Phenolic Composition of the Leaves of Pyrola rotundifolia L. and Their Antioxidant and Cytotoxic Activity
Abstract
1. Introduction
2. Results and Discussion
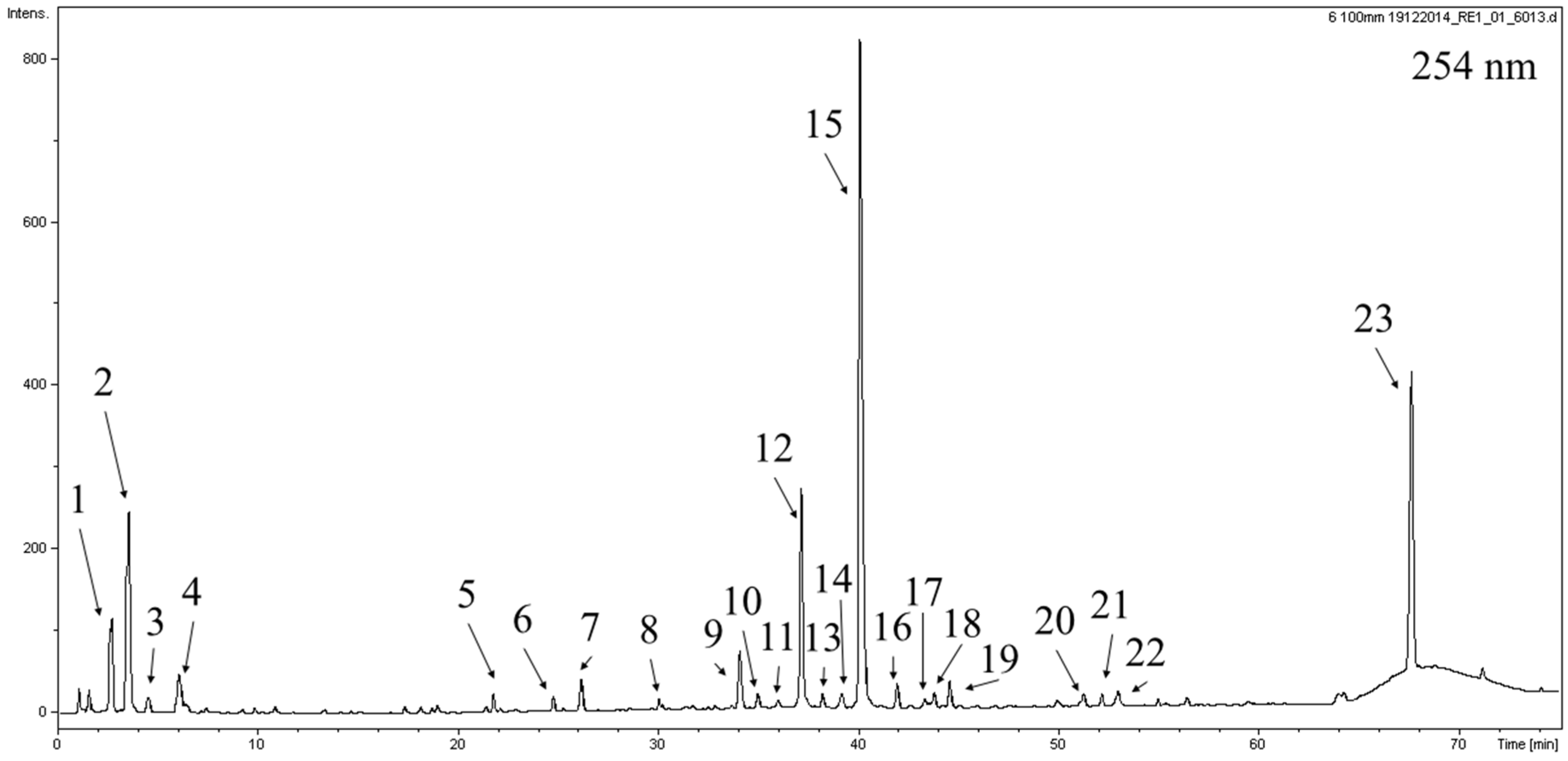
2.1. Phytochemical Analysis
2.2. In Vitro Cytotoxicity Assay
2.3. Antioxidant Activity
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals and Reagents
3.3. Total Phenolic, Flavonoid, and Gallotannin Content
3.4. UHPLC–DAD–MS Analysis
Quantification of Major Flavonoids by UHPLC–DAD
3.5. Cell Lines and Cell Culture
3.6. Analysis of Cell Viability
3.7. Antioxidant Activity Assays
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sayre, L.M.; Smith, M.A.; Perry, G. Chemistry and biochemistry of oxidative stress in neurodegenerative disease. Curr. Med. Chem. 2001, 8, 721–738. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Dhalla, N.S.; Temsah, R.M.; Netticadan, T. Role of oxidative stress in cardiovascular diseases. J. Hypertens. 2000, 18, 655–673. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.; Jialal, I. Oxidative stress and atherosclerosis. Pathophysiology 2006, 13, 129–142. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Van Eeden, S.F.; Sin, D.D. Oxidative stress in chronic obstructive pulmonary disease: A lung and systemic process. Can. Respir. J. 2013, 20, 27–29. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef]
- The Plant List. Available online: http://www.theplantlist.org/tpl1.1/search?q=pyrola (accessed on 7 February 2020).
