Development and Validation of Liquid Chromatography-Tandem Mass Spectrometry Method for Pharmacokinetic Evaluation of 7β-(3-Ethyl-cis-crotonoyloxy)-1α-(2-methylbutyryloxy)-3,14-dehydro-Z-notonipetranon in Rats
Abstract
1. Introduction
2. Results and Discussion
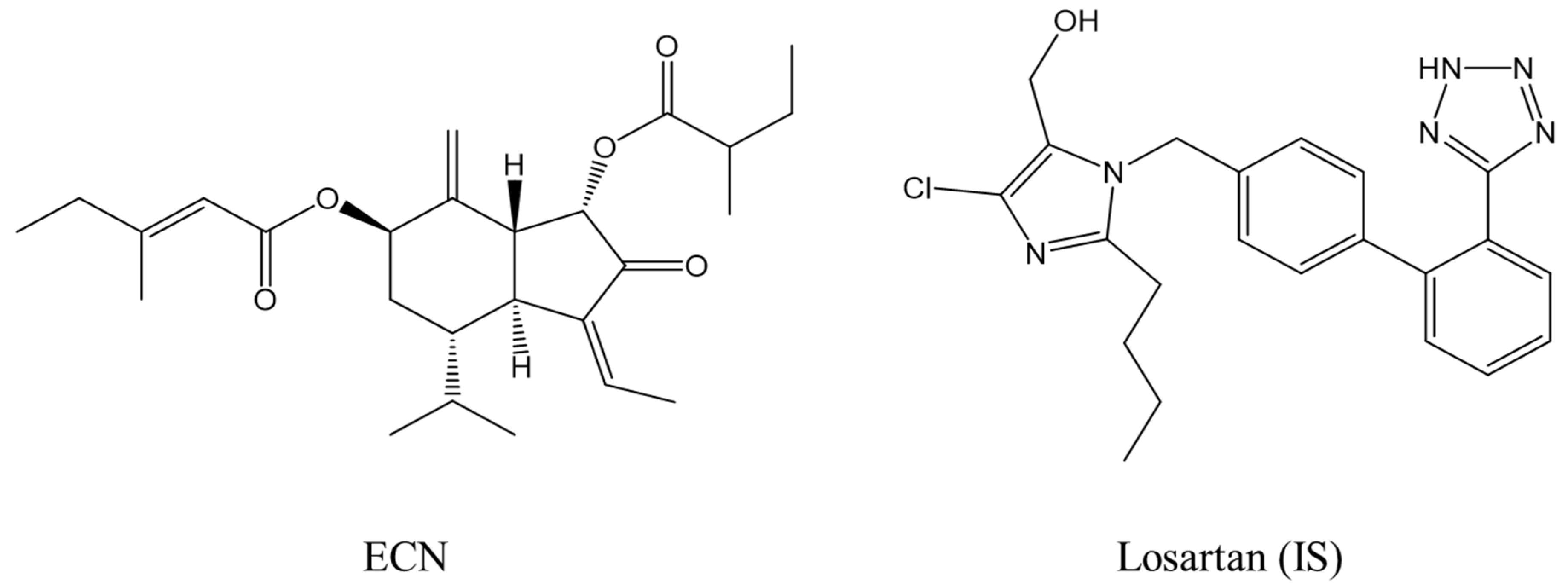
2.1. Optimization of LC-MS/MS Conditions
2.2. Method Validation
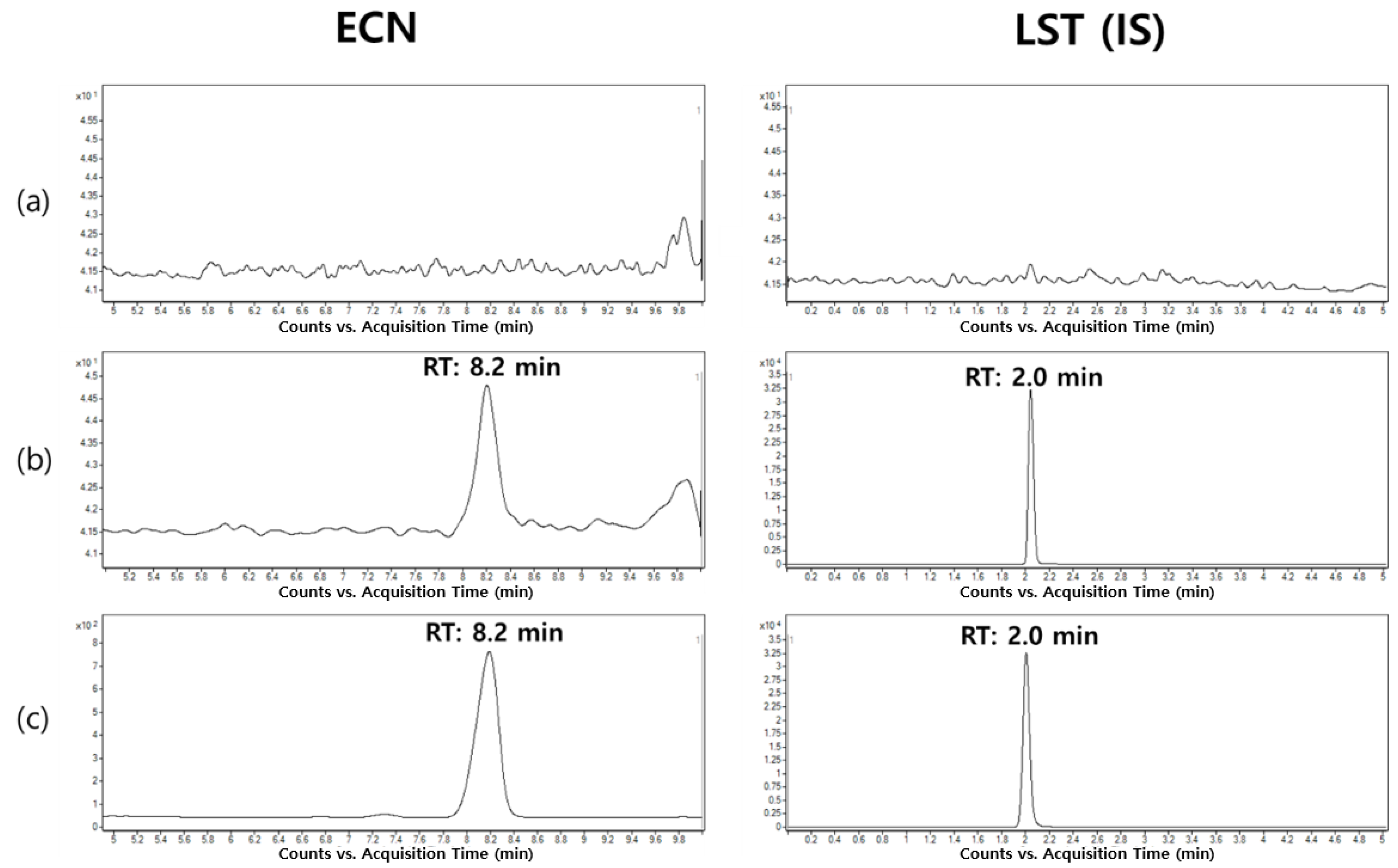
2.2.1. Selectivity
2.2.2. Linearity
2.2.3. Within- and Between-Run Precision and Accuracy
2.2.4. Matrix Effect and Extraction Recovery
2.2.5. Pre- and Post-Preparative Stability
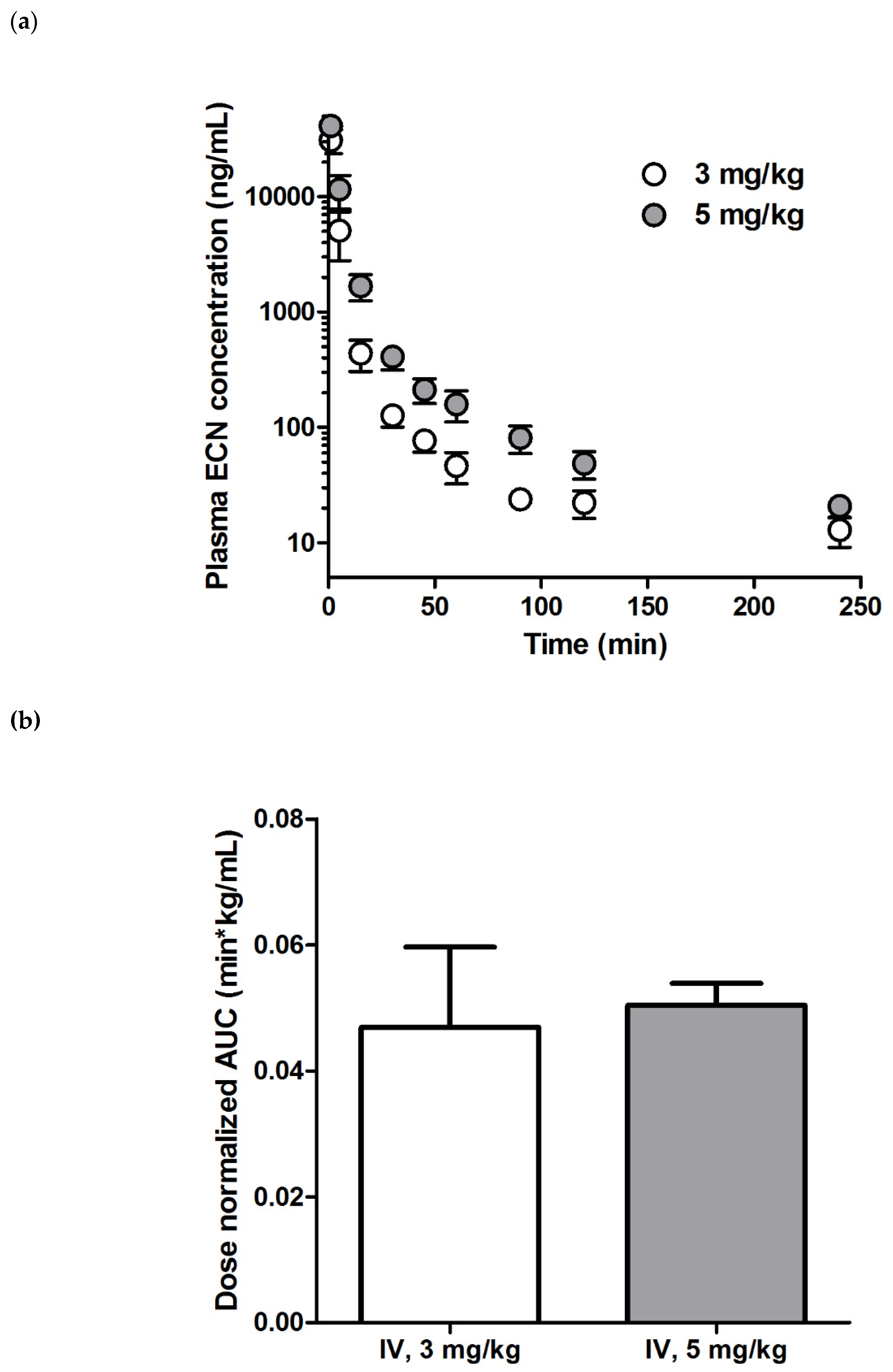
2.3. Pharmacokinetic Studies
3. Materials and Methods
3.1. Materials
3.2. Animals Studies
3.3. Apparatus and Conditions
3.4. Preparation of Calibration Curve and Quality Control Samples
3.5. Sample Pretreatment
3.6. Method Validation
3.6.1. Selectivity
3.6.2. Linearity and LLOQ
3.6.3. Within- and Between-Run Precision and Accuracy
3.6.4. Matrix Effect and Extraction Recovery
3.6.5. Pre- and Post-Preparative Stability
3.7. Sample Dilution
3.8. Pharmacokinetic Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, L.L.; Yang, J.L.; Shi, Y.P. Sesquiterpenoids and other constituents from the flower buds of Tussilago farfara. J. Asian Nat. Prod. Res. 2011, 13, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Zhi, H.J.; Qin, X.M.; Sun, H.F.; Zhang, L.Z.; Guo, X.Q.; Li, Z.Y. Metabolic fingerprinting of Tussilago farfara L. using (1)H-NMR spectroscopy and multivariate data analysis. Phytochem. Anal. 2012, 23, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Fang, L.; Wang, X.; Qiu, J.; Huang, L. Preparative separation and purification of sesquiterpenoids from Tussilago farfara L. by high-speed counter-current chromatography. Quim. Nova. 2011, 34, 804–807. [Google Scholar]
- Kim, M.R.; Lee, J.Y.; Lee, H.H.; Aryal, D.K.; Kim, Y.G.; Kim, S.K.; Woo, E.R.; Kang, K.W. Antioxidative effects of quercetin-glycosides isolated from the flower buds of Tussilago farfara L. Food Chem. Toxicol. 2006, 44, 1299–1307. [Google Scholar] [CrossRef]
