Selected Pharmaceuticals in Different Aquatic Compartments: Part II—Toxicity and Environmental Risk Assessment
Abstract
1. Introduction
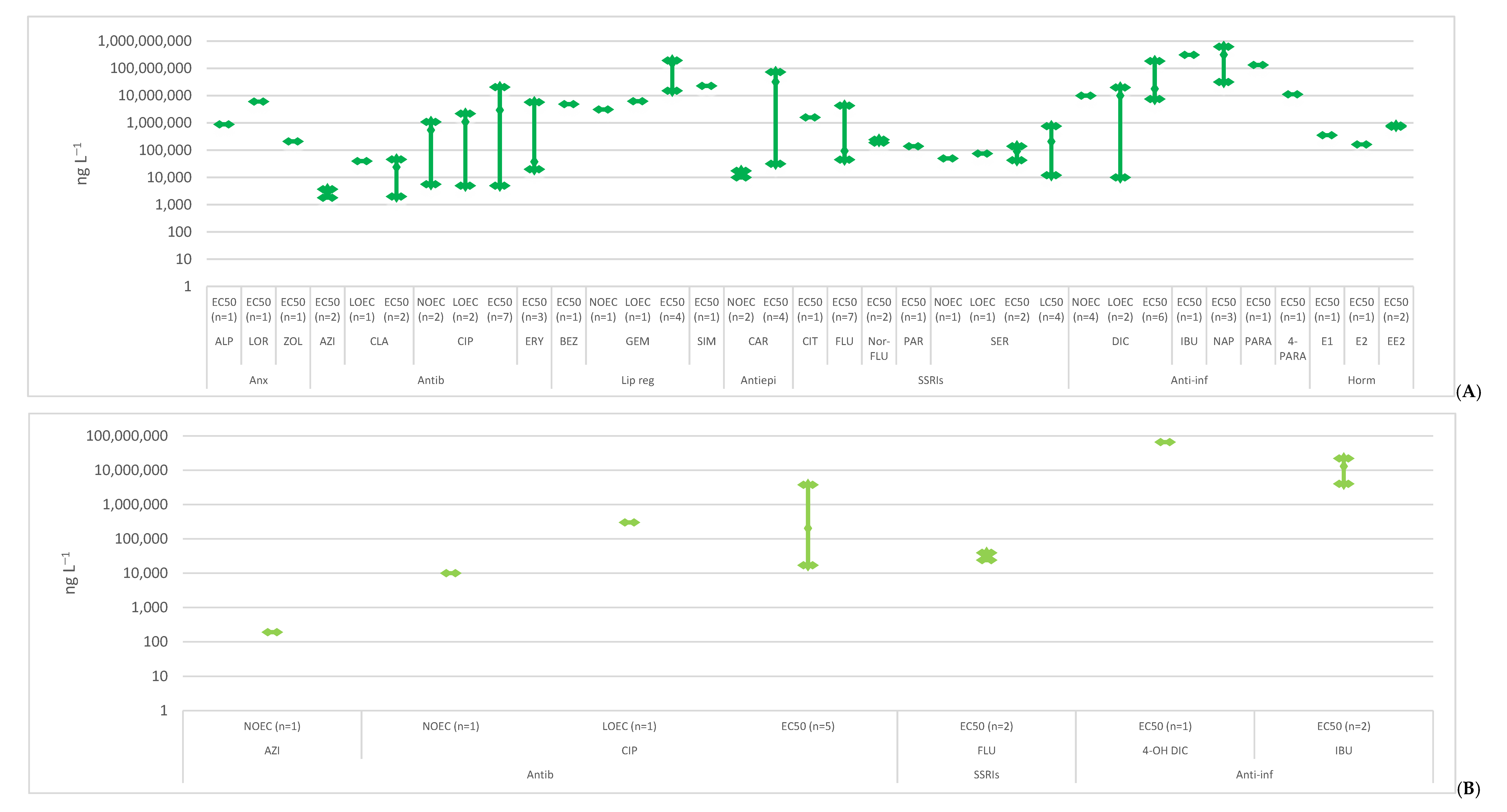
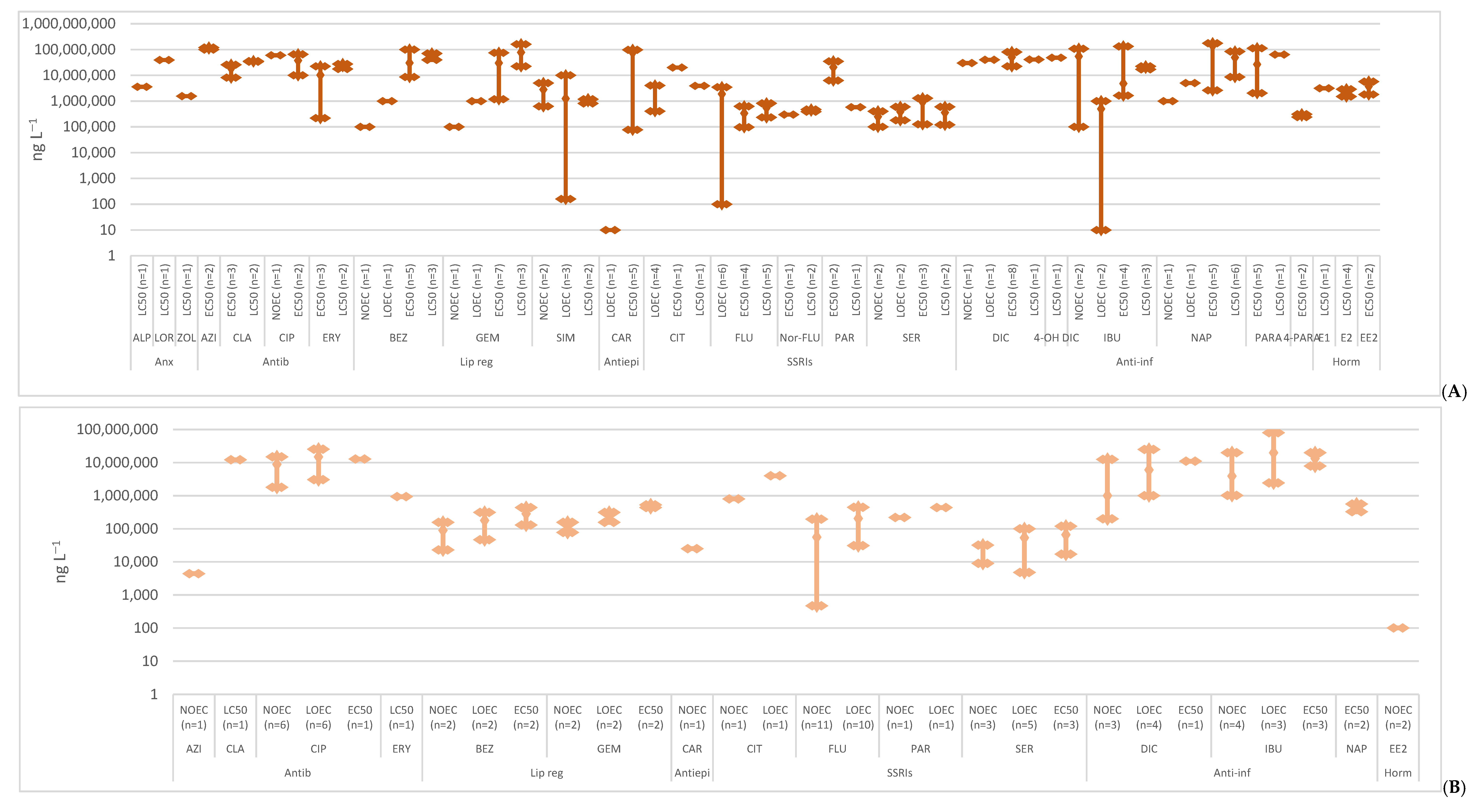
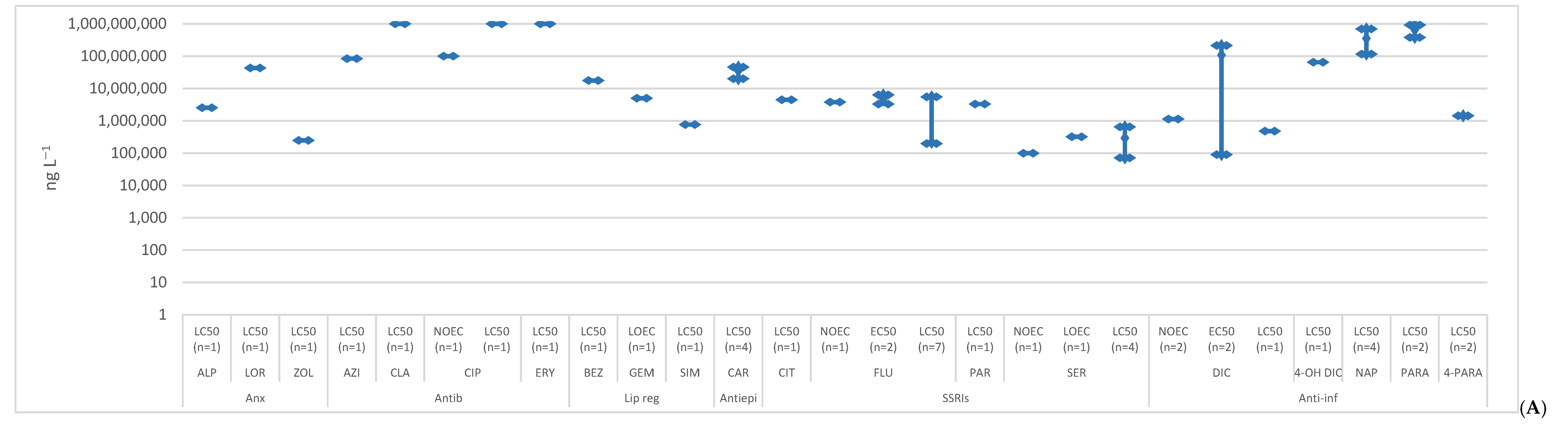
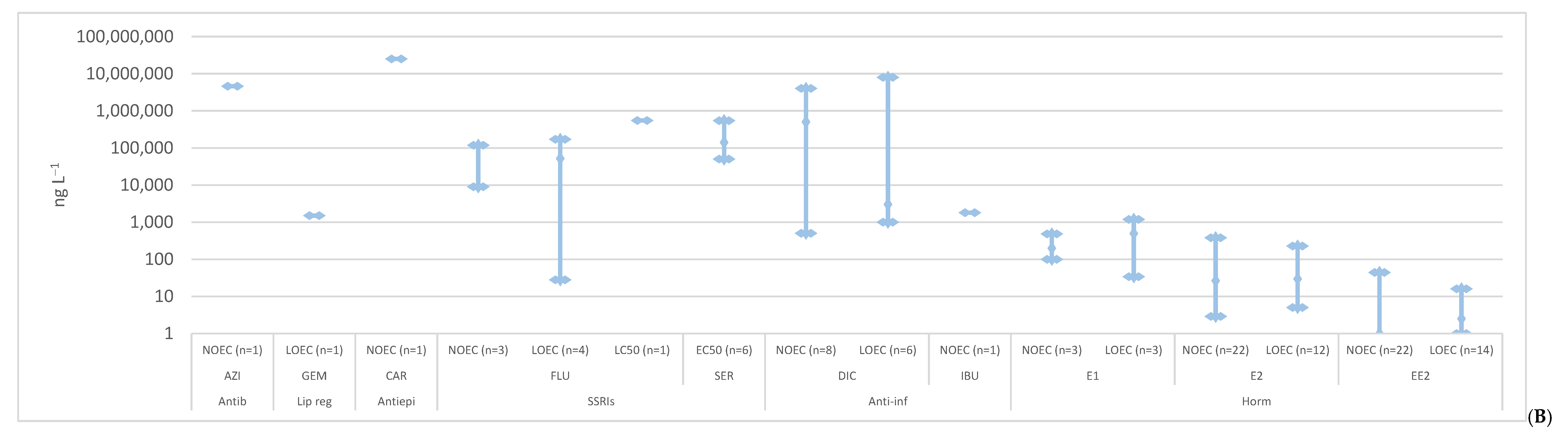
2. Toxicity
2.1. Anxiolytics
2.2. Antibiotics
2.3. Lipid Regulators
2.4. Antiepileptics
2.5. SSRIs
2.6. Anti-Inflammatories
2.7. Hormones
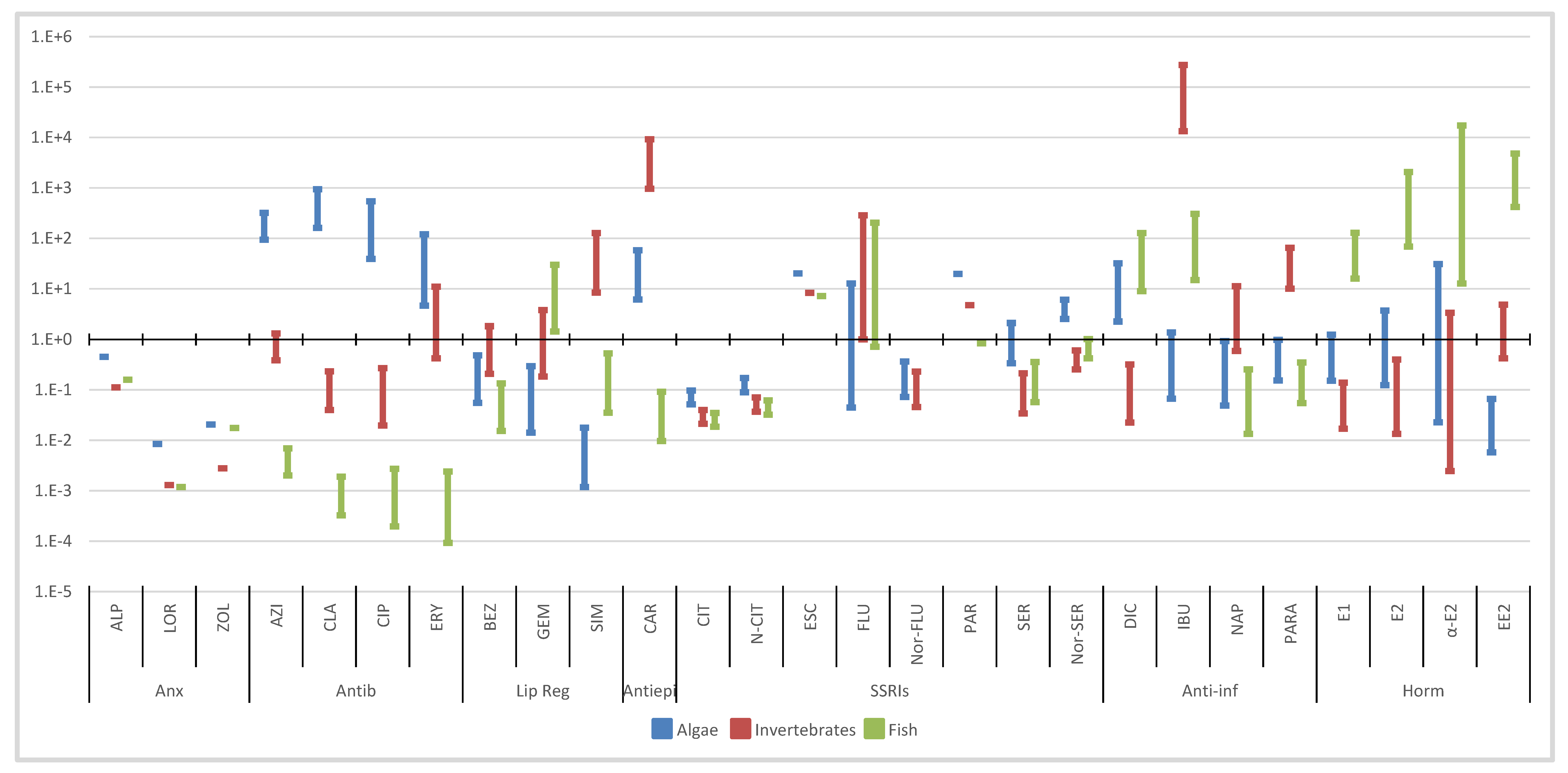
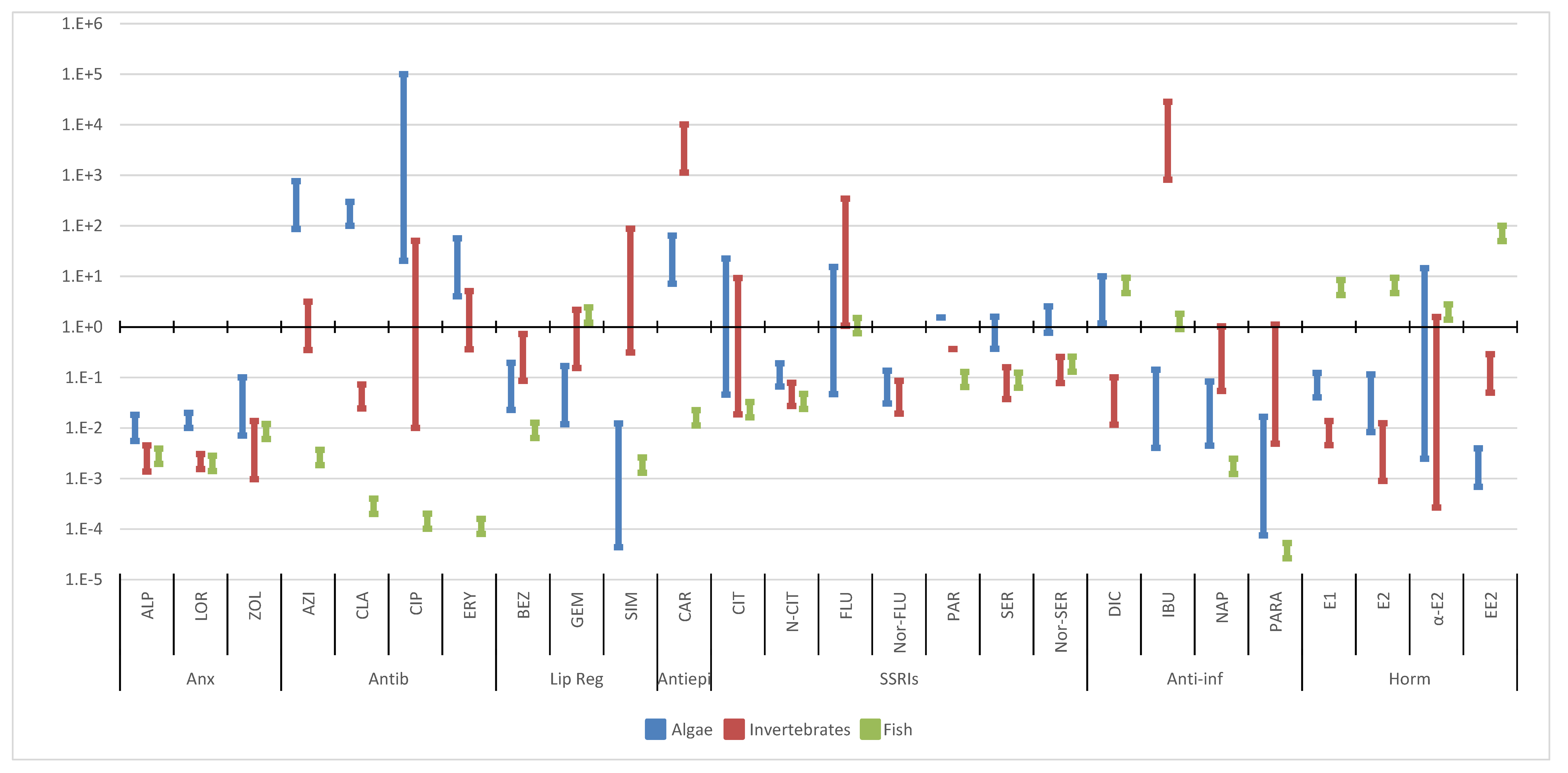
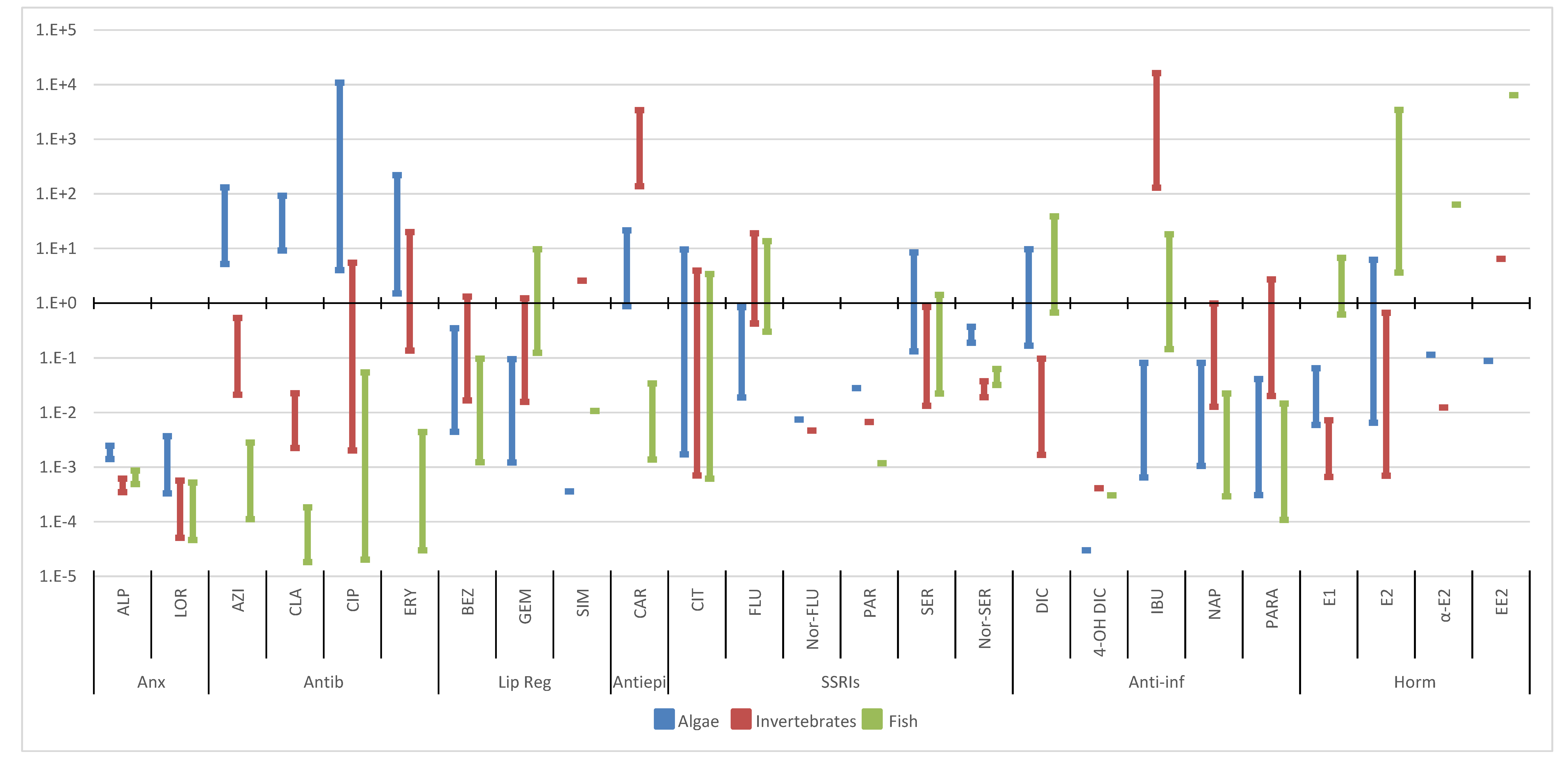
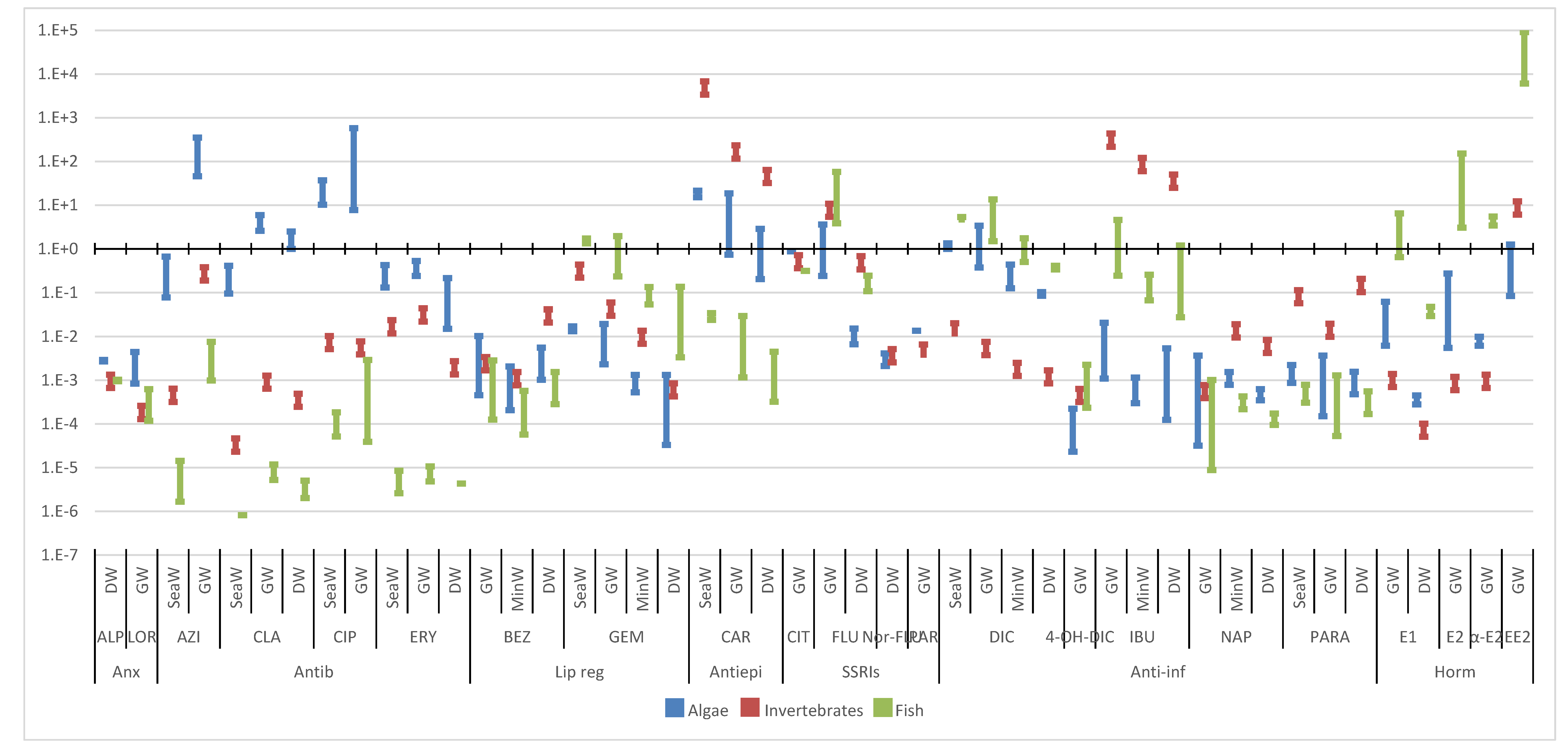
3. Environmental Risk Assessment
3.1. Predicted No-Effect Concentration (PNECs)
3.2. Risk Assessment
3.2.1. Wastewater Influents
3.2.2. Wastewater Effluents
3.2.3. Surface Waters
3.2.4. Other Water Bodies
3.3. Mitigation Measures
4. Final Remarks
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Robles-Molina, J.; Lara-Ortega, F.J.; Gilbert-López, B.; García-Reyes, J.F.; Molina-Díaz, A. Multi-residue method for the determination of over 400 priority and emerging pollutants in water and wastewater by solid-phase extraction and liquid chromatography-time-of-flight mass spectrometry. J. Chromatogr. A 2014, 1350, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.J.G.; Lino, C.M.; Meisel, L.M.; Pena, A. Selective serotonin re-uptake inhibitors (SSRIs) in the aquatic environment: An ecopharmacovigilance approach. Sci. Total Environ. 2012, 437, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rey, M.; Bebianno, M.J. Does selective serotonin reuptake inhibitor (SSRI) fluoxetine affects mussel Mytilus galloprovincialis? Environ. Pollut. 2013, 173, 200–209. [Google Scholar] [CrossRef]
- European Medicines Agency Guideline on the Environmental Risk Assessment of Medicinal Products for Human Use; European Medicines Agency: London, UK, 2006.
- Taylor, D.; Senac, T. Human pharmaceutical products in the environment—The “problem” in perspective. Chemosphere 2014, 115, 95–99. [Google Scholar] [CrossRef]