- Tutin, T.G. Flora Europaea. Diapensiaceae to Myoporaceae; Cambridge University Press: Cambridge, UK, 1972; Volume 3, p. 3. [Google Scholar]
- Yao, X.H.; Zhang, D.Y.; Zu, Y.G.; Fu, Y.; Luo, M.; Gu, C.B.; Li, C.Y.; Efferth, T. Free radical scavenging capability, antioxidant activity and chemical constituents of Pyrola incarnata Fisch. leaves. Ind. Crops Prod. 2013, 49, 247–255. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Luo, M.; Wang, W.; Zhao, C.J.; Gu, C.B.; Zu, Y.G.; Fu, Y.J.; Yao, X.H.; Duan, M.H. Variation of active constituents and antioxidant activity in pyrola [P. incarnata Fisch.] from different sites in Northeast China. Food Chem. 2013, 141, 2213–2219. [Google Scholar] [CrossRef]
- Wang, D.; He, F.; Lv, Z.; Li, D. Phytochemical composition, antioxidant activity and HPLC fingerprinting profiles of three Pyrola species from different regions. PLoS ONE 2014, 9, e96329. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xiao, G.G.; Rong, P.; Zhang, Z.; Dong, J.; Zhao, H.; Li, H.; Li, Y.; Pan, J.; Liu, H.; et al. Therapeutic effects of radix dipsaci, pyrola herb, and cynomorium songaricum on bone metabolism of ovariectomized rats. BMC Complement. Altern. Med. 2012, 12, 67–85. [Google Scholar] [CrossRef] [PubMed]
- Darzuli, N.P.; Vronska, L.V.; Groshovyi, T.A.; Beley, N.M. Development of methods of standarization of medicinal plants—Pyrola rotundifolia leaf. Pharm. Innov. J. 2017, 6, 17–21. [Google Scholar]
- Ho, Y.S.; So, K.F.; Chang, R.C. Anti-aging herbal medicine—How and why can they be used in aging-associated neurodegenerative diseases? Ageing Res Rev. 2009, 9, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Kagawa, K.; Tokura, K.; Uchida, K.; Kakushi, H.; Shike, T.; Nakai, H. Platelet aggregation inhibitors and inotropic constituents in Pyrolae herba. Chem. Pharm. Bull. 1992, 40, 2083–2087. [Google Scholar] [CrossRef] [PubMed]
- Kosuge, T.; Yokota, M.; Sugiyama, K.; Mure, T.; Yamazawa, H.; Yamamoto, T. Studies on bioactive substances in crude drugs used for arthritic diseases in Traditional Chinese Medicine. III. Isolation and identification of anti-inflammatory and analgesic principles from the whole herb of Pyrola rotundifolia L. Chem. Pharm. Bull. 1985, 33, 5355–5357. [Google Scholar] [CrossRef]
- Perry, L.M. Medicinal Plants of East and Southeast Asia: Attributed Properties and Uses; The MIT Press: Cambridge, MA, USA, 1980. [Google Scholar]
- Zhang, D.Y.; Yao, X.H.; Duan, M.H.; Luo, M.; Wang, W.; Fu, Y.J.; Zu, Y.G.; Efferth, T. An effective negative pressure cavitation-microwave assisted extraction for determination of phenolic compounds in P. calliantha H. Andr. Analyst 2013, 138, 4631–4641. [Google Scholar] [CrossRef]
- Yang, X.; Peng, Q.; Liu, Q.; Hu, J.; Tang, Z.; Cui, L.; Lin, Z.; Xu, B.; Lu, K.; Yang, F.; et al. Antioxidant activity against H2O2-induced cytotoxicity of the ethanol extract and compounds from Pyrola decorate leaves. Pharm. Biol. 2017, 55, 1843–1848. [Google Scholar] [CrossRef]
