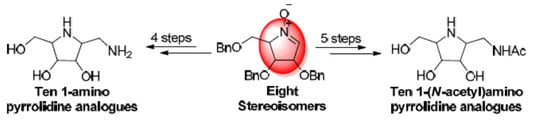
Synthesis of Pyrrolidine Monocyclic Analogues of Pochonicine and Its Stereoisomers: Pursuit of Simplified Structures and Potent β-N-Acetylhexosaminidase Inhibition
Abstract
1. Introduction
2. Results and Discussion
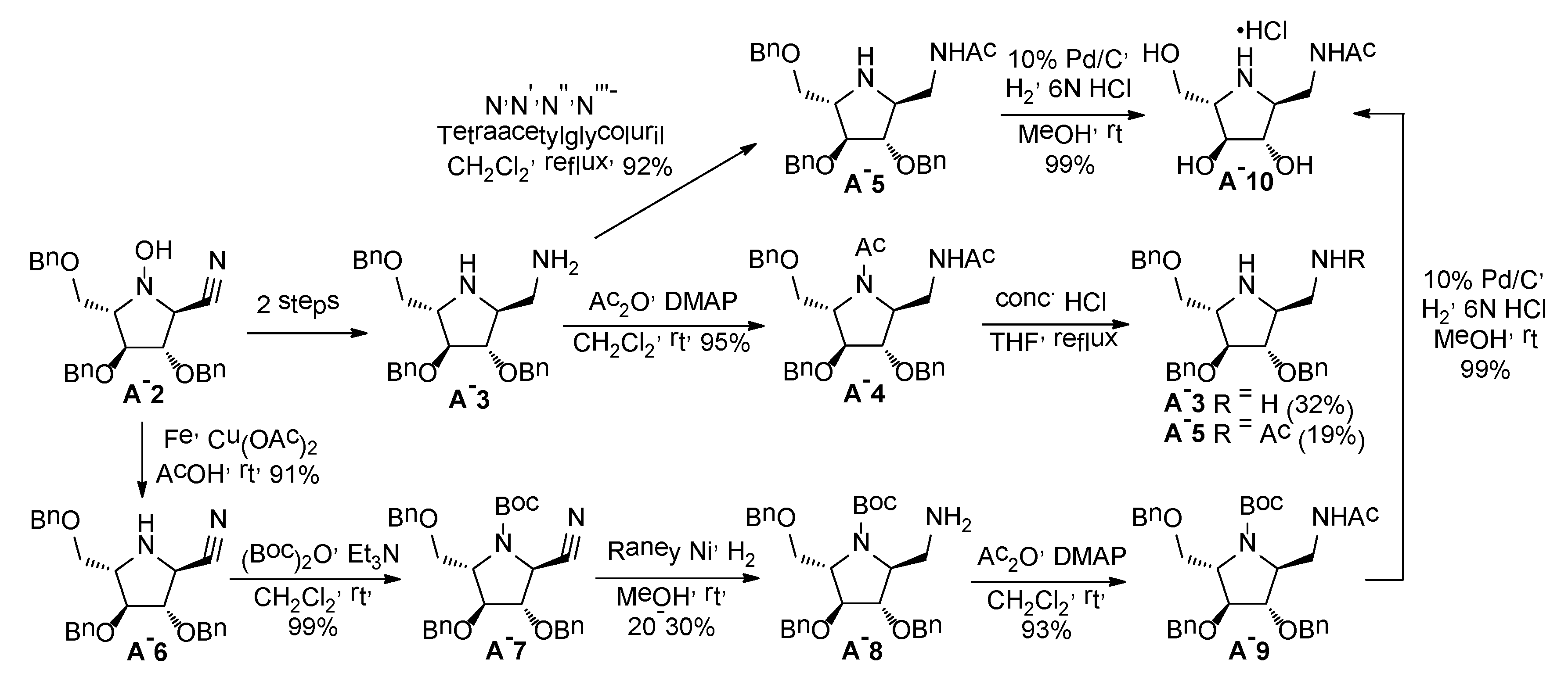
2.1. Synthesis of 1-N-Acetylamino-2,5-Imino-1,2,5-Trideoxy-l-Mannitol hydrochloride (A-10)
2.2. Synthesis of 1-Amino and 1-N-Acetylamino Modified Pyrrolidine Stereoisomers
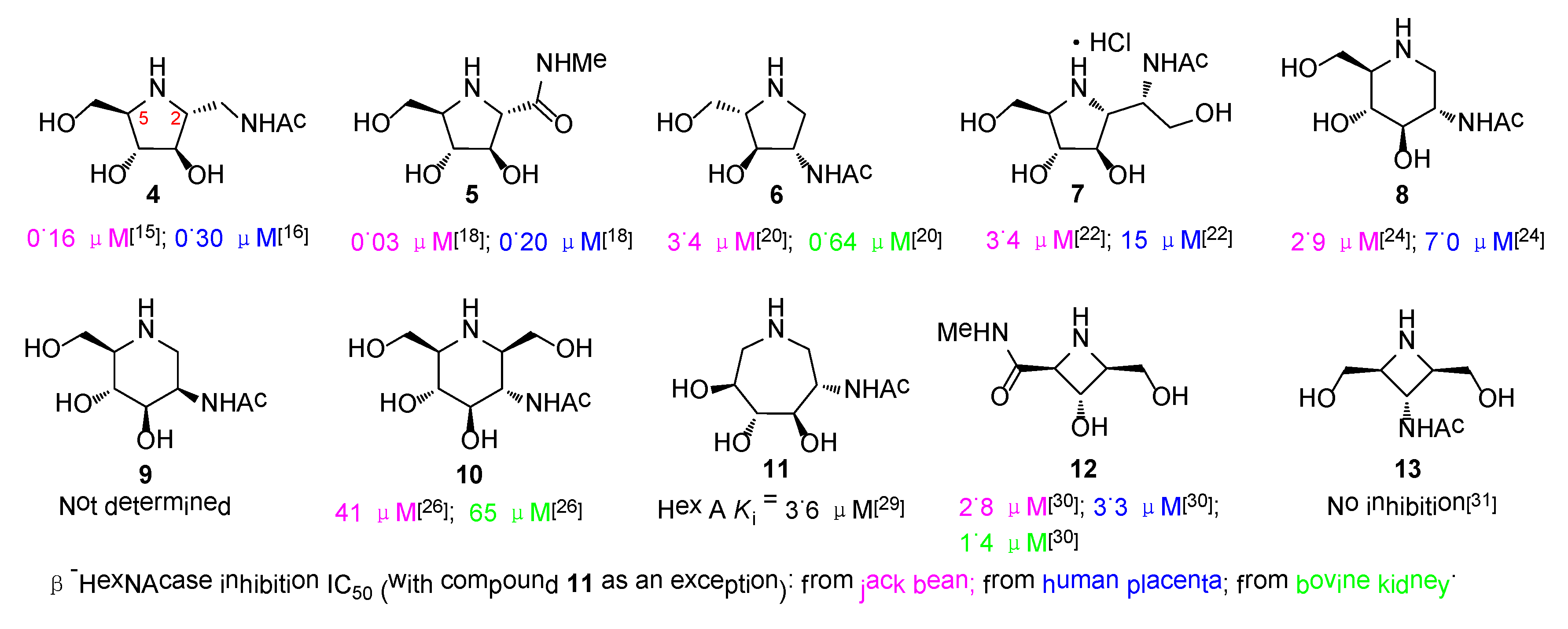
2.3. Glycosidase Inhibition
3. Materials and Methods
3.1. General Methods
3.2. Material and Methods for the Enzyme Inhibition Assay
3.3. Chemistry
3.3.1. General Procedure for Synthesis of Hydroxylamines A-2, B-2, C-2, D-2, E-2a, E-2b, F-2a, F-2b, G-2 and H-2 with A-2 as an Example
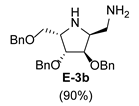
3.3.2. General Procedure for Synthesis of Hydroxylamines A-3, B-3, C-3, D-3, E-3a, E-3b, F-3a, F-3b, G-3 and H-3 with A-3 as an Example
3.3.3. Synthesis of (2S,3S,4S,5S)-3,4-bis(benzyloxy)-5-(benzyloxymethyl)-2-cyano-pyrrolidine (A-6)
3.3.4. Synthesis of tert-butyl-(2S,3S,4S,5S)-2-(aminomethyl)-3,4-bis(benzyloxy)-5-(benzyloxy methyl)pyrrolidine-1-carboxylate (A-8)
3.3.5. Synthesis of tert-butyl-(2S,3S,4S,5S)-2-N-acetylaminomethyl-3,4-bis(benzyloxy)-5-(benzyloxymethyl)pyrrolidine-1-carboxylate (A-9)
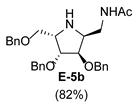
3.3.6. General Procedure for Synthesis of Compounds A-5, B-5, C-5, D-5, E-5a, E-5b, F-5a, F-5b, G-5 and H-5 with A-5 as an Example
3.3.7. General Procedure for Synthesis of 1-N-Acetylamino Derivatives (A-10, 4·HCl, C-10, D-10, E-10a, E-10b, F-10a, F-10b, G-10 and H-10) and 1-Amino Derivatives (A-11, B-11, C-11, D-11, E-11a, E-11b, F-11a, F-11b, G-11 and H-11) with A-10 as an Example
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Usuki, H.; Toyo-oka, M.; Kanzaki, H.; Okuda, T.; Nitoda, T. Pochonicine, a polyhydroxylated pyrrolizidine alkaloid from fungus Pochonia suchlasporia var. suchlasporia TAMA 87 as a potent β-N-acetylglucosaminidase inhibitor. Bioorg. Med. Chem. 2009, 17, 7248–7253. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-S.; Nakagawa, S.; Chen, W.; Adachi, I.; Jia, Y.-M.; Hu, X.-G.; Fleet, G.W.J.; Wilson, F.X.; Nitoda, T.; Horne, G.; et al. Synthesis of Eight Stereoisomers of Pochonicine: Nanomolar Inhibition of β-N-Acetylhexosaminidases. J. Org. Chem. 2013, 78, 10298–10309. [Google Scholar] [CrossRef] [PubMed]
- Stuetz, A.E.; Wrodnigg, T.M. Carbohydrate-Processing Enzymes of the Lysosome: Diseases Caused by Misfolded Mutants and Sugar Mimetics as Correcting Pharmacological Chaperones. In Advances in Carbohydrate Chemistry and Biochemistry; Baker, D.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 73, pp. 225–302. [Google Scholar]
- Rast, D.M.; Baumgartner, D.; Mayer, C.; Hollenstein, G.O. Cell wall-associated enzymes in fungi. Phytochemistry 2003, 64, 339–366. [Google Scholar] [CrossRef]
- Merzendorfer, H.; Zimoch, L. Chitin metabolism in insects: Structure, function and regulation of chitin synthases and chitinases. J. Exp. Biol. 2003, 206, 4393–4412. [Google Scholar] [CrossRef]
- Hart, G.W.; Housley, M.P.; Slawson, C. Cycling of O-linked β-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 2007, 446, 1017–1022. [Google Scholar] [CrossRef]
- Boyd, R.E.; Lee, G.; Rybczynski, P.; Benjamin, E.R.; Khanna, R.; Wustman, B.A.; Valenzano, K.J. Pharmacological Chaperones as Therapeutics for Lysosomal Storage Diseases. J. Med. Chem. 2013, 56, 2705–2725. [Google Scholar] [CrossRef]
