Metabolism and Pharmacokinetics of SP-8356, a Novel (1S)-(−)-Verbenone Derivative, in Rats and Dogs and Its Implications in Humans
Abstract
1. Introduction
2. Results
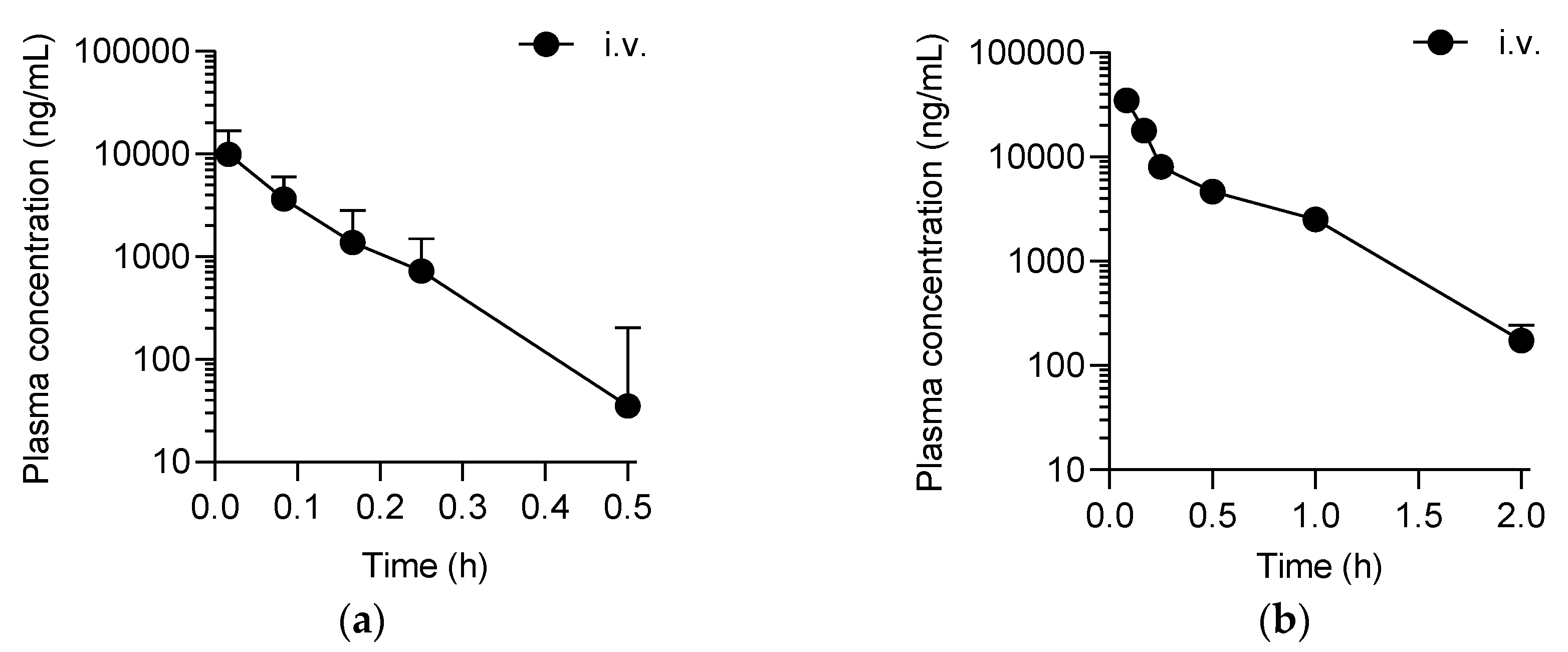
2.1. Pharmacokinetics of SP-8356 in Rats and Dogs
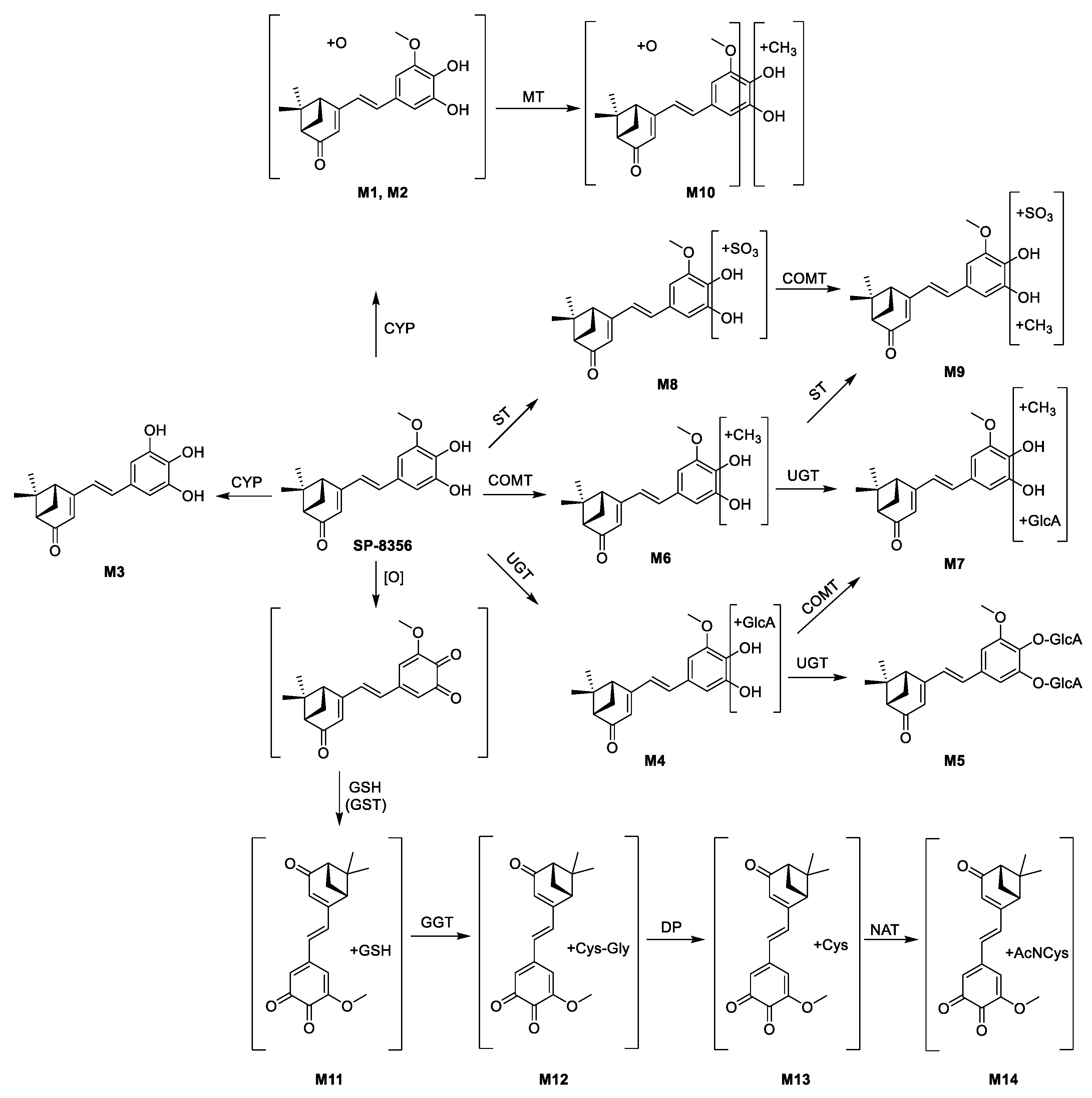
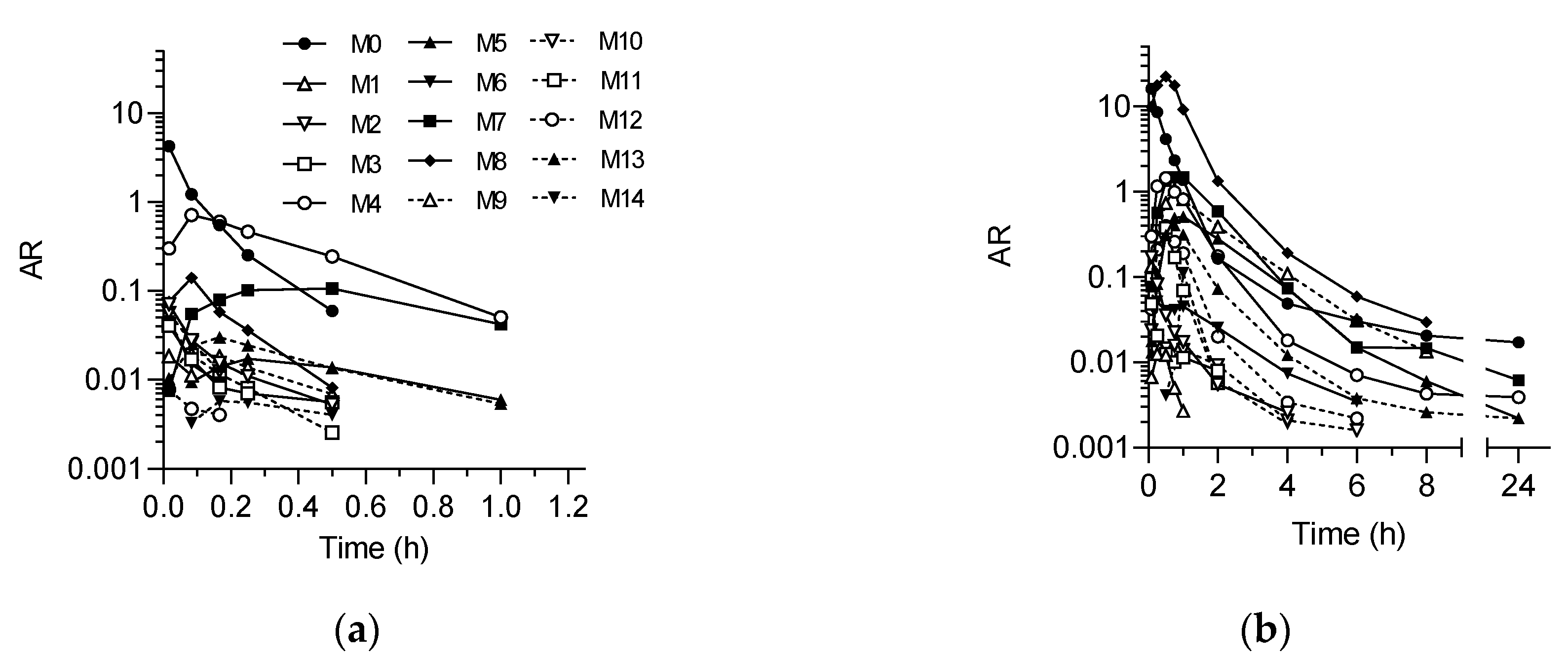
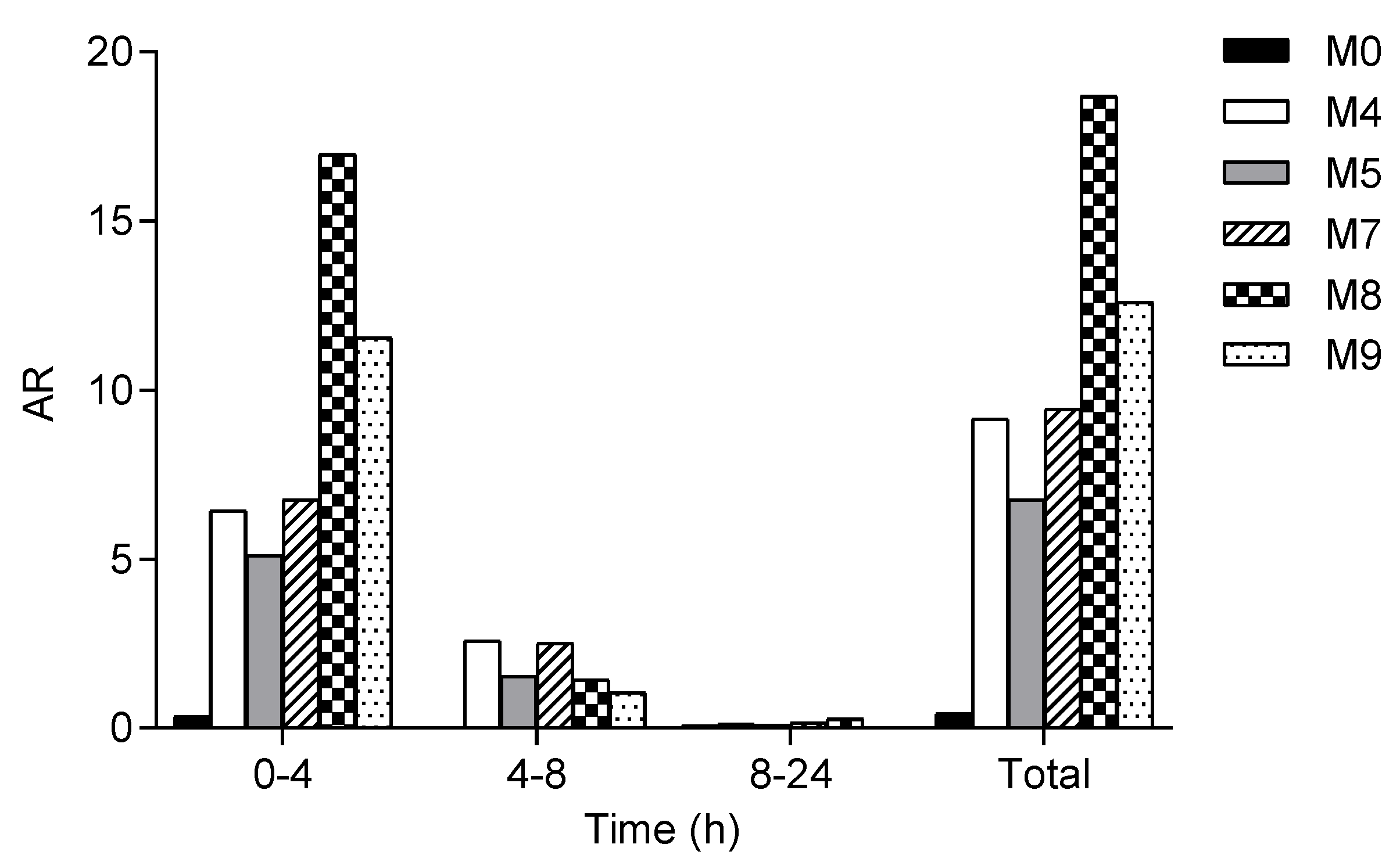
2.2. In Vivo Metabolite Identification in Rats and Dogs
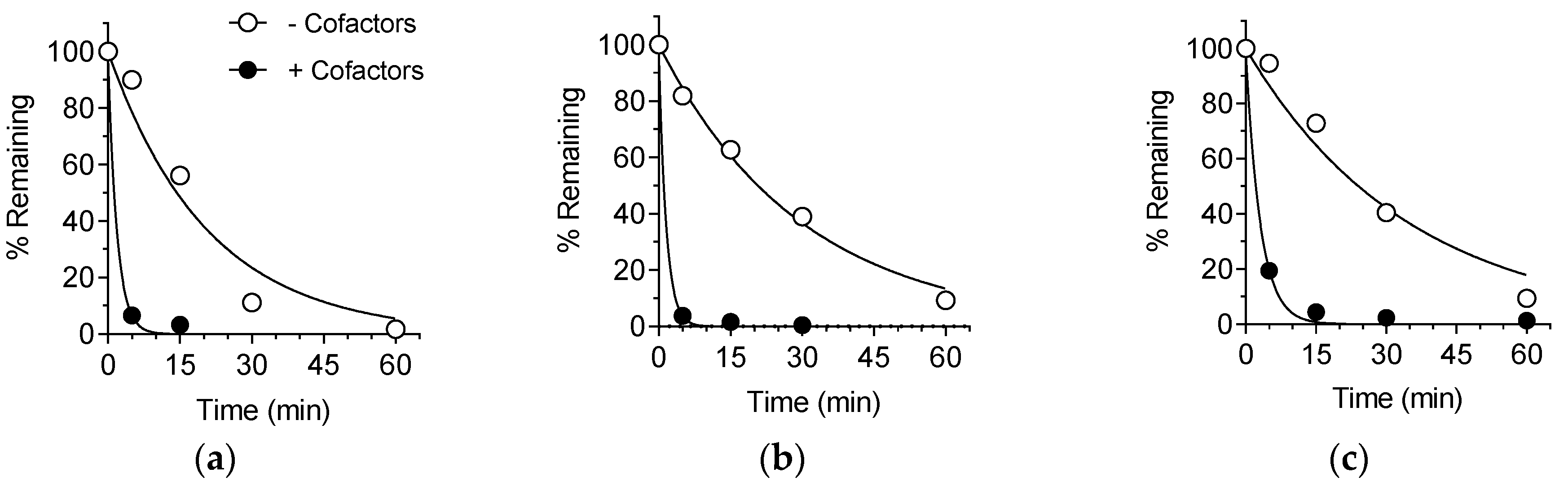
2.3. Bioactivation of SP-8356 in Rat Liver S9 Fraction
2.4. Metabolic Stability in Rat, Dog and Human Liver S9 Fractions
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Pharmacokinetic Studies in Rats
4.3. Pharmacokinetic Studies in Dogs
4.4. In Vivo Metabolite Identification
4.5. Bioactivation Studies in Rat Liver S9 Fraction
4.6. Metabolic Stability in Liver S9 Fractions
Author Contributions
Funding
Conflicts of Interest
References
- Bernardes, W.A.; Lucarini, R.; Tozatti, M.G.; Flauzino, L.G.; Souza, M.G.; Turatti, I.C.; Andrade e Silva, M.L.; Martins, C.H.; da Silva Filho, A.A.; Cunha, W.R. Antibacterial activity of the essential oil from Rosmarinus officinalis and its major components against oral pathogens. Zeitschrift für Naturforschung C 2010, 65, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, S.; Chen, J.; Wu, J.; Suzuki, Y.; Ma, L.; Kumazawa, K. Potent odorants of characteristic floral/sweet odor in Chinese chrysanthemum flower tea infusion. J. Agric. Food Chem. 2017, 65, 10058–10063. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.K.; Ballico, L.J.; Rocha Lapa, F.; Goncalves, H.P.; de Souza, L.M.; Iacomini, M.; Werner, M.F.; Baggio, C.H.; Pereira, I.T.; da Silva, L.M.; et al. Evaluation of the antinociceptive, anti-inflammatory and gastric antiulcer activities of the essential oil from Piper aleyreanum C.DC in rodents. J. Ethnopharmacol. 2012, 142, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Sugie, A.; Shimada, T. Roles of human CYP2A6 and 2B6 and rat CYP2C11 and 2B1 in the 10-hydroxylation of (−)-verbenone by liver microsomes. Drug Metab. Dispos. 2003, 31, 1049–1053. [Google Scholar] [CrossRef] [PubMed]
