Spectroscopic Characterization of Emulsions Generated with a New Laser-Assisted Device
Abstract
1. Introduction
2. Results and Discussions
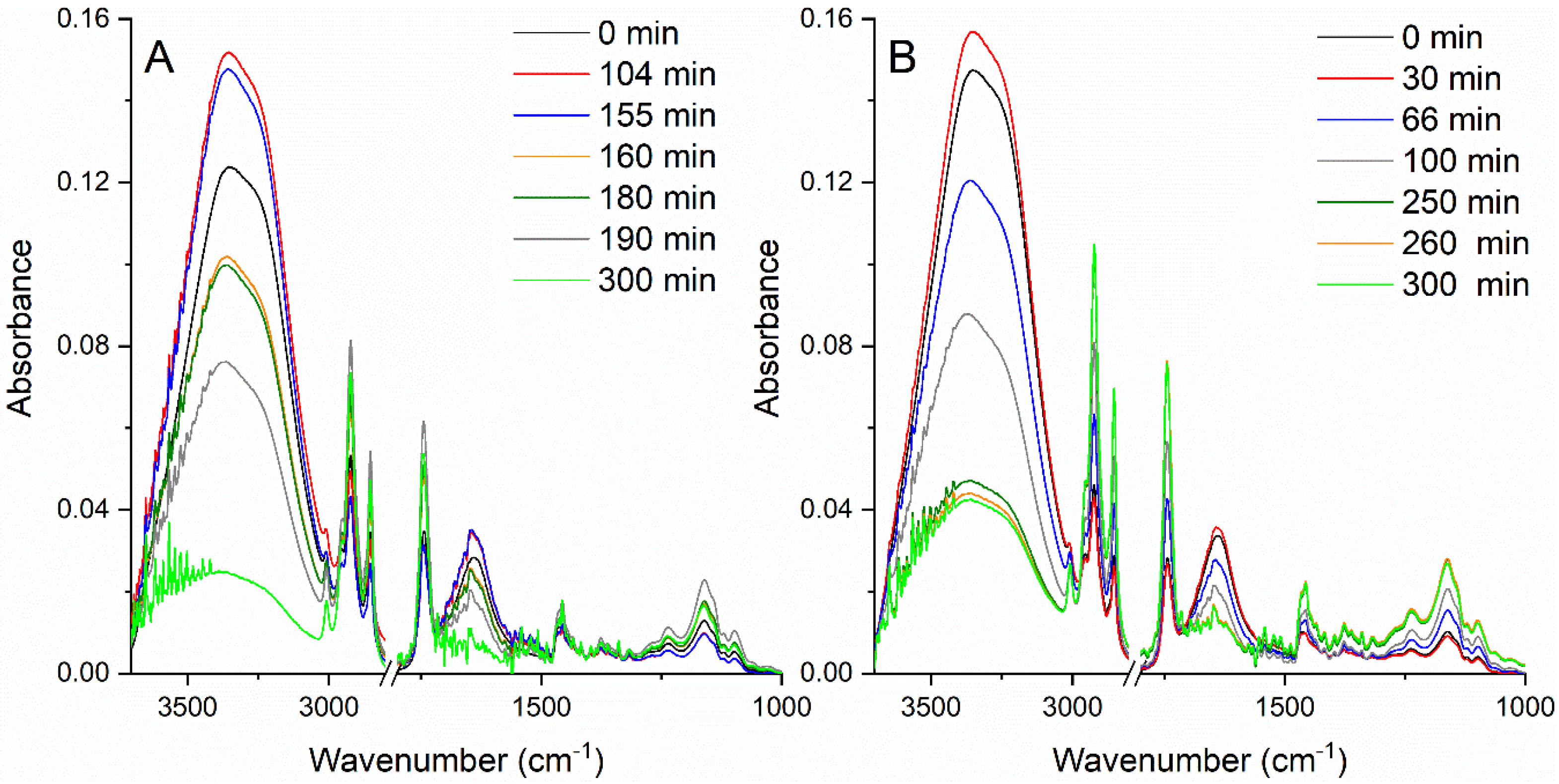
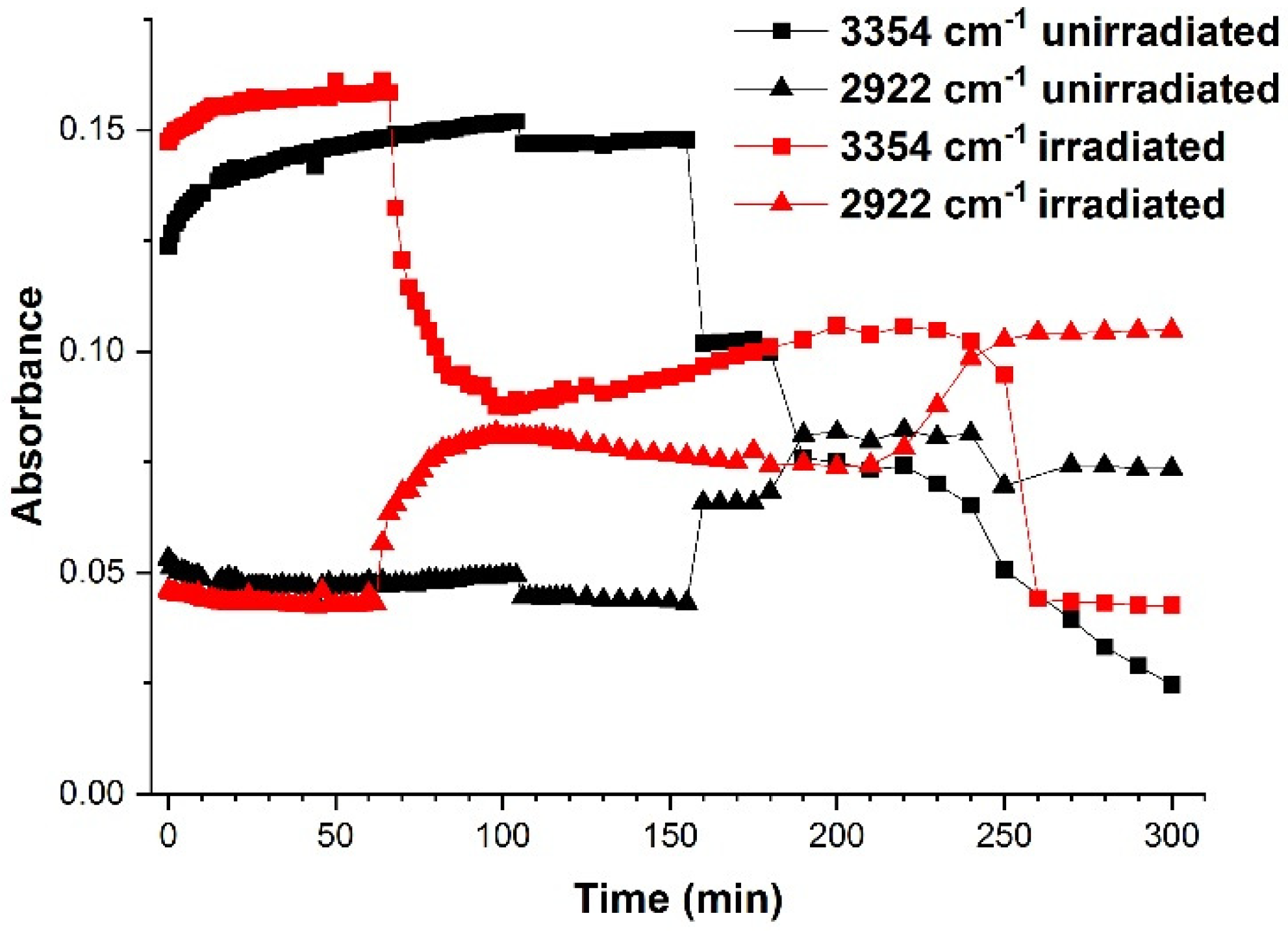
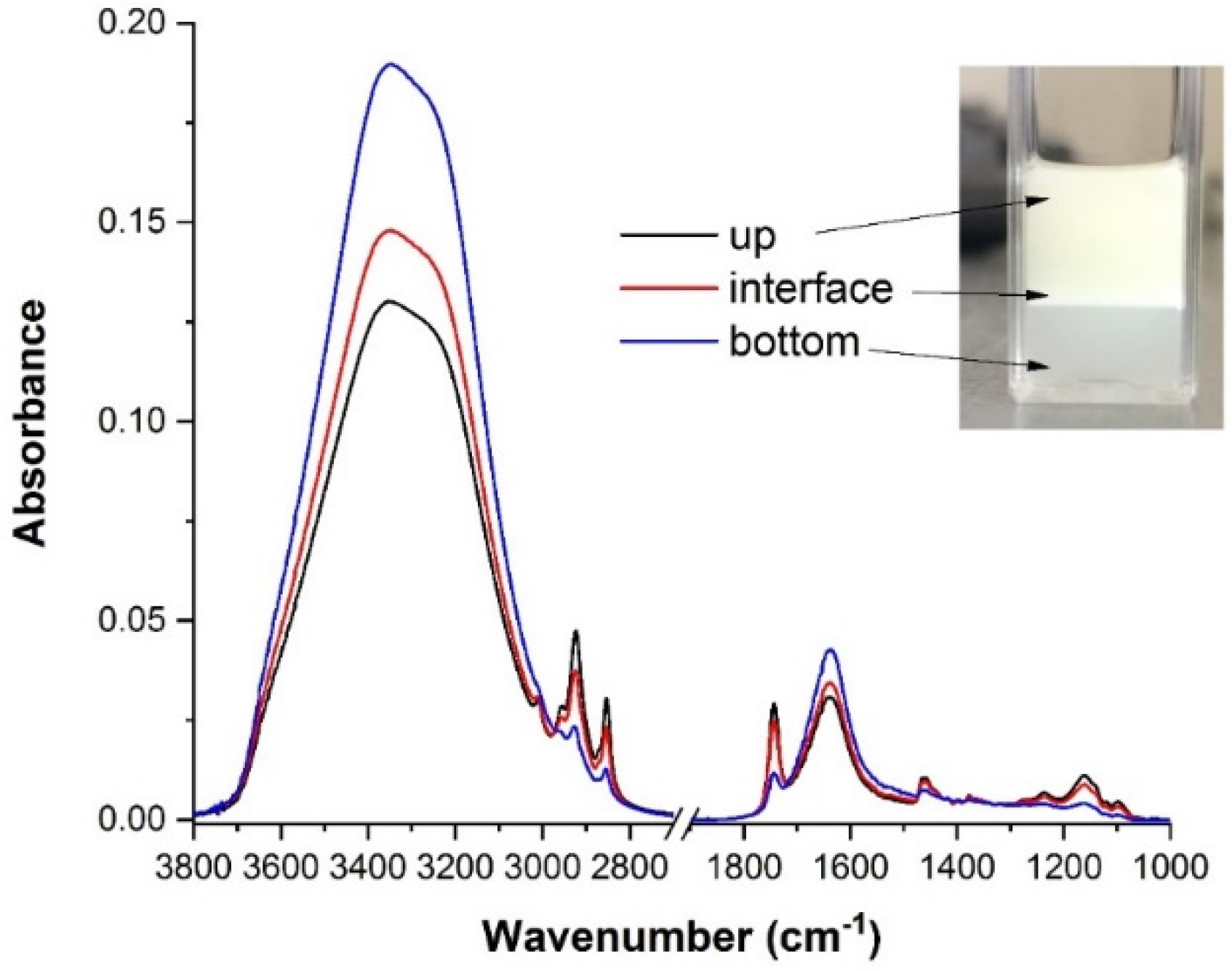
2.1. FTIR Spectroscopy
2.2. Raman Spectroscopy
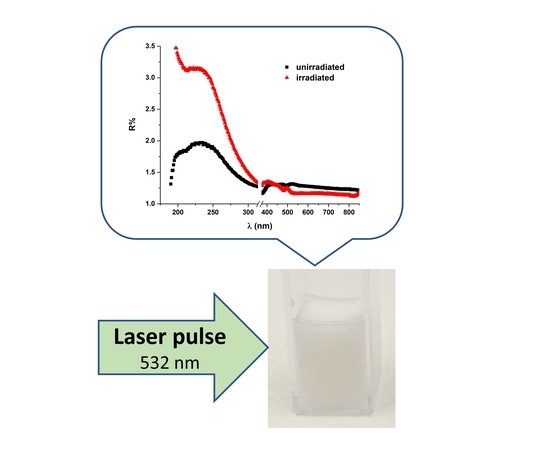
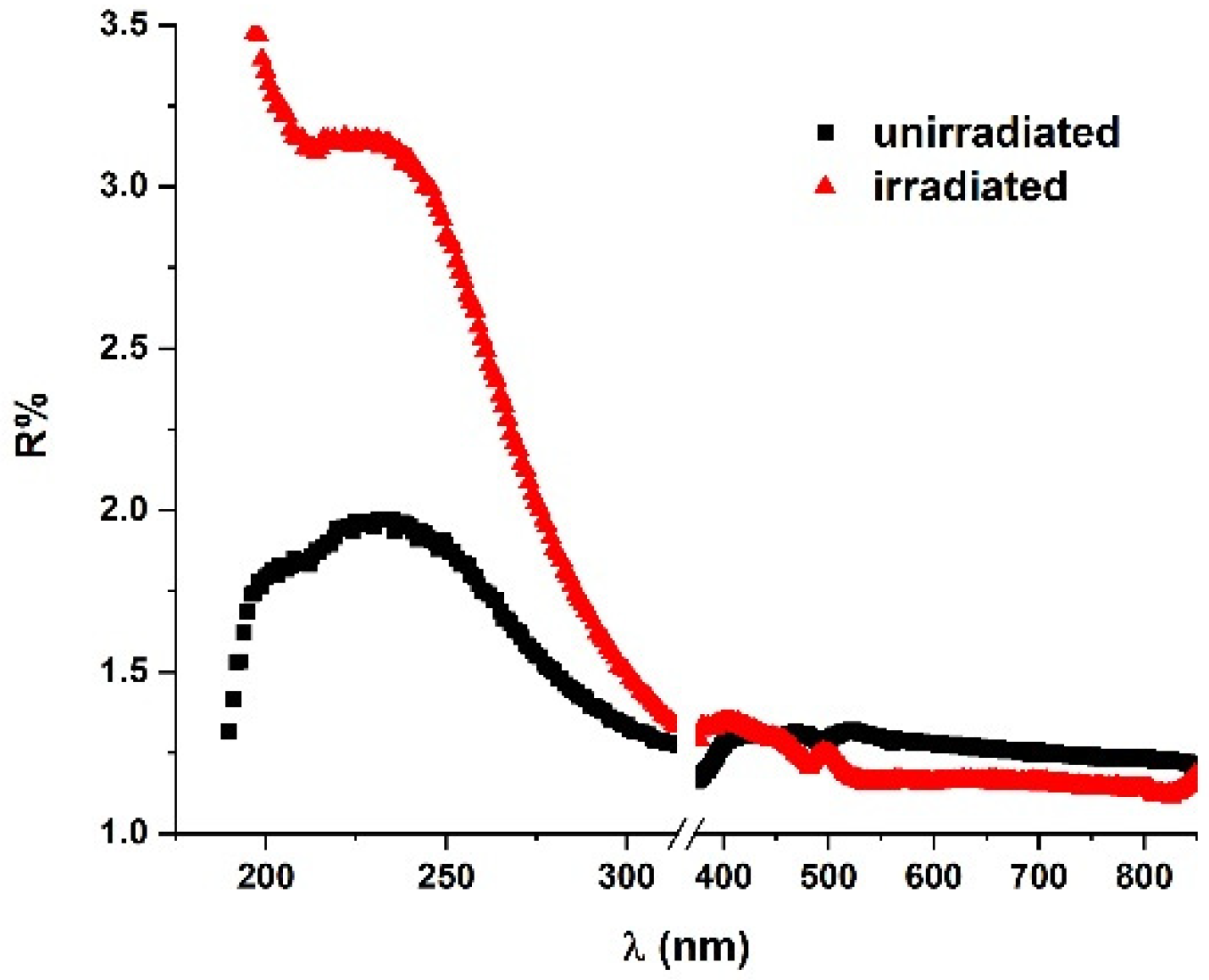
2.3. UV–Vis–NIR Reflectance Spectroscopy
3. Materials and Methods
3.1. Materials
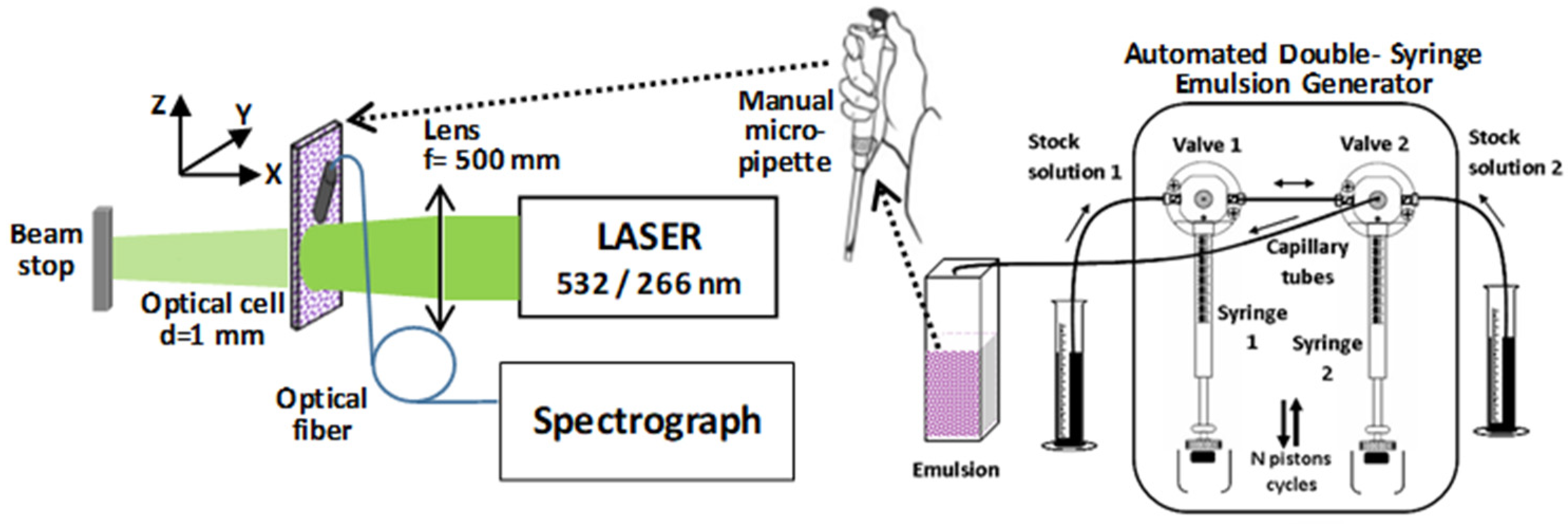
3.2. Experimental System
3.3. Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, Y.; Bolzinger, M.-A. Emulsions stabilized with solid nanoparticles: Pickering emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2013, 439, 23–34. [Google Scholar] [CrossRef]
- Dickinson, E. Food emulsions and foams: Stabilization by particles. Curr. Opin. Colloid Interface Sci. 2010, 15, 40–49. [Google Scholar] [CrossRef]
- Sun, W.; Ma, X.; Wei, X.; Xu, Y. Nano Composite Emulsion for Sustained Drug Release and Improved Bioavailability. Pharm. Res. 2014, 31, 2774–2783. [Google Scholar] [CrossRef]
- Debnath, B.K.; Saha, U.K.; Sahoo, N. A Comprehensive Review on the Application of Emulsions as an Alternative Fuel for Diesel Engines. Renew. Sustain. Energy Rev. 2014, 42, 196–211. [Google Scholar] [CrossRef]
- Kumar, N.; Mandal, A. Surfactant Stabilized Oil-in-Water Nanoemulsion: Stability, Interfacial Tension, and Rheology Study for Enhanced Oil Recovery Application. Energy Fuels 2018, 32, 6452–6466. [Google Scholar] [CrossRef]
- Kogan, A.; Garti, N. Microemulsions as transdermal drug delivery vehicles. Adv. Colloid Interface Sci. 2006, 123–126, 369–385. [Google Scholar] [CrossRef]
