Scrophularia Tenuipes Coss and Durieu: Phytochemical Composition and Biological Activities
Abstract
1. Introduction
2. Results and Discussion
2.1. Phenolic Compounds of Ethyl Acetate (EA) and n-Butanol (Bu) Fractions
2.2. Anti-Inflammatory Activity
2.3. Antioxidant Activity
2.4. α-Glucosidase and α-Amylase Assay
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material
3.3. Extraction
3.4. Characterization of Phenolic Compounds
3.4.1. Total Phenolic Content
3.4.2. Total Flavonoid Content
3.4.3. UHPLC-ESI-DAD-MSn Analysis
3.5. Assessment of Anti-Inflammatory Activity
3.5.1. Carrageenan-Induced Rat Paw Edema
3.5.2. Xylene-Induced Ear Edema
3.5.3. Albumin Denaturation
3.6. Determination of Antioxidant Activity
3.6.1. 2,2 diphenyl-1-picryhydrazyl Free Radical Scavenging Assay
3.6.2. 2,2-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) Cation Radical Decolorization Assay
3.6.3. Cupric -Reducing Antioxidant Capacity (CUPRAC)
3.6.4. Superoxide Radical (O2•−) Scavenging Activity
3.7. Enzyme Inhibitory Activity
3.7.1. α-Amylase
3.7.2. α-Glucosidase
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pasdaran, A.; Hamedi, A. The genus Scrophularia: A Source of Iridoids and Terpenoids with a Diverse Biological Activity. Pharm. Biol. 2017, 55, 2211–2233. [Google Scholar] [CrossRef] [PubMed]
- Dobignard, A.; Chatelain, C. Index Synonymique, Afrique du Nord; Edition des Conservatoires et Jardins Botaniques: Genève, Switzerland, 2013; Volume 5, p. 301. [Google Scholar]
- Valiyari, S.; Baradaran, B.; Delazar, A.; Pasdaran, A.; Zare, F. Dichloromethane and Methanol Extracts of Scrophularia Oxysepala Induces Apoptosis in MCF-7 Human Breast Cancer Cells. Adv. Pharm. Bull. 2012, 2, 223–231. [Google Scholar] [CrossRef] [PubMed]
- De Santos Galíndez, J.; Díaz Lanza, A.-M.; Fernández Matellano, L. Biologically Active Substances from the Genus Scrophularia. Pharm. Biol. 2002, 40, 45–59. [Google Scholar] [CrossRef]
- Crisan, G.; Kiss, B.; Vlase, L.; Balica, G.; Tamas, M. HPLC Determination of Some Phenolic Compounds of Scrophularia Nodosa and S. Scopolii. Chem. Nat. Compd. 2009, 45, 885–888. [Google Scholar] [CrossRef]
- Huang, T.; Chen, N.; Lai, Y. Rapid Determination of Cinnamic Acid and Harpagoside in a Traditional Chinese Medicine of Scrophularia Ningpoensis by Microwave-Assisted Extraction Followed by High Performance Liquid Chromatography (HPLC). J. Med. Plant Res. 2011, 5, 1313–1320. [Google Scholar]
- Diaz, A.; Fernández, L.; Ollivier, E. Reverse-Phase High Pressure Liquid Chromatographic Analysis of Harpagoside, Scorodioside and Verbascoside from Scrophularia Scorodonia: Quantitative Determination of Harpagoside. Planta Med. 1998, 64, 94–95. [Google Scholar] [CrossRef]