- Vosseller, K.; Wells, L.; Lane, M.D.; Hart, G.W. Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3-L1 adipocytes. Proc. Nat. Acad. Sci. USA 2002, 99, 5313–5318. [Google Scholar] [CrossRef]
- Yuzwa, S.A.; Macauley, M.S.; Heinonen, J.E.; Shan, X.; Dennis, R.J.; He, Y.; Whitworth, G.E.; Stubbs, K.A.; McEachern, E.J.; Davies, G.J.; et al. A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat. Chem. Biol. 2008, 4, 483–490. [Google Scholar] [CrossRef]
- Scott, A.Y.; David, J.V. O-GlcNAc Modification and the Tauopathies: Insights from Chemical Biology. Curr. Alzheimer Res. 2009, 6, 451–454. [Google Scholar]
- Cecioni, S.; Vocadlo, D.J. Tools for probing and perturbing O-GlcNAc in cells and in vivo. Curr. Opin. Chem. Biol. 2013, 17, 719–728. [Google Scholar] [CrossRef]
- Aoyama, T.; Naganawa, H.; Suda, H.; Uotani, K.; Aoyagi, T.; Takeuchi, T. The structure of nagstatin, a new inhibitor of N-acetyl-β-d-glucosaminidase. J. Antibiot. 1992, 45, 1557–1558. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, H.; Aoyagi, T.; Komiyama, T.; Morishima, H.; Hamada, M.; Takeuchi, T. Purification and characterization of a sialidase inhibitor, siastatin, produced by streptomyces. J. Antibiot. 1974, 27, 963–969. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tatsuta, K.; Miura, S.; Ohta, S.; Gunji, H. Syntheses and glycosidase inhibiting activities of nagstatin analogs. J. Antibiot. 1995, 48, 286–288. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liang, P.H.; Cheng, W.C.; Lee, Y.L.; Yu, H.P.; Wu, Y.T.; Lin, Y.L.; Wong, C.H. Novel five-membered iminocyclitol derivatives as selective and potent glycosidase inhibitors: New structures for antivirals and osteoarthritis. Chembiochem 2006, 7, 165–173. [Google Scholar] [CrossRef]
- Tsou, E.-L.; Yeh, Y.-T.; Liang, P.-H.; Cheng, W.-C. A convenient approach toward the synthesis of enantiopure isomers of DMDP and ADMDP. Tetrahedron 2009, 65, 93–100. [Google Scholar] [CrossRef]
- Win-Mason, A.L.; Jongkees, S.A.K.; Withers, S.G.; Tyler, P.C.; Timmer, M.S.M.; Stocker, B.L. Stereoselective Total Synthesis of Aminoiminohexitols via Carbamate Annulation. J. Org. Chem. 2011, 76, 9611–9621. [Google Scholar] [CrossRef]
- Ayers, B.J.; Glawar, A.F.G.; Martínez, R.F.; Ngo, N.; Liu, Z.; Fleet, G.W.J.; Butters, T.D.; Nash, R.J.; Yu, C.-Y.; Wormald, M.R.; et al. Nine of 16 Stereoisomeric Polyhydroxylated Proline Amides Are Potent β-N-Acetylhexosaminidase Inhibitors. J. Org. Chem. 2014, 79, 3398–3409. [Google Scholar] [CrossRef]
- Glawar, A.F.G.; Martinez, R.F.; Ayers, B.J.; Hollas, M.A.; Ngo, N.; Nakagawa, S.; Kato, A.; Butters, T.D.; Fleet, G.W.J.; Jenkinson, S.F. Structural essentials for β-N-acetylhexosaminidase inhibition by amides of prolines, pipecolic and azetidine carboxylic acids. Org. Biomol. Chem. 2016, 14, 10371–10385. [Google Scholar] [CrossRef]
- Rountree, J.S.S.; Butters, T.D.; Wormald, M.R.; Dwek, R.A.; Asano, N.; Ikeda, K.; Evinson, E.L.; Nash, R.J.; Fleet, G.W.J. Efficient synthesis from D-lyxonolactone of 2-acetamido-1,4-imino-1,2,4-trideoxy-L-arabinitol LABNAc, a potent pyrrolidine inhibitor of hexosaminidases. Tetrahedron Lett. 2007, 48, 4287–4291. [Google Scholar] [CrossRef]
- Crabtree, E.V.; Martinez, R.F.; Nakagawa, S.; Adachi, I.; Butters, T.D.; Kato, A.; Fleet, G.W.J.; Glawar, A.F.G. Synthesis of the enantiomers of XYLNAc and LYXNAc: Comparison of β-N-acetylhexosaminidase inhibition by the 8 stereoisomers of 2-N-acetylamino-1,2,4-trideoxy-1,4-iminopentitols. Org. Biomol. Chem. 2014, 12, 3932–3943. [Google Scholar] [CrossRef]
