] [CrossRef]
- Santos, M.C.; Navickiene, S.; Gaujac, A. Determination of Tryptamines and β-Carbolines in Ayahuasca Beverage Consumed during Brazilian Religious Ceremonies. J. AOAC Int. 2017, 100, 820–824. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Souza, R.C.Z.; Zandonadi, F.S.; Freitas, D.P.; Tófoli, L.F.F.; Sussulini, A. Validation of an analytical method for the determination of the main ayahuasca active compounds and application to real ayahuasca samples from Brazil. J. Chromatogr. B 2019, 1124, 197–203. [Google Scholar] [CrossRef]
- Simão, A.Y.; Gonçalves, J.; Caramelo, D.; Rosado, T.; Barroso, M.; Restolho, J.; Fernández, N.; Rodilla, J.; Duarte, A.P.; Cristóvão, A.C.; et al. Determination of N,N-dimethyltryptamine and beta-carbolines in plants used to prepare ayahuasca beverages by means of solid-phase extraction and gas-chromatography–mass spectrometry. SN Appl. Sci. 2020, 2, 1–11. [Google Scholar] [CrossRef]
- ASB Standard 036. Standard Practices for Method Validation in Forensic Toxicology, 1st ed.; AAFS Standards Board, LLC: Washington, DC, USA, 2018. [Google Scholar]
- Peters, F.T.; Drummer, O.H.; Musshoff, F. Validation of new methods. Forensic Sci. Int. 2007, 165, 216–224. [Google Scholar] [CrossRef]
- Callaway, J.C. Various alkaloid profiles in decoctions of Banisteriopsis caapi. J. Psychoactive. Drugs 2005, 37, 151–155. [Google Scholar] [CrossRef]
- Aniszewski, T. Chapter 2 - Alkaloid Chemistry. In Alkaloids-Secrets of Life: Aklaloid Chemistry, Biological Significance, Applications and Ecological Role, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2007; p. 80. [Google Scholar] [CrossRef]
- Callaway, J.C.; Raymon, L.P.; Hearn, W.L.; McKenna, D.J.; Grob, C.S.; Brito, G.S.; Mash, D.C. Quantitation of N,N-dimethyltryptamine and harmala alkaloids in human plasma after oral dosing with ayahuasca. J. Anal. Toxicol. 1996, 20, 492–497. [Google Scholar] [CrossRef]
- United Nations Office on Drugs and Crime (UNODC). Guidance for the Validation of Analytical Methodology and Calibration of Equipment Used for Testing of Illicit Drugs in Seized Materials and Biological Specimens; United Nations: New York, NY, USA, 2009. [Google Scholar]
- Rocha, J.M. Efeitos Agudos e Prolongados da Ayahuasca no Reconhecimento de Expressões Faciais de Emoções, Personalidade, Ansiedade e Humor: Um Estudo Randomizado e Controlado com Placebo (Acute and Prolonged Effects of Ayahuasca on the Recognition of Facial Emotional Expressions, Personality, Anxiety and Mood: A Randomized, Placebo-Controlled Study). Master’s Thesis, University of Sao Paulo, Ribeirão Preto-SP, Brazil, 2020. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |

| Sample | Cycle | DMT% | THH% | HRL% | HRM% |
|---|---|---|---|---|---|
| C1 | 1st | −3.9 | 3.8 | −17.4 | * |
| 2nd | 1.3 | 7.7 | −6.1 | * | |
| 3rd | −8.4 | −19.0 | −13.6 | * | |
| C2 | 1st | −6.0 | 3.7 | 4.4 | 4.6 |
| 2nd | −3.5 | 6.5 | 3.6 | 7.9 | |
| 3rd | −16.3 | −13.5 | −14.2 | −9.9 | |
| C3 | 1st | −1.2 | −7.1 | 1.7 | −9.0 |
| 2nd | 1.0 | 18.2 | 8.4 | 16.1 | |
| 3rd | −14.1 | −18.8 | −13.6 | −10.6 |
| Analyte | Retention Time (min.) | Precursor Ion (m/z) | Product Ion (m/z) | Cone Voltage | Collision Energy |
|---|---|---|---|---|---|
| DMT-d6 (IS) | 2.87 | 195.1 | 63.9 1 | 15 | 14 |
| 114.9 | 15 | 36 | |||
| 143.8 | 15 | 22 | |||
| DMT | 2.88 | 188.9 | 57.8 1 | 25 | 11 |
| 116.7 | 25 | 29 | |||
| 143.8 | 25 | 17 | |||
| THH | 4.37 | 217.1 | 172.8 | 25 | 29 |
| 187.9 1 | 25 | 17 | |||
| 200.0 | 25 | 13 | |||
| HRL | 5.27 | 215.2 | 130.4 | 50 | 41 |
| 171.7 1 | 50 | 33 | |||
| 199.9 | 50 | 25 | |||
| HRM | 5.56 | 213.2 | 143.8 | 50 | 41 |
| 169.8 1 | 50 | 33 | |||
| 198.0 | 50 | 25 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira Silveira, G.; Guimarães dos Santos, R.; Rebello Lourenço, F.; Novak Rossi, G.; Hallak, J.E.C.; Yonamine, M. Stability Evaluation of DMT and Harmala Alkaloids in Ayahuasca Tea Samples. Molecules 2020, 25, 2072. https://doi.org/10.3390/molecules25092072
de Oliveira Silveira G, Guimarães dos Santos R, Rebello Lourenço F, Novak Rossi G, Hallak JEC, Yonamine M. Stability Evaluation of DMT and Harmala Alkaloids in Ayahuasca Tea Samples. Molecules. 2020; 25(9):2072. https://doi.org/10.3390/molecules25092072
Chicago/Turabian Stylede Oliveira Silveira, Gabriela, Rafael Guimarães dos Santos, Felipe Rebello Lourenço, Giordano Novak Rossi, Jaime E. C. Hallak, and Mauricio Yonamine. 2020. "Stability Evaluation of DMT and Harmala Alkaloids in Ayahuasca Tea Samples" Molecules 25, no. 9: 2072. https://doi.org/10.3390/molecules25092072
APA Stylede Oliveira Silveira, G., Guimarães dos Santos, R., Rebello Lourenço, F., Novak Rossi, G., Hallak, J. E. C., & Yonamine, M. (2020). Stability Evaluation of DMT and Harmala Alkaloids in Ayahuasca Tea Samples. Molecules, 25(9), 2072. https://doi.org/10.3390/molecules25092072