- Blomquist, G.J.; Figueroa-Teran, R.; Aw, M.; Song, M.; Gorzalski, A.; Abbott, N.L.; Chang, E.; Tittiger, C. Pheromone production in bark beetles. Insect Biochem. Mol. Biol. 2010, 40, 699–712. [Google Scholar] [CrossRef]
- Yang, J.Y.; Lee, H.S. Verbenone structural analogues isolated from Artemesia aucheri as natural acaricides against Dermatophagoides spp. and Tyrophagus putrescentiae. J. Agric. Food Chem. 2013, 61, 12292–12296. [Google Scholar] [CrossRef]
- de Melo, C.G.F.; Salgado, P.R.R.; da Fonseca, D.V.; Braga, R.M.; Filho, M.; de Farias, I.E.V.; de Luna Freire Pessoa, H.; Lima, E.M.; do Amaral, I.P.G.; de Sousa, D.P.; et al. Anticonvulsive activity of (1S)-(−)-verbenone involving RNA expression of BDNF, COX-2 and c-fos. Naunyn-Schmiedeberg‘s Arch. Pharmacol. 2017, 390, 863–869. [Google Scholar] [CrossRef]
- Ju, C.; Song, S.; Kim, M.; Choi, Y.; Kim, W.K. Up-regulation of astroglial heme oxygenase-1 by a synthetic (S)-verbenone derivative LMT-335 ameliorates oxygen-glucose deprivation-evoked injury in cortical neurons. Biochem. Biophys. Res. Commun. 2013, 431, 484–489. [Google Scholar] [CrossRef]
- Ju, C.; Song, S.; Hwang, S.; Kim, C.; Kim, M.; Gu, J.; Oh, Y.K.; Lee, K.; Kwon, J.; Lee, K.; et al. Discovery of novel (1S)-(−)-verbenone derivatives with anti-oxidant and anti-ischemic effects. Bioorg. Med. Chem. Lett. 2013, 23, 5421–5425. [Google Scholar] [CrossRef]
- Mander, S.; Kim, D.H.; Thi Nguyen, H.; Yong, H.J.; Pahk, K.; Kim, E.Y.; Lee, K.; Seong, J.Y.; Kim, W.K.; Hwang, J.I. SP-8356, a (1S)-(−)-verbenone derivative, exerts in vitro and in vivo anti-breast cancer effects by inhibiting NF-kappaB signaling. Sci. Rep. 2019, 9, 6595. [Google Scholar] [CrossRef]
