3.1. General Information
Reagents and solvents were obtained from commercial suppliers and used as received.
1H-NMR spectra were obtained on a 400 MHz Mercury NMR spectrometer (Varian, San Diego, CA, USA). Electrospray ionization (ESI) mass spectra and high-resolution mass spectroscopy (HRMS) were performed with a liquid chromatograph/mass selective detector time-of-flight mass spectrometer (LC/MSD TOF, Agilent Technologies, Santa Clara, CA, USA). Silica gel column chromatography was performed with silica gel 60G (Qingdao Haiyang Chemical, Qingdao, China). Purity was determined using HPLC, LC/MS and NMR spectroscopy (
Supplementary Materials). All of the synthesized compounds have purities over 95%.
3.1.1. Preparation of 4-Fluoro-3-nitro-benzaldehyde (a)
To a solution of sulfuric acid (24 mL) and nitric acid (3 mL) was slowly added 4-fluoro-benzaldehyde (6 g, 48.3 mmol) at −5 °C. The reaction mixture was stirred at room temperature for 2 h, poured onto ice and extracted with ethyl acetate (200 mL). The organic extract was washed with brine (60 mL × 2), dried over anhydrous Na2SO4 and concentrated. The residue was purified by column chromatography (silica gel, PE/EA = 30:1, v/v) to afford compound a as a light yellow solid (7.8 g, 95.4% yield). 1H-NMR (CDCl3): δ 10.05 (s, 1H, CHO), 8.60 (dd, J = 7.0, 1.7 Hz, 1H, H-phenyl), 8.21 (ddd, J = 8.6, 4.2, 2.1 Hz, 1H, H-phenyl), 7.51 (dd, J = 9.9, 8.7 Hz, 1H, H-phenyl). HRMS (ESI) m/z: [M + H]+ calculated for C7H5O3NF, 170.02480; found, 170.02446, Δ −1.99 ppm.
3.1.2. Preparation of 4-(3,5-Dimethylpiperidin-1-yl)-3-nitro-benzaldehyde (b)
To a solution of 4-fluoro-3-nitrobenzaldehyde (a, 5.9 g, 34.9 mmol) in dichloromethane (100 mL) was added 3,5-dimethylpiperidine (5.9 g, 52.1 mmol) and ethylamine (5.3 g, 52.4 mmol) at 0 °C. The resulting mixture was stirred at room temperature for 0.5 h, poured into water (200 mL) and extracted with dichloromethane (200 mL × 2). The combined organic layers were washed with 1N HCl aqueous solution (150 mL × 2), saturated NaCl aqueous solution (150 mL × 2), dried over anhydrous Na2SO4 and concentrated. The residue was purified by column chromatography (silica gel, PE/EA = 10:1, v/v) to afford the product b as a yellow solid (8.6 g, 93.5% yield). 1H-NMR (DMSO-d6): δ 9.82 (s, 1H, CHO), 8.31 (s, 1H, H-phenyl), 7.90 (t, J = 9.8 Hz, 1H, H-phenyl), 7.39 (d, J = 8.6 Hz, 1H, 1H-phenyl), 3.30 (d, J = 12.9 Hz, 2H, CHa-1 and CHa-5), 2.58 (t, J = 12.0 Hz, 2H, CHb-1 and CHb-5), 1.84–1.65 (m, 3H, CHa-3, CH-2, CH-4), 0.92–0.74 (m, 8H, CH3-6, CH3-7 and CHb-3). HRMS (ESI) m/z: [M + H]+ calculated for C14H19O3N2, 263.13902; found, 263.13876, Δ −0.98 ppm.
3.1.3. Preparation of 1-[4-(3,5-Dimethylpiperidin-1-yl)-3-nitro-phenyl]-2,2,2-trifluoroethanol (c)
To a solution of 4-(3,5-dimethylpiperidin-1-yl)-3-nitrobenzaldehyde (b, 4.2 g, 16.0 mmol) and trimethyl(trifluoromethyl)silane (4.6 g, 32.0 mmol) in dimethylformamide (40 mL) was added potassium carbonate (4.4 g, 32.0 mmol) at 0 °C. The resulting mixture was warmed to room temperature and stirred for 12 h. The mixture was then treated with 40 mL of 1 mol/L HCl aqueous solution, stirred for another 30 min, poured into water (200 mL) and extracted with ethyl acetate (200 mL × 2). The organic extract was washed with saturated NaCl aqueous solution (150 mL × 3), dried over anhydrous Na2SO4 and concentrated. The residue was purified by column chromatography (silica gel, PE/EA = 8:1, v/v) to afford the product c as a brown red oil (4.6 g, 86.8% yield). 1H-NMR (DMSO-d6): δ 7.91 (d, J = 1.9 Hz, 1H, H-phenyl), 7.64 (dd, J = 8.6, 1.7 Hz, 1H, H-phenyl), 7.31 (dd, J = 8.7, 2.5 Hz, 1H, H-phenyl), 7.00–6.94 (m, 1H, CH), 5.24 (d, J = 7.0 Hz, 1H, OH), 3.13 (dd, J = 12.0, 1.5 Hz, 2H, CHa-1 and CHa-5), 2.38 (t, J = 11.5 Hz, 2H, CHb-1 and CHb-5), 1.81–1.65 (m, 3H, CHa-3, CH-2, CH-4), 0.85 (d, J = 6.5 Hz, 6H, CH3-6, CH3-7), 0.69 (q, J = 9.6 Hz, 1H, CHb-3). HRMS (ESI) m/z: [M + H]+ calculated for C15H20O3N2F3, 333.14205; found, 333.14246, Δ 1.22 ppm.
3.1.4. Preparation of 3-[4-(3,5-Dimethylpiperidin-1-yl)-3-nitrophenyl]-4,4,4-trifluorobut-2-enoic Acid Ethyl Ester (e)
To a solution of 1-[4-(3,5-dimethylpiperidin-1-yl)-3-nitrophenyl]-2,2,2-trifluoroethanol (c, 4.2 g, 12.6 mmol) in dichloromethane (200 mL) at 0 °C was added sodium bicarbonate (3.2 g, 37.8 mmol) followed by Dess-Martin periodinane (8.0 g, 18.9 mmol). The resulting solution was warmed to room temperature and stirred for 2 h. The reaction mixture was then diluted with saturated NaHCO3 aqueous solution (100 mL) and stirred for another 30 min. The organic layer was separated and washed with saturated NaCl aqueous solution (100 mL × 2), dried over anhydrous Na2SO4 and concentrated. The residue was preliminarily purified by column chromatography (silica gel, PE/EA = 20:1, v/v) to afford crude product d as a yellow solid. The crude product d was used directly without further purification. Next ethyl 2-(diethoxyphosphoryl)acetate (1.1 g, 4.9 mmol) was added to a solution of NaH (2.28 mg, 5.7 mmol) in dry tetrahydrofuran at 0 °C. The reaction mixture became a clear solution after stirring for 30 min. The crude product d in dry tetrahydrofuran was then added. The resulting mixture was warmed to room temperature, stirred for 2 h, quenched with saturated NH4Cl aqueous solution (10 mL). The aqueous layer was further extracted with ethyl acetate (50 mL × 2) and the combined organic extracts were washed with saturated NaCl aqueous solution (50 mL × 2), dried over anhydrous Na2SO4 and concentrated. The residue was purified by column chromatography (silica gel, PE/EA = 40:1, v/v) to afford the product e as an orange yellow oil (1.64 g, 32.5% yield). 1H-NMR (DMSO-d6): δ 7.73 (d, J = 2.2 Hz, 1H, H-phenyl), 7.44 (dd, J = 8.7, 2.0 Hz, 1H, H-phenyl), 7.32 (dd, J = 8.8, 2.8 Hz, 1H, H-phenyl), 6.86 (s, 1H, =CH), 4.04 (q, J = 7.2 Hz, 2H, CH2), 3.21–3.13 (m, 2H, CHa-1 and CHa-5), 2.44 (t, J = 11.7 Hz, 2H, CHb-1 and CHb-5), 1.83–1.64 (m, 3H, CHa-3, CH-2, CH-4), 1.04 (t, J = 7.2 Hz, 3H, CH3), 0.85 (d, J = 6.5 Hz, 6H, CH3-6, CH3-7), 0.73 (q, J = 11.8 Hz, 1H, CHb-3). HRMS (ESI) m/z: [M + H]+ calculated for C15H20O3N2F3, 401.16827; found, 401.16788, Δ −0.97 ppm.
3.1.5. Preparation of 3-[3-Amino-4-(3,5-dimethylpiperidin-1-yl)-phenyl]-4,4,4-trifluorobutyric Acid Ethyl Ester (f)
To a solution of 3-[4-(3,5-dimethylpiperidin-1-yl)-3-nitrophenyl]-4,4,4-trifluorobut-2-enoic acid ethyl ester (e, 2.4 g, 6.0 mmol) in dry tetrahydrofuran (60 mL) was added palladium on carbon (640 mg, 10% Pd/C, 0.6 mmol) and the suspension was hydrogenated (1 atm, balloon) for 2 h. Thin layer chromatography (TLC) indicated completion. The suspension was filtered through a pad of Celite and the filter cake was rinsed with ethyl acetate (50 mL × 3). The combined filtrate and rinses were concentrated and the residue was purified by column chromatography (silica gel, PE/EA = 30:1, v/v) to afford the product f as a colorless oil (945 mg, 42.3% yield). 1H-NMR (DMSO-d6): δ 6.82 (d, J = 8.1 Hz, 1H, H-phenyl), 6.67 (d, J = 1.7 Hz, 1H, H-phenyl), 6.54 (dd, J = 8.0, 1.8 Hz, 1H, H-phenyl), 4.76 (s, 2H, NH2), 4.07–3.93 (m, 2H, CH2), 3.85–3.70 (m, 1H, CH), 3.02–2.76 (m, 4H, CH2, CHa-1 and CHa-5), 2.00 (td, J = 10.9, 4.4 Hz, 2H, CHb-1 and CHb-5), 1.86–1.70 (m, 3H, CHa-3, CH-2, CH-4), 1.08 (t, J = 7.1 Hz, 3H, CH3), 0.85 (d, J = 6.4 Hz, 6H, CH3-6, CH3-7), 0.68–0.56 (m, 1H, CHb-3). HRMS (ESI) m/z: [M + H]+ calculated for C15H20O3N2F3, 373.20974; found, 373.20999, Δ 0.67 ppm.
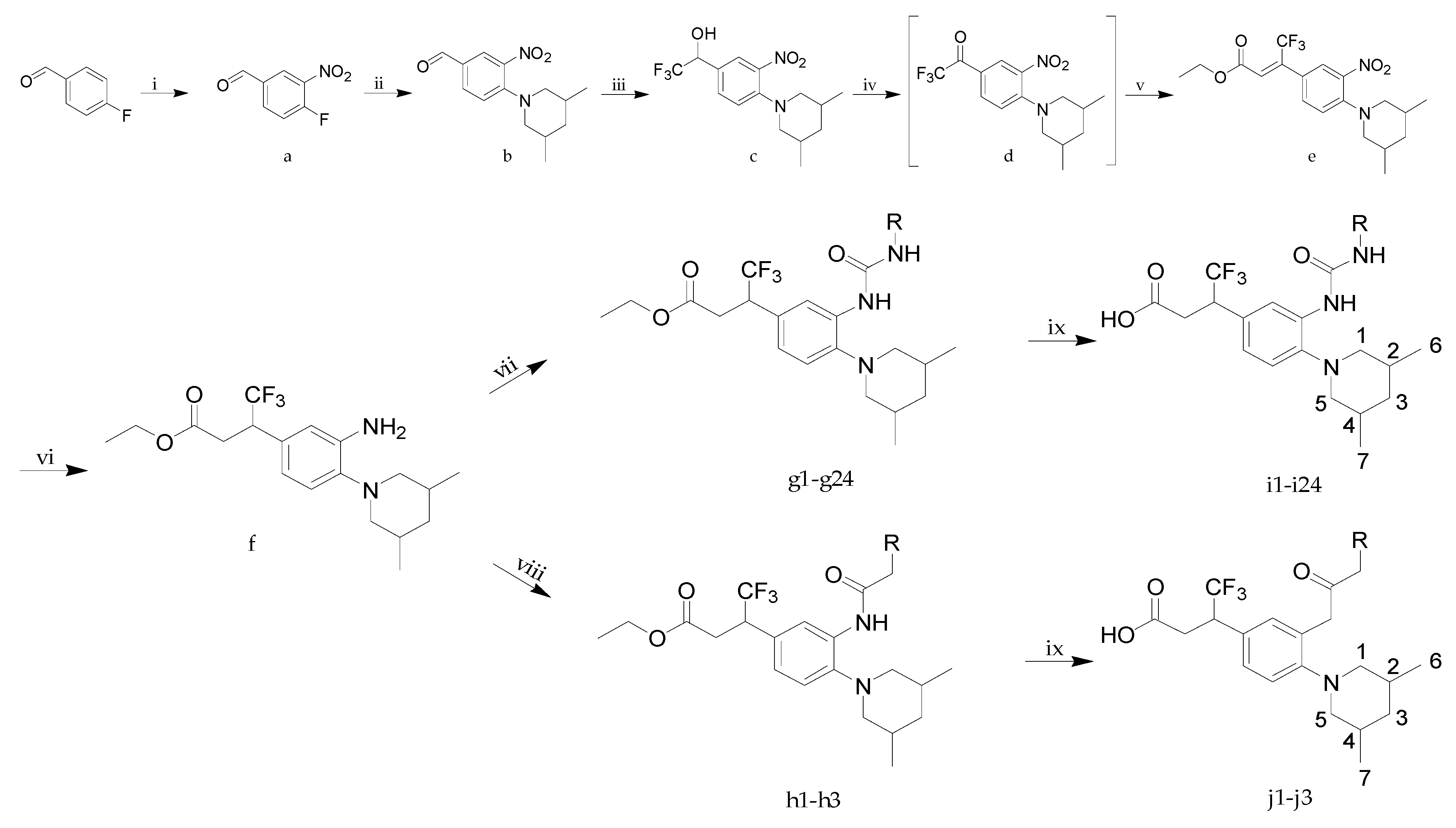
3.1.6. General Procedure A for the Synthesis of g1–g24
To a solution of 3-[3-amino-4-(3,5-dimethylpiperidin-1-yl)-phenyl]-4,4,4-trifluorobutyric acid ethyl ester (f, 1 equiv.) in tetrahydrofuran was added a suitable isocyanate (1 equiv.). The reaction mixture was stirred at room temperature until the starting material disappeared in TLC. The reaction mixture was concentrated and the residue was purified by column chromatography (silica gel, PE/EA = 8:1, v/v) to afford the products g1–g24.