- Chang, J.; Inui, T. Novel phenolic glycoside dimer and trimer from the whole herb of Pyrola rotundifolia. Chem. Pharm. Bull. 2005, 53, 1051–1053. [Google Scholar] [CrossRef][Green Version]
- Park, H.G.; Cha, M.R.; Hwang, J.H.; Kim, J.Y.; Park, M.S.; Choi, S.U.; Park, H.R.; Hwang, Y.I. Antimicrobial activity of the extract from Pyrola japonica against Bacillus subtilis. Int. J. Life Sci. 2006, 16, 989–993. [Google Scholar]
- Odontuya, G.; Hoult, J.R.S.; Houghton, P.J. Structure-activity relationship for antiinflammatory effect of luteolin and its derived glycosides. Phytother. Res. 2005, 19, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Zhang, C.; Ren, R.; Liu, R. Simultaneous determination of seven major triterpenoids in Pyrola decorate H. Andres by LC-MS method. Die Pharm. 2012, 67, 822–826. [Google Scholar] [PubMed]
- Bergeron, C.; Marston, A.; Antus, S.; Gauthier, R.; Hostettmann, K. Flavonoids from Pyrola elliptica. Phytochemistry 1998, 49, 233–236. [Google Scholar] [CrossRef]
- Kim, J.S.; Shim, S.H.; Xu, Y.N.; Kang, S.S.; Son, K.H.; Chang, H.W.; Kim, H.P.; Bae, K. Phenolic glycosides from Pyrola japonica. Chem. Pharm. Bull. 2004, 52, 714–771. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, A.L.; Zhao, M.B.; Tu, P.F. Tetralones and flavonoids from Pyrola calliantha. Chem. Biodivers. 2007, 4, 2932–2937. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Jin, Y.; Yao, F.; Duan, Z.; Wang, Q.; Liu, J. Validated LC-MS/MS method for the simultaneous determination of hyperoside and 2″-O-galloylhyperin in rat plasma: Application to a pharmacokinetic study in rats. Biomed. Chromatogr. 2014, 28, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Yazaki, K.; Shida, S.; Okuda, T. Galloylhomoarbutin and related polyphenols from Pyrola incarnate. Phytochemistry 1989, 28, 607–609. [Google Scholar] [CrossRef]
- Lee, S.M.; An, R.B.; Min, B.S.; Na, M.K.; Lee, C.H.; Kang, S.J.; Maeng, H.Y.; Bae, K.H. A new naphtoquinone from Pyrola japonica. Arch. Pharm. Res. 2001, 24, 522–523. [Google Scholar] [CrossRef] [PubMed]
- Ptitsyn, L.R.; Nomura, K.; Sklyar, I.V.; Ravcheeva, A.B. The 1,4-naphthoquinone derivative from Pyrola rotundifolia activates AMPK phosphorylation in C2C12 myotubes. Fitoterapia 2011, 82, 1285–1289. [Google Scholar] [CrossRef]
- Chen, Y.L.; Tan, C.H.; Tan, J.J.; Qu, S.J.; Wang, H.B.; Zhang, Q.; Jiang, S.H.; Zhu, D.Y. Phenolic and triterpenoid glycosides from Pyrola calliantha. Helv. Chim. Acta 2007, 90, 2421–2426. [Google Scholar] [CrossRef]
- Kirillov, V.; Stikhareva, T.; Atazhanova, G.; Serafimovich, M.; Mukanov, B.; Adekenov, S.; Mukasheva, F.; Yrymgali, M. Chemical composition of the essential oil of the boreal relict of Pyrola rotundifolia L. from northern Kazakhstan. J. Oleo Sci. 2015, 64, 1065–1073. [Google Scholar] [CrossRef][Green Version]