- Richardson, S.J.; Bai, A.; Kulkarni, A.A.; Moghaddam, M.F. Efficiency in drug discovery: Liver S9 fraction assay as a screen for metabolic stability. Drug. Metab. Lett. 2016, 10, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Davies, B.; Morris, T. Physiological parameters in laboratory animals and humans. Pharm. Res. 1993, 10, 1093–1095. [Google Scholar] [CrossRef] [PubMed]
- Bohnert, T.; Patel, A.; Templeton, I.; Chen, Y.; Lu, C.; Lai, G.; Leung, L.; Tse, S.; Einolf, H.J.; Wang, Y.H.; et al. International Consortium for Innovation and Quality in Pharmaceutical Development (IQ) Victim Drug-Drug Interactions Working Group. Evaluation of a new molecular entity as a victim of metabolic drug-drug interactions-an industry perspective. Drug Metab. Dispos. 2016, 44, 1399–1423. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Clifford, M.N. Role of the small intestine, colon and microbiota in determining the metabolic fate of polyphenols. Biochem. Pharmacol. 2017, 139, 24–39. [Google Scholar] [CrossRef]
- Ottaviani, J.I.; Momma, T.Y.; Kuhnle, G.K.; Keen, C.L.; Schroeter, H. Structurally related (−)-epicatechin metabolites in humans: Assessment using de novo chemically synthesized authentic standards. Free Radic. Biol. Med. 2012, 52, 1403–1412. [Google Scholar] [CrossRef]
- Tsoi, C.; Swedmark, S. Sulfation in dog. Curr. Drug Metab. 2005, 6, 275–285. [Google Scholar] [CrossRef]
- Groen, K.; Warrander, A.; Miles, G.S.; Booth, B.S.; Mulder, G.J. Sulphation and glucuronidation of xamoterol in the dog: Dose dependence and site of sulphation. Xenobiotica 1988, 18, 511–518. [Google Scholar] [CrossRef]
- Podder, S.K.; Nakashima, M.; Nakamura, T.; Sasaki, H.; Nakamura, J.; Shibasaki, J. Conjugation of salicylamide in the intestinal wall of dogs and rabbits. J. Pharmacobiodyn. 1986, 9, 917–922. [Google Scholar] [CrossRef]
- Yang, C.H.; Tang, L.; Lv, C.; Ye, L.; Xia, B.J.; Hu, M.; Liu, Z.Q. Sulfation of selected mono-hydroxyflavones by sulfotransferases in vitro: A species and gender comparison. J. Pharm. Pharmacol. 2011, 63, 967–970. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Di, L.; Kerns, E.H. Chapter 7—Solubility. In Drug-Like Properties: Concepts, Structure Design and Methods, 2nd ed.; Di, L., Kerns, E.H., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 61–93. [Google Scholar]