- Tran, A.T.; Luo, B.; Jagadeesh, Y.; Auberger, N.; Désiré, J.; Nakagawa, S.; Kato, A.; Zhang, Y.; Blériot, Y.; Sollogoub, M. Synthesis of pyrrolidine-based analogues of 2-acetamidosugars as N-acetyl-d-glucosaminidase inhibitors. Carbohydr. Res. 2015, 409, 56–62. [Google Scholar] [CrossRef]
- Fleet, G.W.J.; Smith, P.W.; Nash, R.J.; Fellows, L.E.; Parekh, R.B.; Rademacher, T.W. Synthesis of 2-Acetamido-1,5-imino-1,2,5-trideoxy-d-mannitol and of 2-Acetamido-1,5-imino-1,2,5-trideoxy-d-glucitol, a Potent and Specific Inhibitor of a Number of β-N-Acetylglucosaminidases. Chem. Lett. 1986, 15, 1051–1054. [Google Scholar] [CrossRef]
- Glawar, A.F.G.; Best, D.; Ayers, B.J.; Miyauchi, S.; Nakagawa, S.; Aguilar-Moncayo, M.; García Fernández, J.M.; Ortiz Mellet, C.; Crabtree, E.V.; Butters, T.D.; et al. Scalable Syntheses of Both Enantiomers of DNJNAc and DGJNAc from Glucuronolactone: The Effect of N-Alkylation on Hexosaminidase Inhibition. Chem. Eur. J. 2012, 18, 9341–9359. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, A.; Verdaguer, X.; Riera, A. Stereodivergent Syntheses of altro and manno Stereoisomers of 2-Acetamido-1,2-dideoxynojirimycin. Eur. J. Org. Chem. 2017, 47, 7179–7185. [Google Scholar] [CrossRef]
- Blériot, Y.; Tran, A.T.; Prencipe, G.; Jagadeesh, Y.; Auberger, N.; Zhu, S.; Gauthier, C.; Zhang, Y.; Désiré, J.; Adachi, I.; et al. Synthesis of 1,2-trans-2-Acetamido-2-deoxyhomoiminosugars. Org. Lett. 2014, 16, 5516–5519. [Google Scholar] [CrossRef]
- Li, H.; Marcelo, F.; Bello, C.; Vogel, P.; Butters, T.D.; Rauter, A.P.; Zhang, Y.; Sollogoub, M.; Blériot, Y. Design and synthesis of acetamido tri- and tetra-hydroxyazepanes: Potent and selective β-N-acetylhexosaminidase inhibitors. Bioorg. Med. Chem. 2009, 17, 5598–5604. [Google Scholar] [CrossRef]
- Marcelo, F.; He, Y.; Yuzwa, S.A.; Nieto, L.; Jiménez-Barbero, J.; Sollogoub, M.; Vocadlo, D.J.; Davies, G.D.; Blériot, Y. Molecular Basis for Inhibition of GH84 Glycoside Hydrolases by Substituted Azepanes: Conformational Flexibility Enables Probing of Substrate Distortion. J. Am. Chem. Soc. 2009, 131, 5390–5392. [Google Scholar] [CrossRef]
- Mondon, M.; Hur, S.; Vadlamani, G.; Rodrigues, P.; Tsybina, P.; Oliver, A.; Mark, B.L.; Vocadlo, D.J.; Blériot, Y. Selective trihydroxyazepane NagZ inhibitors increase sensitivity of Pseudomonas aeruginosa to β-lactams. Chem. Commun. 2013, 49, 10983–10985. [Google Scholar] [CrossRef]
- Glawar, A.F.G.; Jenkinson, S.F.; Thompson, A.L.; Nakagawa, S.; Kato, A.; Butters, T.D.; Fleet, G.W.J. 3-Hydroxyazetidine Carboxylic Acids: Non-Proteinogenic Amino Acids for Medicinal Chemists. Chemmedchem 2013, 8, 658–666. [Google Scholar] [CrossRef]
- Liu, Z.; Jenkinson, S.F.; Kato, A.; Nakagawa, S.; Wormald, M.R.; Yu, C.-Y.; Fleet, G.W.J. 3-Azidoazetidines as the first scaffolds for β-amino azetidine carboxylic acid peptidomimetics: Azetidine iminosugars containing an acetamido group do not inhibit β-N-acetylhexosaminidases. Tetrahedron Asymmetry 2016, 27, 872–881. [Google Scholar] [CrossRef]
- Harit, V.K.; Ramesh, N.G. Amino-functionalized iminocyclitols: Synthetic glycomimetics of medicinal interest. Rsc Advances 2016, 6, 109528–109607. [Google Scholar] [CrossRef]
- Kitamura, Y.; Koshino, H.; Nakamura, T.; Tsuchida, A.; Nitoda, T.; Kanzaki, H.; Matsuoka, K.; Takahashi, S. Total synthesis of the proposed structure for pochonicine and determination of its absolute configuration. Tetrahedron Lett. 2013, 54, 1456–1459. [Google Scholar] [CrossRef]
- Salunke, R.V.; Ramesh, N.G. A Concise Total Synthesis of the Stereoisomers of (–)-Pochonicine. Eur. J. Org. Chem. 2016, 654–657. [Google Scholar] [CrossRef]