- Zhang, Y.; Chan, H.F.; Leong, K.W. Advanced materials and processing for drug delivery: The past and the future. Adv. Drug Deliv. Rev. 2013, 65, 104–120. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control Release 2017, 252, 28–49. [Google Scholar] [CrossRef]
- Bouchemal, K.; Briançon, S.; Perrier, E.; Fessi, H. Nano-emulsion formulation using spontaneous emulsification: Solvent, oil and surfactant optimisation. Int. J. Pharm. 2004, 280, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Dinache, A.; Smarandache, A.; Andrei, I.R.; Urzica, I.; Nichita, C.; Boni, M.; Nastasa, V.; Pascu, M.L. Laser assisted generation of micro/nanosize emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2019, 577, 265–273. [Google Scholar] [CrossRef]
- Alexandru, T.; Staicu, A.; Pascu, A.; Radu, E.; Stoicu, A.; Nastasa, V.; Dinache, A.; Boni, M.; Amaral, L.; Pascu, M.L. Characterization of mixtures of compounds produced in chlorpromazine aqueous solutions by ultraviolet laser irradiation: Their applications in antimicrobial assays. J. Biomed. Opt. 2014, 20, 051002. [Google Scholar] [CrossRef] [PubMed]
- Tozar, T.; Nastasa, V.; Stoicu, A.; Chifiriuc, M.C.; Popa, M.; Kamerzan, C.; Pascu, M.L. In vitro antimicrobial efficacy of laser exposed chlorpromazine against Gram-positive bacteria in planktonic and biofilm growth state. Microb. Pathog. 2019, 129, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Dinache, A.; Boni, M.; Alexandru, T.; Radu, E.; Stoicu, A.; Andrei, I.R.; Staicu, A.; Liggieri, L.; Nastasa, V.; Pascu, M.L.; et al. Surface properties of Vancomycin after interaction with laser beams. Colloids Surf. A Physicochem. Eng. Asp. 2015, 480, 328–335. [Google Scholar] [CrossRef]