- Di, L.; Kerns, E.H. Chapter 8—Permeability. In Drug-Like Properties: Concepts, Structure Design and Methods, 2nd ed.; Di, L., Kerns, E.H., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 95–111. [Google Scholar]
- Lin, J.H.; Chiba, M.; Baillie, T.A. Is the role of the small intestine in first-pass metabolism overemphasized? Pharmacol. Rev. 1999, 51, 135–158. [Google Scholar] [PubMed]
- Lu, W.; Uetrecht, J.P. Peroxidase-mediated bioactivation of hydroxylated metabolites of carbamazepine and phenytoin. Drug Metab. Dispos. 2008, 36, 1624–1636. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Nikelly, J.G.; Killmer, L., Jr.; Tarloff, J.B. Metabolism of para-aminophenol by rat hepatocytes. Drug Metab. Dispos. 2000, 28, 880–886. [Google Scholar]
- Kalgutkar, A.S.; Gardner, I.; Obach, R.S.; Shaffer, C.L.; Callegari, E.; Henne, K.R.; Mutlib, A.E.; Dalvie, D.K.; Lee, J.S.; Nakai, Y.; et al. A comprehensive listing of bioactivation pathways of organic functional groups. Curr. Drug Metab. 2005, 6, 161–225. [Google Scholar] [CrossRef]
- Bolton, J.L.; Trush, M.A.; Penning, T.M.; Dryhurst, G.; Monks, T.J. Role of quinones in toxicology. Chem. Res. Toxicol. 2000, 13, 135–160. [Google Scholar] [CrossRef]
- Moridani, M.Y.; Scobie, H.; Jamshidzadeh, A.; Salehi, P.; O’Brien, P.J. Caffeic acid, chlorogenic acid and dihydrocaffeic acid metabolism: Glutathione conjugate formation. Drug Metab. Dispos. 2001, 29, 1432–1439. [Google Scholar]
- Dalvie, D.; Kalgutkar, A.S.; Chen, W. Practical approaches to resolving reactive metabolite liabilities in early discovery. Drug Metab. Rev. 2015, 47, 56–70. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- Yang, D.P.; Ji, H.F.; Tang, G.Y.; Ren, W.; Zhang, H.Y. How many drugs are catecholics. Molecules 2007, 12, 878–884. [Google Scholar] [CrossRef]