- Cai, L.; Ye, H.; Li, X.; Lin, Y.; Yu, F.; Chen, J.; Li, H.; Liu, X. Chemical constituents of volatile oil from Pyrolae herba and antiproliferative activity against SW1353 human chondrosarcoma cells. Int. J. Oncol. 2013, 42, 1452–1458. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China, 1st ed.; Chemical Industry Press: Beijing, China, 2005; pp. 226–277. [Google Scholar]
- Tresserra-Rimbau, A.; Lamuela-Raventos, R.M.; Moreno, J.J. Polyphenols, food and pharma. Current knowledge and directions for future research. Biochem. Pharmacol. 2018, 156, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Bogucka-Kocka, A.; Vorobets, N.; Chrząszcz, M.; Pietrzak, W.; Szewczyk, K. Polyphenol composition of extracts of the fruits of Laserpitium krapffii Crantz and their antioxidant and cytotoxic activity. Antioxidants 2019, 8, 363. [Google Scholar] [CrossRef]
- Serrano, J.; Puupponen-Pimiaä, R.; Dauer, A.; Aura, A.M.; Saura-Calixto, F. Tannins: Current knowledge of food sources, intake, bioavailability and biological effects. Mol. Nutr. Food Res. 2009, 53, S310–S329. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Ali-Shtayeh, M.S.; Jamous, R.M.; Arráez-Román, D.; Segura-Carretero, A. HPLC-DAD-ESI-MS/MS screening of bioactive components from Rhus coriaria L. (Sumac) fruits. Food Chem. 2015, 166, 179–191. [Google Scholar] [CrossRef]
- Inouye, H.; Arai, T.; Yaoi, Y.; Ogawa, M. Auftreten von hydrochinontyp-glucosiden, chimaphilin und monotropein in den Pyrolazeen. Chem. Pharm. Bull. 1964, 12, 255–256. [Google Scholar] [CrossRef][Green Version]
- Granica, S.; Hinc, K. Flavonoids in aerial parts of Persicaria mitis (Schrank) Holub. Biochem. Syst. Ecol. 2015, 61, 372–375. [Google Scholar] [CrossRef]
- Yao, X.H.; Zhang, D.Y.; Luo, M.; Zu, Y.G.; Efferth, T.; Fu, Y.J. Negative pressure cavitation-microwave assisted preparation of extract of Pyrola incarnata Fisch. rich in hyperin, 2′-O-galloylhyperin and chimaphilin and evaluation of its antioxidant activity. Food Chem. 2015, 169, 270–276. [Google Scholar] [CrossRef]
- Feng, R.; Ni, H.M.; Wang, S.Y.; Tourkova, I.L.; Shurin, M.R.; Harada, H.; Yin, X.M. Cyanidin-3-rutinoside, a natural polyphenol antioxidant, selectively kills leukemic cells by introduction of oxidative stress. J. Biol. Chem. 2007, 282, 13468–13476. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.; Kim, H.M.; Lee, S. Isolation and evaluation of an antitumor constituent from Pyrolae Herba. Yakhak Hoeji. 1996, 40, 225–229. [Google Scholar]