- Smarandache, A.; Boni, M.; Andrei, I.R.; Handzlik, J.; Kiec-Kononowicz, K.; Staicu, A.; Pascu, M.-L. Spectroscopic investigations of novel pharmaceuticals: Stability and resonant interaction with laser beam. Appl. Surf. Sci. 2017, 417, 143–148. [Google Scholar] [CrossRef]
- Tozar, T.; Smarandache, A.; Pascu, M.L.; Staicu, A.; Pirvulescu, R.A. Laser Modified Phenothiazines and Hydantoins: Photo-products Characterisation and Application on Animal Eyes Pseudo-tumours. LDDD 2018, 15, 687–697. [Google Scholar] [CrossRef]
- Hunyadi, A.; Danko, B.; Boni, M.; Militaru, A.; Alexandru, T.; Nastasa, V.; Andrei, I.R.; Pascu, M.L.; Amaral, L. Rapid, laser-induced conversion of 20-hydroxyecdysone and its diacetonide–experimental set-up of a system for photochemical transformation of bioactive substances. Anticancer Res. 2012, 32, 1291–1297. [Google Scholar]
- Boni, M.; Nastasa, V.; Andrei, I.R.; Staicu, A.; Pascu, M.L. Enhanced fluorescence emitted by microdroplets containing organic dye emulsions. Biomicrofluidics 2015, 9, 014126. [Google Scholar] [CrossRef]
- Mcclements, D.J. Critical Review of Techniques and Methodologies for Characterization of Emulsion Stability. Crit. Rev. Food Sci. Nutr. 2007, 47, 611–649. [Google Scholar] [CrossRef]
- Fuhrmann, P.L.; Sala, G.; Stieger, M.; Scholten, E. Clustering of oil droplets in o/w emulsions: Controlling cluster size and interaction strength. Food Res. Int. 2019, 122, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Celis, M.; Garcia-Rubio, L.H. Characterization of Emulsions: A Systematic Spectroscopy Study. J. Dispers. Sci. Technol. 2008, 29, 20–26. [Google Scholar] [CrossRef]