- Hwangbo, C.; Lee, H.S.; Park, J.; Choe, J.; Lee, J.H. The anti-inflammatory effect of tussilagone, from Tussilago farfara, is mediated by the induction of heme oxygenase-1 in murine macrophages. Int. Immunopharmacol. 2009, 9, 1578–1584. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kang, U.; Seo, E.K.; Kim, Y.S. Heme oxygenase-1-mediated anti-inflammatory effects of tussilagonone on macrophages and 12-O-tetradecanoylphorbol-13-acetate-induced skin inflammation in mice. Int. Immunopharmacol. 2016, 34, 155–164. [Google Scholar] [CrossRef]
- Xu, J.; Sun, X.; Kang, J.; Liu, F.; Wang, P.; Ma, J.; Zhou, H.; Jin, D.Q.; Ohizumi, Y.; Lee, D.; et al. Chemical and biological profiles of Tussilago farfara: Structures, nitric oxide inhibitory activities, and interactions with iNOS protein. J. Funct. Foods. 2017, 32, 37–45. [Google Scholar] [CrossRef]
- Kokoska, L.; Polesny, Z.; Rada, V.; Nepovim, A.; Vanek, T. Screening of some Siberian medicinal plants for antimicrobial activity. J. Ethnopharmacol. 2002, 82, 51–53. [Google Scholar] [CrossRef]
- Zhao, J.; Evangelopoulos, D.; Bhakta, S.; Gray, A.I.; Seidel, V. Antitubercular activity of Arctium lappa and Tussilago farfara extracts and constituents. J. Ethnopharmacol. 2014, 155, 796–800. [Google Scholar] [CrossRef]
- Li, Z.Y.; Zhi, H.J.; Xue, S.Y.; Sun, H.F.; Zhang, F.S.; Jia, J.P.; Xing, J.; Zhang, L.Z.; Qin, X.M. Metabolomic profiling of the flower bud and rachis of Tussilago farfara with antitussive and expectorant effects on mice. J. Ethnopharmacol. 2012, 140, 83–90. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, H.; Wang, S.; Hou, A.; Man, W.; Zhang, J.; Guo, X.; Yang, B.; Kuang, H.; Wang, Q. Discovering the Major Antitussive, Expectorant, and Anti-Inflammatory Bioactive Constituents in Tussilago farfara L. Based on the Spectrum–Effect Relationship Combined with Chemometrics. Molecules. 2020, 25, 620. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Huang, Y.N.; Gao, B.; Xu, P.Y.; Inagaki, C.; Kawabata, J. α-Glucosidase inhibitory effect by the flower buds of Tussilago farfara L. Food Chem. 2008, 106, 1195–1201. [Google Scholar] [CrossRef]