3.1.7. General Procedure B for the Synthesis of i1–i24 and j1–j3
To a solution of compound g1–g24 or h1–h3 (1 equiv.) in tetrahydrofuran (4 volumes), methanol (1 volume) and water (1 volume) was added sodium hydroxide (3 equiv.). The resulting mixture was stirred at room temperature until the starting material disappeared in TLC. Part of the tetrahydrofuran and methanol was removed in vacuo and the crude was diluted with water (2 volumes) and the pH was adjusted to ca. 4 using 1 N HCl solution. The aqueous phase was then extracted with ethyl acetate (15 v × 3) and the combined organic extracts were washed with saturated NaCl solution, dried over anhydrous Na2SO4 and concentrated. The residue was purified by column chromatography (silica gel, DCM/MeOH = 30:1, v/v) to afford the products i1–i24 or j1–j3.
3.1.8. Preparation of Racemic 3-[4-(3,5-dimethylpiperidin-1-yl)-3-(3-phenylureido)-phenyl]-4,4,4-trifluorobutyric Acid Ethyl Ester (g1)
Reaction of compound f and phenyl isocyanate following the general procedure A afforded compound g1 (white solid, 85.3% yield). 1H-NMR (DMSO-d6): δ 9.54 (s, 1H, NH), 8.14 (d, J = 1.6 Hz, 1H, H-phenyl), 8.07 (s, 1H, NH), 7.49 (d, J = 7.9 Hz, 2H, H-phenyl), 7.29 (t, J = 7.8 Hz, 2H, H-phenyl), 7.13 (d, J = 8.2 Hz, 1H, H-phenyl), 7.02–6.95 (m, 2H, H-phenyl), 4.07–3.87 (m, 3H, CH2, CH), 3.07–2.79 (m, 4H, CH2, CHa-1 and CHa-5), 2.14 (t, J = 10.3 Hz, 2H, CHb-1 and CHb-5), 2.06–1.91 (m, 2H, CH-2, CH-4), 1.80 (d, J = 12.7 Hz, 1H, CHa-3), 1.08 (t, J = 7.1 Hz, 3H, CH3), 0.87 (d, J = 6.6 Hz, 6H, CH3-6, CH3-7), 0.67 (q, J = 12.0 Hz, 1H, CHb-3). 13C-NMR (DMSO-d6): δ 169.61, 152.54, 141.92, 139.82, 134.19, 129.17, 128.87 (2C), 128.14, 122.53, 122.04, 120.38, 119.34, 118.56 (2C), 60.48, 59.72 (2C), 45.02, 41.62, 33.81, 30.87 (2C), 19.33 (2C), 13.92. HRMS (ESI) m/z: [M + H]+ calculated for C26H33O3N3F3, 492.24685; found, 492.24582, Δ −2.10 ppm.
3.1.9. Preparation of Racemic 3-[4-(3,5-dimethylpiperidin-1-yl)-3-(3-phenylureido)-phenyl]-4,4,4-trifluorobutyric Acid (i1)
Hydrolysis of compound g1 (80 mg, 0.16 mmol) following the general procedure B afforded compound h1 (white solid, 61 mg, 80.7% yield). mp: 94.1–95.7 °C. 1H-NMR (DMSO-d6): δ 12.61 (s, 1H, COOH), 9.58 (s, 1H, NH), 8.14 (d, J = 1.9 Hz, 1H, H-phenyl), 8.09 (s, 1H, NH), 7.49 (dd, J = 8.6, 1.0 Hz, 2H, H-phenyl), 7.33–7.26 (m, 2H, H-phenyl), 7.13 (d, J = 8.2 Hz, 1H, H-phenyl), 7.02–6.95 (m, 2H, H-phenyl), 3.95–3.83 (m, 1H, CH), 2.97–2.71 (m, 4H, CH2, CHa-1 and CHa-5), 2.14 (td, J = 11.2, 3.7 Hz, 2H, CHb-1 and CHb-5), 2.05–1.91 (m, 2H, CH-2, CH-4), 1.80 (d, J = 12.8 Hz, 1H, CHa-3), 0.87 (d, J = 6.6 Hz, 6H, CH3-6, CH3-7), 0.66 (q, J = 12.4 Hz, 1H, CHb-3). HRMS(ESI) m/z: [M + H]+ calculated for C24H29O3N3F3, 464.21555; found, 464.21466, Δ −1.92 ppm.
3.1.10. Preparation of Racemic 3-[4-(3,5-dimethylpiperidin-1-yl)-3-(3-p-tolylureido)phenyl]-4,4,4-trifluorobutyric Acid Ethyl Ester (g2)
Reaction of compound f and p-tolyl isocyanate following the general procedure A afforded compound g2 (white solid, 86.1% yield). 1H-NMR (DMSO-d6): δ 9.42 (s, 1H, NH), 8.14 (d, J = 2.0 Hz, 1H, H-phenyl), 8.02 (s, 1H, NH), 7.38–7.35 (m, 2H, H-phenyl), 7.15–7.07 (m, 3H, H-phenyl), 6.97 (dd, J = 8.2, 2.0 Hz, 1H, H-phenyl), 4.07–3.88 (m, 3H, CH2, CH), 3.06–2.79 (m, 4H, CH2, CHa-1 and CHa-5), 2.25 (s, 3H, CH3-phenyl), 2.13 (td, J = 11.2, 2.0 Hz, 2H, CHb-1 and CHb-5), 2.02–1.90 (m, 2H, CH-2, CH-4), 1.79 (d, J = 13.1 Hz, 1H, CHa-3), 1.07 (t, J = 6.8 Hz, 3H, CH3), 0.86 (d, J = 6.6 Hz, 6H, CH3-6, CH3-7), 0.66 (q, J = 12.4 Hz, 1H, CHb-3). 13C-NMR (DMSO-d6): δ 169.61, 152.59, 141.81, 137.19, 134.32, 130.95, 129.28 (2C), 129.20, 122.40, 120.39, 119.19, 118.79 (2C), 118.27, 60.48, 59.71 (2C), 44.90, 41.62, 33.81, 30.87 (2C), 20.42, 19.33 (2C), 13.92. HRMS (ESI) m/z: [M + H]+ calculated for C27H35O3N3F3, 506.26250; found, 506.26242, Δ −0.16 ppm.
3.1.11. Preparation of Racemic 3-[4-(3,5-dimethylpiperidin-1-yl)-3-(3-p-tolylureido)phenyl]-4,4,4-trifluorobutyric Acid (i2)
Hydrolysis of compound g2 (140 mg, 0.28 mmol) following the general procedure B afforded compound i2 (white solid, 113 mg, 85.4% yield). mp: 134.3–136.1 °C. 1H-NMR (DMSO-d6): δ 12.50 (s, 1H, COOH), 9.45 (s, 1H, NH), 8.15 (d, J = 1.8 Hz, 1H, H-phenyl), 8.04 (s, 1H, NH), 7.37 (d, J = 8.4 Hz, 2H, ArH-phenyl), 7.15–7.06 (m, 3H, H-phenyl), 6.97 (dd, J = 8.2, 1.8 Hz, 1H, H-phenyl), 3.96–3.82 (m, 1H, CH), 2.99–2.75 (m, 4H, CH2, CHa-1 and CHa-5), 2.25 (s, 3H, -CH3, CH3-phenyl), 2.13 (td, J = 11.1, 3.3 Hz, 2H, CHb-1 and CHb-5), 2.03–1.89 (m, 2H, CH-2, CH-4), 1.79 (d, J = 12.7 Hz, 1H, CHa-3), 0.86 (d, J = 6.6 Hz, 6H, CH3-6, CH3-7), 0.66 (q, J = 12.0 Hz, 1H, CHb-3). 13C-NMR (DMSO-d6): δ 171.05, 152.62, 141.76, 137.22, 134.36, 130.95, 129.48, 129.28 (2C), 126.88, 122.49, 120.39, 119.17, 118.82 (2C), 59.75 (2C), 45.14, 41.65, 33.80, 30.87 (2C), 20.42, 19.33 (2C). HRMS (ESI) m/z: [M + H]+ calculated for C25H31O3N3F3, 478.23120; found, 478.23230, Δ 2.29 ppm.
3.1.12. Preparation of Racemic 3-[3-[3-(4-chlorophenyl)-ureido]-4-(3,5-dimethylpiperidin-1-yl)-phenyl]-4,4,4-trifluorobutyric Acid Ethyl Ester (g3)
Reaction of compound f and 4-chlorophenyl isocyanate following the general procedure A afforded compound g3 (white solid, 83.4% yield). 1H-NMR (DMSO-d6): δ 9.69 (s, 1H, NH), 8.14 (d, J = 1.9 Hz, 1H, H-phenyl), 8.09 (s, 1H, NH), 7.55–7.49 (m, 2H, H-phenyl), 7.37–7.31 (m, 2H, H-phenyl), 7.14 (d, J = 8.2 Hz, 1H, H-phenyl), 7.00 (dd, J = 8.2, 1.9 Hz, 1H, H-phenyl), 4.07–3.88 (m, 3H, CH2, CH), 3.07–2.76 (m, 4H, CH2, CHa-1 and CHa-5), 2.14 (t, J = 10.6 Hz, 2H, CHb-1 and CHb-5), 2.06–1.91 (m, 2H, CH-2, CH-4), 1.80 (d, J = 12.9 Hz, 1H, CHa-3), 1.07 (t, J = 7.2 Hz, 3H, CH3), 0.87 (d, J = 6.6 Hz, 6H, CH3-6, CH3-7), 0.67 (q, J = 12.4 Hz, 1H, CHb-3). 13C-NMR (DMSO-d6): δ 169.59, 152.40, 141.96, 138.84, 134.02, 129.22, 128.72 (2C), 125.51, 122.73, 120.44, 120.21, 119.96 (2C), 119.32, 60.48, 59.72 (2C), 45.00, 41.61, 33.79, 30.88 (2C), 19.32 (2C), 13.92. HRMS (ESI) m/z: [M + H]+ calculated for C26H32O3N3ClF3, 526.20788; found, 526.20862, Δ 1.41 ppm.
3.1.13. Preparation of Racemic 3-[3-[3-(4-chlorophenyl)ureido]-4-(3,5-dimethylpiperidin-1-yl)-phenyl]-4,4,4-trifluorobutyric Acid (i3)
Hydrolysis of compound g3 (134 mg, 0.26 mmol) following the general procedure B afforded compound i3 (light yellow solid, 102 mg, 80.3% yield). mp: 123.5–124.7 °C. 1H-NMR (DMSO-d6): δ 12.51 (s, 1H, COOH), 9.70 (s, 1H, NH), 8.13 (d, J = 1.8 Hz, 1H, H-phenyl), 8.10 (s, 1H, NH), 7.55–7.49 (m, 2H, H-phenyl), 7.36–7.31 (m, 2H, H-phenyl), 7.14 (d, J = 8.2 Hz, 1H, H-phenyl), 6.99 (dd, J = 8.2, 1.9 Hz, 1H, H-phenyl), 3.95–3.83 (m, 1H, CH), 2.98–2.76 (m, 4H, CH2, CHa-1 and CHa-5), 2.15 (td, J = 11.2, 2.9 Hz, 2H, CHb-1 and CHb-5), 2.05–1.92 (m, 2H, CH-2, CH-4), 1.80 (d, J = 12.9 Hz, 1H, CHa-3), 0.87 (d, J = 6.6 Hz, 6H, CH3-6, CH3-7), 0.67 (q, J = 12.4 Hz, 1H, CHb-3). 13C-NMR (DMSO-d6): δ 171.08, 152.47, 141.93, 138.91, 134.13, 129.58, 128.73 (2C), 126.94, 125.52, 122.83, 120.46, 120.01 (2C), 119.34, 59.80 (2C), 45.20, 41.65, 33.86, 30.92 (2C), 19.32 (2C). HRMS (ESI) m/z: [M + H]+ calculated for C24H28O3N3ClF3, 498.17658; found, 498.17761, Δ 2.07 ppm.