- Ma, W.D.; Zou, Y.P.; Wang, P.; Yao, X.H.; Sun, Y.; Duan, M.H.; Fu, Y.J.; Yu, B. Chimaphilin induces apoptosis in human breast cancer MCF-7 cells through a ROS-mediated mitochondrial pathway. Food Chem. Toxicol. 2014, 70, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sak, K. Site-specific anticancer effects of dietary flavonoid quercetin. Nutr. Cancer. 2014, 66, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Shokoohinia, Y.; Rashidi, M.; Hosseinzadeh, L.; Jelodarian, Z. Quercetin-3-O-β-D-glucopyranoside, a dietary flavonoid, protects PC12 cell from H2O2-induced cytotoxicity through inhibition of reactive oxygen species. Food Chem. 2015, 167, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, G.V.; Vysochina, G.I.; Muzyka, N.G.; Samoylova, Z.Y.; Kukushkina, T.A.; Oktyabrsky, O.N. Evaluation of antioxidant properties of medicinal plants using microbial test systems. World J. Microbial. Biotechnol. 2010, 26, 2269–2276. [Google Scholar] [CrossRef]
- Deba, F.; Xuan, T.D.; Yasuda, M.; Tawata, S. Chemical composition and antioxidant, antibacterial and antifungal activities of the essential oils from Bidens pilosa Linn. var. radiata. Food Control. 2008, 19, 346–352. [Google Scholar] [CrossRef]
- Nowak, R.; Szewczyk, K.; Gawlik-Dziki, U.; Rzymowska, J.; Komsta, Ł. Antioxidative and cytotoxic potential of some Chenopodium L. species growing in Poland. Saudi J. Biol. Sci. 2016, 23, 15–23. [Google Scholar] [CrossRef]
- Gazzani, G.; Papetti, A.; Massolini, G.; Daglia, M. Anti- and prooxidant activity of water soluble components of some common diet vegetables and effect of thermal treatment. J. Agric. Food Chem. 1998, 46, 4118–4122. [Google Scholar] [CrossRef]
- Polish Pharmacopoeia IX. PTFarm; Polish Pharmaceutical Society: Warsaw, Poland, 2011; p. 150. [Google Scholar]
- Inoue, K.H.; Hagerman, A.E. Determination of gallotannin with rhodamine. Anal. Biochem. 1988, 169, 363–369. [Google Scholar] [CrossRef]
- Kubrak, T.; Bogucka-Kocka, A.; Komsta, Ł.; Załuski, D.; Bogucki, J.; Gałkowski, D.; Kaczmarczyk, R.; Feldo, M.; Cioch, M.; Kocki, J. Modulation of multidrug resistance gene expression by coumarin derivatives in human leukemic cells. Oxid. Med. Cell. Longev. 2017, 2017, 5647281. [Google Scholar] [CrossRef] [PubMed]
- Olech, M.; Nowacka-Jechalke, N.; Masłyk, M.; Martyna, A.; Pietrzak, W.; Kubiński, K.; Załuski, D.; Nowak, R. Polysaccharide-rich fractions from Rosa rugosa Thunb.—Composition and chemopreventive potential. Molecules 2019, 24, 1354. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.T.; Lee, H.L.; Chiang, S.H.; Lin, H.I.; Chang, C.Y. Antioxidant properties of the extracts from different parts of broccoli in Taiwan. J. Food Drug Anal. 2001, 9, 96–101. [Google Scholar]
- Szewczyk, K.; Grzywa-Celińska, A. Antioxidants and cytotoxic activities of phenolic acids and their role in the anticancer therapies. In Phenolic Acids: Properties, Food Sources and Health Effects; Flores, A., Ed.; Nova Science Publishers: New York, NY, USA, 2016; pp. 61–104. [Google Scholar]
- Sulaiman, G.M.; Hussien, N.N.; Marzoog, T.R.; Awad, H.A. Phenolic content, antioxidant, antimicrobial and cytotoxic activities of ethanolic extract of Salix alba. Am. J. Biochem. Biotechnol. 2013, 9, 41–46. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Phenolic Content | |
|---|---|