- Martinez, S.T.; Belouezzane, C.; Pinto, A.C.; Glasnov, T. Synthetic Strategies towards the Azabicyclo 3.3.0 -Octane Core of Natural Pyrrolizidine Alkaloids. An Overview. Org. Prep. Proced. Int. 2016, 48, 223–253. [Google Scholar] [CrossRef]
- Robertson, J.; Stevens, K. Pyrrolizidine alkaloids: Occurrence, biology, and chemical synthesis. Nat. Prod. Rep. 2017, 34, 62–89. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, S.; Roebuck, Q.P.; Nakagome, I.; Hirono, S.; Kato, A.; Nash, R.; High, S. Characterizing the selectivity of ER α-glucosidase inhibitors. Glycobiology 2019, 29, 530–542. [Google Scholar] [CrossRef]
- Ferhati, X.; Matassini, C.; Fabbrini, M.G.; Goti, A.; Morrone, A.; Cardona, F.; Moreno-Vargas, A.J.; Paoli, P. Dual targeting of PTP1B and glucosidases with new bifunctional iminosugar inhibitors to address type 2 diabetes. Bioorg. Chem. 2019, 87, 534–549. [Google Scholar] [CrossRef]
- Yang, L.-F.; Shimadate, Y.; Kato, A.; Li, Y.-X.; Jia, Y.-M.; Fleet, G.W.J.; Yu, C.-Y. Synthesis and glycosidase inhibition of N-substituted derivatives of DIM. Org. Biomol. Chem. 2020, 18, 999–1011. [Google Scholar] [CrossRef]
- Suzuki, K.; Nakahara, T.; Kanie, O. 3,4-dihydroxypyrrolidine as glycosidase inhibitor. Curr. Top. Med. Chem. 2009, 9, 34–57. [Google Scholar] [CrossRef]
- Takaoka, Y.; Kajimoto, T.; Wong, C.H. Inhibition of N-acetylglucosaminyl transfer enzymes: Chemical-enzymic synthesis of new five-membered acetamido azasugars. J. Org. Chem. 1993, 58, 4809–4812. [Google Scholar] [CrossRef]
- Liu, J.J.; Numa, M.M.D.; Liu, H.T.; Huang, S.J.; Sears, P.; Shikhman, A.R.; Wong, C.H. Synthesis and High-Throughput Screening of N-Acetyl-β-hexosaminidase Inhibitor Libraries Targeting Osteoarthritis. J. Org. Chem. 2004, 69, 6273–6283. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-X.; Kinami, K.; Hirokami, Y.; Kato, A.; Su, J.-K.; Jia, Y.-M.; Fleet, G.W.J.; Yu, C.-Y. Gem-difluoromethylated and trifluoromethylated derivatives of DMDP-related iminosugars: Synthesis and glycosidase inhibition. Org. Biomol. Chem. 2016, 14, 2249–2263. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-Y.; Kinami, K.; Kato, A.; Jia, Y.-M.; Li, Y.-X.; Fleet, G.W.J.; Yu, C.-Y. First total synthesis of (+)-broussonetine W: Glycosidase inhibition of natural product & analogs. Org. Biomol. Chem. 2016, 14, 5157–5174. [Google Scholar] [PubMed]
- Cheng, B.; Hirokami, Y.; Li, Y.-X.; Kato, A.; Jia, Y.-M.; Yu, C.-Y. Synthesis and glycosidase inhibition of C-7 modified casuarine derivatives. Chin. Chem. Lett. 2017, 28, 1701–1704. [Google Scholar] [CrossRef]
- Wu, Q.-K.; Kinami, K.; Kato, A.; Li, Y.-X.; Jia, Y.-M.; Fleet, G.W.J.; Yu, C.-Y. Synthesis and Glycosidase Inhibition of Broussonetine M and Its Analogues. Molecules 2019, 24, 3712. [Google Scholar] [CrossRef]
- Revuelta, J.; Cicchi, S.; Goti, A.; Brandi, A. Enantiopure Cyclic Nitrones: A Useful Class of Building Blocks for Asymmetric Syntheses. Synthesis 2007, 2007, 485–504. [Google Scholar] [CrossRef]
- Murahashi, S.-I.; Imada, Y. Synthesis and Transformations of Nitrones for Organic Synthesis. Chem. Rev. 2019, 119, 4684–4716. [Google Scholar] [CrossRef]
- Holzapfel, C.W.; Crous, R. Synthesis and reactions of chiral cyclic nitrones derived from d-ribose. Heterocycles 1998, 48, 1337–1342. [Google Scholar] [CrossRef]
- Pillard, C.; Desvergnes, V.; Py, S. Diastereoselective addition of alkynylalanes to carbohydrate-derived nitrones. Tetrahedron Lett. 2007, 48, 6209–6213. [Google Scholar] [CrossRef]
