Abstract
Conformational changes in amyloidogenic proteins, such as β-amyloid protein, prion proteins, and α-synuclein, play a critical role in the pathogenesis of numerous neurodegenerative diseases, including Alzheimer’s disease, prion disease, and Lewy body disease. The disease-associated proteins possess several common characteristics, including the ability to form amyloid oligomers with β-pleated sheet structure, as well as cytotoxicity, although they differ in amino acid sequence. Interestingly, these amyloidogenic proteins all possess the ability to bind trace metals, can regulate metal homeostasis, and are co-localized at the synapse, where metals are abundantly present. In this review, we discuss the physiological roles of these amyloidogenic proteins in metal homeostasis, and we propose hypothetical models of their pathogenetic role in the neurodegenerative process as the loss of normal metal regulatory functions of amyloidogenic proteins. Notably, these amyloidogenic proteins have the capacity to form Ca2+-permeable pores in membranes, suggestive of a toxic gain of function. Therefore, we focus on their potential role in the disruption of Ca2+ homeostasis in amyloid-associated neurodegenerative diseases.
1. Introduction
The oligomerization of proteins affects their conformation, shape, and function. The concept of conformational diseases (or protein misfolding diseases), highlighting the importance of protein conformational changes in the pathogenesis of disease, was first proposed in 1997 [1], and has been supported by recent studies [2,3]. Amyloidosis, a conformational disease, is characterized by the accumulation of amyloids, which are protease-resistant insoluble fibrils with β-sheet structures, in various organs [4]. Amyloidosis is associated with various neurodegenerative disorders, including Alzheimer’s disease (AD), prion disease, and Lewy body disease. In these diseases, while the disease-causing amyloidogenic proteins (β-amyloid protein (AβP) in AD, prion protein (PrP) in prion disease, and α-synuclein in Lewy body disease) differ substantially in primary sequence, they have the common ability to form cytotoxic amyloid fibrils composed of oligomers with β-pleated sheets. Furthermore, other amyloidogenic proteins involved in non-neurodegenerative diseases share common characteristics. In type 2 diabetes mellitus (T2DM), the accumulation of the islet amyloid polypeptide (IAPP), also termed amylin, is observed [5]. IAPP is a 37-residue polypeptide and is stored with insulin and Zn2+ in the pancreatic β-cell granules. It is widely accepted that the aggregation of IAPP and the resulting β-cell death contribute to the dysfunction in T2DM. Human amylin (hIAPP) forms amyloid fibrils with β-sheet structures, and is toxic to cultured hippocampal neurons and islet cells [6]. In contrast, rat amylin, which exhibits 95% similarity in primary sequence to human amylin, does not form β-sheet structures, and does not cause cytotoxicity. Moreover, IAPP and AβP exhibit similarity in the mechanism of toxicity. Both peptides activate inflammation in microglial cells, and the response induced by AβP was blocked by a nonamyloidogenic IAPP mimic [7]. Metals such as Zn2+, Cu2+, Fe2+, and Al3+ binds to IAPP and affect its conformation [8,9].
At least two mechanisms likely underlie the neurodegenerative changes observed in these diseases—the loss of normal protective functions and the gain of toxic functions. In this review, we provide an overview of the molecular mechanisms of neurodegeneration in these amyloid diseases, focusing on the common ability of these amyloidogenic proteins to bind metals and form Ca2+-permeable channel-like pores in membranes. Metal is a key determinant of protein conformation and normal brain functions. Interestingly, all of these amyloidogenic proteins are localized at the synapse, where metal ions are abundant, and play critical roles in the regulation of metal homeostasis. Accumulating evidence suggests that the disruption of metal homeostasis is a common feature of neurodegenerative diseases [10,11,12]. Accordingly, we hypothesized that the loss of normal metal regulatory functions may be involved in the pathogenesis of AD, prion diseases, and Lewy body diseases [13]. Here, based on this perspective, we review current studies on the role of these proteins in neurodegenerative diseases. Moreover, we discuss how these amyloidogenic proteins may cause Ca2+ dyshomeostasis by forming channel-like pores in membranes. We hypothesize that this gain of toxic function of amyloid oligomers may be a common neurodegenerative mechanism in amyloidsis [14]. Membrane lipid properties, including membrane fluidity and net membrane surface charge, impact the incorporation of peptides into membranes and channel formation [15]. Metals can modulate these processes by affecting the oligomerization of amyloidogenic proteins and by affecting membrane properties. Therefore, we also discuss the importance of lipid–metal, metal–protein, and lipid–protein crosstalk in this review article. These dual neurodegenerative mechanisms highlight the potential contribution of metals in the pathogenesis of AD, prion diseases, and Lewy body diseases. They may also serve as key targets for drug development for these neurodegenerative diseases.