- Lee, M.-K.; Ok-Gyung, C.H.; Jin-Ho, P. Simultaneous Determination of Four Active Constituents in the Roots of Scrophularia Buergeriana by HPLC-DAD and LC-ESI-MS. J. Sep. Sci. 2007, 30, 2345–2350. [Google Scholar] [CrossRef]
- Nikkhah, E.; Afshar, F.; Babaei, H.; Asgharian, P. Phytochemical Analysis and In-vitro Bioactivity of Scrophularia Umbrosa Rhizome (Scrophulariaceae). Iran. J. Pharm. Res. 2018, 17, 685–694. [Google Scholar]
- Gülin, R.; Büşra, K.; Sercan, Y.; Ahu, R.; Nurdan, Y. Antimicrobial Activity and The Phenolic Profile of Five Scrophularia L. Species Beş Scrophularia L. J. Health Sci. 2018, 27, 10–15. [Google Scholar]
- Guisalberti, E.L. Biological and Pharmacological Activity of Naturally Occurring Iridoids and Secoiridoids. Phytomedicine 1998, 5, 147–163. [Google Scholar] [CrossRef]
- Ahmed, B.; Al-Rehaily, A.-J.; Al-Howiriny, T.-A.; El-Sayed, K.-A.; Ahmad, M.-S. Scropolioside-D2 and Harpagoside-B: Two New Iridoid Glycosides from Scrophularia Deserti and Their Antidiabetic and Anti-inflammatory activity. Biol. Pharm. Bull. 2003, 26, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Garg, H.; Bhandari, S.; Tripathi, S.; Patnaik, G.; Puri, A.; Saxena, R. Antihepatotoxic and Immunostimulant Properties of Iridoid Glycosides of Scrophularia Koelzii. Phytother. Res. 1994, 8, 224–228. [Google Scholar] [CrossRef]
- Kim, H.; Ahn, M.; Lee, S. Isolation and Identification of Phytochemical Constituents from Scrophularia Takesimensis. J. Med. Plant Res. 2012, 6, 3923–3930. [Google Scholar] [CrossRef]
- Lee, E.; Kim, S.; Kim, J.; Kim, Y. Hepatoprotective Phenylpropanoids from Scrophularia Buergeriana Roots against CCl4-Induced Toxicity: Action Mechanism and Structure–Activity Relationship. Planta Med. 2002, 68, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-Y.; Park, J.; Lee, K.-H.; Lee, D.-U.; Kwak, J.-H.; Kim, Y.-S. Ferulic Acid Protects against Carbon Tetrachloride-Induced Liver Injury in Mice. Toxicology 2011, 282, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Quezel, P.; Santa, S. Nouvelle Flore de L’algerie et des Régions Désertiques Méridionales; Editions du Centre National de la Recherche Scientifique: Paris, France, 1963; p. 849. [Google Scholar]
- Battandier, J.-A.; Trabut, L. Flore de l’Algérie; Jourdan, A., Ed.; Biodiversity Heritage Library: Paris, France, 1888; p. 631. [Google Scholar]