| TPC (mg GAE/g DE) | 208.4 ± 1.2 |
| TFC (mg QE/g DE) | 38.9 ± 0.6 |
| GTC (μg GAE/g DE) | 722.9 ± 0.5 |
| No. | Compound Name | Retention Time (min) | UV (nm) | [M − H]− m/z | MS2 Ions | MS3 Ions | NL Detected (amu) | Compound Content (µg/mg of DE) |
|---|---|---|---|---|---|---|---|---|
| 1 | Monotropein t | 2.7 | 237 | 389 | 345, 227b, 209, 191, 179, 165, 147, 135 | - | - | 10.41 ± 0.07 |
| 2 | Galloylglucose isomer I | 3.5 | 278 | 331 | 314, 271, 211, 193, 169b, 125 | 125b | 162 | 13.07 ± 0.11 |
| 3 | Galloylglucose isomer II | 4.5 | 277 | 331 | 271, 207, 169b, 161, 125 | - | - | 0.69 ± 0.05 |
| 4 | Unknown compound | 6.1 | 282 | 331 | 285b, 241, 161, 123 | - | - | 0.91 ± 0.02 |
| 5 | Digalloylglucose isomer | 21.7 | 276 | 483 | 423, 331, 313, 271b, 211, 169 | 271b, 241, 169 | 152 | 0.75 ± 0.03 |
| 6 | 6-O-Galloylhomoarbutin t | 24.7 | 274 | 437 | 313b, 271, 211, 169 | - | - | 0.87 ± 0.01 |
| 7 | Galloylshikimic acid t | 26.1 | 277 | 325 | 205, 169b, 119, 101 | - | - | 1.24 ± 0.05 |
| 8 | Gallic acid derivative | 30.0 | 277 | 725 | 679b, 577, 517, 407, 331 | - | - | 0.61 ± 0.02 |
| 9 | Unknown compound | 34.0 | 224sh, 239, 302 | 349 | 259, 241, 229, 187b, 161 | - | - | 3.82 ± 0.12 |
| 10 | Quercetin-2′′-O-galloylgalactoside s | 35.0 | 250, 261sh, 352 | 615 | 463b, 343, 301 | 343, 301b | 152 | 0.67 ± 0.15 |
| 11 | Epicatechin gallate t | 36.0 | 280 | 441 | 397, 331, 289b, 271, 169 | 245b, 205 | 152 | 0.13 ± 0.01 |
| 12 | Quercetin 3-O-galactoside (hyperoside) s | 37.1 | 254, 263sh, 351 | 463 | 343, 301b, 179 | 271, 255, 179b, 151 | 162 | 9.35 ± 0.75 |
| 13 | Quercetin 3-O-glucoside (isoquercitrin) s | 38.2 | 251, 261sh, 351 | 463 | 343, 301b | - | 162 | 0.72 ± 0.14 |
| 14 | Gallic acid derivative | 39.2 | 270 | 521 | 506, 359b, 169 | 315, 169b | 162 | 1.42 ± 0.07 |
| 15 | Quercetin O-galloylhexoside | 40.0 | 252, 263sh, 352 | 615 | 463, 343, 313, 301b | - | - | 24.90 ± 1.17 |
| 16 | Quercetin-3-O-arabinopyranoside (guajaverin) s | 41.9 | 251, 260sh, 352 | 433 | 343, 301b | 257, 179b, 151 | 132 | 0.89 ± 0.20 |
| 17 | Unknown compound | 43.2 | - | 391 | 259, 241, 229, 187b, 172 | - | - | 0.07 ± 0.01 |
| 18 | Unknown compound | 43.8 | - | 457 | 411b, 379, 337, 301, 217 | - | - | 0.25 ± 0.01 |
| 19 | Unknown compound | 44.5 | 226, 266 | 599 | 435, 313b, 285 | - | - | 0.61 ± 0.03 |
| 20 | Unknown compound | 51.2 | 242sh, 270 | 697 | 584, 535b, 373, 355 | 373b, 355 | 162 | 0.57 ± 0.03 |
| 21 | Unknown compound | 52.2 | - | 963 | 777, 613b, 463, 299 | - | - | 0.49 ± 0.01 |
| 22 | Quercetin-O-galloylpentoside | 52.9 | 251, 263sh, 352 | 585 | 327, 285b, 255 | 505, 433, 301b, 283, 257, 229, 179, 151 | - | 0.78 ± 0.13 |
| 23 | Unknown compound | 67.6 | 224, 255, 269sh | 533 | 515, 501, 472, 443, 384, 371b, 356, 335, 315 | - | - | 11.34 ± 0.05 |
| Cell Line | IC50 (µg/mL) |
|---|---|
| HL-60 | 12.3 ± 2.1 |
| HL-60/MX1 | 3.3 ± 0.4 |
| HL-60/MX2 | 17.8 ± 2.4 |
| CEM/C1 | 16.9 ± 1.9 |