- Patel, K.R.; Andreadi, C.; Britton, R.G.; Horner-Glister, E.; Karmokar, A.; Sale, S.; Brown, V.A.; Brenner, D.E.; Singh, R.; Steward, W.P.; et al. Sulfate metabolites provide an intracellular pool for resveratrol generation and induce autophagy with senescence. Sci. Transl. Med. 2013, 5, 205ra133. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.A.; Moon, R.C.; Anders, M.W.; Fenselau, C.; Shane, B. Enhancement of biological activity by conjugation reactions. J. Nutr. 1992, 122, 615–624. [Google Scholar] [CrossRef]
- Obach, R.S. Pharmacologically active drug metabolites: Impact on drug discovery and pharmacotherapy. Pharmacol. Rev. 2013, 65, 578–640. [Google Scholar] [CrossRef] [PubMed]
- Osborne, R.; Thompson, P.; Joel, S.; Trew, D.; Patel, N.; Slevin, M. The analgesic activity of morphine-6-glucuronide. Br. J. Clin. Pharmacol. 1992, 34, 130–138. [Google Scholar] [CrossRef]
- Volger, K.D. Animal experimental and human pharmacologic studies with phase-II metabolites of triamterene. Arzneimittelforschung 1991, 41, 499–506. [Google Scholar]
- Perez-Vizcaino, F.; Duarte, J.; Santos-Buelga, C. The flavonoid paradox: Conjugation and deconjugation as key steps for the biological activity of flavonoids. J. Sci. Food Agric. 2012, 92, 1822–1825. [Google Scholar] [CrossRef]
- Menendez, C.; Duenas, M.; Galindo, P.; Gonzalez-Manzano, S.; Jimenez, R.; Moreno, L.; Zarzuelo, M.J.; Rodriguez-Gomez, I.; Duarte, J.; Santos-Buelga, C.; et al. Vascular deconjugation of quercetin glucuronide: The flavonoid paradox revealed? Mol. Nutr. Food Res. 2011, 55, 1780–1790. [Google Scholar] [CrossRef]
- Piwowarski, J.P.; Stanislawska, I.; Granica, S.; Stefanska, J.; Kiss, A.K. Phase II Conjugates of urolithins isolated from human urine and potential role of β-glucuronidases in their disposition. Drug Metab. Dispos. 2017, 45, 657–665. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Gonzalez-Sarrias, A.; Garcia-Villalba, R.; Nunez-Sanchez, M.A.; Selma, M.V.; Garcia-Conesa, M.T.; Espin, J.C. Urolithins, the rescue of “old” metabolites to understand a “new” concept: Metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis and host health status. Mol. Nutr. Food Res. 2017, 61, 1500901. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef]