- Hamel, T.; Zaafour, M.; Boumendjel, M. Ethnomedical Knowledge and Traditional Uses of Aromatic and Medicinal Plants of the Wetlands Complex of the Guerbès-Sanhadja Plain (Wilaya of Skikda in Northeastern Algeria). Herb. Med. 2018, 4, 1–3. [Google Scholar] [CrossRef]
- Sha, W.-J.; Lu, H. The rResearch Progress of Astragalus in Regulating Blood Glucose Steady State of Diabetes Patients. Int. J. Trad. Chin. Med. 2015, 37, 87–89. [Google Scholar]
- Gao, Y.; Guo, J.; Ren, S.-P. Effect of Radix Astragali on Blood Glucose, TG, HDL-C, LDL-C and Insulin Levels in Diabetic Rats. Chin. J. Ger. 2008, 28, 1676–1677. [Google Scholar]
- Grzegorczyk-Karolak, I.; Kiss, A. Determination of the Phenolic Profile and Antioxidant Properties of Salvia viridis L. Shoots: A Comparison of Aqueous and Hydroethanolic Extracts. Molecules 2018, 23, 1468. [Google Scholar] [CrossRef]
- Li, C.; Liu, Y.; Abdulla, R.; Aisa, H.-A.; Suo, Y. Characterization and Identification of Chemical Components in Neopicrorhiza Scrphulariiflora Roots by Liquid Chromatography-Electrospray Ionization Quadrupole Time-of-Tlight Tandem Mass Spectrometry. Anal. Methods 2014, 6, 3634–3643. [Google Scholar] [CrossRef]
- Abbas, F.-A.-A. Phenylpropanoid and Phenylethanoid Glycosides from Scrophularia Xanthoglossa and Their Anti-Oxidative and Anti-inflammatory Activities. Biosci. Biotechnol. Res. Asia 2010, 7, 57–64. [Google Scholar]
- Pachaly, P.; Barion, J.; Sin, K.-S. Isolation and Structure Elucidation of New Iridoid Glycosides from Scrophularia Koraiensis. Pharmazie 1994, 49, 150–155. [Google Scholar]
- Han, M.-F.; Zhang, X.; Zhang, L.-Q.; Li, Y.-M. Iridoid and Phenylethanol Glycosides from Scrophularia Umbrosa with Inhibitory Activity on Nitric Oxide Production. Phytochem. Lett. 2018, 28, 37–41. [Google Scholar] [CrossRef]
- Fernández, L.; de Santos, J.; Díaz-Lanza, A.M. Quantitative Determination of Verbascoside in ScrophulariAscorodonia.by High-Performance Liquid Chromatography. Pharm. Biol. 2005, 43, 226–229. [Google Scholar] [CrossRef]
- Fernández, M.A.; García, M.D.; Saenz, M.T. Antibacterial Activity of the Phenolic Acids Fractions of Scrophularia Frutescens and Scrophularia Sambucifolia. J. Ethnopharmacol. 1996, 53, 11–14. [Google Scholar] [CrossRef]
- Fernandez, M.; Saenz, M.; Garcia, M. Natural products: Anti-Inflammatory Activity in Rats and Mice of Phenolic Acids Isolated from Scrophularia Frutescens. J. Pharm. Pharmacol. 1998, 50, 1183–1186. [Google Scholar] [CrossRef]
- Calis, I.; Zor, M.; Basaran, A.A.; Wright, A.D.; Sticher, O. Karsoside and Scropolioside D, Two New Iridoid Glycosides from Scrophularia Ilwensis. J. Nat. Prod. 1993, 56, 606–609. [Google Scholar] [CrossRef]