3.1.14. Preparation of Racemic 3-[3-(3-cyclohexylureido)-4-(3-methylpiperidin-1-yl)-phenyl]-4,4,4-trifluorobutyric Acid Ethyl Ester (g4)
Reaction of compound f and cyclohexyl isocyanate following the general procedure A afforded compound g4 (white solid, 86.3% yield). 1H-NMR (DMSO-d6): δ 8.08 (s, 1H, NH), 7.63 (s, 1H, NH), 7.12–7.02 (m, 2H, H-phenyl), 6.89 (d, J = 8.2 Hz, 1H, H-phenyl), 4.06–3.93 (m, 2H, CH2), 3.92–3.80 (m, 1H, CH), 3.52–3.40 (m, 1H, CH-cyclohexyl), 3.04–2.75 (m, 4H, CH2, CHa-1 and CHa-5), 2.09 (dt, J = 11.0, 5.5 Hz, 2H, CHb-1 and CHb-5), 1.96 (d, J = 6.7 Hz, 2H, CH-2, CH-4), 1.87–1.49 (m, 6H, CHa-3, CH-cyclohexyl), 1.36–0.99 (m, 8H, CH-cyclohexyl, CH3), 0.86 (d, J = 6.5 Hz, 6H, CH3-6, CH3-7), 0.65 (q, J = 12.0 Hz, 1H, CHb-3). HRMS (ESI) m/z: [M + H]+ calculated for C26H39O3N3F3, 498.29380; found, 498.29480, Δ 2.00 ppm.
3.1.15. Preparation of Racemic 3-[3-(3-cyclohexylureido)-4-(3,5-dimethylpiperidin-1-yl)phenyl]-4,4,4-trifluorobutyric Acid (i4)
Hydrolysis of compound g4 (80 mg, 0.16 mmol) following the general procedure B afforded compound i4 (white solid, 53 mg, 70.1% yield). mp: 134.2–135.7 °C. 1H-NMR (DMSO-d6): δ 7.91–7.76 (m, 1H, H-phenyl), 7.45–7.21 (m, 2H, NH, H-phenyl), 7.15–6.98 (m, 1H, H-phenyl), 4.00–3.85 (m, 1H, CH), 3.54–3.42 (m, 1H, CH-cyclohexyl), 3.14–2.75 (m, 4H, CHa-1 and CHa-5, CH2), 2.06–1.90 (m, 2H, CHb-1 and CHb-5), 1.85–1.76 (m, 3H, CH-2, CH-4, CH-cyclohexyl), 1.75–1.62 (m, 3H, CHa-3, CH-cyclohexyl), 1.60–1.48 (m, 1H, CH-cyclohexyl), 1.37–1.11 (m, 6H, CH-cyclohexyl), 0.89 (d, J = 6.6 Hz, 6H, CH3-6, CH3-7), 0.75 (q, J = 12.6 Hz, 1H, CHb-3). HRMS(ESI) m/z: [M + H]+ calculated for C24H35O3N3F3, 470.26250; found, 470.26343, Δ 1.97 ppm.
3.1.16. Preparation of Racemic 3-[3-(3-butylureido)-4-(3,5-dimethylpiperidin-1-yl)-phenyl]-4,4,4-trifluorobutyric Acid Ethyl Ester (g5)
Reaction of compound f and butyl isocyanate following the general procedure A afforded compound g5 (white solid, 84.1% yield). 1H-NMR (DMSO-d6): δ 8.08 (d, J = 1.2 Hz, 1H, H-phenyl), 7.65 (s, 1H, NH), 7.15 (t, J = 5.4 Hz, 1H, NH), 7.06 (d, J = 8.2 Hz, 1H, H-phenyl), 6.92–6.87 (m, 1H, H-phenyl), 4.06–3.93 (m, 2H, CH2), 3.93–3.81 (m, 1H, CH), 3.12–3.05 (m, 2H, CH-butyl), 3.04–2.76 (m, 4H, CH2, CHa-1 and CHa-5), 2.10 (td, J = 11.0, 2.9 Hz, 2H, CHb-1 and CHb-5), 2.03–1.88 (m, 2H, CH-2, CH-4), 1.78 (d, J = 12.7 Hz, 1H, CHa-3), 1.47–1.26 (m, 4H, CH-butyl), 1.07 (t, J = 7.1 Hz, 3H, CH3), 0.93–0.82 (m, 9H, CH- butyl, CH3-6, CH3-7), 0.65 (q, J = 12.0 Hz, 1H, CHb-3). HRMS (ESI) m/z: [M + H]+ calculated for C24H37O3N3F3, 472.27815; found, 472.27792, Δ −0.49 ppm.
3.1.17. Preparation of Racemic 3-[3-(3-butylureido)-4-(3,5-dimethylpiperidin-1-yl)-phenyl]-4,4,4-trifluorobutyric Acid (i5)
Hydrolysis of compound g5 (80 mg, 0.17 mmol) following the general procedure B afforded compound i5 (white solid, 56 mg, 74.3% yield). mp: 131.0–132.9 °C. 1H-NMR (DMSO-d6): δ 8.00–7.80 (m, 1H, -phenyl), 7.36–7.15 (m, 2H, NH, H-phenyl), 7.09–6.96 (m, 1H, H-phenyl), 3.98–3.83 (m, 1H, H-phenyl), 3.11 (t, J = 6.6 Hz, 2H, CH- butyl), 3.04–2.76 (m, 4H, CH2, CHa-1 and CHa-5), 2.46–2.21 (m, 2H, CHb-1 and CHb-5), 2.05–1.89 (m, 2H, CH-2, CH-4), 1.80 (d, J = 12.6 Hz, 1H, CHa-3), 1.49–1.29 (m, 4H, CH-butyl), 0.95–0.83 (m, 9H, CH- butyl, CH3-6, CH3-7), 0.73 (q, J = 11.7 Hz, 1H, CHb-3). HRMS (ESI) m/z: [M + H]+ calculated for C22H33O3N3F3, 444.24685; found, 444.24750, Δ 1.46 ppm.
3.1.18. Preparation of Racemic 3-[4-(3,5-dimethylpiperidin-1-yl)-3-(3-o-tolylureido)phenyl]-4,4,4-trifluorobutyric Acid Ethyl Ester (g6)
Reaction of compound f and o-tolyl isocyanate following the general procedure A afforded compound g6 (white solid, 78.4% yield). 1H-NMR (DMSO-d6): δ 8.62 (s, 1H, NH), 8.07 (s, 1H, NH), 8.04–8.01 (m, 1H, H-phenyl) 7.58–7.54 (m, 1H, H-phenyl), 7.20–7.09 (m, 2H, H-phenyl), 7.06–6.90 (m, 3H, H-phenyl), 4.03–3.80 (m, 3H, CH2, CH), 3.01–2.75 (m, 4H, CH2, CHa-1 and CHa-5), 2.20 (s, 3H, CH3-phenyl), 2.05 (t, J = 10.4 Hz, 2H, CHb-1 and CHb-5), 1.84–1.66 (m, 3H, CH-2, CH-4, CHa-3), 1.03 (t, J = 7.0 Hz, 3H, CH3), 0.79 (d, J = 6.5 Hz, 6H, CH3-6, CH3-7), 0.57 (q, J = 12.0 Hz, 1H, CHb-3). HRMS (ESI) m/z: [M + H]+ calculated for C27H35O3N3F3, 506.26250; found, 506.26297, Δ 0.92 ppm.
3.1.19. Preparation of Racemic 3-[4-(3,5-dimethylpiperidin-1-yl)-3-(3-o-tolylureido)phenyl]-4,4,4-trifluorobutyric Acid (i6)
Hydrolysis of compound g6 (203 mg, 0.40 mmol) following the general procedure B afforded compound i6 (white solid, 156 mg, 81.3% yield). mp: 109.3–111.2 °C. 1H-NMR (DMSO-d6): δ 12.53 (s, 1H, COOH), 8.67 (s, 1H, NH), 8.08 (s, 1H, NH), 8.04 (d, J = 1.0 Hz, 1H, H-phenyl), 7.56 (d, J = 7.7 Hz, 1H, H-phenyl), 7.25–7.14 (m, 2H, H-phenyl), 7.11–6.94 (m, 3H, H-phenyl), 3.96–3.80 (m, 1H, CH), 2.98–2.72 (m, 4H, CHa-1 and CHa-5, CH2), 2.25 (s, 3H, CH3-phenyl), 2.10 (td, J = 11.1, 3.7 Hz, 2H, CHb-1 and CHb-5), 1.86–1.68 (m, 3H, CH-2, CH-4, CHa-3), 0.84 (d, J = 6.4 Hz, 6H, CH3-6, CH3-7), 0.62 (q, J = 12.2 Hz, 1H, CHb-3). 13C-NMR (DMSO-d6): δ 171.12, 153.19, 142.24, 136.94, 133.95, 130.91, 130.47, 129.16, 126.93, 126.34, 124.53, 124.37, 122.80, 120.12, 119.96, 59.50 (2C), 45.13, 41.62, 33.87, 30.88 (2C), 19.31 (2C), 18.02. HRMS (ESI) m/z: [M + H]+ calculated for C25H31O3N3F3, 478.23120; found, 478.23215, Δ 1.98 ppm.
3.1.20. Preparation of Racemic 3-[4-(3,5-dimethylpiperidin-1-yl)-3-(3-m-tolylureido)phenyl]-4,4,4-trifluorobutyric Acid Ethyl Ester (g7)
Reaction of compound f and m-tolyl isocyanate following the general procedure A afforded compound g7 (white solid, 86.1% yield). 1H-NMR (DMSO-d6): δ 9.46 (s, 1H, NH), 8.14 (d, J = 1.9 Hz, 1H, H-phenyl), 8.05 (s, 1H, NH), 7.37–7.33 (m, 1H, H-phenyl), 7.28–7.23 (m, 1H, H-phenyl), 7.20–7.11 (m, 2H, H-phenyl), 6.98 (dd, J = 8.2, 1.9 Hz, 1H, H-phenyl), 6.81 (d, J = 7.4 Hz, 1H, H-phenyl), 4.07–3.87 (m, 3H, CH2, CH), 3.07–2.80 (m, 4H, CH2, CHa-1 and CHa-5), 2.29 (s, 3H, CH3-phenyl), 2.14 (td, J = 11.1, 3.0 Hz, 2H, CHb-1 and CHb-5), 2.04–1.89 (m, 2H, CH-2, CH-4), 1.80 (d, J = 13.2 Hz, 1H, CHa-3), 1.08 (t, J = 7.1, 3H, CH3), 0.87 (d, J = 6.6 Hz, 6H, CH3-6, CH3-7), 0.66 (q, J = 12.4 Hz, 1H, CHb-3). HRMS (ESI) m/z: [M + H]+ calculated for C27H35O3N3F3, 506.26250; found, 506.26297, Δ 0.92 ppm.
3.1.21. Preparation of Racemic 3-[4-(3,5-dimethylpiperidin-1-yl)-3-(3-m-tolylureido)phenyl]-4,4,4-trifluorobutyric Acid (i7)
Hydrolysis of compound g7 (209 mg, 0.41 mmol) following the general procedure B afforded compound i7 (white solid, 148 mg, 75.0% yield). mp: 121.2–122.4 °C. 1H-NMR (DMSO-d6): δ 9.48 (s, 1H, NH), 8.14 (d, J = 1.5 Hz, 1H, H-phenyl), 8.06 (s, 1H, NH), 7.37–7.34 (m, 1H, H-phenyl), 7.26 (d, J = 8.6 Hz, 1H, H-phenyl), 7.20–7.10 (m, 2H, H-phenyl), 6.97 (dd, J = 8.2, 1.6 Hz, 1H, H-phenyl), 6.81 (d, J = 7.4 Hz, 1H, H-phenyl), 3.96–3.82 (m, 1H, CH), 3.00–2.74 (m, 4H, CH2, CHa-1 and CHa-5), 2.29 (s, 3H, CH3-phenyl), 2.14 (td, J = 11.0, 4.2 Hz, 2H, CHb-1 and CHb-5), 2.05–1.91 (m, 2H, CH-2, CH-4), 1.80 (d, J = 12.8 Hz, 1H, CHa-3), 0.87 (d, J = 6.6 Hz, 6H, CH3-6, CH3-7), 0.66 (q, J = 12.0 Hz, 1H, CHb-3). 13C-NMR (DMSO-d6): δ 171.16, 152.57, 141.84, 139.79, 138.09, 134.28, 129.56, 128.75, 126.94, 122.82, 122.60, 120.39, 119.30, 119.17, 115.78, 59.77 (2C), 45.21, 41.67, 33.93, 30.90 (2C), 21.30, 19.35 (2C). HRMS (ESI) m/z: [M + H]+ calculated for C25H31O3N3F3, 478.23120; found, 478.23227, Δ 2.23 ppm.