- Pereira, A.M.P.T.; Silva, L.J.G.; Lino, C.M.; Meisel, L.M.; Pena, A. A critical evaluation of different parameters for estimating pharmaceutical exposure seeking an improved environmental risk assessment. Sci. Total Environ. 2017, 603–604, 226–236. [Google Scholar] [CrossRef]
- Mompelat, S.; Le Bot, B.; Thomas, O. Occurrence and fate of pharmaceutical products and by-products, from resource to drinking water. Environ. Int. 2009, 35, 803–814. [Google Scholar] [CrossRef]
- Van Doorslaer, X.; Dewulf, J.; Van Langenhove, H.; Demeestere, K. Fluoroquinolone antibiotics: An emerging class of environmental micropollutants. Sci. Total Environ. 2014, 500–501, 250–269. [Google Scholar] [CrossRef]
- Boxall, A.B.A.; Keller, V.D.J.; Straub, J.O.; Monteiro, S.C.; Fussell, R.; Williams, R.J. Exploiting monitoring data in environmental exposure modelling and risk assessment of pharmaceuticals. Environ. Int. 2014, 73, 176–185. [Google Scholar] [CrossRef]
- European Commission Implementing Decision (EU) 2018/840 of 5 June 2018 establishing a watch list of substances for Union-wide monitoring in the field of water policy pursuant to Directive 2008/105/EC of the European Parliament and of the Council and repealing 495. Off. J. Eur. Union 2018, L141, 9–12.
- Papageorgiou, M.; Kosma, C.; Lambropoulou, D. Seasonal occurrence, removal, mass loading and environmental risk assessment of 55 pharmaceuticals and personal care products in a municipal wastewater treatment plant in Central Greece. Sci. Total Environ. 2016, 543, 547–569. [Google Scholar] [CrossRef]
- Ter Laak, T.L.; Van der Aa, M.; Houtman, C.J.; Stoks, P.G.; Van Wezel, A.P. Relating environmental concentrations of pharmaceuticals to consumption: A mass balance approach for the river Rhine. Environ. Int. 2010, 36, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Bade, R.; Rousis, N.I.; Bijlsma, L.; Gracia-Lor, E.; Castiglioni, S.; Sancho, J.V.; Hernandez, F. Screening of pharmaceuticals and illicit drugs in wastewater and surface waters of Spain and Italy by high resolution mass spectrometry using UHPLC-QTOF MS and LC-LTQ-Orbitrap MS. Anal. Bioanal. Chem. 2015, 407, 8979–8988. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Nunes, O.C.; Pereira, M.F.R.; Silva, A.M.T. An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched Directive 2013/39/EU. Environ. Int. 2015, 75, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.M.P.T.; Silva, L.J.G.; Meisel, L.M.; Lino, C.M.; Pena, A. Environmental impact of pharmaceuticals from Portuguese wastewaters: Geographical and seasonal occurrence, removal and risk assessment. Environ. Res. 2015, 136, 108–119. [Google Scholar] [CrossRef]
- He, Y.; Sutton, N.B.; Rijnaarts, H.H.H.; Langenhoff, A.A.M. Degradation of pharmaceuticals in wastewater using immobilized TiO2 photocatalysis under simulated solar irradiation. Appl. Catal. B Environ. 2016, 182, 132–141. [Google Scholar] [CrossRef]
- Zenker, A.; Cicero, M.R.; Prestinaci, F.; Bottoni, P.; Carere, M. Bioaccumulation and biomagnification potential of pharmaceuticals with a focus to the aquatic environment. J. Environ. Manag. 2014, 133, 378–387. [Google Scholar] [CrossRef]
- Vergeynst, L.; Haeck, A.; De Wispelaere, P.; Van Langenhove, H.; Demeestere, K. Multi-residue analysis of pharmaceuticals in wastewater by liquid chromatography-magnetic sector mass spectrometry: Method quality assessment and application in a Belgian case study. Chemosphere 2015, 119, S2–S8. [Google Scholar] [CrossRef]
- Johnson, A.C.; Sumpter, J.P. Putting pharmaceuticals into the wider context of challenges to fish populations in rivers. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130581. [Google Scholar] [CrossRef][Green Version]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2014, 72, 3–27. [Google Scholar] [CrossRef]
- Lavén, M.; Alsberg, T.; Yu, Y.; Adolfsson-Erici, M.; Sun, H. Serial mixed-mode cation- and anion-exchange solid-phase extraction for separation of basic, neutral and acidic pharmaceuticals in wastewater and analysis by high-performance liquid chromatography-quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 2009, 1216, 49–62. [Google Scholar] [CrossRef]
- OECD Guidance Document on the Use of the Harmonised System for the Classification of Chemicals Which Are Hazardous for the Aquatic Environment; OECD: Paris, France, 2001.