- Hwang, S.B.; Chang, M.N.; Garcia, M.L.; Han, Q.Q.; Huang, L.; King, V.F.; Kaczorowski, G.J.; Winquist, R.J. L-652,469—A dual receptor antagonist of platelet activating factor and dihydropyridines from Tussilago farfara L. Eur. J. Pharmacol. 1987, 141, 269–281. [Google Scholar] [CrossRef]
- Li, H.; Lee, H.J.; Ahn, Y.H.; Kwon, H.J.; Jang, C.Y.; Kim, W.Y.; Ryu, J.H. Tussilagone suppresses colon cancer cell proliferation by promoting the degradation of b-catenin. Biochem. Biophs. Res. Commun. 2014, 443, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Kim, H.M.; Ryu, J.H.; Jeong, Y.S.; Lee, Y.S.; Jin, C. Neuroprotective and antioxidant effects of the ethyl acetate fraction prepared from Tussilago farfara L. Biol. Pharm. Bull. 2005, 28, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Magesh, S.; Chen, Y.; Hu, L. Small Molecule Modulators of K eap1-N rf2-ARE Pathway as Potential Preventive and Therapeutic Agents. Med. Res. Rev. 2012, 32, 687–726. [Google Scholar] [CrossRef]
- Kumar, H.; Kim, I.S.; More, S.V.; Kim, B.W.; Choi, D.K. Natural product-derived pharmacological modulators of Nrf2/ARE pathway for chronic diseases. Nat. Prod. Rep. 2014, 31, 109–139. [Google Scholar] [CrossRef]
- Lim, H.J.; Dong, G.Z.; Lee, H.J.; Ryu, J.H. In vitro neuroprotective activity of sesquiterpenoids from the flower buds of Tussilago farfara. J. Enzyme Inhib. Med. Chem. 2015, 30, 852–856. [Google Scholar] [CrossRef]
- Lee, J.; Song, K.; Huh, E.; Oh, M.S.; Kim, Y.S. Neuroprotection against 6-OHDA toxicity in PC12 cells and mice through the Nrf2 pathway by a sesquiterpenoid from Tussilago farfara. Redox Biol. 2018, 18, 6–15. [Google Scholar] [CrossRef]