3.1.22. Preparation of Racemic 3-[3-[3-(2-chlorophenyl)-ureido]-4-(3,5-dimethylpiperidin-1-yl)-phenyl]-4,4,4-trifluorobutyric Acid Ethyl Ester (g8)
Reaction of compound f and 2-chlorophenyl isocyanate following the general procedure A afforded compound g8 (white solid, 81.7% yield). 1H-NMR (DMSO-d6): δ 9.03 (s, 1H, NH), 8.43 (s, 1H, NH), 7.97–7.90 (m, 2H, H-phenyl), 7.47 (dd, J = 8.0, 1.2 Hz, 1H, H-phenyl), 7.34–7.27 (m, 1H, H-phenyl), 7.13–6.99 (m, 3H, H-phenyl), 4.07–3.87 (m, 3H, CH2, CH), 3.07–2.82 (m, 4H, CH2, CHa-1 and CHa-5), 2.12 (t, J = 11.0 Hz, 2H, CHb-1 and CHb-5), 2.00–1.86 (m, 2H, CH-2, CH-4), 1.80 (d, J = 12.8 Hz, 1H, CHa-3), 1.08 (t, J = 7.2 Hz, 3H, CH3), 0.86 (d, J = 6.6 Hz, 6H, CH3-6, CH3-7), 0.65 (q, J = 12.0 Hz, 1H, CHb-3). HRMS (ESI) m/z: [M + H]+ calculated for C26H32O3N3ClF3, 526.20788; found, 526.20917, Δ 2.45 ppm.
3.1.23. Preparation of Racemic 3-[3-[3-(2-chlorophenyl)ureido]-4-(3,5-dimethylpiperidin-1-yl)-phenyl]-4,4,4-trifluorobutyric Acid (i8)
Hydrolysis of compound g8 (60 mg, 0.11 mmol) following the general procedure B afforded compound i8 (white solid, 46 mg, 80.7% yield). mp: 111.2–113.7 °C. 1H-NMR (DMSO-d6): δ 12.43 (s, 1H, COOH), 9.01 (s, 1H, NH), 8.40 (s, 1H, NH), 7.95–7.85 (m, 2H, H-phenyl), 7.43 (d, J = 7.9 Hz, 1H, H-phenyl), 7.31–7.22 (m, 1H, H-phenyl), 7.09–6.93 (m, 3H, H-phenyl), 3.89–3.79 (m, 1H, CH), 2.93–2.71 (m, 4H, CH2, CHa-1 and CHa-5), 2.08 (td, J = 11.0, 2.6 Hz, 2H, CHb-1 and CHb-5), 1.96–1.87 (m, 2H, CH-2, CH-4), 1.74 (d, J = 12.7 Hz, 1H, CHa-3), 0.82 (d, J = 6.5 Hz, 6H, CH3-6, CH3-7), 0.61 (q, J = 12.0 Hz, 1H, CHb-3). HRMS (ESI) m/z: [M + H]+ calculated for C24H28O3N3ClF3, 498.17658; found, 498.17786, Δ 2.57 ppm.
3.1.24. Preparation of Racemic 3-[3-[3-(3-chlorophenyl)ureido]-4-(3,5-dimethylpiperidin-1-yl)-phenyl]-4,4,4-trifluorobutyric Acid Ethyl Ester (g9)
Reaction of compound f and 3-chlorophenyl isocyanate following the general procedure A afforded compound g9 (white solid, 79.7% yield). 1H-NMR (DMSO-d6): δ 9.76 (s, 1H, NH), 8.15–8.09 (m, 2H, H-phenyl, NH), 7.79–7.75 (m, 1H, H-phenyl), 7.35–7.25 (m, 2H, H-phenyl), 7.15 (d, J = 8.2 Hz, 1H, H-phenyl), 7.05–6.98 (m, 2H, H-phenyl), 4.06–3.92 (m, 3H, CH2, CH), 3.08–2.79 (m, 4H, CH2, CHa-1 and CHa-5), 2.15 (t, J = 11.0 Hz, 2H, CHb-1 and CHb-5), 2.05–1.90 (m, 2H, CH-2, CH-4), 1.78 (d, J = 12.0 Hz, 1H, CHa-3), 1.08 (t, J = 7.1 Hz, 3H, CH3), 0.87 (d, J = 6.5 Hz, 6H, CH3-6, CH3-7), 0.67 (q, J = 12.4 Hz, 1H, CHb-3). HRMS (ESI) m/z: [M + H]+ calculated for C26H32O3N3ClF3, 526.20788; found, 526.20776, Δ −0.23 ppm.
3.1.25. Preparation of Racemic 3-[3-[3-(3-chlorophenyl)ureido]-4-(3,5-dimethylpiperidin-1-yl)-phenyl]-4,4,4-trifluorobutyric Acid (i9)
Hydrolysis of compound g9 (300 mg, 0.57 mmol) following the general procedure B afforded compound i9 (white solid, 234 mg, 82.5% yield). mp: 129.3–131.7 °C. 1H-NMR (DMSO-d6) δ 12.50 (s, 1H, COOH), 9.77 (s, 1H, NH), 8.14–8.09 (m, 2H, NH, H-phenyl), 7.77 (t, J = 1.9 Hz, 1H, H-phenyl), 7.35–7.25 (m, 2H, H-phenyl), 7.15 (d, J = 8.1 Hz, 1H, H-phenyl), 7.07–6.97 (m, 2H, H-phenyl), 3.98–3.84 (m, 1H, CH), 3.01–2.75 (m, 4H, CH2, CHa-1 and CHa-5), 2.15 (td, J = 11.1, 2.9 Hz, 2H, CHb-1 and CHb-5), 2.06–1.91 (m, 2H, CH-2, CH-4), 1.81 (d, J = 12.9 Hz, 1H, CHa-3), 0.87 (d, J = 6.6 Hz, 6H, CH3-6, CH3-7), 0.67 (q, J = 12.1 Hz, 1H, CHb-3). 13C-NMR (DMSO-d6): δ 171.03, 152.36, 141.97, 141.42, 133.92, 133.33, 130.49, 129.55, 126.86, 122.91, 121.60, 120.48, 119.38, 117.78, 116.74, 59.75 (2C), 45.10, 41.61, 33.78, 30.90 (2C), 19.32 (2C). HRMS (ESI) m/z: [M + H]+ calculated for C24H28O3N3ClF3, 498.17658; found, 498.17770, Δ 2.25 ppm.
3.1.26. Preparation of Racemic 3-[3-[3-(2-cyanophenyl)ureido]-4-(3,5-dimethylpiperidin-1-yl)-phenyl]-4,4,4-trifluorobutyric Acid Ethyl Ester (g10)
Reaction of compound f and 2-cyanophenyl isocyanate following the general procedure A afforded compound g10 (white solid, 79.1% yield). 1H-NMR (DMSO-d6): δ 10.17 (s, 1H, NH), 8.28 (s, 1H, NH), 8.17–8.11 (m, 2H, H-phenyl), 8.03 (dd, J = 8.0, 3.8 Hz, 1H, H-phenyl), 7.63–7.57 (m, 1H, H-phenyl), 7.22–7.01 (m, 3H, H-phenyl), 4.07–3.87 (m, 3H, CH2, CH), 3.08–2.82 (m, 4H, CH2, CHa-1 and CHa-5), 2.13 (t, J = 11.0 Hz, 2H, CHb-1 and CHb-5), 2.03–1.88 (m, 2H, CH-2, CH-4), 1.80 (d, J = 12.8 Hz, 1H, CHa-3), 1.07 (t, J = 7.1 Hz, 3H, CH3), 0.86 (d, J = 6.6 Hz, 6H, CH3-6, CH3-7), 0.65 (q, J = 12.4 Hz, 1H, CHb-3). HRMS (ESI) m/z: [M + H]+ calculated for C27H32O3N4F3, 517.24210; found, 517.24335, Δ 2.41 ppm.
3.1.27. Preparation of Racemic 3-[3-[3-(2-cyanophenyl)ureido]-4-(3,5-dimethylpiperidin-1-yl)-phenyl]-4,4,4-trifluorobutyric Acid (i10)
Hydrolysis of compound g10 (40 mg, 0.08 mmol) following the general procedure B afforded compound i10 (white solid, 30 mg, 79.8% yield). mp: 178.2–179.9 °C. 1H-NMR (DMSO-d6): δ 12.51 (s, 1H, COOH), 10.18 (s, 1H, NH), 8.28 (s, 1H, NH), 8.16–8.10 (m, 2H, H-phenyl), 8.02 (dd, J = 8.1, 3.6 Hz, 1H, H-phenyl), 7.62–7.56 (m, 1H, H-phenyl), 7.22–7.01 (m, 3H, H-phenyl), 3.99–3.84 (m, 1H, CH), 3.02–2.76 (m, 4H, CH2, CHa-1 and CHa-5), 2.14 (t, J = 10.6 Hz, 2H, CHb-1 and CHb-5), 2.04–1.89 (m, 2H, CH-2, CH-4), 1.80 (d, J = 12.8 Hz, 1H, CHa-3), 0.87 (d, J = 6.6 Hz, 6H, CH3-6, CH3-7), 0.66 (q, J = 12.4 Hz, 1H, CHb-3). HRMS (ESI) m/z: [M + H]+ calculated for C25H28O3N4F3, 489.20987; found, 489.21080, Δ −1.90 ppm.
3.1.28. Preparation of Racemic 3-[3-[3-(3-cyanophenyl)ureido]-4-(3,5-dimethylpiperidin-1-yl)-phenyl]-4,4,4-trifluorobutyric Acid Ethyl Ester (g11)
Reaction of compound f and 3-cyanophenyl isocyanate following the general procedure A afforded compound g11 (white solid, 87.5% yield). 1H-NMR (DMSO-d6): δ 9.91 (s, 1H, NH), 8.17 (s, 1H, NH), 8.14–8.11 (m, 1H, H-phenyl), 8.06–8.03 (m, 1H, H-phenyl), 7.66 (d, J = 8.2 Hz, 1H, H-phenyl), 7.54–7.41 (m, 2H, H-phenyl), 7.16 (d, J = 8.1 Hz, 1H, H-phenyl), 7.02 (d, J = 8.4 Hz, 1H, H-phenyl), 4.08–3.88 (m, 3H, CH2, CH), 3.07–2.81 (m, 4H, CH2, CHa-1 and CHa-5), 2.15 (t, J = 10.8 Hz, 2H, CHb-1 and CHb-5), 2.06–1.92 (m, 2H, CH-2, CH-4), 1.81 (d, J = 12.8 Hz, 1H, CHa-3), 1.08 (t, J = 7.2 Hz, 3H, CH3), 0.87 (d, J = 6.5 Hz, 6H, CH3-6, CH3-7), 0.67 (q, J = 12.1 Hz, 1H, CHb-3). HRMS (ESI) m/z: [M + H]+ calculated for C27H32O3N4F3, 517.24210; found, 517.24121, Δ −1.72 ppm.
3.1.29. Preparation of Racemic 3-[3-[3-(3-cyanophenyl)ureido]-4-(3,5-dimethylpiperidin-1-yl)-phenyl]-4,4,4-trifluorobutyric Acid (i11)
Hydrolysis of compound g11 (180 mg, 0.35 mmol) following the general procedure B afforded compound i11 (white solid, 121 mg, 71.2% yield). mp: 128.4–130.2 °C. 1H-NMR (DMSO-d6): δ 12.52 (s, 1H, COOH), 9.94 (s, 1H, NH), 8.01–8.23 (m, 3H, NH, H-phenyl), 7.66 (d, J = 7.3 Hz, 1H, H-phenyl), 7.57–7.39 (m, 2H, H-phenyl), 7.16 (d, J = 8.2 Hz, 1H, H-phenyl), 7.01 (d, J = 8.2 Hz, 1H, H-phenyl), 3.98–3.83 (m, 1H, CH), 3.04–2.74 (m, 4H, CH2, CHa-1 and CHa-5), 2.16 (t, J = 9.9 Hz, 2H, CHb-1 and CHb-5), 2.06–1.92 (m, 2H, CH-2, CH-4), 1.81 (d, J = 11.3 Hz, 1H, CHa-3), 0.87 (d, J = 6.1 Hz, 6H, CH3-6, CH3-7), 0.67 (q, J = 11.6 Hz, 1H, CHb-3). 13C-NMR (DMSO-d6): δ 171.03, 152.38, 142.03, 140.76, 133.79, 130.31, 129.58, 126.90, 125.47, 123.06, 122.96, 120.92, 120.52, 119.40, 118.91, 111.72, 59.75 (2C), 45.10, 41.60, 33.78, 30.90 (2C), 19.33 (2C). HRMS (ESI) m/z: [M + H]+ calculated for C25H28O3N4F3, 489.21080; found, 489.21274, Δ 1.94 ppm.
3.1.30. Preparation of Racemic 3-[3-[3-(4-cyanophenyl)ureido]-4-(3,5-dimethylpiperidin-1-yl)-phenyl]-4,4,4-trifluorobutyric Acid Ethyl Ester (g12)
Reaction of compound f and 4-cyanophenyl isocyanate following the general procedure A afforded compound g12 (white solid, 84.1% yield). 1H-NMR (DMSO-d6): δ 10.05 (s, 1H, NH), 8.21 (s, 1H, NH), 8.12 (d, J = 1.8 Hz, 1H, H-phenyl), 7.77–7.72 (m, 2H, H-phenyl), 7.70–7.66 (m, 2H, H-phenyl), 7.16 (d, J = 8.3 Hz, 1H, H-phenyl), 7.03 (dd, J = 8.1, 2.0 Hz, 1H, H-phenyl), 4.07–3.88 (m, 3H, CH2, CH), 3.08–2.82 (m, 4H, CH2, CHa-1 and CHa-5), 2.15 (t, J = 10.9 Hz, 2H, CHb-1 and CHb-5), 2.05–1.90 (m, 2H, CH-2, CH-4), 1.81 (d, J = 12.7 Hz, 1H, CHa-3), 1.07 (t, J = 7.1 Hz, 3H, CH3), 0.90–0.81 (m, 6H, CH3-6, CH3-7), 0.67 (q, J = 12.8 Hz, 1H, CHb-3). HRMS (ESI) m/z: [M + H]+ calculated for C27H32O3N4F3, 517.24210; found, 517.24298, Δ 1.70 ppm.