- Isidori, M.; Lavorgna, M.; Nardelli, A.; Pascarella, L.; Parrella, A. Toxic and genotoxic evaluation of six antibiotics on non-target organisms. Sci. Total Environ. 2005, 346, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.-H.; Ying, G.-G.; Su, H.-C.; Stauber, J.L.; Adams, M.S.; Binet, M.T. Growth-inhibiting effects of 12 antibacterial agenst and their mixtures on the freshwater microalga Pseudokirchneriella subcapitata. Environ. Toxicol. Chem. 2008, 27, 1201. [Google Scholar] [CrossRef] [PubMed]
- Farré, M.; Ferrer, I.; Ginebreda, A.; Figueras, M.; Olivella, L.; Tirapu, L.; Vilanova, M.; Barceló, D. Determination of drugs in surface water and wastewater samples by liquid chromatography–mass spectrometry: Methods and preliminary results including toxicity studies with Vibrio fischeri. J. Chromatogr. A 2001, 938, 187–197. [Google Scholar] [CrossRef]
- DeLorenzo, M.E.; Fleming, J. Individual and mixture effects of selected pharmaceuticals and personal care products on the marine phytoplankton species Dunaliella tertiolecta. Arch. Environ. Contam. Toxicol. 2008, 54, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, B.; Mons, R.; Vollat, B.; Fraysse, B.; Paxéus, N.; Lo Giudice, R.; Pollio, A.; Garric, J. Environmental risk assessment of six human pharmaceuticals: Are the current environmental risk assessment procedures sufficient for the protection of the aquatic environment? Environ. Toxicol. Chem. 2004, 23, 1344–1354. [Google Scholar] [CrossRef]
- Cleuvers, M. Aquatic ecotoxicity of pharmaceuticals including the assessment of combination effects. Toxicol. Lett. 2003, 142, 185–194. [Google Scholar] [CrossRef]
- Ferrari, B.; Paxéus, N.; Giudice, R.L.; Pollio, A.; Garric, J. Ecotoxicological impact of pharmaceuticals found in treated wastewaters: Study of carbamazepine, clofibric acid, and diclofenac. Ecotoxicol. Environ. Saf. 2003, 55, 359–370. [Google Scholar] [CrossRef]
- Christensen, A.M.; Faaborg-Andersen, S.; Ingerslev, F.; Baun, A. Mixture and single-substance toxicity of selective serotonin reuptake inhibitors toward algae and crustaceans. Environ. Toxicol. Chem. 2007, 26, 85. [Google Scholar] [CrossRef]
- Brooks, B.W.; Turner, P.K.; Stanley, J.K.; Weston, J.J.; Glidewell, E.A.; Foran, C.M.; Slattery, M.; La Point, T.W.; Huggett, D.B. Waterborne and sediment toxicity of fluoxetine to select organisms. Chemosphere 2003, 52, 135–142. [Google Scholar] [CrossRef]
- Brooks, B.W.; Foran, C.M.; Richards, S.M.; Weston, J.; Turner, P.K.; Stanley, J.K.; Solomon, K.R.; Slattery, M.; La Point, T.W. Aquatic ecotoxicology of fluoxetine. Toxicol. Lett. 2003, 142, 169–183. [Google Scholar] [CrossRef]
- Johnson, D.J.; Sanderson, H.; Brain, R.A.; Wilson, C.J.; Solomon, K.R. Toxicity and hazard of selective serotonin reuptake inhibitor antidepressants fluoxetine, fluvoxamine, and sertraline to algae. Ecotoxicol. Environ. Saf. 2007, 67, 128–139. [Google Scholar] [CrossRef]
- Neuwoehner, J.; Fenner, K.; Escher, B.I. Physiological modes of action of fluoxetine and its human metabolites in algae. Environ. Sci. Technol. 2009, 43, 6830–6837. [Google Scholar] [CrossRef]
- Halling-Sorensen, B. Environmental risk assessment of antibiotics: Comparison of mecillinam, trimethoprim and ciprofloxacin. J. Antimicrob. Chemother. 2000, 46, 53–58. [Google Scholar] [CrossRef]
- Minagh, E.; Hernan, R.; O’Rourke, K.; Lyng, F.M.; Davoren, M. Aquatic ecotoxicity of the selective serotonin reuptake inhibitor sertraline hydrochloride in a battery of freshwater test species. Ecotoxicol. Environ. Saf. 2009, 72, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.R.; Swerhone, G.D.W.; Topp, E.; Korber, D.R.; Neu, T.R.; Wassenaar, L.I. Structural and functional responses of river biofilm communities to the nonsteroidal anti-inflammatory diflofenac. Environ. Toxicol. Chem. 2007, 26, 573. [Google Scholar] [CrossRef]
- Ortiz de García, S.; Pinto, G.P.; García-Encina, P.A.; Mata, R.I. Ranking of concern, based on environmental indexes, for pharmaceutical and personal care products: An application to the Spanish case. J. Environ. Manage. 2013, 129, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Pomati, F.; Netting, A.G.; Calamari, D.; Neilan, B.A. Effects of erythromycin, tetracycline and ibuprofen on the growth of Synechocystis sp. and Lemna minor. Aquat. Toxicol. 2004, 67, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Isidori, M.; Lavorgna, M.; Nardelli, A.; Parrella, A.; Previtera, L.; Rubino, M. Ecotoxicity of naproxen and its phototransformation products. Sci. Total Environ. 2005, 348, 93–101. [Google Scholar] [CrossRef]
- Cleuvers, M. Mixture toxicity of the anti-inflammatory drugs diclofenac, ibuprofen, naproxen, and acetylsalicylic acid. Ecotoxicol. Environ. Saf. 2004, 59, 309–315. [Google Scholar] [CrossRef]
- Henschel, K.-P.; Wenzel, A.; Diedrich, M.; Fliedner, A. Environmental Hazard Assessment of Pharmaceuticals. Regul. Toxicol. Pharmacol. 1997, 25, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Onogbosele, C.O. Bioavailability of organic contaminants in rivers. Ph.D. Thesis, Brunel University, London, UK, 2015. [Google Scholar]
- Li, Y.; Zhang, L.; Liu, X.; Ding, J. Ranking and prioritizing pharmaceuticals in the aquatic environment of China. Sci. Total Environ. 2019, 658, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.D.; Soares, E.V. Sensitivity of freshwater and marine green algae to three compounds of emerging concern. J. Appl. Phycol. 2019, 31, 399–408. [Google Scholar] [CrossRef]
- Martins, N.; Pereira, R.; Abrantes, N.; Pereira, J.; Gonçalves, F.; Marques, C.R. Ecotoxicological effects of ciprofloxacin on freshwater species: Data integration and derivation of toxicity thresholds for risk assessment. Ecotoxicology 2012, 21, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Ebert, I.; Bachmann, J.; Kühnen, U.; Küster, A.; Kussatz, C.; Maletzki, D.; Schlüter, C. Toxicity of the fluoroquinolone antibiotics enrofloxacin and ciprofloxacin to photoautotrophic aquatic organisms. Environ. Toxicol. Chem. 2011, 30, 2786–2792. [Google Scholar] [CrossRef]
- Brain, R.A.; Johnson, D.J.; Richards, S.M.; Sanderson, H.; Sibley, P.K.; Solomon, K.R. Effects of 25 pharmaceutical compounds to Lemna gibba using a seven-day static-renewel test. Environ. Toxicol. Chem. 2004, 23, 371–382. [Google Scholar] [CrossRef]
- Robinson, A.A.; Belden, J.B.; Lydy, M.J. Toxicity of fluoroquinolone antibiotics to aquatic organisms. Environ. Toxicol. Chem. 2005, 24, 423. [Google Scholar] [CrossRef]
- Nie, X.; Wang, X.; Chen, J.; Zitko, V.; An, T. Response of the freshwater Alga chlorella vulgaris to trichloroisocyanuric acid and ciprofloxacin. Environ. Toxicol. Chem. 2008, 27, 168–173. [Google Scholar] [CrossRef]
- Isidori, M.; Nardelli, A.; Pascarella, L.; Rubino, M.; Parrella, A. Toxic and genotoxic impact of fibrates and their photoproducts on non-target organisms. Environ. Int. 2007, 33, 635–641. [Google Scholar] [CrossRef]
- Zurita, J.L.; Repetto, G.; Jos, A.; Salguero, M.; López-Artíguez, M.; Cameán, A.M. Toxicological effects of the lipid regulator gemfibrozil in four aquatic systems. Aquat. Toxicol. 2007, 81, 106–115. [Google Scholar] [CrossRef]
- Quinn, B.; Gagné, F.; Blaise, C. An investigation into the acute and chronic toxicity of eleven pharmaceuticals (and their solvents) found in wastewater effluent on the cnidarian, Hydra attenuata. Sci. Total Environ. 2008, 389, 306–314. [Google Scholar] [CrossRef]
- Han, G.H.; Hur, H.G.; Kim, S.D. Ecotoxicological risk of pharmaceuticals from wastewater treatment plants in Korea: Occurrence and toxicity to Daphnia magna. Environ. Toxicol. Chem. 2006, 25, 265–271. [Google Scholar] [CrossRef]
- Key, P.B.; Hoguet, J.; Reed, L.A.; Chung, K.W.; Fulton, M.H. Effects of the statin antihyperlipidemic agent simvastatin on grass shrimp, Palaemonetes pugio. Environ. Toxicol. 2008, 23, 153–160. [Google Scholar] [CrossRef]
- Dahl, U.; Gorokhova, E.; Breitholtz, M. Application of growth-related sublethal endpoints in ecotoxicological assessments using a harpacticoid copepod. Aquat. Toxicol. 2006, 77, 433–438. [Google Scholar] [CrossRef] [PubMed]
- De Lange, H.J.; Noordoven, W.; Murk, A.J.; Lürling, M.; Peeters, E.T.H.M. Behavioural responses of Gammarus pulex (Crustacea, Amphipoda) to low concentrations of pharmaceuticals. Aquat. Toxicol. 2006, 78, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Ishibashi, H.; Yamauchi, R.; Ichikawa, N.; Takao, Y.; Hirano, M.; Koga, M.; Arizono, K. Acute toxicity of pharmaceutical and personal care products on freshwater crustacean (Thamnocephalus platyurus) and fish (Oryzias latipes). J. Toxicol. Sci. 2009, 34, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Choi, K.; Jung, J.; Park, S.; Kim, P.-G.; Park, J. Aquatic toxicity of acetaminophen, carbamazepine, cimetidine, diltiazem and six major sulfonamides, and their potential ecological risks in Korea. Environ. Int. 2007, 33, 370–375. [Google Scholar] [CrossRef]
- Fong, P.P.; Molnar, N. Antidepressants cause foot detachment from substrate in five species of marine snail. Mar. Environ. Res. 2013, 84, 24–30. [Google Scholar] [CrossRef]
- Nałecz-Jawecki, G. Evaluation of the in vitro biotransformation of fluoxetine with HPLC, mass spectrometry and ecotoxicological tests. Chemosphere 2007, 70, 29–35. [Google Scholar] [CrossRef]
- Nentwig, G. Effects of pharmaceuticals on aquatic invertebrates. Part II: The antidepressant drug fluoxetine. Arch. Environ. Contam. Toxicol. 2007, 52, 163–170. [Google Scholar] [CrossRef]
- Péry, A.R.R.; Gust, M.; Vollat, B.; Mons, R.; Ramil, M.; Fink, G.; Ternes, T.; Garric, J. Fluoxetine effects assessment on the life cycle of aquatic invertebrates. Chemosphere 2008, 73, 300–304. [Google Scholar] [CrossRef]
- Gust, M.; Buronfosse, T.; Giamberini, L.; Ramil, M.; Mons, R.; Garric, J. Effects of fluoxetine on the reproduction of two prosobranch mollusks: Potamopyrgus antipodarum and Valvata piscinalis. Environ. Pollut. 2009, 157, 423–429. [Google Scholar] [CrossRef]
- Hazelton, P.D.; Cope, W.G.; Mosher, S.; Pandolfo, T.J.; Belden, J.B.; Barnhart, M.C.; Bringolf, R.B. Fluoxetine alters adult freshwater mussel behavior and larval metamorphosis. Sci. Total Environ. 2013, 445–446, 94–100. [Google Scholar] [CrossRef]
- Cunningham, V.L.; Constable, D.J.C.; Hannah, R.E. Environmental Risk Assessment of Paroxetine. Environ. Sci. Technol. 2004, 38, 3351–3359. [Google Scholar] [CrossRef]
- Lamichhane, K.; Garcia, S.N.; Huggett, D.B.; DeAngelis, D.L.; La Point, T.W. Exposures to a selective serotonin reuptake inhibitor (SSRI), sertraline hydrochloride, over multiple generations: Changes in life history traits in Ceriodaphnia dubia. Ecotoxicol. Environ. Saf. 2014, 101, 124–130. [Google Scholar] [CrossRef]
- Cleuvers, M. Chronic Mixture Toxicity of Pharmaceuticals to Daphnia—The Example of Nonsteroidal Anti-Inflammatory Drugs. In Pharmaceuticals in the Environment; Kümmerer, K., Ed.; Springer: Berlin, Heidelberg, 2006; pp. 277–284. ISBN 978-3-540-74664-5. [Google Scholar]