- Park, H.R.; Yoo, M.Y.; Seo, J.H.; Kim, I.S.; Kim, N.Y.; Kang, J.Y.; Cui, L.; Lee, C.S.; Lee, C.H.; Lee, H.S. Sesquiterpenoids isolated from the flower buds of Tussilago farfara L. inhibit diacylglycerol acyltransferase. J. Agric. Food Chem. 2008, 56, 10493–10497. [Google Scholar] [CrossRef]
- Song, K.; Lee, K.J.; Kim, Y.S. Development of an efficient fractionation method for the preparative separation of sesquiterpenoids from Tussilago farfara by counter-current chromatography. J. Chromatogr. A. 2017, 1489, 107–114. [Google Scholar] [CrossRef]
- Karra, V.K.; Pilli, N.R.; Inamadugu, J.K.; Rao, J.V.L.N.S. Simultaneous determination of losartan, losartan acid and amlodipine in human plasma by LC-MS/MS and its application to a human pharmacokinetic study. Pharm. Methods. 2012, 3, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Liao, M.; Diao, X.; Sun, Y.; Zhang, L. Screening and identification of metabolites of two kinds of main active ingredients and hepatotoxic pyrrolizidine alkaloids in rat after lavage Farfarae Flos extract by UHPLC-Q-TOF-MS mass spectrometry. Biomed. Chromatogr. 2018, 32, e4047. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, T.A.; Winger, B.E. Mass spectrometry for small molecule pharmaceutical product development: A review. Mass Spectrum. Rev. 2011, 30, 479–490. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Guidance for Industry: Bioanalytical Method Validation. 2018. Available online: https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf (accessed on 24 May 2018).
- Nandi, U.; Dan, S.; Pal, T.K. Development and validation of a liquid chromatography–mass spectrometry method for simultaneous determination of metoprolol and telmisartan in rat plasma and its application to pharmacokinetic study. J. Pharm. Investig. 2015, 45, 329–340. [Google Scholar] [CrossRef]
- Eeckhaut, A.V.; Lanckmans, K.; Sarre, S.; Smolders, I.; Michotte, Y. Validation of bioanalytical LC–MS/MS assays: Evaluation of matrix effects. J. Chromatogr. B 2009, 877, 2198–2207. [Google Scholar] [CrossRef]
- Gavhane, Y.N.; Yadav, A.V. Loss of orally administered drugs in GI tract. Saudi Pharm. J. 2012, 20, 331–344. [Google Scholar] [CrossRef]