3.1.31. Preparation of Racemic 3-[3-[3-(4-cyanophenyl)ureido]-4-(3,5-dimethylpiperidin-1-yl)-phenyl]-4,4,4-trifluorobutyric Acid (i12)
Hydrolysis of compound g12 (331 mg, 0.64 mmol) following the general procedure B afforded compound i12 (white solid, 271 mg, 86.5% yield). mp: 172.3–172.9 °C. 1H-NMR (DMSO-d6): δ 10.18 (s, 1H, NH), 8.27 (s, 1H, NH), 8.12 (d, J = 1.4 Hz, 1H, H-phenyl), 7.76–7.67 (m, 4H, H-phenyl), 7.16 (dd, J = 8.0, 4.2 Hz, 1H, H-phenyl), 7.02 (dd, J = 8.2, 1.8 Hz, 1H, H-phenyl), 3.98–3.83 (m, 1H, CH), 3.03–2.75 (m, 4H, CH2, CHa-1 and CHa-5), 2.16 (td, J = 11.1, 1.9 Hz, 2H, CHb-1 and CHb-5), 2.06–1.94 (m, 2H, CH-2, CH-4), 1.80 (d, J = 12.7 Hz, 1H, CHa-3), 0.86 (d, J = 6.5 Hz, 6H, CH3-6, CH3-7), 0.67 (q, J = 12.4 Hz, 1H, CHb-3). 13C-NMR (DMSO-d6): δ 170.92, 152.17, 144.35, 141.88, 133.53, 133.25 (2C), 129.64, 126.77 (q, Jc-f = 280.8 Hz), 123.29, 120.49, 119.75, 119.31, 118.11 (2C), 103.25, 59.73 (2C), 44.97 (q, J = 27.3 Hz), 41.45, 33.67, 30.62 (2C), 19.21 (2C). HRMS (ESI) m/z: [M + H]+ calculated for C25H28O3N4F3, 489.21080; found, 489.20990, Δ −1.84 ppm.
3.1.32. Preparation of Racemic 3-{4-(3,5-dimethylpiperidin-1-yl)-3-[3-(2-methoxyphenyl)ureido]-phenyl}-4,4,4-trifluorobutyric Acid Ethyl Ester (g13)
Reaction of compound f and 2-methoxyphenyl isocyanate following the general procedure A afforded compound g13 (white solid, 85.9% yield). 1H-NMR (DMSO-d6): δ 8.91 (s, 1H, NH), 8.42 (s, 1H, NH), 8.00–7.93 (m, 2H, H-phenyl), 7.10–6.96 (m, 4H, H-phenyl), 6.93–6.86 (m, 1H, H-phenyl), 4.07–3.87 (m, 3H, CH2, CH), 3.85 (s, 3H, OCH3-phenyl), 3.05–2.83 (m, 4H, CH2, CHa-1 and CHa-5), 2.10 (td, J = 11.1, 2.1 Hz, 2H, CHb-1 and CHb-5), 2.02–1.87 (m, 2H, CH-2, CH-4), 1.77 (d, J = 12.8 Hz, 1H, CHa-3), 1.07 (t, J = 7.1 Hz, 3H, CH3), 0.85 (d, J = 6.6 Hz, 6H, CH3-6, CH3-7), 0.64 (q, J = 12.4 Hz, 1H, CHb-3). HRMS (ESI) m/z: [M + H]+ calculated for C27H35O4N3F3, 522.25742; found, 522.25598, Δ −2.75 ppm.
3.1.33. Preparation of Racemic 3-{4-(3,5-dimethylpiperidin-1-yl)-3-[3-(2-methoxyphenyl)ureido]-phenyl}-4,4,4-trifluorobutyric Acid (i13)
Hydrolysis of compound g13 (80 mg, 0.15 mmol) following the general procedure B afforded compound i13 (white solid, 61 mg, 80.8% yield). mp: 119.3–120.2 °C. 1H-NMR (DMSO-d6): δ 12.64 (s, 1H, COOH), 8.91 (s, 1H, NH), 8.42 (s, 1H, NH), 7.99–7.93 (m, 2H, H-phenyl), 7.10–6.86 (m, 5H, H-phenyl), 3.94–3.81 (m, 4H, CH, OCH3-phenyl), 2.93–2.65 (m, 4H, CHa-1 and CHa-5, CH2), 2.10 (td, J = 11.2, 4.6 Hz, 2H, CHb-1 and CHb-5), 2.01–1.86 (m, 2H, CH-2, CH-4), 1.77 (d, J = 13.3 Hz, 1H, CHa-3), 0.85 (d, J = 6.5 Hz, 6H, CH3-6, CH3-7), 0.63 (q, J = 12.4 Hz, 1H, CHb-3). HRMS (ESI) m/z: [M + H]+ calculated for C25H31O4N3F3, 494.22612; found, 494.22455, Δ −3.17 ppm.
3.1.34. Preparation of Racemic 3-{4-(3,5-dimethylpiperidin-1-yl)-3-[3-(3-methoxyphenyl)ureido]-phenyl}-4,4,4-trifluorobutyric Acid Ethyl Ester (g14)
Reaction of compound f and 3-methoxyphenyl isocyanate following the general procedure A afforded compound g14 (white solid, 83.9% yield). 1H-NMR (DMSO-d6): δ 9.55 (s, 1H, NH), 8.13 (d, J = 1.9 Hz, 1H, H-phenyl), 8.07 (s, 1H, NH), 7.22–7.17 (m, 2H, H-phenyl), 7.13 (d, J = 8.2 Hz, 1H, H-phenyl), 7.03–6.96 (m, 2H, H-phenyl), 6.59–6.55 (m, 1H, H-phenyl), 4.07–3.88 (m, 3H, CH2, CH), 3.75 (s, 3H, OCH3-phenyl), 3.06–2.75 (m, 4H, CH2, CHa-1 and CHa-5), 2.13 (td, J = 11.2, 1.6 Hz, 2H, CHb-1 and CHb-5), 2.04–1.91 (m, 2H, CH-2, CH-4), 1.80 (d, J = 12.7 Hz, 1H, CHa-3), 1.10-1.06 (t, J = 7.2 Hz, 3H, CH3), 0.87 (d, J = 6.6 Hz, 6H, CH3-6, CH3-7), 0.66 (q, J = 12.4 Hz, 1H, CHb-3). HRMS (ESI) m/z: [M + H]+ calculated for C27H35O4N3F3, 522.25742; found, 522.25769, Δ 0.52 ppm.
3.1.35. Preparation of Racemic 3-{4-(3,5-dimethylpiperidin-1-yl)-3-[3-(3-methoxyphenyl)ureido]-phenyl}-4,4,4-trifluorobutyric Acid (i14)
Hydrolysis of compound g14 (245 mg, 0.47 mmol) following the general procedure B afforded compound i14 (white solid, 178 mg, 76.7% yield). mp: 112.3–113.7 °C. 1H-NMR (DMSO-d6): δ 12.52 (s, 1H, COOH), 9.56 (s, 1H, NH), 8.14 (d, J = 1.6 Hz, 1H, H-phenyl), 8.08 (s, 1H, NH), 7.22–7.11 (m, 3H, H-phenyl), 7.03–6.96 (m, 2H, H-phenyl), 6.57 (dd, J = 8.2, 2.5 Hz, 1H, H-phenyl), 3.96–3.81 (m, 1H, CH), 3.75 (s, 3H, OCH3-phenyl), 3.00–2.77 (m, 4H, CH2, CHa-1 and CHa-5), 2.14 (td, J = 11.0, 2.1 Hz, 2H, CHb-1 and CHb-5), 2.05–1.92 (m, 2H, CH-2, CH-4), 1.80 (d, J = 12.9 Hz, 1H, CHa-3), 0.87 (d, J = 6.6 Hz, 6H, CH3-6, CH3-7), 0.67 (q, J = 12.0 Hz, 1H, CHb-3). 13C-NMR (DMSO-d6): δ 171.08, 159.82, 152.50, 141.89, 141.09, 134.19, 129.66, 129.52, 126.90, 122.58, 120.44, 119.38, 110.87, 107.65, 104.23, 59.74 (2C), 55.06, 45.15, 41.64, 33.83, 30.88 (2C), 19.34 (2C). HRMS (ESI) m/z: [M + H]+ calculated for C25H31O4N3F3, 494.22612; found, 494.22699, Δ 1.77 ppm.
3.1.36. Preparation of Racemic 3-{4-(3,5-dimethylpiperidin-1-yl)-3-[3-(4-methoxyphenyl)ureido]-phenyl}-4,4,4-trifluorobutyric Acid Ethyl Ester (g15)
Reaction of compound f and 4-methoxyphenyl isocyanate following the general procedure A afforded compound g15 (light yellow solid, 81.3% yield). 1H-NMR (DMSO-d6): δ 9.34 (s, 1H, NH), 8.16 (d, J = 1.9 Hz, 1H, H-phenyl), 7.99 (s, 1H, NH), 7.41–7.36 (m, 2H, H-phenyl), 7.12 (d, J = 8.2 Hz, 1H, H-phenyl), 6.96 (dd, J = 8.2, 1.9 Hz, 1H, H-phenyl), 6.91–6.87 (m, 2H, H-phenyl), 4.05–3.88 (m, 3H, CH2, CH), 3.73 (s, 3H, OCH3-phenyl), 3.06–2.80 (m, 4H, CH2, CHa-1 and CHa-5), 2.12 (dt, J = 11.2, 5.6 Hz, 2H, CHb-1 and CHb-5), 1.99–1.86 (m, 2H, CH-2, CH-4), 1.78 (d, J = 13.0 Hz, 1H, CHa-3), 1.08 (t, J = 7.2 Hz, 3H, CH3), 0.86 (d, J = 6.8 Hz, 6H, CH3-6, CH3-7), 0.66 (q, J = 12.0 Hz, 1H, CHb-3). HRMS (ESI) m/z: [M + H]+ calculated for C27H35O4N3F3, 522.25665; found, 522.25742, Δ −1.47 ppm.
3.1.37. Preparation of Racemic 3-{4-(3,5-dimethylpiperidin-1-yl)-3-[3-(4-methoxyphenyl)ureido]-phenyl}-4,4,4-trifluorobutyric Acid (i15)
Hydrolysis of compound g15 (230 mg, 0.44 mmol) following the general procedure B afforded compound i15 (white solid, 181 mg, 81.8% yield). mp: 108.9–120.1 °C. 1H-NMR (DMSO-d6): δ 12.50 (s, 1H, COOH), 9.32 (s, 1H, NH), 8.15 (d, J = 1.9 Hz, 1H, H-phenyl), 7.99 (s, 1H, NH), 7.40–7.36 (m, 2H, H-phenyl), 7.12 (d, J = 8.3, 1H, H-phenyl), 6.95 (dd, J = 8.2, 1.9 Hz, 1H, H-phenyl), 6.90–6.86 (m, 2H, H-phenyl), 3.94–3.83 (m, 1H, CH), 3.73 (s, 3H, OCH3-phenyl), 2.98–2.75 (m, 4H, CH2, CHa-1 and CHa-5), 2.13 (td, J = 11.1, 3.1 Hz, 2H, CHb-1 and CHb-5), 1.98-1.85 (d, J = 6.6 Hz, 2H, CH-2, CH-4), 1.78 (d, J = 12.8 Hz, 1H, CHa-3), 0.86 (d, J = 6.6 Hz, 6H, CH3-6, CH3-7), 0.66 (q, J = 12.4 Hz, 1H, CHb-3). HRMS (ESI) m/z: [M + H]+ calculated for C25H31O4N3F3, 494.22612; found, 494.22815, Δ 4.11 ppm.
3.1.38. Preparation of Racemic 3-[3-[3-(2,4-difluorophenyl)ureido]-4-(3,5-dimethylpiperidin-1-yl)-phenyl]-4,4,4-trifluorobutyric Acid Ethyl Ester (g16)
Reaction of compound f and 2,4-difluorophenyl isocyanate following the general procedure A afforded compound g16 (white solid, 80.8% yield). 1H-NMR (DMSO-d6): δ 9.36 (s, 1H, NH), 8.34 (s, 1H, NH), 8.09–7.98 (m, 2H, H-phenyl), 7.31 (ddd, J = 11.6, 8.9, 2.9 Hz, 1H, H-phenyl), 7.14–6.97 (m, 3H, H-phenyl), 4.07–3.85 (m, 3H, CH2, CH), 3.06–2.82 (m, 4H, CH2, CHa-1 and CHa-5), 2.13 (t, J = 10.8 Hz, 2H, CHb-1 and CHb-5), 2.04–1.89 (m, 2H, CH-2, CH-4), 1.79 (d, J = 13.0 Hz, 1H, CHa-3), 1.07 (t, J = 7.1 Hz, 3H, CH3), 0.86 (d, J = 6.6 Hz, 6H, CH3-6, CH3-7), 0.66 (q, J = 12.4 Hz, 1H, CHb-3). HRMS (ESI) m/z: [M + H]+ calculated for C26H31O3N3F5, 528.22801; found, 528.22894, Δ 1.76 ppm.