- Nalecz-Jawecki, G.; Persoone, G. Toxicity of selected pharmaceuticals to the Anostracan crustacean Thamnocephalus platyurus—Comparison of sublethal and lethal effect levels with the 1 h Rapidtoxkit and the 24 h Thamnotoxkit microbiotests. Environ. Sci. Pollut. Res. Int. 2006, 13, 22–27. [Google Scholar] [CrossRef]
- Haap, T.; Triebskorn, R.; Köhler, H.-R. Acute effects of diclofenac and DMSO to Daphnia magna: Immobilisation and hsp70-induction. Chemosphere 2008, 73, 353–359. [Google Scholar] [CrossRef]
- Heckmann, L.-H.; Callaghan, A.; Hooper, H.L.; Connon, R.; Hutchinson, T.H.; Maund, S.J.; Sibly, R.M. Chronic toxicity of ibuprofen to Daphnia magna: Effects on life history traits and population dynamics. Toxicol. Lett. 2007, 172, 137–145. [Google Scholar] [CrossRef]
- Pounds, N.; Maclean, S.; Webley, M.; Pascoe, D.; Hutchinson, T. Acute and chronic effects of ibuprofen in the mollusc Planorbis carinatus (Gastropoda: Planorbidae). Ecotoxicol. Environ. Saf. 2008, 70, 47–52. [Google Scholar] [CrossRef]
- Dave, G.; Herger, G. Determination of detoxification to Daphnia magna of four pharmaceuticals and seven surfactants by activated sludge. Chemosphere 2012, 88, 459–466. [Google Scholar] [CrossRef]
- Sherer, J.T. Pharmaceuticals in the environment. Am. J. Heal. Pharm. 2006, 63, 174–178. [Google Scholar] [CrossRef]
- El-Bassat, R.; Touliabah, H.; Harisa, G. Toxicity of four pharmaceuticals from different classes to isolated plankton species. African J. Aquat. Sci. 2012, 37, 71–80. [Google Scholar] [CrossRef]
- Li, M.-H. Acute toxicity of 30 pharmaceutically active compounds to freshwater planarians, Dugesia japonica. Toxicol. Environ. Chem. 2013, 95, 1157–1170. [Google Scholar] [CrossRef]
- Kühn, R.; Pattard, M.; Pernak, K.; Winter, A. Results of the harmful effects of selected water pollutants (anilines, phenols, aliphatic compounds) to Daphnia magna. Water Res. 1989, 23, 495–499. [Google Scholar] [CrossRef]
- Li, M. Acute toxicity of industrial endocrine-disrupting chemicals, natural and synthetic sex hormones to the freshwater planarian, Dugesia japonica. Toxicol. Environ. Chem. 2013, 95, 984–991. [Google Scholar] [CrossRef]
- Jaser, W. Effects of 17α-ethinylestradiol on the reproduction of the cladoceran species Ceriodaphnia reticulata and Sida crystallina. Environ. Int. 2003, 28, 633–638. [Google Scholar] [CrossRef]
- Caldwell, D.J.; Mastrocco, F.; Hutchinson, T.H.; Länge, R.; Heijerick, D.; Janssen, C.; Anderson, P.D.; Sumpter, J.P. Derivation of an Aquatic Predicted No-Effect Concentration for the Synthetic Hormone, 17α-Ethinyl Estradiol. Environ. Sci. Technol. 2008, 42, 7046–7054. [Google Scholar] [CrossRef]
- Vandenbergh, G.F.; Adriaens, D.; Verslycke, T.; Janssen, C.R. Effects of 17α-ethinylestradiol on sexual development of the amphipod Hyalella azteca. Ecotoxicol. Environ. Saf. 2003, 54, 216–222. [Google Scholar] [CrossRef]
- Jobling, S.; Casey, D.; Rodgers-Gray, T.; Oehlmann, J.; Schulte-Oehlmann, U.; Pawlowski, S.; Baunbeck, T.; Turner, A.; Tyler, C. Comparative responses of molluscs and fish to environmental estrogens and an estrogenic effluent. Aquat. Toxicol. 2004, 66, 207–222. [Google Scholar] [CrossRef]
- Sidhu, H.; O’Connor, G.; McAvoy, D. Risk assessment of biosolids-borne ciprofloxacin and azithromycin. Sci. Total Environ. 2019, 651, 3151–3160. [Google Scholar] [CrossRef]
- Henry, T.B.; Kwon, J.W.; Armbrust, K.L.; Black, M.C. Acute and chronic toxicity of five selective serotonin reuptake inhibitors in Ceriodaphnia dubia. Environ. Toxicol. Chem. 2004, 23, 2229–2233. [Google Scholar] [CrossRef]
- Mimeault, C.; Trudeau, V.L.; Moon, T.W. Waterborne gemfibrozil challenges the hepatic antioxidant defense system and down-regulates peroxisome proliferator-activated receptor beta (PPARbeta) mRNA levels in male goldfish (Carassius auratus). Toxicology 2006, 228, 140–150. [Google Scholar] [CrossRef]
- Raldúa, D.; André, M.; Babin, P.J. Clofibrate and gemfibrozil induce an embryonic malabsorption syndrome in zebrafish. Toxicol. Appl. Pharmacol. 2008, 228, 301–314. [Google Scholar]
- Caminada, D.; Escher, C.; Fent, K. Cytotoxicity of pharmaceuticals found in aquatic systems: Comparison of PLHC-1 and RTG-2 fish cell lines. Aquat. Toxicol. 2006, 79, 114–123. [Google Scholar] [CrossRef]
- Richards, S.M.; Cole, S.E. A toxicity and hazard assessment of fourteen pharmaceuticals to Xenopus laevis larvae. Ecotoxicology 2006, 15, 647–656. [Google Scholar] [CrossRef]
- Stanley, J.K.; Ramirez, A.J.; Chambliss, C.K.; Brooks, B.W. Enantiospecific sublethal effects of the antidepressant fluoxetine to a model aquatic vertebrate and invertebrate. Chemosphere 2007, 69, 9–16. [Google Scholar] [CrossRef]
- Henry, T.B.; Black, M.C. Acute and chronic toxicity of fluoxetine (selective serotonin reuptake inhibitor) in western mosquitofish. Arch. Environ. Contam. Toxicol. 2008, 54, 325–330. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yamamoto, H.; Sekizawa, J.; Kondo, T.; Hirai, N.; Tatarazako, N. The effects of pH on fluoxetine in Japanese medaka (Oryzias latipes): Acute toxicity in fish larvae and bioaccumulation in juvenile fish. Chemosphere 2008, 70, 865–873. [Google Scholar] [CrossRef]
- Valenti, T.W.; Hurtado, P.P.; Chambliss, C.K.; Brooks, B.W. Aquatic toxicity of sertraline to Pimephales promelas at environmentally relevant surface water pH. Environ. Toxicol. Chem. 2009, 28, 2685–2694. [Google Scholar] [CrossRef]
- Schwaiger, J.; Ferling, H.; Mallow, U.; Wintermayr, H.; Negele, R.D. Toxic effects of the non-steroidal anti-inflammatory drug diclofenac. Part I: Histopathological alterations and bioaccumulation in rainbow trout. Aquat. Toxicol. 2004, 68, 141–150. [Google Scholar] [CrossRef]
- Triebskorn, R.; Casper, H.; Heyd, A.; Eikemper, R.; Köhler, H.-R.; Schwaiger, J. Toxic effects of the non-steroidal anti-inflammatory drug diclofenac. Part II: Cytological effects in liver, kidney, gills and intestine of rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol. 2004, 68, 151–166. [Google Scholar] [CrossRef]
- Hoeger, B.; Köllner, B.; Dietrich, D.R.; Hitzfeld, B. Water-borne diclofenac affects kidney and gill integrity and selected immune parameters in brown trout (Salmo trutta f. fario). Aquat. Toxicol. 2005, 75, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Memmert, U.; Peither, A.; Burri, R.; Weber, K.; Schmidt, T.; Sumpter, J.P.; Hartmann, A. Diclofenac: New data on chronic toxicity and bioconcentration in fish. Environ. Toxicol. Chem. 2013, 32, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Mehinto, A.C.; Hill, E.M.; Tyler, C.R. Uptake and biological effects of environmentally relevant concentrations of the nonsteroidal anti-inflammatory pharmaceutical diclofenac in rainbow trout (Oncorhynchus mykiss). Environ. Sci. Technol. 2010, 44, 2176–2182. [Google Scholar] [CrossRef]
- Oaks, J.L.; Gilbert, M.; Virani, M.Z.; Watson, R.T.; Meteyer, C.U.; Rideout, B.A.; Shivaprasad, H.L.; Ahmed, S.; Iqbal Chaudhry, M.J.; Arshad, M.; et al. Diclofenac residues as the cause of vulture population decline in Pakistan. Nature 2004, 427, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, P.; Chen, L.; Gao, H.; Wu, L. Acute toxicity and histopathological effects of naproxen in zebrafish (Danio rerio) early life stages. Environ. Sci. Pollut. Res. 2016, 23, 18832–18841. [Google Scholar] [CrossRef]
- Sun, L.W.; Qu, M.M.; Li, Y.Q.; Wu, Y.L.; Chen, Y.G.; Kong, Z.M.; Liu, Z.T. Toxic effects of aminophenols on aquatic life using the Zebrafish embryo test and the comet assay. Bull. Environ. Contam. Toxicol. 2004, 73, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, C.D.; Metcalfe, T.L.; Kiparissis, Y.; Koenig, B.G.; Khan, C.; Hughes, R.J.; Croley, T.R.; March, R.E.; Potter, T. Estrogenic potency of chemicals detected in sewage treatment plant effluents as determined by in vivo assays with Japanese medaka ( Oryzias latipes ). Environ. Toxicol. Chem. 2001, 20, 297–308. [Google Scholar] [CrossRef]
- Imai, S.; Koyama, J.; Fujii, K. Effects of estrone on full life cycle of Java medaka (Oryzias javanicus), a new marine test fish. Environ. Toxicol. Chem. 2007, 26, 726–731. [Google Scholar] [CrossRef]
- Thorpe, K.L.; Benstead, R.; Hutchinson, T.H.; Tyler, C.R. Associations between altered vitellogenin concentrations and adverse health effects in fathead minnow (Pimephales promelas). Aquat. Toxicol. 2007, 85, 176–183. [Google Scholar] [CrossRef]
- Kang, I.J.; Yokota, H.; Oshima, Y.; Tsuruda, Y.; Yamaguchi, T.; Maeda, M.; Imada, N.; Tadokoro, H.; Honjo, T. Effect of 17β-estradiol on the reproduction of Japanese medaka (Oryzias latipes). Chemosphere 2002, 47, 71–80. [Google Scholar] [CrossRef]
- Caldwell, D.J.; Mastrocco, F.; Anderson, P.D.; Länge, R.; Sumpter, J.P. Predicted-no-effect concentrations for the steroid estrogens estrone, 17β-estradiol, estriol, and 17α-ethinylestradiol. Environ. Toxicol. Chem. 2012, 31, 1396–1406. [Google Scholar] [CrossRef] [PubMed]
- Kramer, V.J.; Miles-Richardson, S.; Pierens, S.L.; Giesy, J.P. Reproductive impairment and induction of alkaline-labile phosphate, a biomarker of estrogen exposure, in fathead minnows (Pimephales promelas) exposed to waterborne 17β-estradiol. Aquat. Toxicol. 1998, 40, 335–360. [Google Scholar] [CrossRef]
- Shappell, N.W.; Elder, K.H.; West, M. Estrogenicity and nutrient concentration of surface waters surrounding a large confinement dairy operation using best management practices for land application of animal wastes. Environ. Sci. Technol. 2010, 44, 2365–2371. [Google Scholar] [CrossRef]
- Seki, M.; Fujishima, S.; Nozaka, T.; Maeda, M.; Kobayashi, K. Comparison of response to 17β-estradiol and 17β-trenbolone among three small fish species. Environ. Toxicol. Chem. 2006, 25, 2742. [Google Scholar] [CrossRef] [PubMed]
- Brion, F.; Tyler, C.; Palazzi, X.; Laillet, B.; Porcher, J.; Garric, J.; Flammarion, P. Impacts of 17β-estradiol, including environmentally relevant concentrations, on reproduction after exposure during embryo-larval-, juvenile- and adult-life stages in zebrafish (Danio rerio). Aquat. Toxicol. 2004, 68, 193–217. [Google Scholar] [PubMed]
- Van der Ven, L.T.M.; Van den Brandhof, E.-J.; Vos, J.H.; Wester, P.W. Effects of the estrogen agonist 17β-estradiol and antagonist tamoxifen in a partial life-cycle assay with with zebrafish (danio rerio). Environ. Toxicol. Chem. 2007, 26, 92. [Google Scholar] [CrossRef] [PubMed]
- Nash, J.P.; Kime, D.E.; Van der Ven, L.T.M.; Wester, P.W.; Brion, F.; Maack, G.; Stahlschmidt-Allner, P.; Tyler, C.R. Long-term exposure to environmental concentrations of the pharmaceutical ethynylestradiol causes reproductive failure in fish. Environ. Health Perspect. 2004, 112, 1725–1733. [Google Scholar] [CrossRef]
- Hirai, N.; Nanba, A.; Koshio, M.; Kondo, T.; Morita, M.; Tatarazako, N. Feminization of Japanese medaka (Oryzias latipes) exposed to 17β-estradiol: Formation of testis–ova and sex-transformation during early-ontogeny. Aquat. Toxicol. 2006, 77, 78–86. [Google Scholar] [CrossRef]