- Jorgensen, L.; Weert, M.V.D.; Vermehren, C.; Bjerregaard, S.; Frokjaer, S. Probing Structural Changes of Proteins Incorporated into Water-in-Oil Emulsions. J. Pharm. Sci. 2004, 93, 1847–1859. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.-W.; Li, G.-Z.; Chen, W.-J. Fourier Transform Infrared Investigation on Water States and the Conformations of Aerosol-OT in Reverse Microemulsions. Langmuir 2002, 18, 4566–4571. [Google Scholar] [CrossRef]
- Sechler, T.D.; DelSole, E.M.; Deák, J.C. Measuring properties of interfacial and bulk water regions in a reverse micelle with IR spectroscopy: A volumetric analysis of the inhomogeneously broadened OH band. J. Colloid Interface Sci. 2010, 346, 391–397. [Google Scholar] [CrossRef]
- Nickolov, Z.S.; Paruchuri, V.; Shah, D.O.; Miller, J.D. FTIR–ATR studies of water structure in reverse micelles during the synthesis of oxalate precursor nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2004, 232, 93–99. [Google Scholar] [CrossRef]
- Kiefer, J.; Frank, K.; Schuchmann, H.P. Attenuated Total Reflection Infrared (ATR-IR) Spectroscopy of a Water-in-Oil Emulsion. Appl. Spectrosc. 2011, 65, 1024–1028. [Google Scholar] [CrossRef]
- Kiefer, J.; Frank, K.; Zehentbauer, F.; Schuchmann, H. Infrared Spectroscopy of Bilberry Extract Water-in-Oil Emulsions: Sensing the Water-Oil Interface. Biosensors 2016, 6, 13. [Google Scholar] [CrossRef]