- Zhou, Y.; Choi, Y.J.; Kim, E.; Oh, M.H.; Shin, H.J.; Kim, S.K.; Lee, K. Pharmacokinetics and metabolism of streptochlorin and its synthetic derivative, 5-hydroxy-2’-isobutyl streptochlorin, in mice. Biol. Pharm. Bull. 2018, 41, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Shi, R.; Gee, W.; Bonderud, R. Regulated drug bioanalysis for human pharmacokinetic studies and therapeutic drug management. Bioanalysis 2012, 4, 1919–1931. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huo, M.; Zhou, J.; Xie, S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Programs Biomed. 2010, 99, 306–314. [Google Scholar] [CrossRef]
- Nishimuta, H.; Houston, J.B.; Galetin, A. Hepatic, intestinal, renal and plasma hydrolysis of prodrugs in human, cynomolgus monkey, dog and rat: Implications for in vitro-in vivo extrapolation of clearance of prodrugs. Drug Metab. Dispos. 2014, 42, 1522–1531. [Google Scholar] [CrossRef]
- Houston, J.B.; Galetin, A. Methods for predicting in vivo pharmacokinetics using data from in vitro assays. Curr. Drug Metab. 2008, 9, 940–951. [Google Scholar] [CrossRef]
Sample Availability: Sample of SP-8356 is available from the authors. |
| Parameter | Rat | Dog | ||
|---|---|---|---|---|
| i.v. | p.o. | i.v. | p.o. | |
| Dose (mg/kg) | 20 | 20 | 50 | 50 |
| t1/2 (h) | 0.07 ± 0.02 | ND | 0.03 ± 0.04 | ND |
| AUClast (ng∙h/mL) | 980.9 ± 314.3 | ND | 12,361.9 ± 1027.4 | ND |
| AUCinf (ng∙h/mL) | 1034.1 ± 314.4 | ND | 12,442.9 ± 1012.2 | ND |
| CL (L/h/kg) | 21.0 ± 6.5 | NA | 4.0 ± 0.3 | NA |
| Vz (L/kg) | 1.9 ± 0.6 | NA | 1.8 ± 0.3 | NA |
| Vss (L/kg) | 1.6 ± 0.5 | NA | 1.3 ± 0.3 | NA |
| Metabolite (Biotransformation) | tr (min) | Molecular Formula | Precursor ion (m/z, [M−H]−) | Product Ions (m/z) | ||
|---|---|---|---|---|---|---|