- Seki, M.; Yokota, H.; Maeda, M.; Kobayashi, K. Fish full life-cycle testing for 17beta-estradiol on medaka (Oryzias latipes). Environ. Toxicol. Chem. 2005, 24, 1259–1266. [Google Scholar] [CrossRef]
- Jukosky, J.A.; Watzin, M.C.; Leiter, J.C. The effects of environmentally relevant mixtures of estrogens on Japanese medaka (Oryzias latipes) reproduction. Aquat. Toxicol. 2008, 86, 323–331. [Google Scholar] [CrossRef]
- Imai, S.; Koyama, J.; Fujii, K. Effects of 17β-estradiol on the reproduction of Java-medaka (Oryzias javanicus), a new test fish species. Mar. Pollut. Bull. 2005, 51, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Cripe, G.M.; Hemmer, B.L.; Goodman, L.R.; Fournie, J.W.; Raimondo, S.; Vennari, J.C.; Danner, R.L.; Smith, K.; Manfredonia, B.R.; Kulaw, D.H.; et al. Multigerational exposure of the estuarine Sheepshead minnow (cyprinodon variegatus) to 17β-estradiol. I. Organism-level effects over three generations. Environ. Toxicol. Chem. 2009, 28, 2397. [Google Scholar] [CrossRef] [PubMed]
- Toft, G.; Baatrup, E. Altered sexual characteristics in guppies (Poecilia reticulata) exposed to 17β-estradiol and 4-tert-octylphenol during sexual development. Ecotoxicol. Environ. Saf. 2003, 56, 228–237. [Google Scholar] [CrossRef]
- Liao, T.; Guo, Q.L.; Jin, S.W.; Cheng, W.; Xu, Y. Comparative responses in rare minnow exposed to 17β-estradiol during different life stages. Fish Physiol. Biochem. 2009, 35, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.D.; Brown, E.; Craft, J.A.; Davies, I.M.; Megginson, C.; Miller, C.; Moffat, C.F. Bioindicators and reproductive effects of prolonged 17β-oestradiol exposure in a marine fish, the sand goby (Pomatoschistus minutus). Aquat. Toxicol. 2007, 81, 397–408. [Google Scholar] [CrossRef]
- Pollino, C.A.; Georgiades, E.; Holdway, D.A. Use of the Australian crimson-spotted rainbowfish (Melanotaenia fluviatilis) as a model test species for investigating the effects of endocrine disruptors. Environ. Toxicol. Chem. 2007, 26, 2171. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, S.; van Aerle, R.; Tyler, C.; Braunbeck, T. Effects of 17α-ethinylestradiol in a fathead minnow (Pimephales promelas) gonadal recrudescence assay. Ecotoxicol. Environ. Saf. 2004, 57, 330–345. [Google Scholar] [CrossRef]
- Örn, S.; Holbech, H.; Madsen, T.H.; Norrgren, L.; Petersen, G.I. Gonad development and vitellogenin production in zebrafish (Danio rerio) exposed to ethinylestradiol and methyltestosterone. Aquat. Toxicol. 2003, 65, 397–411. [Google Scholar] [CrossRef]
- Zha, J.; Sun, L.; Zhou, Y.; Spear, P.A.; Ma, M.; Wang, Z. Assessment of 17α-ethinylestradiol effects and underlying mechanisms in a continuous, multigeneration exposure of the Chinese rare minnow (Gobiocypris rarus). Toxicol. Appl. Pharmacol. 2008, 226, 298–308. [Google Scholar] [CrossRef]
- Schäfers, C.; Teigeler, M.; Wenzel, A.; Maack, G.; Fenske, M.; Segner, H. Concentration- and time-dependent effects of the synthetic estrogen, 17α-ethinylestradiol, on reproductive capabilities of the Zebrafish, Danio rerio. J. Toxicol. Environ. Heal. Part A 2007, 70, 768–779. [Google Scholar] [CrossRef]
- Van den Belt, K.; Berckmans, P.; Vangenechten, C.; Verheyen, R.; Witters, H. Comparative study on the in vitro/in vivo estrogenic potencies of 17β-estradiol, estrone, 17α-ethynylestradiol and nonylphenol. Aquat. Toxicol. 2004, 66, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yang, J.; Wang, Y.; Jiang, Q.; Chen, H.; Song, H. Exposure to 17α-ethynylestradiol impairs reproductive functions of both male and female zebrafish (Danio rerio). Aquat. Toxicol. 2008, 88, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.; Coimbra, A.M.; Reis-Henriques, M.A.; Monteiro, N.M.; Vieira, M.N.; Oliveira, J.M.A.; Guedes-Dias, P.; Fontaínhas-Fernandes, A.; Parra, S.S.; Carvalho, A.P. Disruption of zebrafish (Danio rerio) embryonic development after full life-cycle parental exposure to low levels of ethinylestradiol. Aquat. Toxicol. 2009, 95, 330–338. [Google Scholar] [CrossRef]
- Parrott, J.L.; Blunt, B.R. Life-cycle exposure of fathead minnows (Pimephales promelas) to an ethinylestradiol concentration below 1 ng/L reduces egg fertilization success and demasculinizes males. Environ. Toxicol. 2005, 20, 131–141. [Google Scholar] [CrossRef]
- Länge, R.; Hutchinson, T.H.; Croudace, C.P.; Siegmund, F.; Schweinfurth, H.; Hampe, P.; Panter, G.H.; Sumpter, J.P. Effects of the synthetic estrogen 17 alpha-ethinylestradiol on the life-cycle of the fathead minnow (Pimephales promelas). Environ. Toxicol. Chem. 2001, 20, 1216–1227. [Google Scholar] [CrossRef]
- Fenske, M.; Maack, G.; Schäfers, C.; Segner, H. An environmentally relevant concentration of estrogen induces arrest of male gonad development in zebrafish, Danio rerio. Environ. Toxicol. Chem. 2005, 24, 1088–1098. [Google Scholar] [CrossRef]
- Scholz, S. 17-α-ethinylestradiol affects reproduction, sexual differentiation and aromatase gene expression of the medaka (Oryzias latipes). Aquat. Toxicol. 2000, 50, 363–373. [Google Scholar] [CrossRef]
- Balch, G.C.; Mackenzie, C.A.; Metcalfe, C.D. Alterations of gonadal development and reproductive success in Japanese medaka (Oryzias latipes) exposed to 17α-ethinylestradiol. Environ. Toxicol. Chem. 2004, 23, 782. [Google Scholar] [CrossRef]
- Schultz, I.R.; Skillman, A.; Nicolas, J.-M.; Cyr, D.G.; Nagler, J.J. Short-term exposure to 17α-ethynylestradiol decreases the fertility of sexually maturing male rainbow trout ( Oncorhynchus mykiss ). Environ. Toxicol. Chem. 2003, 22, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Zillioux, E.J.; Johnson, I.C.; Kiparissis, Y.; Metcalfe, C.D.; Wheat, J.V.; Ward, S.G.; Liu, H. The sheepshead minnow as an in vivo model for endocrine disruption in marine teleosts: A partial life-cycle test with 17α-ethynylestradiol. Environ. Toxicol. Chem. 2001, 20, 1968–1978. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, T.; Baatrup, E.; Bayley, M. 17α-Ethinylestradiol Reduces the Competitive Reproductive Fitness of the Male Guppy (Poecilia reticulata)1. Biol. Reprod. 2005, 72, 150–156. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lange, A.; Katsu, Y.; Ichikawa, R.; Paull, G.C.; Chidgey, L.L.; Coe, T.S.; Iguchi, T.; Tyler, C.R. Altered sexual development in roach (Rutilus rutilus) exposed to environmental concentrations of the pharmaceutical 17α-Ethinylestradiol and associated expression dynamics of aromatases and estrogen receptors. Toxicol. Sci. 2008, 106, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xu, X.; Wei, X.; Wan, J.; Zhang, Y. Biomarker effects in carassius auratus exposure to ofloxacin, sulfamethoxazole and ibuprofen. Int. J. Environ. Res. Public Health 2019, 16, 1628. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Pharmaceuticals in the Environment. Annu. Rev. Environ. Resour. 2010, 35, 57–75. [Google Scholar] [CrossRef]
- Oliveira, T.S.; Murphy, M.; Mendola, N.; Wong, V.; Carlson, D.; Waring, L. Characterization of Pharmaceuticals and Personal Care products in hospital effluent and waste water influent/effluent by direct-injection LC-MS-MS. Sci. Total Environ. 2015, 518–519, 459–478. [Google Scholar] [CrossRef] [PubMed]
- Backhaus, T.; Faust, M. Predictive environmental risk assessment of chemical mixtures: A conceptual framework. Environ. Sci. Technol. 2012, 46, 2564–2573. [Google Scholar] [CrossRef]
- Escher, B.I.; Baumgartner, R.; Koller, M.; Treyer, K.; Lienert, J.; McArdell, C.S. Environmental toxicology and risk assessment of pharmaceuticals from hospital wastewater. Water Res. 2011, 45, 75–92. [Google Scholar] [CrossRef]
- Jones-Lepp, T.L.; Sanchez, C.; Alvarez, D.A.; Wilson, D.C.; Taniguchi-Fu, R.L. Point sources of emerging contaminants along the Colorado River Basin: Source water for the arid Southwestern United States. Sci. Total Environ. 2012, 430, 237–245. [Google Scholar] [CrossRef]
- McEachran, A.D.; Shea, D.; Bodnar, W.; Nichols, E.G. Pharmaceutical occurrence in groundwater and surface waters in forests land-applied with municipal wastewater. Environ. Toxicol. Chem. 2016, 35, 898–905. [Google Scholar] [CrossRef]
- Stalter, D.; Magdeburg, A.; Wagner, M.; Oehlmann, J. Ozonation and activated carbon treatment of sewage effluents: Removal of endocrine activity and cytotoxicity. Water Res. 2011, 45, 1015–1024. [Google Scholar] [CrossRef]
- Magdeburg, A.; Stalter, D.; Oehlmann, J. Whole effluent toxicity assessment at a wastewater treatment plant upgraded with a full-scale post-ozonation using aquatic key species. Chemosphere 2012, 88, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Silva, L.; Laranjeiro, C.; Lino, C.; Pena, A. Selected Pharmaceuticals in Different Aquatic Compartments: Part I—Source, Fate and Occurrence. Molecules 2020, 25, 1026. [Google Scholar] [CrossRef] [PubMed]
- Benotti, M.J.; Trenholm, R.A.; Vanderford, B.J.; Holady, J.C.; Stanford, B.D.; Snyder, S.A. Pharmaceuticals and endocrine disrupting compounds in U.S. drinking water. Environ. Sci. Technol. 2009, 43, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Crouse, B.A.; Ghoshdastidar, A.J.; Tong, A.Z. The presence of acidic and neutral drugs in treated sewage effluents and receiving waters in the Cornwallis and Annapolis River watersheds and the Mill CoveSewage Treatment Plant in Nova Scotia, Canada. Environ. Res. 2012, 112, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Tuševljak, N.; Dutil, L.; Rajić, A.; Uhland, F.C.; McClure, C.; St-Hilaire, S.; Reid-Smith, R.J.; McEwen, S.A. Antimicrobial Use and Resistance in Aquaculture: Findings of a Globally Administered Survey of Aquaculture-Allied Professionals. Zoonoses Public Health 2013, 60, 426–436. [Google Scholar] [CrossRef]
- Kostich, M.S.; Batt, A.L.; Lazorchak, J.M. Concentrations of prioritized pharmaceuticals in effluents from 50 large wastewater treatment plants in the US and implications for risk estimation. Environ. Pollut. 2014, 184, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Khan, G.A.; Berglund, B.; Khan, K.M.; Lindgren, P.E.; Fick, J. Occurrence and Abundance of Antibiotics and Resistance Genes in Rivers, Canal and near Drug Formulation Facilities—A Study in Pakistan. PLoS ONE 2013, 8, e62712. [Google Scholar] [CrossRef]
- Zuccato, E.; Castiglioni, S.; Bagnati, R.; Melis, M.; Fanelli, R. Source, occurrence and fate of antibiotics in the Italian aquatic environment. J. Hazard. Mater. 2010, 179, 1042–1048. [Google Scholar] [CrossRef]
- Rizzo, L.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Ploy, M.C.; Michael, I.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Sci. Total Environ. 2013, 447, 345–360. [Google Scholar] [CrossRef]
- Fick, J.; Söderström, H. Contamination of surface, ground, and drinking water from pharmaceutical production. Environ. Toxicol. Chem. 2009, 28, 2522–2527. [Google Scholar] [CrossRef]
- EAHC Study on the Environmental Risks of Medicinal Products Executive Agency for Health and Consumers Document Information; Executive Agency for Health and Consumers: Luxembourg, 2013.