- Ferrer, E.G.; Gómez, A.V.; Añón, M.C.; Puppo, M.C. Structural changes in gluten protein structure after addition of emulsifier. A Raman spectroscopy study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 278–281. [Google Scholar] [CrossRef]
- Elizalde, O.; Azpeitia, M.; Reis, M.M.; Asua, J.M.; Leiza, J.R. Monitoring Emulsion Polymerization Reactors: Calorimetry Versus Raman Spectroscopy. Ind. Eng. Chem. Res. 2005, 44, 7200–7207. [Google Scholar] [CrossRef]
- Skowyra, M.; Falguera, V.; Azman, N.; Segovia, F.; Almajano, M. The Effect of Perilla frutescens Extract on the Oxidative Stability of Model Food Emulsions. Antioxidants 2014, 3, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Osaki, T.; Soejima, E. Quadratic scaling functions for obtaining normal vibrational wavenumbers from the B3LYP calculation. Res. Bull. Fukuoka Inst. Technol. 2010, 42, 129–134. [Google Scholar]
- Yoshida, H.; Ehara, A.; Matsuura, H. Density functional vibrational analysis using wavenumber-linear scale factors. Chem. Phys. Lett. 2000, 325, 477–483. [Google Scholar] [CrossRef]
- Dinache, A.; Smarandache, A.; Simon, A.; Nastasa, V.; Tozar, T.; Pascu, A.; Enescu, M.; Khatyr, A.; Sima, F.; Pascu, M.-L.; et al. Photosensitized cleavage of some olefins as potential linkers to be used in drug delivery. Appl. Surf. Sci. 2017, 417, 136–142. [Google Scholar] [CrossRef]
- Delmas, T.; Piraux, H.; Couffin, A.-C.; Texier, I.; Vinet, F.; Poulin, P.; Cates, M.E.; Bibette, J. How To Prepare and Stabilize Very Small Nanoemulsions. Langmuir 2011, 27, 1683–1692. [Google Scholar] [CrossRef]
- Zhang, J.; Severtson, S.J.; Houtman, C.J. Characterizing the Distribution of Sodium Alkyl Sulfate Surfactant Homologues in Water-Based, Acrylic Pressure-Sensitive Adhesive Films. J. Phys. Chem. B 2011, 115, 8138–8144. [Google Scholar] [CrossRef]