3.1.39. Preparation of Racemic 3-[3-[3-(2,4-difluorophenyl)ureido]-4-(3,5-dimethylpiperidin-1-yl)-phenyl]-4,4,4-trifluorobutyric Acid (i16)
Hydrolysis of compound g16 (190 mg, 0.36 mmol) following the general procedure B afforded compound i16 (white solid, 149 mg, 82.0% yield). mp: 119.6–120.6 °C. 1H-NMR (DMSO-d6): δ 9.38 (s, 1H, -NH-), 8.36 (s, 1H, -NH-), 8.08–7.97 (m, 2H, H-phenyl), 7.31 (ddd, J = 11.6, 8.9, 2.9 Hz, 1H, H-phenyl), 7.14–6.95 (m, 3H, H-phenyl), 4.07–3.86 (m, 1H, CH), 2.94–2.70 (m, 4H, CH2, CHa-1 and CHa-5), 2.13 (td, J = 11.0, 3.8 Hz, 2H, CHb-1 and CHb-5), 2.04–1.88 (m, 2H, CH-2, CH-4), 1.79 (d, J = 12.6 Hz, 1H, CHa-3), 0.86 (d, J = 6.5 Hz, 6H, CH3-6, CH3-7), 0.65 (q, J = 12.0 Hz, 1H, CHb-3). 13C-NMR (DMSO-d6): δ 171.31, 155.16 (2C), 152.71, 142.28, 133.78, 129.47, 126.97, 123.85, 123.82, 123.10, 120.20, 120.08, 111.07, 103.92, 59.65 (2C), 45.23, 41.65, 34.18, 30.77 (2C), 19.33 (2C). HRMS (ESI) m/z: [M + H]+ calculated for C24H27O3N3F5, 500.19671; found, 500.19440, Δ −4.62 ppm.
3.1.40. Preparation of Racemic 3-[3-[3-(2,4-dichlorophenyl)ureido]-4-(3,5-dimethylpiperidin-1-yl)-phenyl]-4,4,4-trifluorobutyric Acid Ethyl Ester (g17)
Reaction of compound f and 2,4-dichlorophenyl isocyanate following the general procedure A afforded compound g17 (white solid, 81.3% yield). 1H-NMR (DMSO-d6): δ 9.12 (s, 1H, NH), 8.50 (s, 1H, NH), 8.00 (d, J = 8.9 Hz, 1H, H-phenyl), 7.93 (d, J = 1.8 Hz, 1H, H-phenyl), 7.63 (d, J = 2.5 Hz, 1H, H-phenyl), 7.39 (dd, J = 8.9, 2.5 Hz, 1H, H-phenyl), 7.10 (d, J = 8.3 Hz, 1H, H-phenyl), 7.03 (dd, J = 8.2, 1.9 Hz, 1H, H-phenyl), 4.07–3.86 (m, 3H, CH2, CH), 3.06–2.82 (m, 4H, CH2, CHa-1 and CHa-5), 2.12 (t, J = 11.1 Hz, 2H, CHb-1 and CHb-5), 2.02–1.88 (m, 2H, CH-2, CH-4), 1.79 (d, J = 12.7 Hz, 1H, CHa-3), 1.07 (t, J = 7.1 Hz, 3H, CH3), 0.86 (d, J = 6.6 Hz, 6H, CH3-6, CH3-7), 0.65 (q, J = 12.4 Hz, 1H, CHb-3). HRMS (ESI) m/z: [M + H]+ calculated for C26H31O3N3Cl2F3, 560.16891; found, 560.17065, Δ 3.11 ppm.
3.1.41. Preparation of Racemic 3-[3-[3-(2,4-dichlorophenyl)ureido]-4-(3,5-dimethylpiperidin-1-yl)-phenyl]-4,4,4-trifluorobutyric Acid (i17)
Hydrolysis of compound g17 (80 mg, 0.14 mmol) following the general procedure B afforded compound i17 (white solid, 61 mg, 80.1% yield). mp: 119.3–110.5 °C. 1H-NMR (DMSO-d6): δ 9.14 (s, 1H, NH), 8.50 (s, 1H, NH), 8.00 (d, J = 8.9 Hz, 1H, H-phenyl), 7.92 (d, J = 1.7 Hz, 1H, H-phenyl), 7.63 (d, J = 2.4 Hz, 1H, H-phenyl), 7.38 (dd, J = 8.9, 2.5 Hz, 1H, H-phenyl), 7.09 (d, J = 8.3 Hz, 1H, H-phenyl), 7.01 (dd, J = 8.2, 1.8 Hz, 1H, H-phenyl), 3.96–3.82 (m, 1H, CH), 2.96–1.71 (m, 4H, CHa-1 and CHa-5, CH2), 2.12 (td, J = 11.1, 3.9 Hz, 2H, CHb-1 and CHb-5), 2.03–1.87 (m, 2H, CH-2, CH-4), 1.83–1.75 (m, 1H, CHa-3), 0.86 (d, J = 6.6 Hz, 6H, CH3-6, CH3-7), 0.65 (q, J = 12.4 Hz, 1H, CHb-3). 13C-NMR (DMSO-d6): δ 171.29, 152.61, 143.00, 135.17, 133.12, 129.07, 128.72, 127.56, 127.24, 126.97, 125.07, 124.91, 123.65, 121.31, 119.86, 59.44 (2C), 45.12, 41.66, 34.15, 30.75 (2C), 19.33 (2C). HRMS (ESI) m/z: [M + H]+ calculated for C24H27O3N3Cl2F3, 532.13761; found, 532.13892, Δ 2.47 ppm.
3.1.42. Preparation of Racemic 3-{4-(3,5-dimethylpiperidin-1-yl)-3-[3-(4-fluorophenyl)ureido]-phenyl}-4,4,4-trifluorobutyric Acid Ethyl Ester (g18)
Reaction of compound f and 4-fluorophenyl isocyanate following the general procedure A afforded compound g18 (white solid, 81.3% yield). 1H-NMR (DMSO-d6): δ 9.57 (s, 1H, NH), 8.13 (d, J = 1.8 Hz, 1H, H-phenyl), 8.04 (s, 1H, NH), 7.54–7.46 (m, 2H, H-phenyl), 7.18–7.09 (m, 3H, H-phenyl), 6.98 (dd, J = 8.2, 1.9 Hz, 1H, H-phenyl), 4.08–3.85 (m, 3H, CH2, CH), 3.07–2.79 (m, 4H, CH2, CHa-1 and CHa-5), 2.14 (t, J = 10.6 Hz, 2H, CHb-1 and CHb-5), 2.05–1.88 (m, 2H, CH-2, CH-4), 1.80 (d, J = 12.8 Hz, 1H, CHa-3), 1.07 (t, J = 7.1 Hz, 3H, CH3), 0.87 (d, J = 6.6 Hz, 6H, CH3-6, CH3-7), 0.67 (q, J = 12.4 Hz, 1H, CHb-3). 13C-NMR (DMSO-d6): δ 169.60, 158.68, 156.31, 152.59, 141.88, 136.13, 134.17, 129.20, 122.57, 120.34 (2C), 120.33, 119.25, 115.38 (2C), 60.47, 59.71 (2C), 45.02, 41.61, 33.79, 30.90 (2C), 19.32 (2C), 13.92. HRMS (ESI) m/z: [M + H]+ calculated for C26H32O3N3F4, 510.23743; found, 510.23688, Δ −1.08 ppm.
3.1.43. Preparation of Racemic 3-{4-(3,5-dimethylpiperidin-1-yl)-3-[3-(4-fluorophenyl)ureido]-phenyl}-4,4,4-trifluorobutyric Acid (i18)
Hydrolysis of compound g18 (456 mg, 0.89 mmol) following the general procedure B afforded compound i18 (white solid, 371 mg, 86.3% yield). mp: 125.1–126.4 °C. 1H-NMR (DMSO-d6): δ 12.50 (s, 1H, COOH), 9.76 (s, 1H, NH), 8.14 (d, J = 1.8 Hz, 1H, H-phenyl), 8.11 (s, 1H, NH), 7.55–7.48 (m, 2H, H-phenyl), 7.16–7.09 (m, 3H, H-phenyl), 6.98 (dd, J = 8.2, 1.9 Hz, 1H, H-phenyl), 3.96–3.79 (m, 1H, CH), 2.99–2.74 (m, 4H, CH2, CHa-1 and CHa-5), 2.14 (td, J = 11.0, 2.8 Hz, 2H, CHb-1 and CHb-5), 2.08–1.93 (m, 2H, CH-2, CH-4), 1.79 (d, J = 12.8 Hz, 1H, CHa-3), 0.86 (d, J = 6.5 Hz, 6H, CH3-6, CH3-7), 0.66 (q, J = 12.4 Hz, 1H, CHb-3). HRMS (ESI) m/z: [M + H]+ calculated for C24H28O3N3F4, 482.20613; found, 482.20630, Δ 0.35 ppm.
3.1.44. Preparation of Racemic 3-[3-[3-(4-bromophenyl)ureido]-4-(3,5-dimethylpiperidin-1-yl)-phenyl]-4,4,4-trifluorobutyric Acid Ethyl Ester (g19)
Reaction of compound f and 4-bromophenyl isocyanate following the general procedure A afforded compound g19 (white solid, 100 mg, 81.5% yield). 1H-NMR (DMSO-d6): δ 9.69 (s, 1H, NH), 8.13 (d, J = 1.7 Hz, 1H, H-phenyl), 8.09 (s, 1H, NH), 7.50–7.44 (m, 4H, H-phenyl), 7.14 (d, J = 8.2 Hz, 1H, H-phenyl), 7.00 (dd, J = 8.2, 1.8 Hz, 1H, H-phenyl), 4.07–3.87 (m, 3H, CH2, CH), 3.07–2.80 (m, 4H, CH2, CHa-1 and CHa-5), 2.14 (t, J = 10.7 Hz, 2H, CHb-1 and CHb-5), 2.05–1.91 (m, 2H, CH-2, CH-4), 1.80 (d, J = 12.6 Hz, 1H, CHa-3), 1.07 (t, J = 7.1 Hz, 3H, CH3), 0.87 (d, J = 6.6 Hz, 6H, CH3-6, CH3-7), 0.67 (q, J = 12.0 Hz, 1H, CHb-3). HRMS (ESI) m/z: [M + H]+ calculated for C26H33O3N3BrF3, 570.15737; found, 570.15735, Δ −0.03 ppm.
3.1.45. Preparation of Racemic 3-[3-[3-(4-bromophenyl)ureido]-4-(3,5-dimethylpiperidin-1-yl)-phenyl]-4,4,4-trifluorobutyric Acid (i19)
Hydrolysis of compound g19 (100 mg, 0.18 mmol) following the general procedure B afforded compound i19 (white solid, 71 mg, 74.8% yield). mp: 136.9–137.7 °C. 1H-NMR (DMSO-d6): δ 12.58 (s, 1H, COOH), 9.75 (s, 1H, -NH-), 8.15–8.09 (m, 2H, H-phenyl, NH), 7.51–7.42 (m, 4H, H-phenyl), 7.14 (d, J = 8.2 Hz, 1H, H-phenyl), 6.98 (dd, J = 8.1, 1.6 Hz, 1H, H-phenyl), 3.96–3.82 (m, 1H, CH), 2.98–2.71 (m, 4H, CH2, CHa-1 and CHa-5), 2.14 (td, J = 11.0, 3.3 Hz, 2H, CHb-1 and CHb-5), 2.06–1.91 (m, 2H, CH-2, CH-4), 1.80 (d, J = 13.7 Hz, 1H, CHa-3), 0.86 (d, J = 6.5 Hz, 6H, CH3-6, CH3-7), 0.66 (q, J = 12.4 Hz, 1H, CHb-3). 13C-NMR (DMSO-d6): δ 171.14, 152.47, 141.95, 139.41, 134.10, 131.57 (2C), 129.57, 126.92, 122.81, 120.42, 120.34 (2C), 119.36, 113.30, 59.79 (2C), 45.20, 41.68, 33.98, 30.79 (2C), 19.34 (2C). HRMS (ESI) m/z: [M + H]+ calculated for C24H28O3N3BrF3, 542.12607; found, 542.12616, Δ 0.17 ppm.
3.1.46. Preparation of Racemic 3-{4-(3,5-dimethylpiperidin-1-yl)-3-[3-(4-ethylphenyl)ureido]phenyl}-4,4,4-trifluorobutyric Acid Ethyl Ester (g20)
Reaction of compound f and 4-ethylphenethyl isocyanate following the general procedure A afforded compound g20 (white solid, 83.7% yield). 1H-NMR (DMSO-d6): δ 9.42 (s, 1H, NH), 8.15 (d, J = 1.9 Hz, 1H, H-phenyl), 8.03 (s, 1H, NH), 7.41–7.37 (m, 2H, H-phenyl), 7.16–7.10 (m, 3H, H-phenyl), 6.97 (dd, J = 8.2, 2.0 Hz, 1H, H-phenyl), 4.08–3.86 (m, 3H, CH2, CH), 3.06–2.78 (m, 4H, CH2, CHa-1 and CHa-5), 2.55 (q, J = 7.6 Hz, 2H, CH3CH2-phenyl), 2.13 (td, J = 11.2, 2.3 Hz, 2H, CHb-1 and CHb-5), 2.01–1.87 (m, 2H, CH-2, CH-4), 1.78 (d, J = 12.8 Hz, 1H, CHa-3), 1.16 (t, J = 7.6 Hz, 3H, CH3CH2-phenyl), 1.07 (t, J = 7.6 Hz, 3H, CH3), 0.86 (d, J = 6.6 Hz, 6H, CH3-6, CH3-7), 0.66 (q, J = 12.4 Hz, 1H, CHb-3). HRMS (ESI) m/z: [M + H]+ calculated for C28H37O3N3F3, 520.27815; found, 520.27795, Δ −0.39 ppm.