- Lewenhofer, V.; Schweighofer, L.; Ledermüller, T.; Eichsteininger, J.; Kählig, H.; Zehl, M.; Nguyen, C.H.; Krupitza, G.; Özmen, A.; Krenn, L. Chemical Composition of Scrophularia Lucida and the Effects on Tumor Invasiveness in Vitro. Front Pharmacol. 2018, 9, 304. [Google Scholar] [CrossRef]
- Sowemimoa, A.; Onakoyaa, M.; Fageyinbob, M.; Fadojua, T. Studies on the Anti-inflammatory and Anti-Nociceptive Properties of Blepharis Maderaspatensis Leaves. Rev. Bras. Farmacogn. 2013, 23, 830–835. [Google Scholar] [CrossRef]
- Mizushima, Y.; Kobayashi, M. Interaction of Anti-Inflammatory Drugs with Serum Preoteins, Especially with some Biologically Active Proteins. J. Pharm. Pharmacol. 1968, 20, 169–173. [Google Scholar] [CrossRef]
- Hafeez, A.; Upendra, J.; Pinky, S.; Sirish, S. Evaluation of Carrageenan Induced Anti-Iinflammatory Activity of Ethanolic Extract of Bark of Ficus Virens Linn. in Swiss Albino Mice. Phytopharmacol. J. 2013, 2, 39–43. [Google Scholar]
- Zhao, J.; Maitituersun, A.; Li, C.; Li, Q.; Xu, F.; Liu, T. Evaluation on Analgesic and Anti-Inflammatory Activities of Total Flavonoids from Juniperus Sabina. Evid-Based Complement. Altern. Med. 2018, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tatli, I.-I.; Akdemir, Z.-S.; Yesilada, E.; Küpeli, E. Anti-Inflammatory and Antinociceptive Potential of Major Phenolics from Verbascum Salviifolium Boiss. Z Naturforsch C J Biosci. 2008, 63, 196–202. [Google Scholar] [CrossRef]
- Küpeli, E.; Harput, U.-S.; Varel, M.; Yesilada, E.; Saracoglu, I. Bioassay-Guided Isolation of Iridoid Glucosides with Antinociceptive and Anti-inflammatory Activities from Veronica Anagallis-Aquatica L. J. Ethnopharmacol. 2005, 102, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Kondamudi, N.; Turner, M.-W.; McDougal, O.M. Harpagoside Content in Devil’s Claw Extracts. Nat. Prod. Commun. 2016, 11, 1215–1216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, T.; Qian, F.; Xu, J.; Dorje, G. Iridoid Glycosides Isolated from Scrophularia Dentata Royle Ex Benth. and Their Anti-Inflammatory Activity. Fitoterapia 2014, 98, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Giner, R.; Villalba, M.-L. Anti-Inflammatory Glycoterpenoids from Scrophularia Auriculata. Eur. J. Pharmacol. 2000, 389, 243–252. [Google Scholar] [CrossRef]
- Azadmehr, A.; Maliji, G.; Hajiaghaee, R.; Shahnazi, M.; Afaghi, A. Inhibition of Pro-inflammatory Cytokines by Ethyl Acetate Extract of Scrophularia Striata. Trop. J. Pharm. Res. 2013, 11, 893–897. [Google Scholar] [CrossRef]