- Yang Y, h.; Aloysius, H.; Inoyama, D.; Chen, Y.; Hu, L. Enzyme-mediated hydrolytic activation of prodrugs. Acta Pharm. Sin. B 2011, 1, 143–159. [Google Scholar] [CrossRef]
- Liu, Y.F.; Yang, X.W.; Lu, W.; Xin, X.L. Determination and pharmacokinetic study of tussilagone in rat plasma by RP-HPLC method. Biomed. Chromatogr. 2008, 22, 1194–1200. [Google Scholar] [CrossRef]
Sample Availability: Samples of 7β-(3-ethyl-cis-crotonoyloxy)-1α-(2-methylbutyryloxy)-3,14-dehydro-Z-notonipetranone is available from the authors. |

| Nominal Concentration (ng/mL) | Within-Run (n = 6) | Between-Run (n = 3) | ||||
|---|---|---|---|---|---|---|
| Concentration Determined (ng/mL) | RSDa (%) | REb (%) | Concentration Determined (ng/mL) | RSD (%) | RE (%) | |
| 10.0 | 9.24 | 9.90 | −7.58 | 9.27 | 5.97 | −7.33 |
| 25.0 | 24.3 | 5.12 | −2.89 | 23.0 | 7.57 | −8.13 |
| 3750 | 3766 | 3.11 | 0.42 | 3542 | 5.61 | −5.56 |
| 7500 | 7215 | 2.59 | −3.80 | 6977 | 3.74 | −6.98 |
| Nominal Concentration (ng/mL) | Matrix Effect (%) a | Extraction Recovery (%) b |
|---|---|---|
| ECN | ||
| 10.0 | 83.3 ± 7.65 | 62.6 ± 4.56 |
| 25.0 | 86.7 ± 4.28 | 58.5 ± 3.88 |
| 3750 | 89.6 ± 1.50 | 73.4 ± 1.41 |
| 7500 | 93.1 ± 0.86 | 77.7 ± 5.03 |
| Losartan (IS) | ||
| 80.0 | 70.3 ± 1.10 | 99.5 ± 0.93 |
| Nominal Concentration (ng/mL) | Pre-preparative Stability (n = 4) | Post-preparative Stability (n = 4) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4°C Stability a | Long-term Stability b | Freeze-thaw Stability c | Autosampler Stabilityd | |||||||||
| Mean | RSD (%) | RE (%) | Mean | RSD (%) | RE (%) | Mean | RSD (%) | RE (%) | Mean | RSD (%) | RE (%) | |
| 10.0 | 9.89 | 10.3 | −1.13 | 7.35 | 20.4 | −26.5 | 9.05 | 18.0 | −9.50 | 10.5 | 3.94 | 4.88 |
| 25.0 | 24.7 | 4.06 | −1.25 | 16.3 | 8.43 | −34.8 | 23.0 | 18.1 | −7.90 | 23.8 | 10.5 | −4.87 |
| 3750 | 3575 | 1.95 | −4.67 | 2581 | 3.65 | −31.2 | 3508 | 1.36 | −6.47 | 3351 | 2.04 | −10.6 |