2. Neurometals and Amyloidogenic Proteins
2.1. Neurometals in the Brain
In the brain, essential trace elements, such as iron (Fe), zinc (Zn) and copper (Cu), play critical roles in various brain functions, including neurotransmitter synthesis, neural transmission, myelination, and protection against reactive oxygen species (ROS). Deficiency of these neurometals is known to impair brain functions and cause learning and memory disorders. The levels and distributions of these neurometals differ in the various brain regions [16]. In whole-brain, Fe is the most abundant neurometal, with a concentration of ~200 μg/g (wet weight). Fe functions as a cofactor in numerous enzymes involved in important cellular processes, including oxidative phosphorylation, mitochondrial energy transfer, the synthesis of neurotransmitters (e.g., dopamine), and myelination [17]. In Fe-deficient children, retardation of mental and physical development is observed [18]. Fe is a redox-active metal, and forms ferric iron (Fe3+) and ferrous iron (Fe2+). Thus, Fe can generate ROS and can be neurotoxic. Fe in foods is absorbed as Fe2+ by binding with divalent metal transporter-1 (DMT1) in the gastrointestinal pathway. Ferroxidases, such as ceruloplasmin, convert Fe2+ to Fe3+, which is then transported in blood after binding transferrin. Thereafter, Fe3+ enters neurons or glial cells via transferrin receptors. Because several enzymes require Fe2+ as a cofactor, ferrireductases convert Fe3+ to Fe2+. Therefore, the ratio of Fe2+ to Fe3+, as well as total Fe levels, need to be tightly controlled for normal brain functioning.
Zn is the second most abundant element in the brain. Its level is estimated to be ~80 μg/g in total brain. Zn is particularly abundant in the hippocampus, amygdala, cerebral cortex, thalamus, and olfactory cortex [19]. Although much Zn is associated with metalloproteins or metal-related enzymes, a considerable amount of Zn is stored in the presynaptic vesicles of glutamatergic neurons as free Zn ions (Zn2+). These neurons secrete Zn2+ with glutamate into the synaptic cleft when activated. The secreted synaptic Zn2+ binds to various receptors, including N-methyl-d-aspartate (NMDA)-type glutamate receptors, γ-aminobutyric acid (GABA) receptors and glycine receptors. Moreover, accumulating evidence suggests that the synaptic Zn2+ plays important roles in neural information processing, synaptic plasticity, and memory formation [20]. Zn deficiency causes dwarfism and immune dysfunction in children, as well as delayed mental and physical development [21,22,23]. Moreover, Zn deficiency in adults produces learning, taste, and odor disorders.
There are two types of Zn2+ transporters; ZnT transporters, which facilitate Zn2+ efflux when in excess, and Zrt-, Irt-like protein (ZIP) transporters, which facilitate Zn2+ influx when deficient [24]. At least 14 different ZnT transporters are present in mammals. Among these, ZnT-1 has a major role in the excretion of excess Zn2+, and ZnT-3 regulates Zn2+ levels in synaptic vesicles. ZIP transporters control Zn2+ influx into cells or subcellular organs such as the Golgi apparatus or endoplasmic reticulum (ER). Disorders of Zn2+ transporters have been shown to cause Ehlers–Danlos syndrome and other human diseases [25]. Metallothioneins (MTs), such as MT1, MT2, and MT3, also regulate Zn2+ homeostasis [26]. MT3 is of particular interest because it is expressed in the brain, and is decreased in AD patient brains [27,28].
Cu is the third most abundant element in the brain. Cu plays a critical role as a cofactor in key enzymatic reactions in the brain. Cu is a component of ferroxidase (ceruloplasmin) and Cu/Zn superoxide dismutase (Cu/Zn-SOD) [29]. Thus, Cu is essential for the maintenance of Fe homeostasis and protection against ROS. Moreover, some neurons have been reported to contain free Cu2+ in synaptic vesicles, as well as Zn2+. The synaptic Cu2+ is released into synaptic clefts during neuronal firing and binds to NMDA-type glutamate receptors and other receptors. Cu is present as Cu2+ and Cu+, and therefore, excess free Cu is strictly regulated because it participates in the production of ROS, which can result in cytotoxicity. Both Cu deficiency and overaccumulation caused by a perturbation in Cu transport can lead to severe neurodegenerative disorders, as in Wilson’s disease and Menkes disease [30].
Although the levels of these neurometals in the cerebrospinal fluid (CSF) are reportedly about 1 μM or less [31,32], it is plausible that these metals can be concentrated in synaptic clefts. Because each synaptic cleft is regarded as a small compartment with a 120-nm radius and a 20-nm height, and that the total volume of the synaptic clefts is estimated to be about 1% of the extracellular space of the brain [33]. Indeed, Zn and Cu levels in the synaptic clefts are estimated to be 1–100 μM and ~15 μM, respectively [34,35].
2.2. Alzheimer’s Disease and Neurometals
It is widely accepted that the disruption of neurometal homeostasis is involved in the pathogenesis of AD, prion diseases, dementia with Lewy bodies (DLB), and vascular dementia (VD) [10,11,12,36]. Among these, AD, VD, and DLB are classified as senile-type dementia. AD is characterized by the loss of neurons and synapses in the hippocampus or cerebral cortex, the extracellular accumulation of senile plaques, and intraneuronal neurofibrillary tangles (NFTs) [37]. The major constituent of senile plaques is AβP, while that in NFTs is phosphorylated tau protein. Although the cause of AD still remains unclear, the amyloid cascade hypothesis posits that AβP-induced neurodegeneration plays a major role in AD pathogenesis, and is supported by numerous studies [38].