- Ishii, F.; Nii, T. Lipid emulsions and lipid vesicles prepared from various phospholipids as drug carriers. In Colloid and Interface Science in Pharmaceutical Research and Development; Elsevier: Amsterdam, The Netherlands, 2014; pp. 469–501. ISBN 978-0-444-62614-1. [Google Scholar]
- Jenkinson, H.A.; Wilmas, K.M.; Silapunt, S. Sodium Tetradecyl Sulfate: A Review of Clinical Uses. Dermatol. Surg. 2017, 43, 1313–1320. [Google Scholar] [CrossRef]
- Alakailly, X.; Kummoona, R.; Quereshy, F.A.; Baur, D.A.; González, A.E. The Use of Sodium Tetradecyl Sulphate for the Treatment of Venous Malformations of the Head and Neck. J. Maxillofac. Oral Surg. 2015, 14, 332–338. [Google Scholar] [CrossRef]
- Bai, G.; Nichifor, M.; Lopes, A.; Bastos, M. Thermodynamic Characterization of the Interaction Behavior of a Hydrophobically Modified Polyelectrolyte and Oppositely Charged Surfactants in Aqueous Solution: Effect of Surfactant Alkyl Chain Length. J. Phys. Chem. B 2005, 109, 518–525. [Google Scholar] [CrossRef]
- Tanumihardjo, S.A.; Russell, R.M.; Stephensen, C.B.; Gannon, B.M.; Craft, N.E.; Haskell, M.J.; Lietz, G.; Schulze, K.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND)—Vitamin A Review. J. Nutr. 2016, 146, 1816S–1848S. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.E.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Leimkuhler, B. New Algorithms for Macromolecular Simulation; Springer: Berlin, Germany; New York, NY, USA, 2006; ISBN 978-3-540-31618-3. [Google Scholar]