| Calculated | Observed | ∆m (ppm) | ||||
| M0 (SP-8356) | 4.00 | C18H20O4 | 299.1289 | 299.1298 | 3.0 | 255, 283, 284 |
| M1 (+O) | 3.73 | C18H20O5 | 315.1268 | 315.1247 | −6.7 | ND |
| M2 (+O) | 3.94 | C18H20O5 | 315.1268 | 315.1225 | −13.6 | ND |
| M3 (−CH3) | 3.91 | C17H18O4 | 285.1132 | 285.1102 | −10.5 | ND |
| M4 (+GlcA) | 3.88 | C24H28O10 | 475.1610 | 475.1631 | 4.4 | 85, 113, 175, 255, 284, 299 |
| M5 (+2 × GlcA) | 3.76 | C30H36O16 | 651.1931 | 651.1945 | 2.1 | 85, 113, 175, 284, 299, 475 |
| M6 (+CH3) | 4.06 | C19H22O4 | 313.1445 | 313.1426 | −6.1 | ND |
| M7 (+CH3 + GlcA) | 3.86 | C25H30O10 | 489.1766 | 489.1755 | −2.2 | 85, 113, 175, 283, 298, 313 |
| M8 (+SO3) | 4.25 | C18H20O7S | 379.0857 | 379.0878 | 5.5 | 284, 299 |
| M9 (+CH3 + SO3) | 4.18 | C19H22O7S | 393.1013 | 393.1030 | 4.3 | 283, 298, 313 |
| M10 (+CH3 + O) | 3.91 | C19H22O5 | 329.1359 | 329.1356 | −0.9 | ND |
| M11 (−H2 + GSH) | 3.79 | C28H35N3O10S | 604.1970 | 604.1986 | 2.6 | 272, 316, 331 |
| M12 (−H2 + Cys-Gly) | 3.78 | C23H28N2O7S | 475.1544 | 475.1566 | 4.6 | 316, 331 |
| M13 (−H2 + Cys) | 3.79 | C21H25NO6S | 418.1330 | 418.1347 | 4.1 | 316, 331 |
| M14 (−H2 + AcNCys) | 3.90 | C23H27NO7S | 460.1435 | 460.1449 | 3.0 | ND |
| Metabolite | Rat | Dog | ||||
|---|---|---|---|---|---|---|
| AUClast | tmax | AUClast | tmax | |||
| (AR∙h) × 102 | (%) | (h) | (AR∙h) × 102 | (%) | (h) | |
| M0 (SP-8356) | 36.22 | 26.48 | NA | 698.5 | 17.66 | NA |
| M1 | 0.11 | 0.08 | 0.017 | 0.8 | 0.02 | 0.25 |
| M2 | 0.88 | 0.64 | 0.017 | 7.5 | 0.19 | 0.083 |
| M3 | 0.55 | 0.40 | 0.017 | 2.7 | 0.07 | 0.083 |
| M4 | 60.52 | 44.24 | 0.083 | 177.7 | 4.49 | 0.5 |
| M5 | 1.14 | 0.84 | 0.25 | 120.5 | 3.05 | 1.0 |
| M6 | 0.34 | 0.25 | 0.017 | 12.2 | 0.31 | 0.25 |
| M7 | 30.97 | 22.64 | 0.5 | 300.1 | 7.59 | 0.75 |
| M8 | 2.55 | 1.86 | 0.083 | 2307.0 | 58.33 | 0.5 |
| M9 | 0.62 | 0.45 | 0.083 | 191.6 | 4.84 | 0.75 |
| M10 | 0.49 | 0.35 | 0.017 | 4.3 | 0.11 | 0.083 |
| M11 | 0.58 | 0.43 | 0.017 | 26.7 | 0.68 | 0.5 |
| M12 | 0.08 | 0.06 | 0.017 | 37.1 | 0.94 | 0.5 |