- Rana, M.-G.; Katbamna, R.-V.; Padhya, A.-A. In Vitro Antioxidant and Free Radical Scavenging Studies of Alcoholic Extract of Medicago Sativa L. Rom. J. Biol. 2010, 55, 15–22. [Google Scholar]
- Jeong, E.-J.; Lee, K.-Y.; Kim, K.-S. Cognitive-Enhancing and Antioxidant Activities of Iridoid Glycosides from Scrophularia Buergeriana in Scopolamine-Treated Mice. Eur. J. Pharmacol. 2008, 588, 78–84. [Google Scholar] [CrossRef]
- Gray, D.-M. Carbohydrate Digestion and Absorption—Role of Small Intestine. N. Engl. J. Med. 1995, 29, 1225–1230. [Google Scholar] [CrossRef]
- Krentz, A.-J.; Bailey, C.-J. Oral Antidiabetic Agents: Current Role in Type 2 Diabetes Mellitus. Drugs 2005, 65, 385–411. [Google Scholar] [CrossRef] [PubMed]
- McCue, P.-P.; Shetty, K. Inhibitory Effects of Rosmarinic Acid Extracts on Porcine Pancreatic Amylase in vitro. Asia Pac. J. Clin. Nutr. 2004, 13, 1. [Google Scholar]
- Gholamhoseinian, A.; Fallah, H.; Sharifi-far, F.; Mirtajaddini, M. The Inhibitory Effect of Some Iranian Plants Extracts on the Alpha Glucosidase. Iran. J. Basic Med. Sci. 2008, 11, 1–9. [Google Scholar]
- Müller, L.; Gnoyke, S.; Popken, A.-M.; Böhm, V. Antioxidant Capacity and Related Parameters of Different Fruit Formulations. LWT Food Sci. Technol. 2010, 43, 992–999. [Google Scholar] [CrossRef]
- Park, Y.-K.; Koo, M.-H.; Ikegaki, M.; Contado, J.-L. Comparison of the Flavonoid aglycone contents of Apis Mellifera ropolis from Various Regions of Brazil. Arq. Biol. Technol. 1997, 40, 97–106. [Google Scholar]
- Bouaoudia-Madi, N.; Boulekbache-Makhlouf, L.; Madani, K.; Silva, A.-M.-S.; Dairi, S.; Oukhmanou–Bensidhoum, S.; Cardoso, S.-M. Optimization of Ultrasound-Assisted Extraction of Polyphenols from Myrtus communis L. Pericarp. Antioxidants 2019, 8, 205. [Google Scholar] [CrossRef]
- Azadmehr, A.; Hajiaghaee, R.; Zohal, M.-A.; Maliji, G. Protective effects of Scrophularia striata in Ovalbumin-induced mice asthma model. Daru 2013, 21, 56. [Google Scholar] [CrossRef]
- Winter, C.-A.; Risley, E.-A.; Nuss, G.-W. Carregeenin-Induced Edema in Hind Paw of the Rat as Assay for Anti-Inflammatory Drugs. Proc. Soc. Exp. Biol. Med. 1962, 11, 544–547. [Google Scholar] [CrossRef]
- Tang, X.; Lin, Z.; Cai, W.; Chen, N.; Shen, L. Anti-Inflammatory Effect of 3-Acethylaconitine. Acta. Pharmacol. Sin. 1984, 5, 85–89. [Google Scholar]
- Sakat, S.; Juvekar, A.-R.; Gambhire, M.-N. In Vitro Antioxidant and Anti-Inflammatory Activity of Methanol Extract of Oxalis corniculata Linn. Int. J. Pharm. Pharm. Sci. 2010, 2, 146–155. [Google Scholar] [CrossRef]