AβP is derived from a large precursor protein (amyloid precursor protein, APP) as a small 39–43-amino acid peptide. The excision of the N-terminal of AβP from APP is regulated by β-secretase (BACE), while cleavage of the C-terminal is controlled by γ-secretase. The APP gene is mutated in early-onset cases of AD [39]. Presenilin, a γ-secretase, has been shown to control the production of various truncated AβPs [40]. Mutations in presenilin genes impact the production of truncated AβPs, and are found in cases of early-onset familial AD [41].
AβP(1–40), the first 40 amino acid residues of AβP, was reported in 1991 to cause neurotoxicity in vitro as well as in vivo [42]. AβP tends to form oligomers with AβP-pleated sheet structure in solution. Although the solutions of freshly prepared AβP(1–40) are non-toxic, the solutions of aged AβP(1–40) (aggregated by incubation at 37 °C for several days) cause severe neurodegeneration in cultured rat cortical neurons [43]. The β-sheet content in AβP is correlated with the degree of neurotoxicity [44]. Truncated AβP, such as AβP(1–42), has been shown to seed aggregates and enhance the oligomerization of AβP(1–40) [45].
Recent studies using size-exclusion chromatography, gel electrophoresis and atomic force microscopy have demonstrated that monomers and amyloid fibrils are non-toxic, and that soluble oligomers are synaptotoxic and neurotoxic [46,47]. AβP oligomers derived from the CSF of AD patients induce the loss of dendritic spines and synapses, and the blockade of long term potentiation (LTP). Given that synapses play a key role in memory formation, these observations suggest that synaptic dysfunction induced by AβP oligomers, but not monomers or fibrils, is a critical pathogenetic event in cognitive impairment in AD.
The levels of AβP in the CSF of non-demented elderly subjects and AD patients are similar; however, AβP is also present in the CSF of young individuals [48]. Therefore, factors that influence oligomerization are important for AβP toxicity. These factors include the composition of solvents, temperature, metals, and the oxidation state and racemization of AβP [49,50]. Among the acceleratory factors, metals are known to cause conformational changes in various proteins by binding to amino acids such as His, Tyr, and Arg or with phosphorylated amino acids. Using western blotting and HPLC, we showed that aluminum (Al) significantly enhances AβP oligomerization compared with other metals [51,52]. Zn2+ and Cu2+ also reportedly bind AβP and accelerate its aggregation [53,54]. Moreover, Mn2+ also binds N-terminal domain of AβP, enhancing oligomerization [55]. Interestingly, senile plaques are rarely detected in the rodent brain, compared with primate brain [56], despite the fact that primate and rodent AβP proteins differ only in three amino acids (Arg5, Tyr10, and His13). Given that all three of these amino acids are involved in metal binding, it is possible that metals contribute significantly to the oligomerization of AβP and the formation of senile plaques.
APP is also a metal-binding protein, and possesses two Zn2+ and/or Cu2+ binding domains in its N-terminal, and reportedly can convert oxidized Cu2+ to the reduced Cu+ form [57]. Both Zn2+ and Cu2+ are implicated in the dimerization and trafficking of APP [58], as well as APP expression, processing, and the production of AβP [59]. Additionally, APP reportedly regulates Fe2+ efflux from cells by binding to ferroportin, an iron transporter [60]. Notably, transferrin C2 genotype, iron transporters, and the hemochromatosis gene are important risk factors for AD [61]. The supplementation of Fe has reportedly beneficial roles for cognitive functions in AD patients [62]. Furthermore, Fe regulates the expression of several proteins that possess the iron-response element (IRE) sequence in their mRNAs by binding to iron regulatory proteins (IRPs) [63]. The mRNA for APP contains an IRE domain similar to ferritin and other Fe-binding proteins [64]. Therefore, APP can regulate the levels of these neurometals, and vice versa, these neurometals can control APP expression. Other AD-related proteins, presenilins, are also reportedly metal-binding proteins that are involved in the uptake of Zn2+ and Cu2+ [60]. Zn2+ and Cu2+ affect several functions of the presenilins, including trafficking, γ-secretase activity, and APP processing [65].
2.3. Prion Diseases and Neurometals
Prion diseases include scrapie in sheep, bovine spongiform encephalopathy (BSE) in cow, Creutzfeldt–Jakob disease (CJD), Gerstmann–Sträussler–Scheinker syndrome (GSS), and Kuru in human. In patients with prion diseases, the spongiform degeneration of glial cells and neurons, and the accumulation of the abnormal scrapie-type isoform of PrP (PrPSc) are commonly observed [66]. Normal prion protein (PrPC), a 30–35-kDa cell surface glycoprotein, is widely distributed in the brain. PrPC and PrPSc share the same chemical characteristics with the same primary sequence, except that PrPSc is protease-resistant, insoluble, and with a high β-sheet content. The contamination of PrPSc from foods or iatrogenic factors can promote normal PrPC to misfold and aggregate as PrPSc, resulting in disease. Thus, these diseases are also called transmissible spongiform encephalopathies (TSE), and the conformational change to PrPSc is crucial for disease pathogenesis.