| M13 | 1.52 | 1.11 | 0.17 | 59.8 | 1.51 | 0.75 |
| M14 | 0.22 | 0.16 | 0.17 | 8.4 | 0.21 | 1.0 |
| Cofactors | SP-8356 (% Remaining at 60 min) | M11 (AR) |
|---|---|---|
| None | 20.3 ± 1.9 | NA |
| AA | 105.9 ± 2.6 | NA |
| NADPH | 21.6 ± 2.0 | NA |
| GSH | 29.1 ± 6.9 | 0.20 ± 0.01 |
| GSH + AA | 92.1 ± 1.7 | 0.009 ± 0.001 |
| GSH + NADPH | 18.0 ± 1.9 | 0.32 ± 0.009 |
| GSH + UDPGA + PAPS + SAM | 1.3 ± 0.2 | 0.012 ± 0.0002 |
| Species | −Cofactors | +Cofactors | |||
|---|---|---|---|---|---|
| t1/2 (min) | t1/2 (min) | CLint (mL/min/kg) | CLH (mL/min/kg) | EH | |
| Rat | 14.4 | 1.3 | 2899.9 | 54.0 | 0.98 |
| Dog | 20.7 | 1.1 | 2433.3 | 29.6 | 0.98 |
| Human | 24.1 | 2.1 | 1035.6 | 19.6 | 0.99 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Oh, M.H.; Kim, Y.J.; Kim, E.-y.; Kang, J.; Chung, S.; Ju, C.; Kim, W.-K.; Lee, K. Metabolism and Pharmacokinetics of SP-8356, a Novel (1S)-(−)-Verbenone Derivative, in Rats and Dogs and Its Implications in Humans. Molecules 2020, 25, 1775. https://doi.org/10.3390/molecules25081775
Zhou Y, Oh MH, Kim YJ, Kim E-y, Kang J, Chung S, Ju C, Kim W-K, Lee K. Metabolism and Pharmacokinetics of SP-8356, a Novel (1S)-(−)-Verbenone Derivative, in Rats and Dogs and Its Implications in Humans. Molecules. 2020; 25(8):1775. https://doi.org/10.3390/molecules25081775
Chicago/Turabian StyleZhou, Yuanyuan, Mun Hwan Oh, Yeon Joon Kim, Eun-yeong Kim, Jinhong Kang, Sung Chung, Chung Ju, Won-Ki Kim, and Kiho Lee. 2020. "Metabolism and Pharmacokinetics of SP-8356, a Novel (1S)-(−)-Verbenone Derivative, in Rats and Dogs and Its Implications in Humans" Molecules 25, no. 8: 1775. https://doi.org/10.3390/molecules25081775
APA StyleZhou, Y., Oh, M. H., Kim, Y. J., Kim, E.-y., Kang, J., Chung, S., Ju, C., Kim, W.-K., & Lee, K. (2020). Metabolism and Pharmacokinetics of SP-8356, a Novel (1S)-(−)-Verbenone Derivative, in Rats and Dogs and Its Implications in Humans. Molecules, 25(8), 1775. https://doi.org/10.3390/molecules25081775