- Blois, M.-S. Antioxidant Determinations by the Use of a Stable Free Radic. Nature 1958, 4617, 1119–1200. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Apak, R.; Guclü, K.; Ozyurek, M.; Karademir, S.-E. Novel Total Antioxidant Capacity Index for Dietary Polyphenols and Vitamins C and E, Using their Cupric Ion Reducing Capability in the Presence of Neocuproine: CUPRAC Method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- Elizabeth, K.; Rao, M.-N.-A. Oxygen Radical Scavenging Activity of Curcumin. Int. J. Pharm. 1990, 58, 237–240. [Google Scholar] [CrossRef]
- Zengin, G.; Sarikurkcu, C.; Aktumsek, A.; Ceylan, O. A Comprehensive Study on Phytochemical Characterization of Haplophyllum myrtifolium Boiss. Endemic to Turkey and its Inhibitory Potential against Key Enzymes Involved in Alzheimer, Skin Diseases and Type II Tiabetes. Ind. Crop. Prod. 2014, 53, 244–251. [Google Scholar] [CrossRef]
- Sinéad Lordan, A.; Thomas, J.; Smyth, B. The a-Amylase and a-Glucosidase Inhibitory Effects of Irish Seaweed Extracts. Food Chem. 2013, 141, 2170–2176. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
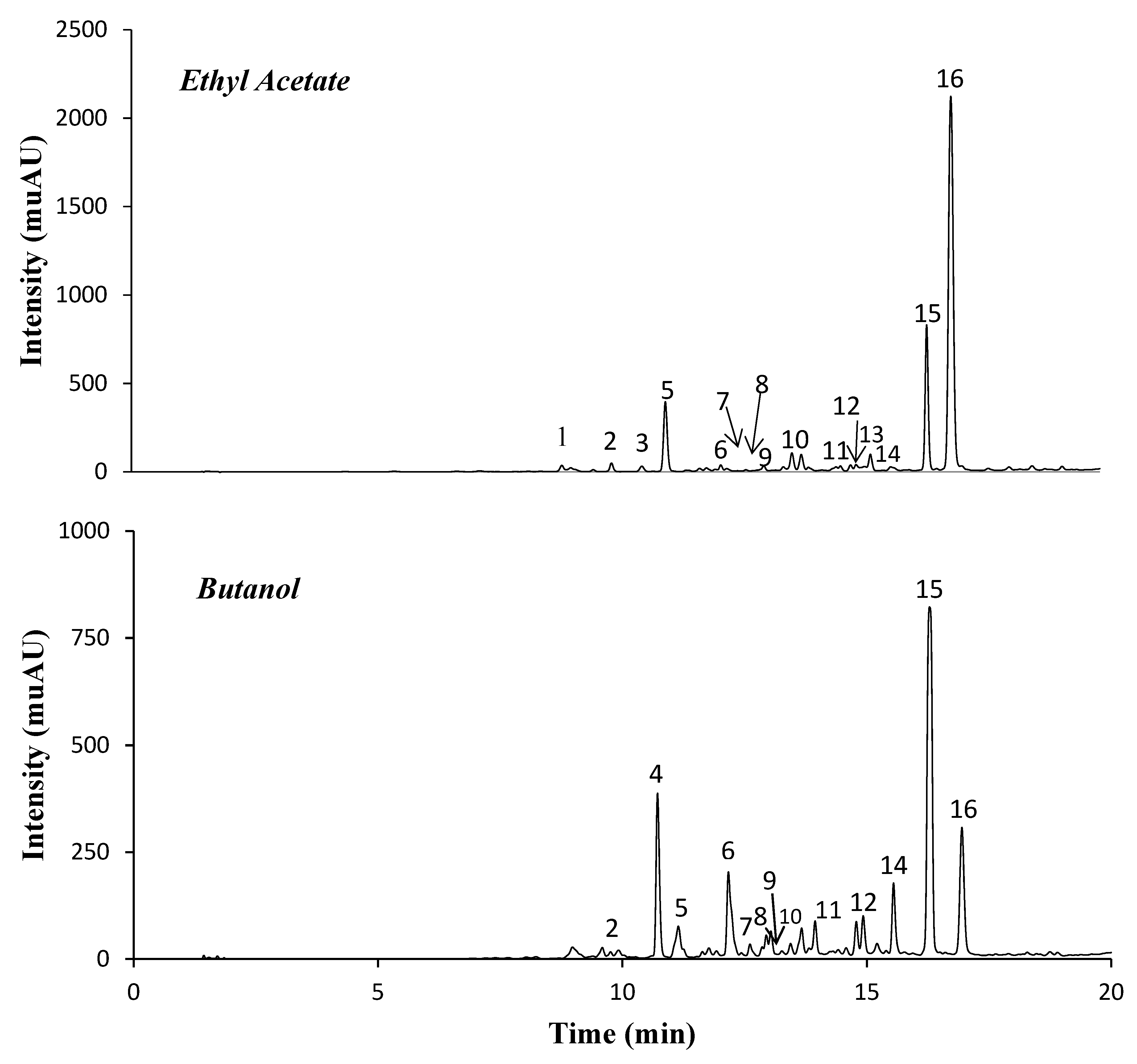
| Peak | Rt (min) | λmax | [M − H]− | ESI MS/MS Product Ions | Probable Compound | EA (mg/g) | Bu (mg/g) |
|---|---|---|---|---|---|---|---|
| 1 | 8.9 | 294sh, 320 | 179 | 135 | Caffeic acid | d | - |
| 2 | 9.9 | 312 | 309 | 187,118,163 | Coumaroyl-O-rhamnoside | d | d |
| 3 | 10.5 | 277, 320 | 367 | 349,307,203,161,289,245,191,173 | Feruloylquinic acid | d | - |
| 4 | 10.7 | 272, 334 | 609 | 447, 285,489,429,255 | Kaempferol-O-dihexoside | - | 68.0 ± 4.0 |
| 5 | 11.0 | 309 | 163 | 119 | Coumaric acid | 21.4 ± 2.5 | 7.5 ± 0.9 |
| 6 | 12.2 | 282, 334 | 447 | 285,284,327,255; MS3 [285]: 267, 241, 185 | Kampferol-O-hexoside | 2.2 ± 0.3 | 39.3 ± 3.0 |
| 7 | 12.3 | 313 | 509 | 307, 265, 163, 235 | Coumaric ac derivative | d | d |
| 8 | 13.0 | 272, 331 | 447 | 285, 327,255; MS3[285]: 267, 241/239 | Kaempferol-O-hexoside | d | d |
| 9 | 13.6 | 313 | 351 | 333,229,187,273,163,119 | Coumaroyl-O-acetyl-rhamnoside (isom 1) | d | d |
| 10 | 13.8 | 313 | 351 | 187, 229, 333, 163, 119 | Coumaroyl-O-acetyl-rhamnoside (isom 2) | d | d |
| 11 | 14.9 | 314 | 679 | 637, 499, 351,619, 229, 333, 273 | Coumaroyl-O-acetyl-rhamnoside derivative (isom 1) | 1.5 ± 0.1 | 6.8 ± 0.7 |
| 12 | 15.0 | 314 | 679 | 499,351,637,619,333,229,273,517 | Coumaroyl-O-acetyl-rhamnoside derivative (isom 2) | 1.5 ±0.2 | 7.7 ± 0.9 |
| 13 | 15.3 | 313 | 351 | 163, 187, 333, 119 | Coumaroyl-O-acetyl-rhamnoside (isom 3) | 3.7 ± 0.4 | - |
| 14 | 15.7 | 281 | 493 | 345, 179, 181 | Harpagoside (isom 1) | 7.2 ± 0.6 | 40.4 ± 4.0 |