- Wang, W.-B.; Huang, M.-H.; Li, Y.-X.; Rui, P.-X.; Hu, X.-G.; Zhang, W.; Su, J.-K.; Zhang, Z.-L.; Zhu, J.-S.; Xu, W.-H.; et al. A Practical Synthesis of Sugar-derived Cyclic Nitrones: Powerful Synthons for the Synthesis of Iminosugars. Synlett 2010, 3, 488–492. [Google Scholar]
- Messire, G.; Massicot, F.; Vallee, A.; Vasse, J.-L.; Behr, J.-B. Aza-Henry Reaction with Nitrones, an Under-Explored Transformation. Eur. J. Org. Chem. 2019, 7, 1659–1668. [Google Scholar] [CrossRef]
- Cheng, W.-C.; Wang, J.-H.; Yun, W.-Y.; Li, H.-Y.; Hu, J.-M. Rapid preparation of (3R,4S,5R) polyhydroxylated pyrrolidine-based libraries to discover a pharmacological chaperone for treatment of Fabry disease. Eur. J. Med. Chem. 2017, 126, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-G.; Bartholomew, B.; Nash, R.J.; Wilson, F.X.; Fleet, G.W.J.; Nakagawa, S.; Kato, A.; Jia, Y.-M.; Well, R.V.; Yu, C.-Y. Synthesis and Glycosidase Inhibition of the Enantiomer of (-)-Steviamine, the First Example of a New Class of Indolizidine Alkaloid. Org. Lett. 2010, 12, 2562–2565. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-X.; Huang, M.-H.; Yamashita, Y.; Kato, A.; Jia, Y.-M.; Wang, W.-B.; Fleet, G.W.J.; Nash, R.J.; Yu, C.-Y. L-DMDP, L-homoDMDP and their C-3 fluorinated derivatives: Synthesis and glycosidase-inhibition. Org. Biomol. Chem. 2011, 9, 3405–3414. [Google Scholar] [CrossRef]
- Tice, C.M.; Ganem, B. The chemistry of naturally occurring polyamines. 6. Efficient syntheses of N1- and N8-acetylspermidine. J. Org. Chem. 1983, 48, 2106–2108. [Google Scholar] [CrossRef]
- Welter, A.; Jadot, J.; Dardenne, G.; Marlier, M.; Casimir, J. 2,5-Dihydroxymethyl 3,4-dihydroxypyrrolidine dans les feuilles de Derris elliptica. Phytochemistry 1976, 15, 747–749. [Google Scholar] [CrossRef]
- Kessler, M.; Acuto, O.; Storelli, C.; Murer, H.; Semenza, G.A. A modified procedure for the rapid preparation of efficiently transporting vesicles from small intestinal brush border membranes. Their use in investigating some properties of d-glucose and choline transport systems. Biochim. Biophys. Acta 1978, 506, 136–154. [Google Scholar] [CrossRef]
- Ayers, B.J.; Ngo, N.; Jenkinson, S.F.; Martínez, R.F.; Shimada, Y.; Adachi, I.; Weymouth-Wilson, A.C.; Kato, A.; Fleet, G.W.J. Glycosidase Inhibition by All 10 Stereoisomeric 2,5-Dideoxy-2,5-iminohexitols Prepared from the Enantiomers of Glucuronolactone. J. Org. Chem. 2012, 77, 7777–7792. [Google Scholar] [CrossRef]
- Cheng, T.-J.R.; Chan, T.-H.; Tsou, E.-L.; Chang, S.-Y.; Yun, W.-Y.; Yang, P.-J.; Wu, Y.-T.; Cheng, W.-C. From Natural Product-Inspired Pyrrolidine Scaffolds to the Development of New Human Golgi α-Mannosidase II Inhibitors. Chem. Asian J. 2013, 8, 2600–2604. [Google Scholar] [CrossRef]
- Cheng, W.-C.; Wang, J.-H.; Li, H.-Y.; Lu, S.-J.; Hu, J.-M.; Yun, W.-Y.; Chiu, C.-H.; Yang, W.-B.; Chien, Y.-H.; Hwu, W.-L. Bioevaluation of sixteen ADMDP stereoisomers toward alpha-galactosidase A: Development of a new pharmacological chaperone for the treatment of Fabry disease and potential enhancement of enzyme replacement therapy efficiency. Eur. J. Med. Chem. 2016, 123, 14–20. [Google Scholar] [CrossRef]
- Takebayashi, M.; Hiranuma, S.; Kanie, Y.; Kajimoto, T.; Kanie, O.; Wong, C.H. A Versatile Synthetic Strategy for the Preparation and Discovery of New Iminocyclitols as Inhibitors of Glycosidases. J. Org. Chem. 1999, 64, 5280–5291. [Google Scholar] [CrossRef]
- Kang, S.H.; Ryu, D.H. Intramolecular cyclization of C2 symmetric and meso-iodo amino alcohols: A synthetic approach to azasugars. Tetrahedron Lett. 1997, 38, 607–610. [Google Scholar] [CrossRef]