The link between PrPC and metals is of great interest. Decreased levels of Cu and reduced activity of Cu-dependent enzymes have been reported in the brain of PrP-knockout mice [67]. PrPC attenuates hydrogen peroxide-induced neurotoxicity, as PrPC mimics Cu/Zn-SOD [68]. PrPC reportedly binds four Cu atoms in the octarepeat domain (-PHGGGWGQ-) in its N-terminal. Cu can also bind to His96 and His111 [69]. Huang et al. found that PrPC binds to α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA)-type and/or NMDA-type glutamate receptors and affects their function in a Cu-dependent manner [70]. Cu reportedly also affects the expression and cellular trafficking of PrPC [71], as well as physiological functions such as neurite outgrowth [72].
Other metal ions, such as Zn2+, Mn2+ and Ni2+ can bind to PrPC [69]. PrPC is evolutionarily related to ZIP-type Zn2+ transporters [73]. PrPC binds to the AMPA-type glutamate receptor in postsynaptic membranes and enhances the cellular uptake of Zn2+, and may function as a Zn2+ sensor at the synapse [74]. Additionally, PrPC functions as a ferrireductase that converts Fe3+ to Fe2+ [75], which can be then transported by the DMT1/ZIP14 complex [76]. Indeed, altered Fe metabolism and Fe deficiency are observed in PrP-knockout mice [77], and altered levels of ferroxidase and transferrin in the CSF of CJD patients have also been reported [78]. Intriguingly, several studies suggest that Mn may facilitate prion diseases. Mn enhances the stability of PrP in soils and increases its infectivity [79], and an epidemiological relationship between the pathogenesis of CJD and an imbalance in Mn has been suggested [80].
2.4. Lewy Body Diseases and Neurometals
Lewy body diseases include Parkinson’s disease (PD), DLB, and multiple system atrophy. The pathological hallmarks of Lewy body diseases are the abnormal intracellular accumulation of Lewy bodies, which are composed of α-synuclein [81]. α-Synuclein is a 140-amino acid protein that is abundantly localized to presynaptic terminals. α-Synuclein has been shown to play critical roles in synaptic functions and in the maintenance of synaptic plasticity. It was also shown that non-amyloid component (NAC), the α-synuclein fragment peptide, co-accumulates with AβP in senile plaques in AD [82]. Therefore, the oligomerization of α-synuclein is considered to contribute to the pathogenesis of Lewy body diseases.
The link between α-synuclein and metals has been extensively studied, particularly as Fe-rich regions (such as the substantia nigra) are vulnerable in PD, and patients with Mn toxicity exhibits Parkinson-like symptoms. α-Synuclein reportedly binds Cu2+ and other metal ions in its N and C-terminal domains [83]. The His50 residue plays a key role in the interaction between Cu2+ and α-synuclein [84], which is intriguing, given that mutation of this residue is implicated in familial PD. α-Synuclein can also bind to other metals, including Al and Mn, which enhance its oligomerization [85]. Moreover, α-synuclein is a ferrireductase that converts Fe3+ to Fe2+, similar to PrPC [86]. This indicates that α-synuclein controls dopamine synthesis by providing bioavailable Fe2+ to tyrosine hydroxylase and other enzymes. Indeed, the levels of Fe as well as the ratio of Fe2+ to Fe3+ are reportedly altered in the brains of PD patients [87]. The expression of α-synuclein is controlled by Fe levels, because its mRNA possesses an IRE domain similar to APP and ferritin [88]. Mn reportedly induces the overexpression of α-synuclein [89].
2.5. Hypothesis: Loss of Normal Regulatory Functions of Amyloidogenic Proteins in the Synapse
As described in the previous section, amyloidogenic proteins share several common characteristics. All of these amyloidogenic proteins or related proteins (APP or presenilins) possess metal-binding domains and play major roles in the maintenance of metal ion homeostasis. Furthermore, all of these proteins are reportedly co-localized at the synapse, where metals are abundantly present, and which is a major site of degenerative change in these diseases. Although some APP reportedly is present in the postsynaptic membrane and regulates spine formation [90], it is primarily localized to the presynaptic membrane [91]. PrPC is localized to the postsynaptic membrane with receptors [92], and α-synuclein is mainly found in the presynaptic cytosol. Presenilins are mainly localized in the ER, and in the presynaptic and postsynaptic membranes.
We have developed a hypothetical model of the crosstalk between these amyloidogenic proteins and metals (Figure 1). During neuronal excitation, Zn2+ and/or Cu2+ are released into the synaptic clefts. The level of synaptic Zn2+ is mainly controlled by ZnT-1 transporter, which is primarily localized at postsynaptic membranes [93]. PrPC acts as an analogue of ZIP transporters and also contributes to the regulation of synaptic Zn2+ levels. APP and PrPC regulate the levels of Fe2+ and Cu2+ in the pre-synapse and post-synapse, respectively. Presenilins also participates in the maintenance of Cu2+ and Zn2+. α-Synuclein controls the Cu2+ or Mn2+ levels in the presynaptic domains. PrPC functions as a ferrireductase to convert Fe3+ to Fe2+ in the postsynaptic domain, and regulates Fe2+ influx through the DMT1/ZIP14 complex [76]. α-Synuclein acts as a ferrireductase presynaptically. Both proteins control neurotransmitter synthesis and other Fe2+-requiring functions in the pre-synapse and post-synapse. In turn, the expression of these amyloidogenic proteins is controlled by the levels of these neurometals.