- Cabello, F.C. Heavy use of prophylactic antibiotics in aquaculture: A growing problem for human and animal health and for the environment. Environ. Microbiol. 2006, 8, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Segura, P.A.; Takada, H.; Correa, J.A.; El Saadi, K.; Koike, T.; Onwona-Agyeman, S.; Ofosu-Anim, J.; Sabi, E.B.; Wasonga, O.V.; Mghalu, J.M.; et al. Global occurrence of anti-infectives in contaminated surface waters: Impact of income inequality between countries. Environ. Int. 2015, 80, 89–97. [Google Scholar] [CrossRef]
- Kreke, N.; Dietrich, D.R. Physiological endpoints for potential SSRI interactions in fish. Crit. Rev. Toxicol. 2008, 38, 215–247. [Google Scholar] [CrossRef] [PubMed]
- Bossus, M.C.; Guler, Y.Z.; Short, S.J.; Morrison, E.R.; Ford, A.T. Behavioural and transcriptional changes in the amphipod Echinogammarus marinus exposed to two antidepressants, fluoxetine and sertraline. Aquat. Toxicol. 2014, 151, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Lajeunesse, A.; Gagnon, C.; Sauvé, S. Determination of Basic Antidepressants and Their N-Desmethyl Metabolites in Raw Sewage and Wastewater Using Solid-Phase Extraction and Liquid Chromatography-Tandem Mass Spectrometry. Anal. Chem. 2008, 80, 5325–5333. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, J.; Klaper, R. Environmental concentrations of the selective serotonin reuptake inhibitor fluoxetine impact specific behaviors involved in reproduction, feeding and predator avoidance in the fish Pimephales promelas (fathead minnow). Aquat. Toxicol. 2014, 151, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Celiz, M.D.; Tso, J.; Aga, D.S. Pharmaceutical metabolites in the environment: Analytical challenges and ecological risks. Environ. Toxicol. Chem. 2009, 28, 2473. [Google Scholar] [CrossRef]
- Laganà, A.; Bacaloni, A.; De Leva, I.; Faberi, A.; Fago, G.; Marino, A. Analytical methodologies for determining the occurrence of endocrine disrupting chemicals in sewage treatment plants and natural waters. Anal. Chim. Acta 2004, 501, 79–88. [Google Scholar] [CrossRef]
- Fent, K.; Weston, A.A.; Caminada, D. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef]
- Corcoran, J.; Winter, M.J.; Tyler, C.R. Pharmaceuticals in the aquatic environment: A critical review of the evidence for health effects in fish. Crit. Rev. Toxicol. 2010, 40, 287–304. [Google Scholar] [CrossRef]
- Pereira, A.M.P.T.; Silva, L.J.G.; Laranjeiro, C.S.M.; Meisel, L.M.; Lino, C.M.; Pena, A. Human pharmaceuticals in Portuguese rivers: The impact of water scarcity in the environmental risk. Sci. Total Environ. 2017, 609, 1182–1191. [Google Scholar] [CrossRef] [PubMed]
- Bound, J.P.; Voulvoulis, N. Predicted and measured concentrations for selected pharmaceuticals in UK rivers: Implications for risk assessment. Water Res. 2006, 40, 2885–2892. [Google Scholar] [CrossRef] [PubMed]
- Holm, G.; Snape, J.R.; Murray-Smith, R.; Talbot, J.; Taylor, D.; Sörme, P. Implementing ecopharmacovigilance in practice: Challenges and potential opportunities. Drug Saf. 2013, 36, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.H.M.L.M.; Gros, M.; Rodriguez-Mozaz, S.; Delerue-Matos, C.; Pena, A.; Barceló, D.; Montenegro, M.C.B.S.M. Contribution of hospital effluents to the load of pharmaceuticals in urban wastewaters: Identification of ecologically relevant pharmaceuticals. Sci. Total Environ. 2013, 461–462, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Roig, P.; Andreu, V.; Blasco, C.; Picó, Y. Risk assessment on the presence of pharmaceuticals in sediments, soils and waters of the Pego-Oliva Marshlands (Valencia, eastern Spain). Sci. Total Environ. 2012, 440, 24–32. [Google Scholar] [CrossRef]
- Pereira, A.M.P.T.; Silva, L.J.G.; Lino, C.M.; Meisel, L.M.; Pena, A. Assessing environmental risk of pharmaceuticals in Portugal: An approach for the selection of the Portuguese monitoring stations in line with Directive 2013/39/EU. Chemosphere 2016, 144, 2507–2515. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, G.; Zheng, Q.; Tang, J.; Chen, Y.; Xu, W.; Zou, Y.; Chen, X. Occurrence and risks of antibiotics in the Laizhou Bay, China: Impacts of river discharge. Ecotoxicol. Environ. Saf. 2012, 80, 208–215. [Google Scholar] [CrossRef]
- Acuña, V.; Schiller, D.; García-Galán, M.J.; Rodríguez-Mozaz, S.; Corominas, L.; Petrovic, M.; Poch, M.; Barceló, D.; Sabater, S. Occurrence and in-stream attenuation of wastewater-derived pharmaceuticals in Iberian rivers. Sci. Total Environ. 2015, 503–504, 133–141. [Google Scholar] [CrossRef]
- Simazaki, D.; Kubota, R.; Suzuki, T.; Akiba, M.; Nishimura, T.; Kunikane, S. Occurrence of selected pharmaceuticals at drinking water purification plants in Japan and implications for human health. Water Res. 2015, 76, 187–200. [Google Scholar] [CrossRef]
- Henry, T.B.; Black, M.C. Mixture and single-substance acute toxicity of selective serotonin reuptake inhibitors in Ceriodaphnia dubia. Environ. Toxicol. Chem. 2007, 26, 1751–1755. [Google Scholar] [CrossRef]
- Vestel, J.; Caldwell, D.J.; Constantine, L.; D’Aco, V.J.; Davidson, T.; Dolan, D.G.; Millard, S.P.; Murray-Smith, R.; Parke, N.J.; Ryan, J.J.; et al. Use of acute and chronic ecotoxicity data in environmental risk assessment of pharmaceuticals. Environ. Toxicol. Chem. 2016, 35, 1201–1212. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.M.; Painter, M.M.; Bartell, S.E.; Logue, A.; Furlong, E.T.; Werner, S.L.; Schoenfuss, H.L. Selective uptake and biological consequences of environmentally relevant antidepressant pharmaceutical exposures on male fathead minnows. Aquat. Toxicol. 2011, 104, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Minguez, L.; Pedelucq, J.; Farcy, E.; Ballandonne, C.; Budzinski, H.; Halm-Lemeille, M.P. Toxicities of 48 pharmaceuticals and their freshwater and marine environmental assessment in northwestern France. Environ. Sci. Pollut. Res. 2016, 23, 4992–5001. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Li, Z.; Gibson, M.; Gao, H. Ecological risk assessment of nonylphenol in coastal waters of China based on species sensitivity distribution model. Chemosphere 2014, 104, 113–119. [Google Scholar] [CrossRef]