- Ganesan, M.; Madhukarrao, R.V.; Ramesh, N.G. Design and synthesis of new amino-modified iminocyclitols: Selective inhibitors of α-galactosidase. Org. Biomol. Chem. 2010, 8, 1527–1530. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

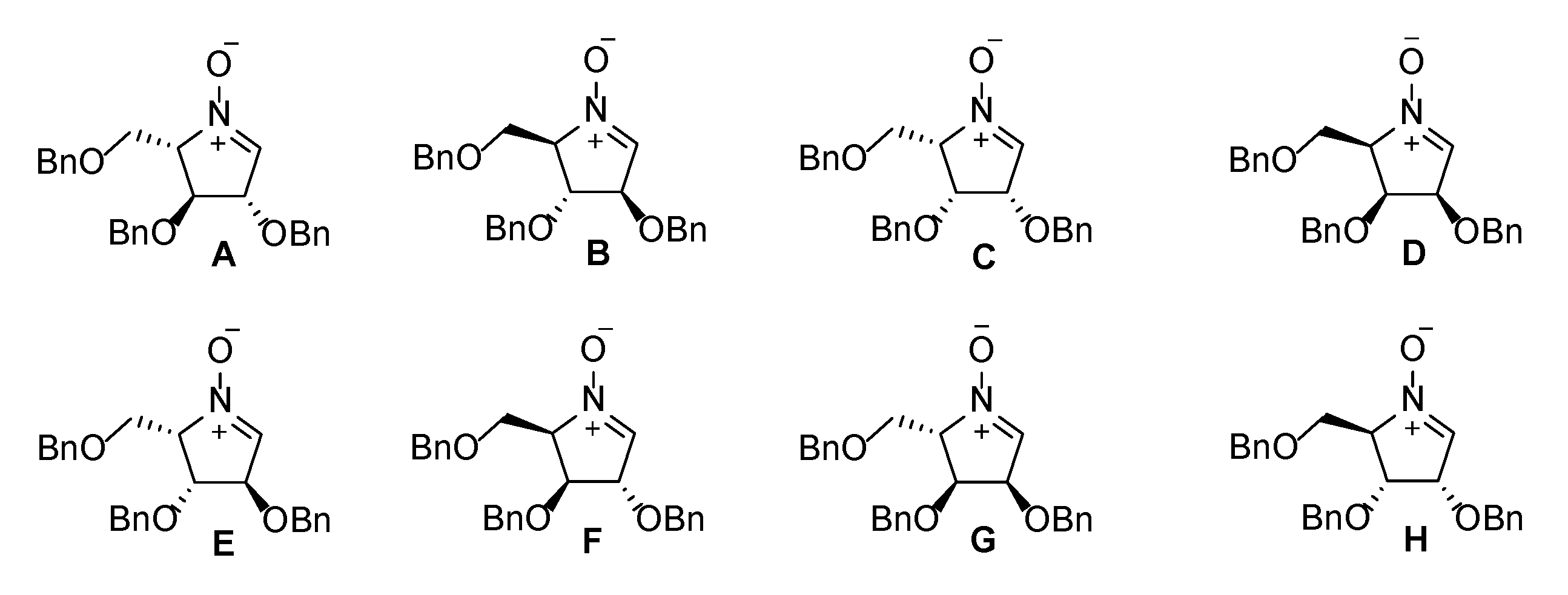
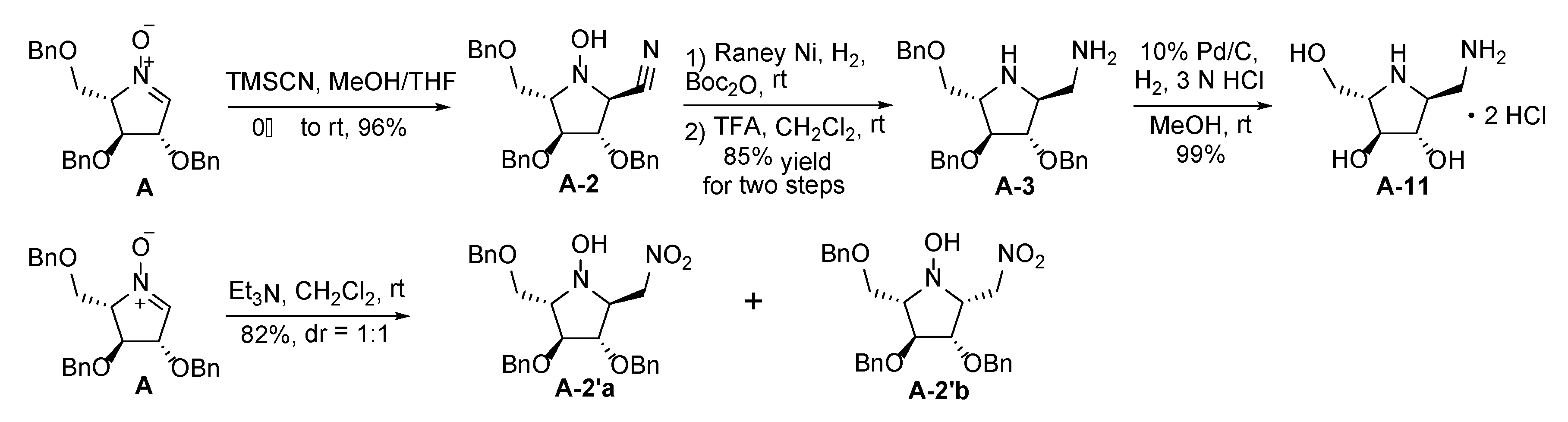
| Entry | Cyclic Nitrone | Hydroxylamine | Diamine | Monoacetylated Pyrrolidine | 1-Amino Product | 1-N-Acetylamino Product |
|---|---|---|---|---|---|---|
| 1 | 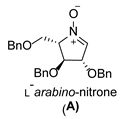 | 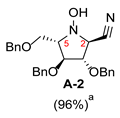 |  | 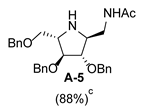 | 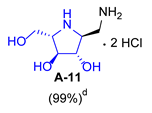 | 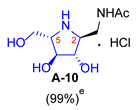 |
| 2 |  |  | 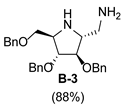 | 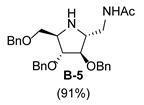 |  |  |
| 3 | 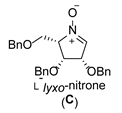 |  | 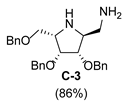 |  | 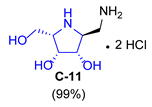 | 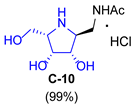 |
| 4 | 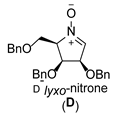 | 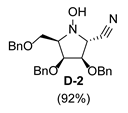 |  |  | 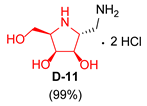 | 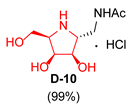 |
| 5 | 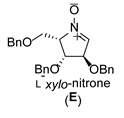 | 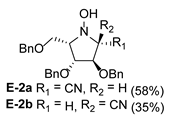 | 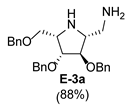 | 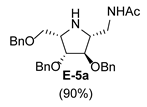 | 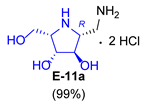 |  |
 |  |  | 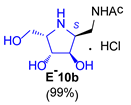 | |||
| 6 | 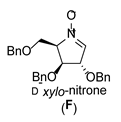 | 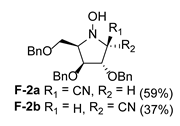 | 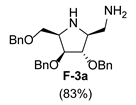 | 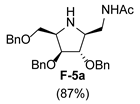 | 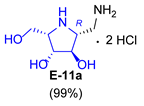 | 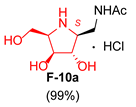 |
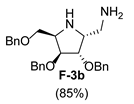 | 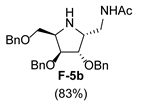 |  | 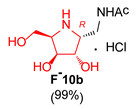 | |||
| 7 | 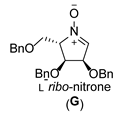 |  |  | 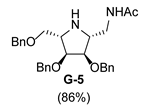 |  | 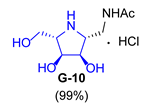 |
| 8 | 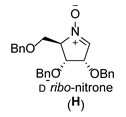 | 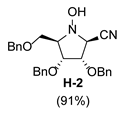 |  |  |  |  |
| Enzyme | IC50 (μM) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
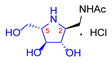 | 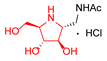 | 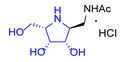 | 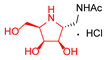 | 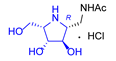 | 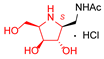 |  | 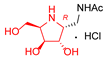 |  | 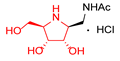 | 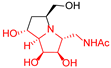 | |
| A-10 | 4·HCl | C-10 | D-10 | E-10a | F-10a | E-10b | F-10b | G-10 | H-10 | Pochonicine (1) | |
| α-Glucosidase | |||||||||||
| Yeast | NIa (0.5%)b | 450 | NI (0%) | NI (0%) | NI (0%) | NI (6.91%) | NI (0%) | NI (0%) | NI (0%) | NI (0%) | —c |
| Rice | 568 | 1000 | NI (18.2%) | NI (26.1%) | NI (15.9%) | NI (12.5%) | NI (0%) | NI (0%) | NI (0%) | NI (5.6%) | — |
| Rat intestinal maltase | 92 | 211 | NI (35.4%) | NI (9.24%) | NI (12.7%) | NI (14.6%) | NI (0%) | NI (12.7%) | NI (16.0%) | NI (13.6%) | — |
| β-Glucosidase | |||||||||||
| Almond | NI (16.1%) | 170 | NI (0%) | NI (41.0%) | NI (37.9%) | NI (11.6%) | NI (1.2%) | NI (19.8%) | NI (15.3%) | NI (17.1%) | — |
| Bovine liver | NI (2.0%) | NI (33.8%) | NI (1.0%) | NI (2.4%) | NI (4.1%) | NI (0.3%) | NI (0%) | NI (0%) | NI (0%) | NI (1.7%) | — |
| α-Galactosidase | |||||||||||
| Coffee beans | NI (0%) | NI (1.1%) | NI (0.36%) | 380 | NI (0.36%) | NI (0.36%) | NI (0%) | NI (0%) | NI (0%) | NI (0%) | — |