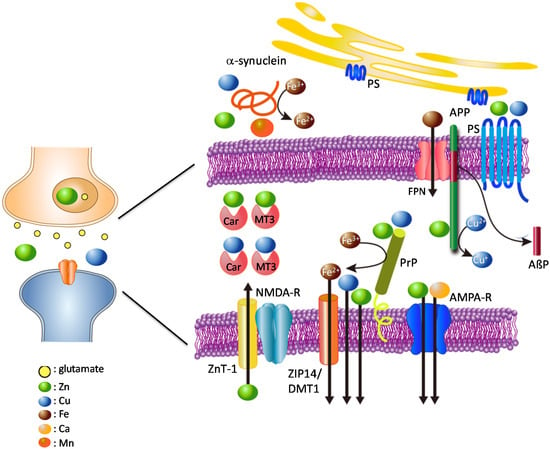
Figure 1.
Hypothesis: loss of normal functions of amyloidogenic proteins at the synapse.
Given the narrow distance between the pre- and postsynaptic membranes (~20 nm), it is plausible that these proteins can interact with each other. Indeed, PrPC reportedly binds to AβP oligomers and controls its toxicity [94]. α-Synuclein influences the processing of APP as well as AβP secretion [95]. Meanwhile, AβP promotes seeding and spreading α-synuclein [96]. It is also possible that PrPC affects the production of AβP by providing Cu to APP. Carnosine (β-alanyl histidine), which is synthesized and secreted from glial cells [97,98], and MT3 [27,28] may also participate in the maintenance of metal homeostasis at the synapse.
The crosstalk between metals and amyloidogenic proteins described here is highly complex. Therefore, when crosstalk is perturbed by genetic or environmental factors, the resulting disruption of metal homeostasis can trigger various adverse changes and cause neurodegenerative diseases. For example, pathogenetic PrPSc from contaminated foods causes the depletion of neuroprotective PrPC, and initiates oxidative damage by increasing Cu and Fe, thereby increasing susceptibility to ROS, causing the depletion of neurotransmitters, and inducing the degeneration of neurons and glial cells. The metal imbalance caused by excess Mn2+ triggers the toxic accumulation of Fe by affecting IRE–IRP binding and by inhibiting the expression of APP and ferritin [99].
APP is mainly localized to the presynaptic membrane. PrPC is located in the postsynaptic membrane bound to receptors. APP binds to Cu2+ and/or Zn2+ and has the ability to convert Cu2+ to Cu+. APP also regulates Fe2+ efflux from cells via ferroportin. Presenilins function as γ-secretases, and affect the uptake of Zn2+ and Cu2+. PrPC binds to Cu, Zn, and Fe and regulates their levels at the synapse. Additionally, PrPC acts as a ZIP Zn2+ transporter analogue, and ZnT-1 Zn2+ transporter is also localized to postsynaptic membranes; both control Zn2+ levels at the synapse. α-Synuclein is mainly localized to the presynaptic domain, and binds Cu, Mn, and Fe. Both PrPC and α-synuclein have ferrireductase activity, and provide bioavailable Fe2+ to enzymes at the pre- and post-synapse, respectively. Fe2+ is transported into cells by the complex of ZIP-14 and DMT-1. Other metal-binding factors such as MT3 and carnosine (Car) are secreted into synaptic clefts and play critical roles in the maintenance of metal homeostasis.
NMDA-R; NMDA-type glutamate receptor, AMPA-R; AMPA-type glutamate receptor, FPN: ferroportin; PS: presenilins; colored circles represent Zn, Cu, Fe, Mn, Ca, and glutamate.
3. Neurotoxicity by Amyloidogenic Protein Oligomers
3.1. AβP-Induced Ca Dyshomeostasis
The preceding discussion focused on neurodegeneration caused by the loss of the normal function of amyloidogenic proteins (Figure 1). Now, we focus on neurodegeneration caused by the gain of neurotoxic function of the amyloid oligomers of these proteins. Oligomerized AβP reportedly has neurotoxic effects, including synaptotoxicity [100]. PrPSc as well as its peptide fragment (PrP106–126) cause synaptotoxicity and cellular toxicity in vitro and in vivo [101]. Oligomerized α-synuclein is also toxic, and its fragment peptide (NAC) causes neurotoxicity [102].
The molecular mechanism of neurodegeneration induced by oligomerized proteins is of considerable interest. Numerous studies have demonstrated that AβP induces various adverse changes, such as ROS production, cytokine induction, induction of ER stress, and disruption of Ca2+ homeostasis [103,104]. Among these, the disruption of Ca2+ homeostasis, especially the abnormal increase in intracellular Ca2+ levels ([Ca2+]i) is considered a key pathogenetic event, because Ca2+ ions play critical roles in normal brain functions. Perturbed Ca2+ homeostasis leads to various adverse events, including the activation of the calpain and caspase pathways, and disruption of numerous key enzymatic reactions. Ca2+ is also involved in the processing of APP, as well as tau phosphorylation [105]. The Ca2+ dyshomeostasis ultimately leads to membrane disruption, lipid peroxidation, ROS production, and apoptosis. These changes are also observed after exposure to AβP. The disruption of Ca2+ homeostasis and the elevated [Ca2+]i have been reported in AD patients and in AβP-exposed neurons [106,107,108,109].