3.1.47. Preparation of Racemic 3-{4-(3,5-dimethylpiperidin-1-yl)-3-[3-(4-ethylphenyl)ureido]phenyl}-4,4,4-trifluorobutyric Acid (i20)
Hydrolysis of compound g20 (134 mg, 0.26 mmol) following the general procedure B afforded compound i20 (white solid, 103 mg, 81.2% yield). mp: 122.1–122.9 °C. 1H-NMR (DMSO-d6): δ 9.44 (s, 1H, NH), 8.14 (d, J = 1.6 Hz, 1H, H-phenyl), 8.04 (s, 1H, NH), 7.39 (d, J = 8.4 Hz, 2H, H-phenyl), 7.16–7.10 (m, 3H, H-phenyl), 6.96 (dd, J = 8.2, 1.5 Hz, 1H, H-phenyl), 3.95–3.81 (m, 1H, CH), 2.96–2.73 (m, 4H, CH2, CHa-1 and CHa-5), 2.55 (q, J = 7.6 Hz, 2H, CH3CH2-phenyl), 2.13 (td, J = 11.0, 3.8 Hz, 2H, CHb-1 and CHb-5), 2.00–1.88 (m, 2H, CH-2, CH-4), 1.78 (d, J = 12.7 Hz, 1H, CHa-3), 1.16 (t, J = 7.6 Hz, 3H, CH3CH2-phenyl), 0.86 (d, J = 6.6 Hz, 6H, CH3-6, CH3-7), 0.65 (q, J = 12.4 Hz, 1H, CHb-3). 13C-NMR (DMSO-d6): δ 171.21, 152.63, 141.72, 137.54, 137.40, 134.33, 129.60, 128.10 (2C), 126.93, 122.49, 120.36, 119.19, 118.97 (2C), 59.74 (2C), 45.18, 41.64, 34.00, 30.86 (2C), 27.60, 19.32 (2C), 15.85. HRMS (ESI) m/z: [M + H]+ calculated for C26H33O3N3F3, 492.24685; found, 492.24774, Δ 1.80 ppm.
3.1.48. Preparation of Racemic 3-{4-(3,5-dimethylpiperidin-1-yl)-3-[3-(4-isopropylphenyl)ureido]-phenyl}-4,4,4-trifluorobutyric Acid Ethyl Ester (g21)
Reaction of compound f and 4-isopropylphenyl isocyanate following the general procedure A afforded compound g21 (white solid, 80.7% yield). 1H-NMR (DMSO-d6): δ 9.42 (s, 1H, NH), 8.15 (d, J = 1.9 Hz, 1H, H-phenyl), 8.03 (s, 1H, NH), 7.42–7.37 (m, 2H, H-phenyl), 7.14–7.20 (m, 2H, H-phenyl), 7.12 (d, J = 8.4 Hz, 1H, H-phenyl), 6.97 (dd, J = 8.2, 1.9 Hz, 1H, H-phenyl), 4.08–3.86 (m, 3H, CH2, CH), 3.07–2.79 (m, 5H, CH2, CHa-1 and CHa-5, (CH3)2CH-phenyl), 2.13 (td, J = 11.1, 2.5 Hz, 2H, CHb-1 and CHb-5), 2.00–1.85 (m, 2H, CH-2, CH-4), 1.78 (d, J = 12.9 Hz, 1H, CHa-3), 1.18 (d, J = 6.8 Hz, 6H, (CH3)2CH-phenyl), 1.08 (t, J = 7.2 Hz, 3H, CH3), 0.86 (d, J = 6.6 Hz, 6H, CH3-6, CH3-7), 0.65 (q, J = 12.4 Hz, 1H, CHb-3). HRMS (ESI) m/z: [M + H]+ calculated for C29H39O3N3F3, 534.29380; found, 534.29602, Δ 4.15 ppm.
3.1.49. Preparation of Racemic 3-{4-(3,5-dimethylpiperidin-1-yl)-3-[3-(4-isopropylphenyl)ureido]-phenyl}-4,4,4-trifluorobutyric Acid (i21)
Hydrolysis of compound g21 (200 mg, 0.35 mmol) following the general procedure B afforded compound i21 (white solid, 142 mg, 81.4% yield). mp: 121.7–122.7 °C. 1H-NMR (DMSO-d6): δ 9.43 (s, 1H, NH), 8.15 (d, J = 1.6 Hz, 1H, H-phenyl), 8.04 (s, 1H, NH), 7.39 (d, J = 8.4 Hz, 2H, H-phenyl), 7.16 (d, J = 8.5 Hz, 2H, H-phenyl), 7.12 (d, J = 8 Hz, 1H, H-phenyl), 6.96 (dd, J = 8.3, 1.7 Hz, 1H, H-phenyl), 3.95–3.82 (m, 1H, CH), 2.98–2.73 (m, 5H, CH2, CHa-1 and CHa-5, (CH3)2CH-phenyl), 2.13 (td, J = 11.0, 3.9 Hz, 2H, CHb-1 and CHb-5), 2.00–1.85 (m, 2H, CH-2, CH-4), 1.77 (d, J = 12.7 Hz, 1H, CHa-3), 1.18 (t, J = 7.1 Hz, 6H, (CH3)2CH-phenyl), 0.85 (d, J = 6.6 Hz, 6H, CH3-6, CH3-7), 0.65 (q, J = 12.4 Hz, 1H, CHb-3). 13C-NMR (DMSO-d6): δ 171.13, 152.64, 142.30, 141.72, 137.44, 134.34, 129.56, 126.92, 126.62 (2C), 122.47, 120.37, 119.18, 119.09 (2C), 59.73 (2C), 45.19, 41.63, 33.92, 32.87, 30.87 (2C), 24.09 (3C), 19.32 (2C). HRMS (ESI) m/z: [M + H]+ calculated for C27H35O3N3F3, 506.26250; found, 506.26373, Δ 2.42 ppm.
3.1.50. Preparation of Racemic 3-{4-(3,5-dimethylpiperidin-1-yl)-3-[3-(4-trifluoromethoxyphenyl)-ureido]phenyl}-4,4,4-trifluorobutyric Acid Ethyl Ester (g22)
Reaction of compound f and 4-fluorophenyl isocyanate following the general procedure A afforded compound g22 (white solid, 81.5% yield). 1H-NMR (DMSO-d6): δ 9.75 (s, 1H, NH), 8.14 (d, J = 1.9 Hz, 1H, H-phenyl), 8.10 (s, 1H, NH), 7.62–7.58 (m, 2H, H-phenyl), 7.30 (d, J = 8.5 Hz, 2H, H-phenyl), 7.14 (d, J = 8.2 Hz, 1H, H-phenyl), 7.00 (dd, J = 8.2, 1.9 Hz, 1H, H-phenyl), 4.07–3.88 (m, 3H, CH2, CH), 3.07–2.81 (m, 4H, CH2, CHa-1 and CHa-5), 2.15 (t, J = 10.4 Hz, 2H, CHb-1 and CHb-5), 2.05–1.90 (m, 2H, CH-2, CH-4), 1.80 (d, J = 12.8 Hz, 1H, CHa-3), 1.07 (t, J = 7.1 Hz, 3H, CH3), 0.87 (d, J = 6.6 Hz, 6H, CH3-6, CH3-7), 0.67 (q, J = 12.8 Hz, 1H, CHb-3). HRMS (ESI) m/z: [M + H]+ calculated for C27H32O4N3F6, 576.22915; found, 576.22772, Δ −2.49 ppm.
3.1.51. Preparation of Racemic 3-{4-(3,5-dimethylpiperidin-1-yl)-3-[3-(4-trifluoromethoxyphenyl)-ureido]phenyl}-4,4,4-trifluorobutyric Acid (i22)
Hydrolysis of compound g22 (586 mg, 1.02 mmol) following the general procedure B afforded compound i22 (white solid, 455 mg, 81.5% yield). mp: 196.2–197.8 °C. 1H-NMR (DMSO-d6): δ 12.37 (s, 1H, COOH), 9.78 (s, 1H, NH), 8.17–8.08 (m, 2H, H-phenyl, NH), 7.64–7.56 (m, 2H, H-phenyl), 7.30 (d, J = 8.7 Hz, 2H, H-phenyl), 7.15 (d, J = 8.2 Hz, 1H, H-phenyl), 7.00 (dd, J = 8.2, 1.5 Hz, 1H, H-phenyl), 3.97–3.82 (m, 1H, -CH-), 3.01–2.74 (m, 4H, CH2, CHa-1 and CHa-5), 2.15 (td, J = 11.0, 2.8 Hz, 2H, CHb-1 and CHb-5), 2.07–1.91 (m, 2H, CH-2, CH-4), 1.80 (d, J = 12.6 Hz, 1H, CHa-3), 0.87 (d, J = 6.5 Hz, 6H, CH3-6, CH3-7), 0.67 (q, J = 12.4 Hz, 1H, CHb-3). 13C-NMR (DMSO-d6): δ 171.02, 152.47, 142.73, 141.93, 139.14, 134.00, 129.51, 126.86, 122.87, 121.79 (2C), 120.44, 120.28, 119.65 (2C), 119.32, 59.74 (2C), 45.09, 41.62, 33.77, 30.89 (2C), 19.32 (2C). HRMS (ESI) m/z: [M + H]+ calculated for C25H28O4N3F6, 548.19785; found, 548.19781, Δ −0.08 ppm.
3.1.52. Preparation of Racemic 3-{4-(3,5-dimethylpiperidin-1-yl)-3-[3-(4-trifluoromethylphenyl)-ureido]phenyl}-4,4,4-trifluorobutyric Acid Ethyl Ester (g23)
Reaction of compound f and 4-(trifluoromethyl)phenyl isocyanate following the general procedure A afforded compound g23 (white solid, 80.6% yield). 1H-NMR (DMSO-d6): δ 9.96 (s, 1H, NH), 8.26–8.12 (m, 2H, NH, H-phenyl), 7.81–7.61 (m, 4H, H-phenyl), 7.16 (d, J = 8.2 Hz, 1H, H-phenyl), 7.02 (d, J = 8.2 Hz, 1H, H-phenyl), 4.12–3.89 (m, 3H, CH2, CH), 3.13–2.79 (m, 4H, CH2, CHa-1 and CHa-5), 2.15 (t, J = 10.6 Hz, 2H, CHb-1 and CHb-5), 2.08–1.92 (m, 2H, CH-2, CH-4), 1.81 (d, J = 12.1 Hz, 1H, CHa-3), 1.14–1.03 (m, 3H, CH3), 0.87 (d, J = 6.4 Hz, 6H, CH3-6, CH3-7), 0.67 (q, J = 12.0 Hz, 1H, CHb-3). HRMS (ESI) m/z: [M + H]+ calculated for C27H32O3N3F6, 560.23424; found, 560.23425, Δ 0.02 ppm.
3.1.53. Preparation of Racemic 3-{4-(3,5-dimethylpiperidin-1-yl)-3-[3-(4-trifluoromethylphenyl)-ureido]phenyl}-4,4,4-trifluorobutyric Acid (i23)
Hydrolysis of compound g23 (350 mg, 0.63 mmol) following the general procedure B afforded compound i23 (white solid, 270 mg, 80.6% yield). mp: 179.7–180.5 °C. 1H-NMR (DMSO-d6): δ 12.50 (s, 1H, COOH), 9.97 (s, 1H, NH), 8.19 (s, 1H, NH), 8.14 (d, J = 1.9 Hz, 1H, H-phenyl), 7.74–7.62 (m, 4H, H-phenyl), 7.16 (d, J = 8.2 Hz, 1H, H-phenyl), 7.01 (dd, J = 8.3, 2.0 Hz, 1H, H-phenyl), 3.98–3.82 (m, 1H, CH), 3.00–2.76 (m, 4H, CH2, CHa-1 and CHa-5), 2.16 (td, J = 11.1, 3.1 Hz, 2H, CHb-1 and CHb-5), 2.07–1.92 (m, 2H, CH-2, CH-4), 1.81 (d, J = 12.8 Hz, 1H, CHa-3), 0.87 (d, J = 6.6 Hz, 6H, CH3-6, CH3-7), 0.67 (q, J = 12.4 Hz, 1H, CHb-3). HRMS (ESI) m/z: [M + H]+ calculated for C25H28O3N3F6, 532.20294; found, 532.20508, Δ 4.03 ppm.
3.1.54. Preparation of Racemic 3-{4-(3,5-dimethylpiperidin-1-yl)-3-[3-(4-nitrophenyl)ureido]phenyl}-4,4,4-trifluorobutyric Acid Ethyl Ester (g24)
Reaction of compound f and 4-nitrophenyl isocyanate following the general procedure A afforded compound g24 (yellow solid, 86.5% yield). 1H-NMR (DMSO-d6): δ 10.28 (s, 1H, NH), 8.29 (s, 1H, NH), 8.24–8.19 (m, 2H, H-phenyl), 8.15 (d, J = 1.8 Hz, 1H, H-phenyl), 7.78–7.73 (m, 2H, H-phenyl), 7.17 (d, J = 8.2 Hz, 1H, H-phenyl), 7.04 (dd, J = 8.2, 1.9 Hz, 1H, H-phenyl), 4.07–3.91 (m, 3H, CH2, CH), 3.08–2.82 (m, 4H, CH2, CHa-1 and CHa-5), 2.15 (dd, J = 16.1, 6.1 Hz, 2H, CHb-1 and CHb-5), 2.07–1.92 (m, 2H, CH-2, CH-4), 1.81 (d, J = 12.8 Hz, 1H, CHa-3), 1.08 (t, J = 7.1 Hz, 3H, CH3), 0.87 (d, J = 6.6 Hz, 6H, CH3-6, CH3-7), 0.67 (q, J = 12.4 Hz, 1H, CHb-3). HRMS (ESI) m/z: [M + H]+ calculated for C26H32O5N4F3, 537.23193; found, 537.23126, Δ −1.25 ppm.