| β-Galactosidase | |||||||||||
| Bovine liver | NI (1.6%) | 511 | NI (1.3%) | NI (3.3%) | NI (4.9%) | NI (4.2%) | NI (3.9%) | NI (4.2%) | NI (2.9%) | NI (3.6%) | — |
| α-Mannosidase | |||||||||||
| Jack bean | NI (0%) | NI (0%) | NI (0.4%) | 205 | NI (0.7%) | NI (0%) | NI (3.8%) | NI (1.6%) | NI (4.0%) | NI (9.5%) | — |
| β-Mannosidase | |||||||||||
| Snail | NI (0%) | 296 | NI (0%) | NI (0%) | NI (25.8%) | NI (3.23%) | NI (0%) | NI (0%) | NI (0%) | NI (0%) | — |
| α-l-Fucosidase | |||||||||||
| Bovine kidney | NI (0%) | NI (1.5%) | NI (4.9%) | NI (1.7%) | NI (41.7%) | NI (8.4%) | NI (20.5%) | NI (3.9%) | NI (11.5%) | NI (22.3%) | — |
| α,α-Trehalase | |||||||||||
| Porcine kidney | NI (4.3%) | NI (20.3%) | NI (0%) | NI (0%) | NI (5.6%) | NI (3.0%) | NI (0%) | NI (0%) | NI (5.6%) | NI (40.9%) | — |
| Amyloglucosidase | |||||||||||
| A. niger | NI (5.5%) | NI (40.6%) | NI (1.9%) | NI (3.8%) | NI (4.0%) | NI (2.8%) | NI (6.4%) | NI (5.7%) | NI (7.6%) | NI (8.3%) | — |
| α-l-Rhamnosidase | |||||||||||
| P. decumbens | NI (10.1%) | NI (6.5%) | NI (3.6%) | NI (8.3%) | NI (11.8%) | NI (5.3%) | NI (30.8%) | NI (3.0%) | NI (9.5%) | NI (7.7%) | — |
| β-Glucuronidase | |||||||||||
| E. coli | NI (0%) | NI (0.6%) | NI (0.3%) | NI (0%) | NI (0%) | NI (0%) | NI (0%) | NI (0%) | NI (0%) | NI (0%) | — |
| Bovine liver | NI (4.6%) | NI (1.7%) | NI (0%) | NI (1.3%) | NI (0%) | NI (0%) | NI (4.3%) | NI (0%) | NI (0%) | NI (0%) | — |
| β-N Acetylhexosaminidase | |||||||||||
| Bovine liver | 943 | 4.7 | NI (36.3%) | 2.8 | 95 | NI (23.2%) | NI (38.4%) | 652 | 299 | NI (33.7%) | 0.021 [2] |
| HL60 | NI (12.7%) | 34 | NI (3.9%) | 10 | 591 | NI (0%) | NI (4.5%) | NI (16.6%) | NI (18.8%) | NI (0.3%) | 0.018 [2] |
| Jack bean | 202 | 0.21 | 129 | 0.12 | 10 | NI (42%) | 115 | 98 | 26 | 241 | 0.0016 [2] |
| A. oryzae | — | — | — | — | — | — | — | — | — | — | 0.33 [2] |
| Human placenta | — | — | — | — | — | — | — | — | — | — | 0.012 [2] |
| β-N-Acetylgalactosaminidase | |||||||||||
| HL60 | NI (13.0%) | 9.5 | NI (1.4%) | 8.8 | 490 | NI (0.9%) | NI (10.1%) | NI (36.1%) | NI (15.9%) | NI (5.3%) | 0.049 [2] |
| α-N-Acetylgalactosaminidase | |||||||||||
| Chicken liver | NI (0%) | NI (3.3%) | NI (6.1%) | 65.3 | NI (1.4%) | NI (0%) | NI (0.5%) | NI (2.3%) | NI (2.3%) | NI (6.1%) | NI (9.0%) [2] |
| Enzyme | IC50 (μM) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
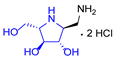 | 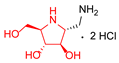 |  | 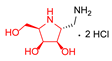 |  | 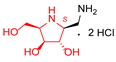 | 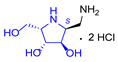 |  | 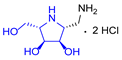 |  | |
| A-11 | B-11 | C-11 | D-11 | E-11 | F-11a | E-11b | F-11b | G-11 | H-11 | |
| α-Glucosidase | ||||||||||
| Yeast | NIa (0%)b | NI (42.7%) | NI (9.55%) | NI (4.52%) | NI (9.55%) | NI (10.1%) | NI (0%) | NI (0%) | NI (0%) | NI (0%) |
| Rice | 286 | 725 | NI (0%) | NI (8.4%) | NI (15.7%) | NI (31.5%) | NI (12.9%) | NI (16.9%) | NI (11.8%) | NI (3.4%) |
| Rat intestinal maltase | 68 | 251 | NI (37.1%) | NI (4.02%) | NI (9.95%) | NI (48.0%) | NI (10.9%) | NI (20.8%) | NI (%2.64) | NI (10.1%) |
| β-Glucosidase | ||||||||||
| Almond | NI (0%) | NI (44.4%) | NI (13.0%) | 419 | NI (12.4%) | NI (6.0%) | NI (4.8%) | NI (13.5%) | NI (2.3%) | NI (18.8%) |
| Bovine liver | NI (0%) | NI (16.2%) | NI (0.7%) | NI (3.1%) | NI (0%) | NI (0%) | NI (0%) | NI (0%) | NI (0%) | NI (3.8%) |
| α-Galactosidase | ||||||||||