| 15 | 16.4 | 280 | 493 | 345, 179, 201,147 | Harpagoside (isom 2) | 142.0 ± 15.1 | 316.0 ± 8.0 |
| 16 | 16.9 | 277 | 693 | 651, 633, 505, 517, 475, 457 | Acetyl martynoside | 416.5 ± 17.7 | 90.7 ± 10 |
| TPC (mg GAE/g) | 225.5 ± 0.9 | 181.4 ± 0.5 | |||||
| TF (mg QE/g) | 21.7 ± 3.2 | 64.6 ± 4.8 |
| Treatment | Paw-Edema Volume (mL) | Ear-Edema Weight (mg) | BSA Denaturation (% Inhibition) | ||
|---|---|---|---|---|---|
| 1 h | 2 h | 3 h | |||
| Control | 2.40 ± 0.01a | 1.68 ± 0.02a | 1.40 ± 0.02a | 4.00 ± 0.03a | - |
| EA | 1.19 ± 0.32b | 0.91 ± 0.09b | 0.52 ± 0.14b | 1.90 ± 0.05b | 80.72 ± 0.75a |
| Bu | 1.96 ± 0.21c | 1.33 ± 0.49c | 1.08 ± 0.54c | 3.00 ± 0.07c | NA |
| Diclofenac | 0.54 ± 0.05d | 0.3 ± 0.01d | 0.25 ± 0.05d | 1.00 ± 0.05d | 94.04 ± 0.49b |
| ABTS•+ (IC50, µg/mL) | DPPH• (IC50, µg/mL) | O2•– (IC50, µg/mL) | Cupric Reduction (A0.50, µg/mL) | |
|---|---|---|---|---|
| EA | 20.3 ± 0.3a | 111.2 ± 1.4a | 18.9 ± 1.2a | 53.4 ± 0.6a |
| Bu | 18.7 ± 0.5b | 69.0 ± 0.2b | 18.5 ± 2.8a | 43.3 ± 1.3b |
| Diclofenac | - | - | - | - |
| BHA | 1.8 ± 0.1c | 5.7 ± 0.4c | - | 3.6 ± 0.2c |
| BHT | 1.3 ± 0.3d | 22.3 ± 1.2d | - | 9.6 ± 0.9d |
| α-tocopherol | - | - | ˂3.1b | - |
| Tanic acid | - | - | ˂3.1b | - |
| α-Amylase (IC50, µg/mL) | α-Glucosidase (IC50, µg/mL) | |
|---|---|---|
| EA | 8.3 ± 0.2a | ≥1000a |
| Bu | 11.0 ± 0.5b | ≥1000a |
| Acarbose | 45.2 ± 1.3c | 275.4 ± 1.6b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaibeddra, Z.; Akkal, S.; Ouled-Haddar, H.; Silva, A.M.S.; Zellagui, A.; Sebti, M.; Cardoso, S.M. Scrophularia Tenuipes Coss and Durieu: Phytochemical Composition and Biological Activities. Molecules 2020, 25, 1647. https://doi.org/10.3390/molecules25071647
Chaibeddra Z, Akkal S, Ouled-Haddar H, Silva AMS, Zellagui A, Sebti M, Cardoso SM. Scrophularia Tenuipes Coss and Durieu: Phytochemical Composition and Biological Activities. Molecules. 2020; 25(7):1647. https://doi.org/10.3390/molecules25071647
Chicago/Turabian StyleChaibeddra, Zeyneb, Salah Akkal, Houria Ouled-Haddar, Artur M. S. Silva, Ammar Zellagui, Mohamed Sebti, and Susana M. Cardoso. 2020. "Scrophularia Tenuipes Coss and Durieu: Phytochemical Composition and Biological Activities" Molecules 25, no. 7: 1647. https://doi.org/10.3390/molecules25071647
APA StyleChaibeddra, Z., Akkal, S., Ouled-Haddar, H., Silva, A. M. S., Zellagui, A., Sebti, M., & Cardoso, S. M. (2020). Scrophularia Tenuipes Coss and Durieu: Phytochemical Composition and Biological Activities. Molecules, 25(7), 1647. https://doi.org/10.3390/molecules25071647