3.1.55. Preparation of Racemic 3-{4-(3,5-dimethylpiperidin-1-yl)-3-[3-(4-nitrophenyl)ureido]phenyl}-4,4,4-trifluorobutyric Acid (i24)
Hydrolysis of compound g24 (160 mg, 0.30 mmol) following the general procedure B afforded compound i24 (yellow solid, 121 mg, 79.3% yield). mp: 151.3–153.0 °C. 1H-NMR (DMSO-d6): δ 12.51 (s, 1H, COOH), 10.29 (s, 1H, NH), 8.29 (s, 1H, NH), 8.23–8.19 (m, 2H, H-phenyl), 8.14 (d, J = 1.6 Hz, 1H, H-phenyl), 7.77–7.72 (m, 2H, H-phenyl), 7.17 (d, J = 8.3 Hz, 1H, H-phenyl), 7.04 (dd, J = 8.2, 1.7 Hz, 1H, H-phenyl), 3.98–3.85 (m, 1H, CH), 3.01–2.77 (m, 4H, CH2, CHa-1 and CHa-5), 2.16 (td, J = 11.0, 2.8 Hz, 2H, CHb-1 and CHb-5), 2.08-1.93 (m, 2H, CH–2, CH-4), 1.81 (d, J = 12.8 Hz, 1H, CHa-3), 0.86 (t, J = 8.4 Hz, 6H, CH3-6, CH3-7), 0.68 (q, J = 12.4 Hz, 1H, CHb-3). 13C-NMR (DMSO-d6): δ 171.00, 152.05, 146.58, 142.19, 141.09, 133.52, 129.62, 126.84, 125.24 (2C), 123.49, 120.59, 119.65, 117.62 (2C), 59.79 (2C), 45.04, 41.59, 33.74, 30.80 (2C), 19.32 (2C). HRMS (ESI) m/z: [M + H]+ calculated for C24H28O5N4F3, 509.20063; found, 509.20267, Δ 4.00 ppm.
3.1.56. Preparation of Racemic 3-[3-[2-(4-cyanophenyl)acetylamino]-4-(3,5-dimethylpiperidin-1-yl)-phenyl]-4,4,4-trifluorobutyric Acid Ethyl Ester (h1)
To a solution of racemic 3-[3-amino-4-(3,5-dimethylpiperidin-1-yl)-phenyl]-4,4,4-trifluoro-butyric acid ethyl ester (f, 120 mg, 0.32 mmol) in N,N-dimethylformamide (4 mL) was added 4-cyanophenylacetic acid (52 mg, 0.32 mmol), 1-hydroxybenzotriazole (43 mg, 0.32 mmol), N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride (61 mg, 0.32 mmol) and N,N-diisopropylethylamine (106 μL, 0.64 mmol). The reaction mixture was stirred at room temperature until the starting material disappeared in TLC. The reaction mixture was then diluted with ethyl acetate (100 mL), washed with 1 N HCl aqueous solution (40 mL × 2), saturated NaCl aqueous solution (40 mL × 2), dried over anhydrous Na2SO4 and concentrated. The residue was purified by column chromatography (silica gel, PE/EA = 10:1, v/v) to afford the product as a colorless oil (117 mg, 70.5% yield). 1H-NMR (DMSO-d6): δ 8.85 (s, 1H, NH), 8.04 (s, 1H, H-phenyl), 7.84 (d, J = 8.1 Hz, 2H, H-phenyl), 7.59 (d, J = 8.1 Hz, 2H, H-phenyl), 7.11 (d, J = 8.0 Hz, 2H, H-phenyl), 4.05–3.86 (m, 5H, CH2, CH, CH2-phenyl), 3.06–2.82 (m, 2H, CH2), 2.73–2.63 (m, 2H, CHa-1 and CHa-5), 2.04 (t, J = 11.1 Hz, 2H, CHb-1 and CHb-5), 1.67 (d, J = 12.0 Hz, 1H, CHa-3), 1.61–1.45 (m, 2H, CH-2, CH-4), 1.05 (t, J = 7.1 Hz, 3H, CH3), 0.77 (d, J = 6.6 Hz, 6H, CH3-6, CH3-7), 0.57 (q, J = 12.0 Hz, 1H, CHb-3). HRMS (ESI) m/z: [M + H]+ calculated for C28H33O3N3F3, 516.24685; found, 516.24878, Δ 3.73 ppm.
3.1.57. Preparation of Racemic 3-[3-[2-(4-cyanophenyl)acetylamino]-4-(3,5-dimethylpiperidin-1-yl)-phenyl]-4,4,4-trifluorobutyric Acid (j1)
Hydrolysis of compound h1 (70 mg, 0.14 mmol) following the general procedure B afforded compound j1 (white solid, 49 mg, 73.9% yield). mp: 108.2–108.9 °C. 1H-NMR (DMSO-d6): δ 8.83 (s, 1H), 8.07 (s, 1H, H-phenyl), 7.84 (d, J = 6.1 Hz, 2H, H-phenyl), 7.59 (d, J = 6.6 Hz, 2H, H-phenyl), 7.13–7.03 (m, 2H, H-phenyl), 3.98–3.82 (m, 3H, CH, CH2-phenyl), 2.91–2.61 (m, 4H, CH2, CHa-1 and CHa-5), 2.03 (t, J = 11.0 Hz, 2H, CHb-1 and CHb-5), 1.68 (d, J = 12.4 Hz, 1H, CHa-3), 1.59–1.43 (m, 2H, CH-2, CH-4), 0.79–0.68 (m, 6H, CH3-6, CH3-7), 0.57 (q, J = 12.0 Hz, 1H, CHb-3). 13C-NMR (DMSO-d6): δ 168.08, 143.22, 141.42, 132.58 (2C), 132.40, 130.58 (2C), 129.58, 127.02, 125.04, 121.43, 120.35, 118.84, 109.88, 59.35 (2C), 45.16, 43.55, 41.42, 34.36, 31.00 (2C), 19.16 (2C). HRMS (ESI) m/z: [M + H]+ calculated for C26H29O3N3F3, 488.21555; found, 488.21490, Δ −1.34 ppm.
3.1.58. Preparation of Racemic 3-{4-(3,5-dimethylpiperidin-1-yl)-3-[2-(4-trifluoromethylphenyl)-acetylamino]phenyl}-4,4,4-trifluorobutyric Acid Ethyl Ester (h2)
Reaction of compound f and 4-(trifluoromethyl)phenylacetic acid following the similar procedure described for the preparation of h1 afforded h2 as a colorless oil (128 mg, 71.2%). 1H-NMR (DMSO-d6): δ 8.78 (s, 1H, NH), 8.12 (s, 1H, H-phenyl), 7.74 (d, J = 8.2 Hz, 2H, H-phenyl), 7.63 (d, J = 8.2 Hz, 2H, H-phenyl), 7.10 (s, 2H, H-phenyl), 4.05–3.92 (m, 3H, CH, CH2), 3.90 (s, 2H, CH2-phenyl), 3.06–2.82 (m, 2H, CH2), 2.59–2.69 (m, 2H, CHa-1 and CHa-5), 2.01 (t, J = 11.1 Hz, 2H, CHb-1 and CHb-5), 1.62 (d, J = 13.0 Hz, 1H, CHa-3), 1.47-1.34 (dt, J = 22.8, 11.6 Hz, 2H, CH-2, CH-4), 1.06 (t, J = 7.1 Hz, 3H, CH3), 0.73 (d, J = 6.6 Hz, 6H, CH3-6, CH3-7), 0.53 (q, J = 12.4 Hz, 1H, CHb-3). HRMS (ESI) m/z: [M + H]+ calculated for C28H33O3N2F6, 559.23899; found, 559.24072, Δ 3.10 ppm.
3.1.59. Preparation of Racemic 3-{4-(3,5-dimethylpiperidin-1-yl)-3-[2-(4-trifluoromethylphenyl)-acetylamino]phenyl}-4,4,4-trifluorobutyric Acid (j2)
Hydrolysis of compound h2 (80 mg, 0.14 mmol) following the general procedure B afforded compound j2 (white solid, 49 mg, 77.8% yield). mp: 93.2–93.6 °C. 1H-NMR (DMSO-d6): δ 8.77 (s, 1H, NH), 8.13 (s, 1H, H-phenyl), 7.74 (d, J = 8.1 Hz, 2H, H-phenyl), 7.63 (d, J = 8.0 Hz, 2H, H-phenyl), 7.13–7.02 (m, 2H, H-phenyl), 3.98–3.82 (m, 3H, CH, CH2), 2.90–2.58 (m, 4H, CH2, CHa-1 and CHa-5), 2.01 (td, J = 11.1, 4.2 Hz, 2H, CHb-1 and CHb-5), 1.61 (d, J = 12.6 Hz, 1H, CHa-3), 1.46–1.30 (m, 2H, CH-2, CH-4), 0.73 (d, J = 6.6 Hz, 6H, CH3-6, CH3-7), 0.53 (q, J = 12.0 Hz, 1H, CHb-3). 13C-NMR (DMSO-d6): δ 171.22, 168.28, 143.04, 140.23, 132.59, 130.44 (2C), 129.50, 127.98-125.48 (4C), 124.89, 123.05, 120.92, 120.49, 59.36 (2C), 45.02, 43.47, 41.29, 33.96, 30.91 (2C), 19.07 (2C). HRMS (ESI) m/z: [M + H]+ calculated for C26H29O3N2F6, 531.20769; found, 531.20703, Δ −1.24 ppm.
3.1.60. Preparation of Racemic 3-{4-(3,5-dimethylpiperidin-1-yl)-3-[2-(4-nitrophenyl)acetylamino]-phenyl}-4,4,4-trifluorobutyric Acid Ethyl Ester (h3)
Reaction of compound f and 4-nitrophenylacetic acid following the similar procedure described for the preparation of h1 afforded h3 as a light yellow oil (99 mg, 68.7%). 1H-NMR (DMSO-d6): δ 8.91 (s, 1H, NH), 8.24 (d, J = 8.6 Hz, 2H, H-phenyl), 8.03 (s, 1H, H-phenyl), 7.67 (d, J = 8.5 Hz, 2H, H-phenyl), 7.11 (s, 2H, H-phenyl), 4.05–3.88 (m, 5H, CH2, CH, CH2-phenyl), 3.06–1.99 (m, 2H, CH2), 2.75–2.65 (m, 2H, CHa-1 and CHa-5), 2.04 (t, J = 11.1 Hz, 2H, CHb-1 and CHb-5), 1.70–1.50 (m, 3H, CHa-3, CH-2, CH-4), 1.05 (t, J = 7.1 Hz, 3H, CH3), 0.75 (d, J = 6.6 Hz, 6H, CH3-6, CH3-7), 0.56 (q, J = 12.0 Hz, 1H, CHb-3). HRMS (ESI) m/z: [M + H]+ calculated for C27H33O5N3F3, 536.23668; found, 536.23749, Δ 1.51 ppm.
3.1.61. Preparation of Racemic 3-{4-(3,5-dimethylpiperidin-1-yl)-3-[2-(4-nitrophenyl)acetylamino]-phenyl}-4,4,4-trifluorobutyric Acid (j3)
Hydrolysis of compound h3 (70 mg, 0.13 mmol) following the general procedure B afforded compound j3 (yellow solid, 52 mg, 78.2% yield). mp: 92.1–92.7 °C. 1H-NMR (DMSO-d6): δ 12.51 (s, 1H, COOH), 8.90 (s, 1H, NH), 8.24 (d, J = 8.7 Hz, 2H, H-phenyl), 8.04 (s, 1H, H-phenyl), 7.67 (d, J = 8.6 Hz, 2H, H-phenyl), 7.11 (s, 2H, H-phenyl), 3.99–3.84 (m, 3H, CH2-phenyl, CH), 2.94–2.64 (m, 4H, CH2, CHa-1 and CHa-5), 2.04 (t, J = 9.9 Hz, 2H, CHb-1 and CHb-5), 1.69–1.48 (m, 3H, CHa-3, CH-2, CH-4), 0.75 (d, J = 6.6 Hz, 6H, CH3-6, CH3-7), 0.56 (q, J = 12.4 Hz, 1H, CHb-3). 13C-NMR (DMSO-d6): δ 170.95, 168.00, 146.64, 143.69, 132.37, 130.95, 130.83 (2C), 129.17, 126.78, 125.12, 123.73 (2C), 121.62, 120.45, 59.34 (2C), 45.85, 43.20, 41.37, 33.66, 30.96 (2C), 19.09 (2C). HRMS (ESI) m/z: [M + H]+ calculated for C25H29O5N3F3, 508.20538; found, 508.20618, Δ 1.57 ppm.