- Richards, S.M.; Wilson, C.J.; Johnson, D.J.; Castle, D.M.; Lam, M.; Mabury, S.A.; Sibley, P.K.; Solomon, K.R. Effects of pharmaceutical mixtures in aquatic microcosms. Environ. Toxicol. Chem. 2004, 23, 1035–1042. [Google Scholar] [CrossRef]
- Goolsby, E.W.; Mason, C.M.; Wojcik, J.T.; Jordan, A.M.; Black, M.C. Acute and chronic effects of diphenhydramine and sertraline mixtures in Ceriodaphnia dubia. Environ. Toxicol. Chem. 2013, 32, 2866–2869. [Google Scholar] [CrossRef]
- Pereira, A.M.P.T.; Silva, L.J.G.; Meisel, L.M.; Pena, A. Fluoroquinolones and Tetracycline Antibiotics in a Portuguese Aquaculture System and Aquatic Surroundings: Occurrence and Environmental Impact. J. Toxicol. Environ. Heal. Part A 2015, 78, 959–975. [Google Scholar] [CrossRef]
- Altenburger, R.; Ait-Aissa, S.; Antczak, P.; Backhaus, T.; Barceló, D.; Seiler, T.-B.; Brion, F.; Busch, W.; Chipman, K.; de Alda, M.L.; et al. Future water quality monitoring — Adapting tools to deal with mixtures of pollutants in water resource management. Sci. Total Environ. 2015, 512–513, 540–551. [Google Scholar] [CrossRef]
| Therapeutic Group | Compound and Chemical Structure | |||
|---|---|---|---|---|
| Anxiolytics (Anx) | Alprazolam (ALP) | Lorazepam (LOR) | Zolpidem (ZOL) | |
 | 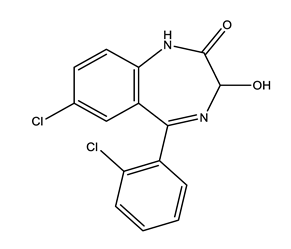 | 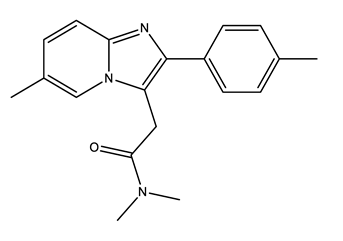 | ||
| Antibiotics (Antib) | Azithromycin (AZI) | Ciprofloxacin (CIP) | Clarithromycin (CLA) | Erythromycin (ERY) |
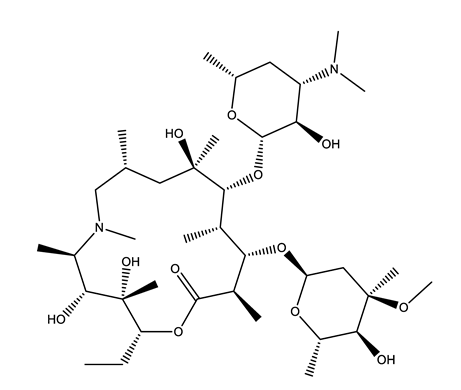 |  |  | 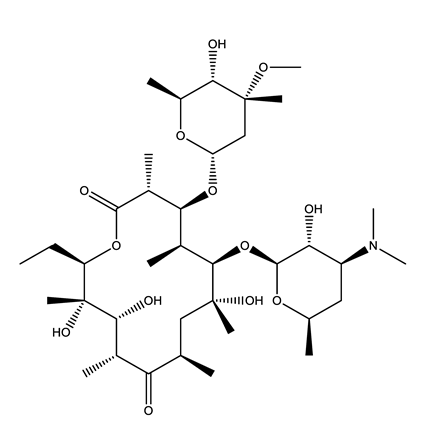 | |
| Lipid regulators (Lip Reg) | Bezafibrate (BEZ) | Gemfibrozil (GEM) | Simvastatin (SIM) | |
 | 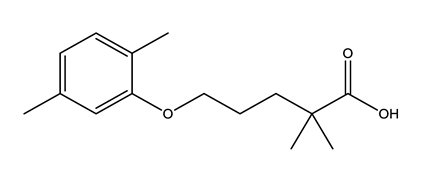 |  | ||
| Antiepileptic (Antiepi) | Carbamazepine (CAR) | |||
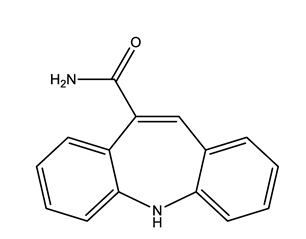 | ||||
| Selective serotonin reuptake inhibitors (SSRIs) | Citalopram (CIT) | Desmethylcitalopram (N-Cit) (metabolite) | Escitalopram (ESC) | Fluoxetine (FLU) |
 | 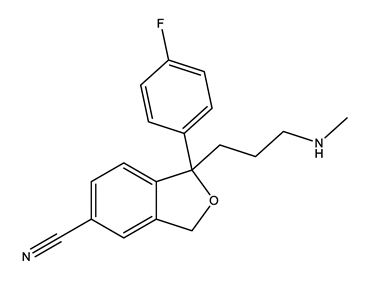 | 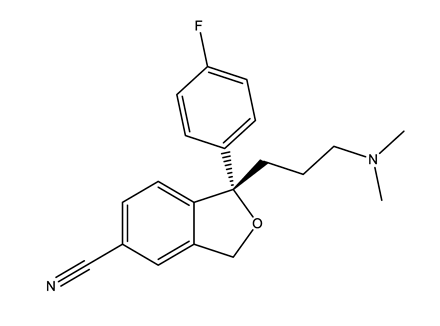 | 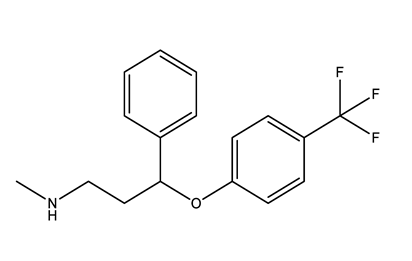 | |
| Norfluoxetine (Nor-FLU) (metabolite) | Paroxetine (PAR) | Sertraline (SER) | Desmethylsertraline (Nor-SER) (metabolite) | |
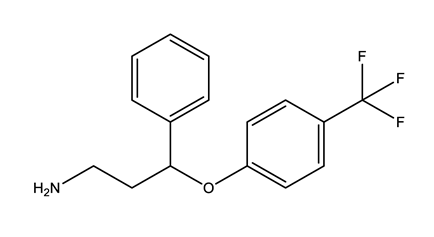 |  | 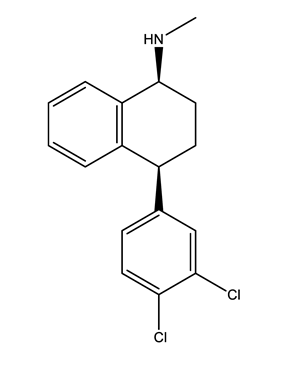 | 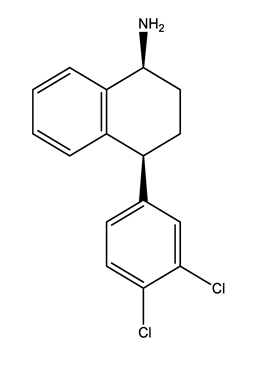 | |
| Anti-inflammatories (Anti-inf) | Diclofenac (DIC) | 4-hydroxydiclofenac (4-OH-DIC) (metabolite) | Ibuprofen (IBU) | Naproxen (NAP) |
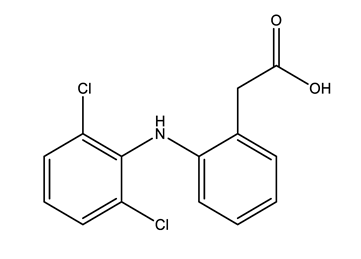 | 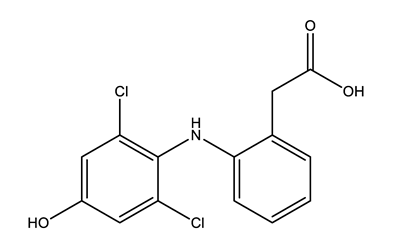 | 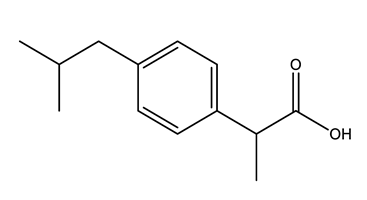 | 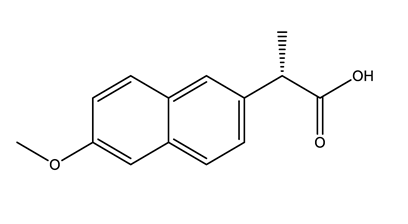 | |
| Paracetamol (PARA) | 4-aminophenol (4-PARA) (transformation product) | |||
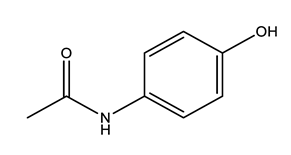 | 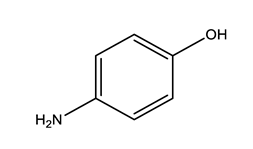 | |||
| Hormones (Horm) | Estrone (E1) (natural hormone/metabolite) | 17β-estradiol (E2) | 17α-ethinylestradiol (EE2) | |
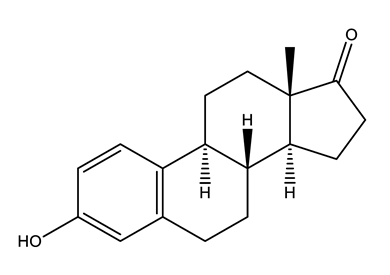 | 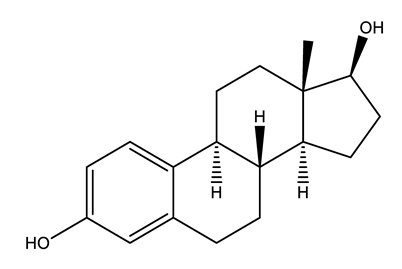 |  | ||
| Therapeutic Group | Pharmaceutical | PNEC (ng L−1) Algae | PNEC (ng L−1) Invertebrates | PNEC (ng L−1) Fish |
|---|---|---|---|---|
| Anx | ALP | 892 a,b | 3590 b,c | 2540 b,c |
| LOR | 6070 a,b | 39,400 b,c | 43,100 b,c | |
| ZOL | 211 a,b | 1550 b,c | 248 b,c | |
| Antib | AZI | 1.8 b [176] | 440 e,g [83] | 84,000 b |
| CLA | 2 b [23] | 8160 b [23] | 1,000,000 b [23] | |
| CIP | 5 b [35] | 10,000 b [44] | 1,000,000 b [44] | |
| ERY | 20 b [23] | 220 b [23] | 1,000,000 b [23] | |
| Lip reg | BEZ | 4870 a,b | 1300 e,f [51] | 17,600 b,c |
| GEM | 15,190 b [51] | 1180 b [53] | 150 e,g [85] | |
| SIM | 22,800 b [26] | 3.2 d [56] | 765 b,c | |
| Antiepi | CAR | 31.6 b [27] | 0.2 d [57] | 20,000 b [44] |
| SSRIs | CIT | 1600 b [30] | 3900 b [58] | 4470 b,c |
| FLU | 44.99 b [33] | 2 d [57] | 2.8 e,g [177] | |
| Nor-FLU (M) | 189 b [34] | 300 b [61] | n.a | |
| PAR | 140 b [30] | 580 b [58] | 3290 b,c | |
| SER | 12.10 b [33] | 120 b [58] | 72 b [92] | |
| Anti-inf | DIC | 200 d [37] | 20,000 e,g [70] | 50 e,g [95] |
| 4-OH-DIC (M) | 660,300 e,f [38] | 48,200 b,c | 65,200 b,c | |
| IBU | 40,100 e,f [39] | 0.2 d [57] | 180 e,g | |
| NAP | 31,820 b [40] | 2620 b [53] | 115,200 b [99] | |
| PARA | 134,000 b [59] | 2040 b [73] | 378,000 b [42] | |
| 4-PARA (TP) | 11,300 a,b | 240 b [77] | 1430 b [100] | |
| Horm | E1 (NH/M) | 355 a,b | 3160 b,c | 3.4 e,g [103] |
| E2 | 162 a,b | 1500 b [78] | 0.29 e,g [113] | |
| EE2 | 730 b [43] | 10 e,g [81,82] | 0.01 e,g [123] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, A.; Silva, L.; Laranjeiro, C.; Lino, C.; Pena, A. Selected Pharmaceuticals in Different Aquatic Compartments: Part II—Toxicity and Environmental Risk Assessment. Molecules 2020, 25, 1796. https://doi.org/10.3390/molecules25081796
Pereira A, Silva L, Laranjeiro C, Lino C, Pena A. Selected Pharmaceuticals in Different Aquatic Compartments: Part II—Toxicity and Environmental Risk Assessment. Molecules. 2020; 25(8):1796. https://doi.org/10.3390/molecules25081796
Chicago/Turabian StylePereira, André, Liliana Silva, Célia Laranjeiro, Celeste Lino, and Angelina Pena. 2020. "Selected Pharmaceuticals in Different Aquatic Compartments: Part II—Toxicity and Environmental Risk Assessment" Molecules 25, no. 8: 1796. https://doi.org/10.3390/molecules25081796
APA StylePereira, A., Silva, L., Laranjeiro, C., Lino, C., & Pena, A. (2020). Selected Pharmaceuticals in Different Aquatic Compartments: Part II—Toxicity and Environmental Risk Assessment. Molecules, 25(8), 1796. https://doi.org/10.3390/molecules25081796