- Parr, R.G. Density Functional Theory of Atoms and Molecules. In Horizons of Quantum Chemistry; Fukui, K., Pullman, B., Eds.; Springer: Dordrecht, The Netherlands, 1980. [Google Scholar]
- Boni, M.; Nastasa, V.; Militaru, A.; Smarandache, A.; Andrei, I.R.; Staicu, A.; Pascu, M.L. Laser beams interaction with liquids in optofluidic experiments. Rom. Rep. Phys. 2012, 64, 1179–1194. [Google Scholar]
- Smarandache, A.; Staicu, A.; Moraga, J.M.; Trelles, M.; Pascu, M.-L. Applications of Polidocanol in Varicose vein Treatment Assisted by Exposure to Nd: YAG Laser Radiation; INTECH Open Access Publisher: London, UK, 2012. [Google Scholar]
- Smarandache, A. Laser Beams Interaction with Polidocanol Foam: Molecular Background. Photomed. Laser Surg. 2012, 30, 262–267. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds Sodium tetradecyl sulfate, vitamin A and generated emulsions are available from the authors. |
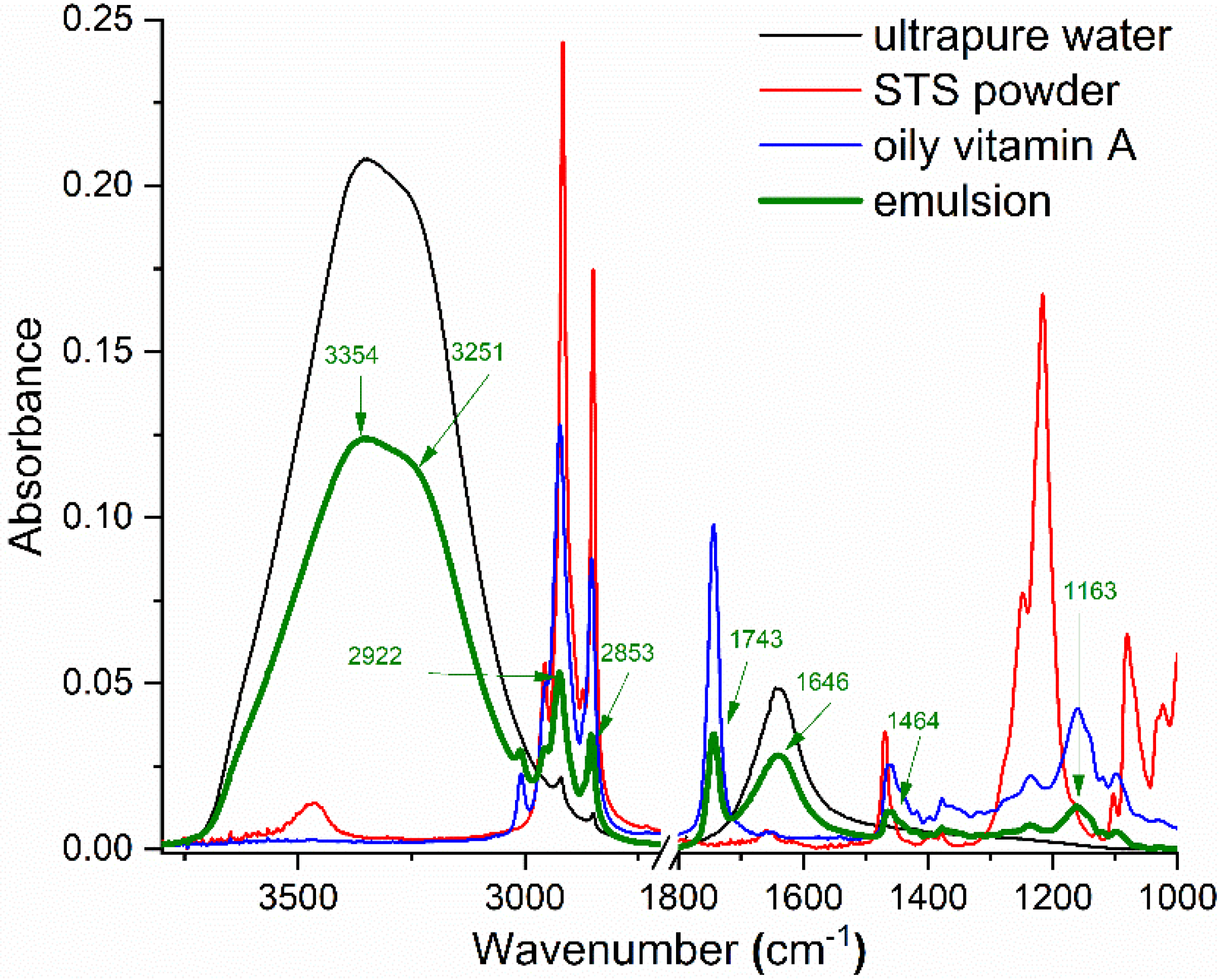

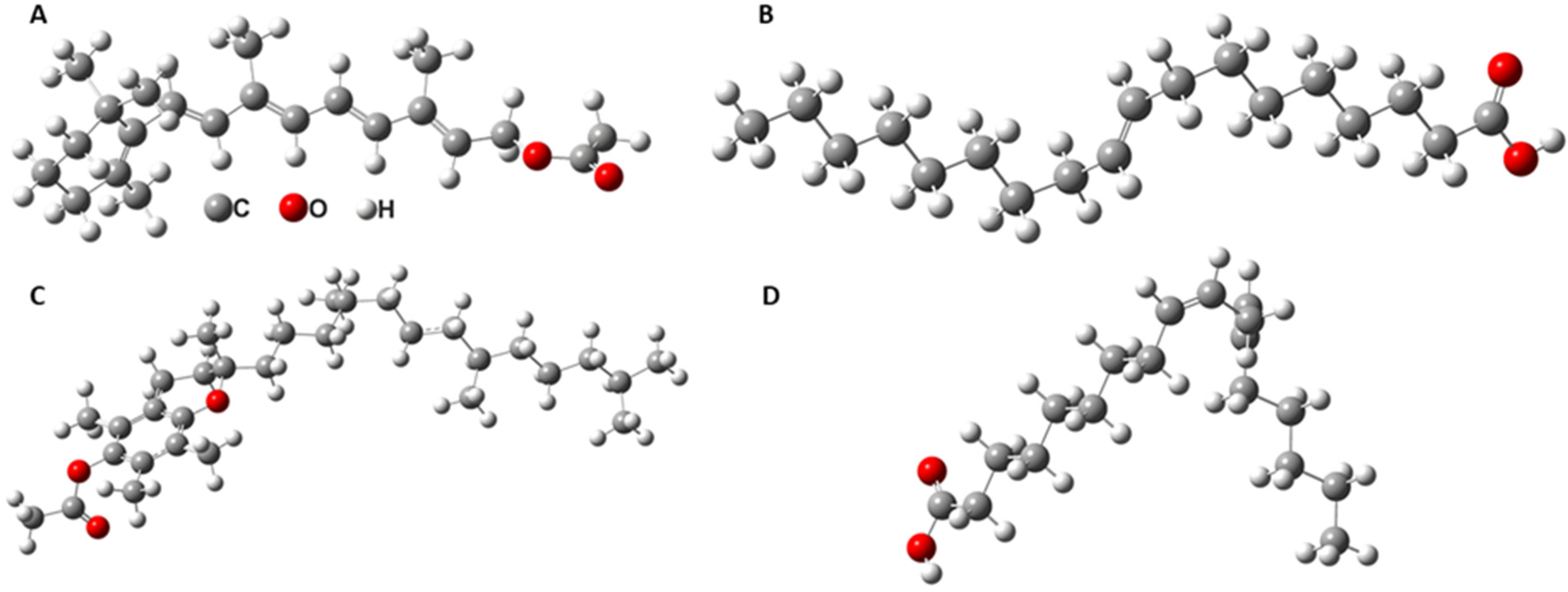


| Calculated ν (cm−1) | Experimental ν (cm−1) | Assigned Vibrations |
|---|---|---|
| 2960 | 2955 | C–H asymmetrical stretching from CH3 from aliphatic chain |
| 2939 | 2916 | C–H asymmetrical stretching from CH2 from aliphatic chain |
| 2897 | 2849 | C–H symmetrical stretching from CH2 from aliphatic chain |
| 1393 | 1247 | CH2 out of plane bending from aliphatic chain |
| 1373 | 1215 | CH2 out of plane bending from aliphatic chain |
| Calculated ν (cm−1) | Experimental ν (cm−1) | Assigned Vibrations |
|---|---|---|
| 3008 | 3009 | C–H asymmetrical stretching from CH3 groups |
| 2932 | 2953 | C–H asymmetrical stretching from CH3 groups |