Studies by others and our group suggest that AβPs directly incorporate into the membrane and form channels (pores) that allow the passage of Ca2+ and other cations, and that the resulting change in [Ca2+]i may be a primary pathogenetic event in AβP neurotoxicity [110,111,112]. Neurotoxic peptide fragments of AβP, including AβP(1–40), AβP(25–35) and AβP(1–42), have been demonstrated to incorporate into artificial planar lipid membranes and form giant multi-level pores (~5 nS) with cation (including Ca2+) selectivity [113,114,115]. To assess the validity of this amyloid channel hypothesis, we performed an electrophysiological study to examine whether AβPs form channels on neuronal cell membranes as well as on liposomes. For this experiment, we employed membrane patches from immortalized hypothalamic neurons (GT1–7 cells), which are derived from murine hypothalamic neurons [116]. Within 3–30 min of the addition of AβP(1–40), a channel current was detected across the excised membranes as well as the liposome membranes. The activity of the channel in both preparations was inhibited by the addition of Zn2+, and recovered by a Zn2+ chelator, o-phenanthroline [117,118]. AβP(1–40), as 5–8-mer oligomers, forms pore-like structures in the membranes, as revealed by 3-D computer simulations [119]. Nuclear magnetic resonance (NMR) studies show that AβP(1–42) pores are composed of tetrameric and hexameric oligomers [120]. Voelker et al. demonstrated that AβP(1–40) as well as AβP(1–42) formed trimers to pentamers that could form ion channels in water [121]. AβPs were shown to form pores by high resolution atomic force microscopy (AFM) as well [122]. Lee et al. observed the formation of AβP channels on membranes from brain total lipid extracts using AFM [123]. Pores or pore-like structures consisting of AβP have also been observed in AD patients [124,125,126].
We also demonstrated that exposure to AβP causes an increase in [Ca2+]i in GT1–7 cells using multi-site fluorometry with fura-2, a Ca2+-sensitive fluorescent dye [15,127,128,129,130]. A robust increase in [Ca2+]i occurs in many GT1–7 cells after exposure to AβP(1–40) (Figure 2A). Figure 2B shows the temporal changes in [Ca2+]i in these cells during the exposure. Exposure to both AβP(1–40) (line (a)) and AβP(1–42) (line (c)) causes a marked increase in [Ca2+]i. However, AβP(40–1), a reverse-sequence peptide with no neurotoxicity, caused no change in [Ca2+]i (line (b)).
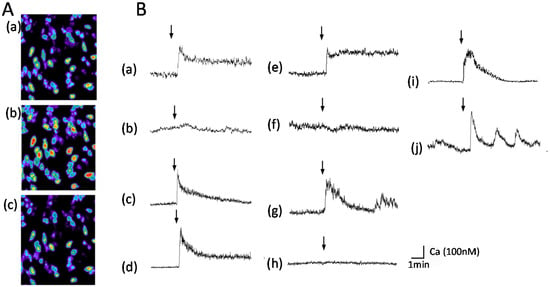
Figure 2.
Temporal changes [Ca2+]i in GT1-7 cells after exposure to amyloidogenic proteins. (A) Temporal changes in [Ca2+]i shown as pseudocolor images. AβP(1–40) (10 µM) was applied to GT1–7 cells, and temporal changes in fluorescence intensities corresponding to changes in [Ca2+]i were analyzed by multi-site fluorometry using fura-2. (a): 1 min before exposure to AβP(1–40); (b): 20 s after exposure; (c): 5 min after exposure. (B) Typical time course of [Ca2+]i changes before and after exposure to each amyloidogenic protein (10 µM). (a) AβP(1–40); (b) AβP(40–1); (c) AβP(1–42); (d) D-AβP(1–40); (e) PrP106–126; (f) scramble PrP106–126; (g) human amylin (hIAPP); (h) rat amylin; (i) NAC; (j) magainin 2. The arrow indicates the time of peptide addition (modified from Ref. No. 15 with permission).
Despite the empirical evidence, the amyloid channel hypothesis still remains controversial. There are several other mechanisms by which AβPs could interact with neurons and disrupt calcium homeostasis. These include the activation of some type of cell surface receptor coupled to Ca2+ influx, and the disruption of membrane integrity [107]. AβPs were reported to bind to NMDA and AMPA glutamate receptors [130], as well as nicotinic acetylcholine receptors [131], and all of these receptors are highly Ca2+-permeable. Furthermore, AβP reportedly influences voltage-gated Ca2+ channels and the inositol triphosphate (IP3) receptor [132]. Furthermore, presenilins have been implicated in capacitative Ca2+ entry, ER Ca2+ signaling, and mitochondrial Ca2+ signaling, and that mutations in these proteins affect Ca2+-regulated functions [133].