| Coffee beans | NI (0%) | NI (0%) | NI (1.9%) | NI (42.2%) | NI (0%) | NI (0%) | NI (0%) | NI (0%) | NI (0%) | NI (0%) |
| β-Galactosidase | ||||||||||
| Bovine liver | NI (1.0%) | NI (36.3%) | NI (2.6%) | NI (6.5%) | NI (2.0%) | NI (0%) | NI (0%) | NI (2.6%) | NI (0%) | NI (4.2%) |
| α-Mannosidase | ||||||||||
| Jack bean | NI (0%) | NI (0%) | NI (0%) | 54 | NI (0.32%) | NI (0.97%) | NI (12.1%) | NI (0%) | NI (5.19%) | NI (3.81%) |
| β-Mannosidase | ||||||||||
| Snail | NI (0%) | NI (0%) | NI (3.35%) | NI (2.23%) | NI (2.6%) | NI (1.9%) | NI (0%) | NI (0%) | NI (0%) | NI (1.2%) |
| α-l-Fucosidase | ||||||||||
| Bovine kidney | NI (13.1%) | NI (0%) | NI (39.9%) | NI (8.0%) | NI (21.2%) | NI (46.4%) | NI (19.9%) | NI (16.2%) | NI (16.2%) | NI (16.2%) |
| α,α-Trehalase | ||||||||||
| Porcine kidney | NI (0%) | NI (4.7%) | NI (0.9%) | NI (0%) | NI (4.7%) | NI (0%) | NI (2.2%) | NI (0.6%) | NI (3.8%) | NI (0%) |
| Amyloglucosidase | ||||||||||
| A. niger | NI (0.9%) | 589 | NI (0%) | NI (0.7%) | NI (9.1%) | NI (0%) | NI (0%) | NI (0%) | NI (1.1%) | NI (2.1%) |
| α-l-Rhamnosidase | ||||||||||
| P. decumbens | NI (0%) | NI (0%) | NI (0%) | NI (0%) | NI (0%) | NI (0%) | NI (0%) | NI (1.6%) | NI (1.6%) | NI (1.3%) |
| β-Glucuronidase | ||||||||||
| E. coli | NI (0%) | NI (0%) | NI (0%) | NI (0%) | NI (0%) | NI (0%) | NI (0%) | NI (0%) | NI (0%) | NI (0%) |
| Bovine liver | NI (0.8%) | NI (0%) | NI (0%) | NI (0%) | NI (0%) | NI (5.7%) | NI (4.1%) | NI (0%) | NI (0%) | NI (4.9%) |
| β-N-Acetylhexosaminidase | ||||||||||
| Bovine liver | NI (2.1%) | NI (0%) | NI (0%) | NI (0%) | NI (0%) | NI (0%) | NI (0%) | NI (1.6%) | NI (1.6%) | NI (1.3%) |
| HL60 | NI (0%) | NI (0%) | NI (0%) | NI (3.1%) | NI (1.3%) | NI (2.7%) | NI (0%) | NI (0%) | NI (0%) | NI (0%) |
| Jack bean | NI (21.1%) | NI (14.5%) | NI (14.5%) | 99 | NI (19.3%) | NI (30.7%) | NI (23.5%) | 264 | NI (29.5%) | NI (7.2%) |
| β-N-Acetylgalactosaminidase | ||||||||||
| HL60 | NI (5.2%) | NI (3.8%) | NI (2.9%) | NI (14.8%) | NI (4.8%) | NI (6.0%) | NI (11.9%) | NI (8.1%) | NI (1.9%) | NI (0%) |
| α-N-Acetylgalactosaminidase | ||||||||||
| Chicken liver | NI (4.7%) | NI (7.5%) | NI (0.5%) | 44 | NI (0%) | NI (5.1%) | NI (0%) | NI (0%) | NI (13.6%) | NI (2.8%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Shimadate, Y.; Kato, A.; Li, Y.-X.; Jia, Y.-M.; Fleet, G.W.J.; Yu, C.-Y. Synthesis of Pyrrolidine Monocyclic Analogues of Pochonicine and Its Stereoisomers: Pursuit of Simplified Structures and Potent β-N-Acetylhexosaminidase Inhibition. Molecules 2020, 25, 1498. https://doi.org/10.3390/molecules25071498
Yan X, Shimadate Y, Kato A, Li Y-X, Jia Y-M, Fleet GWJ, Yu C-Y. Synthesis of Pyrrolidine Monocyclic Analogues of Pochonicine and Its Stereoisomers: Pursuit of Simplified Structures and Potent β-N-Acetylhexosaminidase Inhibition. Molecules. 2020; 25(7):1498. https://doi.org/10.3390/molecules25071498
Chicago/Turabian StyleYan, Xin, Yuna Shimadate, Atsushi Kato, Yi-Xian Li, Yue-Mei Jia, George W. J. Fleet, and Chu-Yi Yu. 2020. "Synthesis of Pyrrolidine Monocyclic Analogues of Pochonicine and Its Stereoisomers: Pursuit of Simplified Structures and Potent β-N-Acetylhexosaminidase Inhibition" Molecules 25, no. 7: 1498. https://doi.org/10.3390/molecules25071498
APA StyleYan, X., Shimadate, Y., Kato, A., Li, Y.-X., Jia, Y.-M., Fleet, G. W. J., & Yu, C.-Y. (2020). Synthesis of Pyrrolidine Monocyclic Analogues of Pochonicine and Its Stereoisomers: Pursuit of Simplified Structures and Potent β-N-Acetylhexosaminidase Inhibition. Molecules, 25(7), 1498. https://doi.org/10.3390/molecules25071498