| 7500 | 7073 | 3.11 | −5.70 | 5502 | 2.77 | −26.6 | 6953 | 1.50 | −7.30 | 6537 | 0.97 | −12.9 |
| Parameter | IV (3 mg/kg) | IV (5 mg/kg) |
|---|---|---|
| AUClast (μg∙min/mL) | 149 ± 42 | 253 ± 21 |
| AUCinf (μg∙min/mL) | 152 ± 42 | 256 ± 21 |
| t1/2 (min) | 80.0 ± 6.9 | 84.4 ± 15.5 |
| CLp (mL/min/kg) | 21.0 ± 6.3 | 19.7 ± 1.6 |
| Vd,ss (mL/kg) | 203 ± 25 | 252 ± 51 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, N.-W.; Lee, J.-Y.; Song, K.; Kim, M.-H.; Yoon, S.; Nguyen, D.-T.; Kim, S.; Kim, Y.S.; Kim, D.-D. Development and Validation of Liquid Chromatography-Tandem Mass Spectrometry Method for Pharmacokinetic Evaluation of 7β-(3-Ethyl-cis-crotonoyloxy)-1α-(2-methylbutyryloxy)-3,14-dehydro-Z-notonipetranon in Rats. Molecules 2020, 25, 1774. https://doi.org/10.3390/molecules25081774
Kang N-W, Lee J-Y, Song K, Kim M-H, Yoon S, Nguyen D-T, Kim S, Kim YS, Kim D-D. Development and Validation of Liquid Chromatography-Tandem Mass Spectrometry Method for Pharmacokinetic Evaluation of 7β-(3-Ethyl-cis-crotonoyloxy)-1α-(2-methylbutyryloxy)-3,14-dehydro-Z-notonipetranon in Rats. Molecules. 2020; 25(8):1774. https://doi.org/10.3390/molecules25081774
Chicago/Turabian StyleKang, Nae-Won, Jae-Young Lee, Kwangho Song, Min-Hwan Kim, Soyeon Yoon, Duy-Thuc Nguyen, Sungho Kim, Yeong Shik Kim, and Dae-Duk Kim. 2020. "Development and Validation of Liquid Chromatography-Tandem Mass Spectrometry Method for Pharmacokinetic Evaluation of 7β-(3-Ethyl-cis-crotonoyloxy)-1α-(2-methylbutyryloxy)-3,14-dehydro-Z-notonipetranon in Rats" Molecules 25, no. 8: 1774. https://doi.org/10.3390/molecules25081774
APA StyleKang, N.-W., Lee, J.-Y., Song, K., Kim, M.-H., Yoon, S., Nguyen, D.-T., Kim, S., Kim, Y. S., & Kim, D.-D. (2020). Development and Validation of Liquid Chromatography-Tandem Mass Spectrometry Method for Pharmacokinetic Evaluation of 7β-(3-Ethyl-cis-crotonoyloxy)-1α-(2-methylbutyryloxy)-3,14-dehydro-Z-notonipetranon in Rats. Molecules, 25(8), 1774. https://doi.org/10.3390/molecules25081774