| 2910 | 2922 | C–H symmetrical stretching from CH3 groups |
| 2856 | 2853 | C–H symmetrical stretching from CH3 groups |
| 1747 | 1743 | C=O stretching |
| 1466 | 1464 | C–H out of plane bending (wagging) from CH3 groups |
| 1377 | 1377 | C–H out of plane bending (wagging) from CH3 groups |
| 1163 | 1163 | C–H out of plane bending (twisting) from CH3 groups |
| 1025 | 1097 | C–O stretching |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinache, A.; Tozar, T.; Smarandache, A.; Andrei, I.R.; Nistorescu, S.; Nastasa, V.; Staicu, A.; Pascu, M.-L.; Romanitan, M.O. Spectroscopic Characterization of Emulsions Generated with a New Laser-Assisted Device. Molecules 2020, 25, 1729. https://doi.org/10.3390/molecules25071729
Dinache A, Tozar T, Smarandache A, Andrei IR, Nistorescu S, Nastasa V, Staicu A, Pascu M-L, Romanitan MO. Spectroscopic Characterization of Emulsions Generated with a New Laser-Assisted Device. Molecules. 2020; 25(7):1729. https://doi.org/10.3390/molecules25071729
Chicago/Turabian StyleDinache, Andra, Tatiana Tozar, Adriana Smarandache, Ionut Relu Andrei, Simona Nistorescu, Viorel Nastasa, Angela Staicu, Mihail-Lucian Pascu, and Mihaela Oana Romanitan. 2020. "Spectroscopic Characterization of Emulsions Generated with a New Laser-Assisted Device" Molecules 25, no. 7: 1729. https://doi.org/10.3390/molecules25071729
APA StyleDinache, A., Tozar, T., Smarandache, A., Andrei, I. R., Nistorescu, S., Nastasa, V., Staicu, A., Pascu, M.-L., & Romanitan, M. O. (2020). Spectroscopic Characterization of Emulsions Generated with a New Laser-Assisted Device. Molecules, 25(7), 1729. https://doi.org/10.3390/molecules25071729