Nonetheless, despite the controversy, we have demonstrated that D-AβP(1–40), composed of D-amino acid residues only, also caused an increase in [Ca2+]i, similar to L-AβP(1–40) (line (d)). D-AβP(1–40) reportedly possess similar toxicity to L-AβP(1–40) [134], as well as the ability to form pores [135]. The AβP-induced increase in [Ca2+]i was not influenced by the addition of the Na+ channel blocker tetrodotoxin, the Ca2+ channel blocker nifedipine, an antagonist of the NMDA receptor (D-APV), or an antagonist of the GABA receptor (bicuculline) [15]. Moreover, our detailed quantitative analysis using multi-site Ca2+ fluorometry suggests the AβP-induced rise in [Ca2+]i was highly heterogeneous among the GT1-7 cells, although this cell line is genetically homogenous. As shown in Figure 2A, the exposure to the same peptide solution produced different changes in the magnitude and latency in [Ca2+]i in cells in the same field of view [15,129]. This suggests that AβP-induced [Ca2+]i changes are mediated by unregulated amyloid channels, and not by endogenous receptor-mediated pathways.
Membrane properties, such as the surface net charge and fluidity, influence the interactions between proteins and membranes, and accordingly, channel formation [136]. In particular, the negative charge of the membrane outer surface is critical for the incorporation of AβP, as the peptide normally possesses a positive charge [137]. The ratio of cholesterol to phospholipids alters the membrane fluidity and influences the oligomerization process as well. We have demonstrated that the pre-administration of several compounds that affect membrane fluidity and membrane potential, including cholesterol, phloretin, and estradiol, markedly inhibit AβP-induced [Ca2+]i increases in GT1-7 cells [128,129]. Gangliosides, which possess a negative charge (from their sialic acid residues), may influence these processes. GM1 gangliosides were reported to bind to AβP in AD brains [138]. It was also suggested that GM1-bound AβP behaves as a “seed” for oligomers and enhances AβP oligomerization [139,140]. Our AFM imaging study demonstrated the deposition of AβP(1–40) on ganglioside (GM1)/phospholipid monolayers [15].
3.2. Channel Formation by Other Amyloidogenic Proteins
The ability to form Ca2+-permeable pores is also found in certain toxins and venoms. The α-toxin of Staphylococcus aureus possesses hemolytic activity by forming pore-like hexamers composed of 33-kDa subunits with β-sheet structures, which allow Ca2+ influx [141]. Other antimicrobial peptides, such as magainin 2 (a 26-residue antimicrobial peptide obtained from the skin of Xenopus laevis), melittin (a 28-residue peptide from bee venom), and antibiotics such as amphotericin and gramicidin, were also reported to form Ca2+-permeable pores on cell membranes and to cause cell lysis [142]. The electrophysiological activity of gramicidin and amphotericin on membranes excised from hippocampal neurons was shown to be similar to that of AβP(1–40) [143]. Thus, it is possible that AβP has similarities to these pore-forming venoms, toxins, and antibiotics. Indeed, AβP reportedly possesses antimicrobial activity [144].
Accumulating evidence demonstrates that other amyloidogenic proteins, including PrPSc and α-synuclein, form amyloid channels (pores). Similar to AβP, PrP106–126 reportedly forms cation-permeable pores in artificial lipid bilayers [145]. Kourie et al. demonstrated that channels formed by PrP106–126 are Cu-sensitive cation channels [146]. Lashule et al. observed that α-synuclein forms annular pore-like structures similar to AβP(1–40) by electron microscopy [147]. The pore formation by α-synuclein on mitochondrial membranes was reportedly accelerated by cardiolipin, a membrane lipid [148].
Other amyloidogenic proteins involved in non-neurodegenerative diseases can form channel structures as well. Human amylin (hIAPP) reportedly forms ion channels on liposomes, meanwhile non- toxic rat amylin does not form ion channels [149]. Similar to AβP, the membrane composition such as cholesterol and raft affects fiber growth of IAPP and the membrane damage [150,151]. It was also demonstrated that the membrane curvature plays critical roles in the amyloid formation of IAPP and the resulting membrane damages [152]. Calcitonin, linked with medullary thyroid carcinoma, also forms pore-like structures on membranes [153]. The AFM imaging study demonstrated that α-synuclein, hIAPP, and other amyloidogenic proteins form annular pore-like structures on bilayer membranes, similar to AβP [154]. We found that these amyloidogenic proteins also cause an increase in [Ca2+]i, similar to AβP(1–40), as shown in Figure 2B. A marked increase in [Ca2+]i is observed after exposure to PrP106–126 (line (e)), hIAPP (line (g)), NAC(line (i)), and magainin 2 (line (j)). However, control peptides such as scrambled PrP106–126 (a peptide with a random sequence; line (f)) and rat amylin (line (h)) did not cause notable changes. Furthermore, PrP106–126, hIAPP and AβP(1–40) induce membrane disruption in liposomes [15].
3.3. Hypothesis: The Gain of Toxic Functions of Amyloid Oligomers at the Synapse
Based on the findings described above, we propose the amyloid channel hypothesis for AD pathogenesis (schematically shown in Figure 3). We also posit that similar mechanisms may underlie other diseases, such as prion diseases and Lewy body diseases, given their commonalities.