| CCRF/CEM | 6.2 ± 0.7 |
| r (p) for | HL-60 (IC50) | HL-60/MX1 (IC50) | HL-60/MX2 (IC50) | CCRF-CEM (IC50) | CEM/C1 (IC50) |
|---|---|---|---|---|---|
| HL-60 (IC50) | X | 0.9983 (0.002) | 0.9992 (0.001) | 0.9981 (0.002) | 0.6715 (0.329) |
| HL-60/MX1 (IC50) | 0.9983 (0.002) | X | 0.9998 (0.000) | 0.9999 (0.000) | 0.627 (0.373) |
| HL-60/MX2 (IC50) | 0.9992 (0.001) | 0.9998 (0.000) | X | 0.9998 (0.000) | 0.6421 (0.358) |
| CCRF-CEM (IC50) | 0.9981 (0.002) | 0.9999 (0.000) | 0.9998 (0.000) | X | 0.6249 (0.375) |
| CEM/C1 (IC50) | 0.6715 (0.329) | 0.627 (0.373) | 0.6421 (0.358) | 0.6249 (0.375) | X |
| TPC (GAE) | −0.8053 (0.195) | −0.8386 (0.161) | −0.8278 (0.172) | −0.8401 (0.160) | −0.1014 (0.899) |
| TFC (QE) | −0.4165 (0.583) | −0.4165 (0.583) | −0.3987 (0.601) | −0.4189 (0.581) | 0.4471 (0.553) |
| GTC (GAE) | 0.9972 (0.003) | 0.9999 (0.000) | 0.9994 (0.001) | 0.9999 (0.000) | 0.6146 (0.385) |
| Antioxidant Activity | |
|---|---|
| DPPH (EC50 mg/mL) | 0.2 ± 0.01 |
| ABTS (TEAC mmol Trolox/g DE) | 0.6 ± 0.03 |
| CHEL (EC50 mg/mL) | 1.4 ± 0.20 |
| β-Carotene/linoleic acid (EC50 mg/mL) | 0.1 ± 0.03 |
| r (p) for | DPPH (EC50) | ABTS (TEAC) | CHEL (EC50) | ß-C/LA (EC50) |
|---|---|---|---|---|
| DPPH (EC50) | X | 0.6612 (0.339) | −0.6292 (0.371) | 0.6595 (0.340) |
| ABTS (TE) | 0.6612 (0.339) | X | 0.1672 (0.833) | −0.1279 (0.872) |
| CHEL (EC50) | −0.6292 (0.371) | 0.1672 (0.833) | X | −0.9992 (0.001) |
| ß-C/LA (EC50) | 0.6595 (0.340) | −0,1279 (0.872) | −0.9992 (0.001) | X |
| TPC (GAE) | 0.9300 (0.070) | 0.8906 (0.109) | −0.2994 (0.701) | 0.3371 (0.663) |
| TFC (QE) | 0.9822 (0.018) | 0.5087 (0.491) | −0.7638 (0.236) | 0.7888 (0.211) |
| GTC (GAE) | −0.5925 (0.408) | −0.9961 (0.004) | −0.2534 (0.747) | 0.2148 (0.785) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szewczyk, K.; Bogucka-Kocka, A.; Vorobets, N.; Grzywa-Celińska, A.; Granica, S. Phenolic Composition of the Leaves of Pyrola rotundifolia L. and Their Antioxidant and Cytotoxic Activity. Molecules 2020, 25, 1749. https://doi.org/10.3390/molecules25071749
Szewczyk K, Bogucka-Kocka A, Vorobets N, Grzywa-Celińska A, Granica S. Phenolic Composition of the Leaves of Pyrola rotundifolia L. and Their Antioxidant and Cytotoxic Activity. Molecules. 2020; 25(7):1749. https://doi.org/10.3390/molecules25071749
Chicago/Turabian StyleSzewczyk, Katarzyna, Anna Bogucka-Kocka, Natalia Vorobets, Anna Grzywa-Celińska, and Sebastian Granica. 2020. "Phenolic Composition of the Leaves of Pyrola rotundifolia L. and Their Antioxidant and Cytotoxic Activity" Molecules 25, no. 7: 1749. https://doi.org/10.3390/molecules25071749
APA StyleSzewczyk, K., Bogucka-Kocka, A., Vorobets, N., Grzywa-Celińska, A., & Granica, S. (2020). Phenolic Composition of the Leaves of Pyrola rotundifolia L. and Their Antioxidant and Cytotoxic Activity. Molecules, 25(7), 1749. https://doi.org/10.3390/molecules25071749