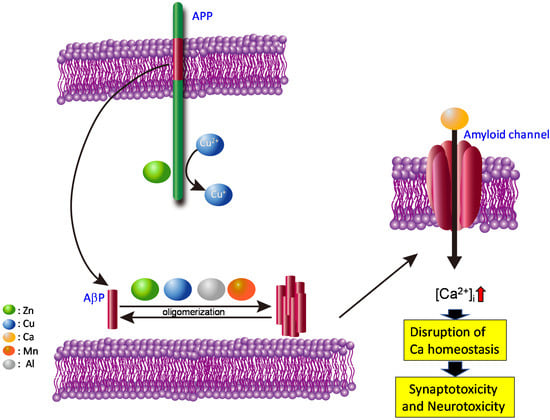
Figure 3.
Hypothesis: gain of toxic functions of amyloid oligomers at the synapse.
AβPs are secreted from APP into synaptic clefts, and are usually degraded rapidly by proteases. However, increased AβP secretion or an altered ratio of AβP(1–42) to AβP(1–40), caused by mutations in APP and/or presenilins, may increase the half-life (and accordingly, the concentration) of AβP in the brain. The contamination of toxic Al causes the up-regulation of APP and the increased amount of AβP [155]. The accumulated AβP peptides then aggregate and form pore-like channels on membranes. Because these AβP channels are unregulated, Ca2+ flows freely through them, eventually triggering various neurodegenerative processes including ROS formation, disturbances in capacitive Ca2+ in the ER, and tau phosphorylation. Additionally, the elevation in [Ca2+]i can result in the overproduction of APP and the increased production of AβP, ultimately inducing a vicious cycle of unregulated Ca2+ influx.
Our hypothesis can explain the long delay in AD development. Although AβP is normally secreted in young and in non-demented elderly subjects, AD occurs only in senile subjects. Net charges on the membrane outer surface influence the rate of channel formation by affecting the binding of proteins. Given that positively charged and neutral phospholipids (e.g., phosphatidylcholine) are normally present on the outer membrane surface, the membrane in normal and young brains seldom bind AβP, because it possesses a positive charge. However, when the asymmetric distribution is disrupted during apoptotic death, and the negatively charged phospholipids, such as phosphatidylserine, are exposed on the surface, AβP peptides can easily bind membranes and form channels. This hypothesis may also explain the contribution of various environmental factors as well as genetic factors to AD pathogenesis. It is well known that the ratio of cholesterol to phospholipids is altered by various environmental and genetic factors (e.g., foods, apolipoprotein E genotype), and is linked with AD pathogenesis. Cholesterol alters membrane fluidity and influences protein oligomerization, thereby impacting channel formation. Polyunsaturated fatty acids such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) also affect these processes by altering membrane fluidity. We demonstrated that dehydroepiandrosterone (DHEA), an endogenous testosterone, attenuates the AβP-induced increase in [Ca2+]i by influencing membrane fluidity [129].
Because AβP dimers form toxic protofibrils rapidly, compared with monomeric AβP [156], it is possible that metals such as Al3+, Zn2+, Cu2+ and Mn2+ accelerate oligomerization and thereby increase the rate of channel formation. The involvement of metals in pore formation by AβP and neurotoxicity is complex. Recent studies demonstrate that not all oligomers are equally neurotoxic, although all possess AβP-pleated sheet structure. The characteristics (size and shape) of AβP oligomers formed in the presence of Al3+, Zn2+, Cu2+ and Fe3+ are unique by morphological analysis using AFM [157]. Moreover, Sharma et al. revealed that Zn2+-aggregated AβPs are less toxic than Cu2+-aggregated AβP [158]. Although Cu2+ accelerates the oligomerization of AβP, it reportedly inhibits the oligomerization of human amylin and PrP106-126 [9,159]. In addition, our group and others have demonstrated that the amyloid channel is blocked by Zn2+ [116,117]. Given that Zn2+ is secreted into synaptic clefts in an activity-dependent manner, Zn2+ may therefore protect neurons against AβP toxicity [160]. Similar biphasic effects of Zn2+ is observed also in T2DM. Zn2+, which is co-secreted with insulin and IAPP from the pancreatic β cells, has a dual effect on the aggregation and cell toxicity of IAPP in a concentration-dependent manner [161]. Zn2+ at the physiological concentration inhibits the aggregation of IAPP, meanwhile, atypical (low or high) concentrations of Zn2+ enhance the aggregation.
Metals modulate the interaction between lipids and amyloidogenic proteins. Because gangliosides are negatively charged, the lipid raft may provide a platform for oligomerization. Indeed, Zn2+ or Cu2+ reportedly influences membrane fluidity [162,163]. Curtain et al. showed that Zn2+ and Cu2+ affects the membrane insertion of AβP peptides [164]. Membrane-bound metal ions such as Ca2+, Mn2+ and Zn2+ are essential for the toxicity of pore-forming toxins [165].
In pathological conditions, such as the genetic overexpression of APP accumulated AβP peptides can directly incorporate into membranes and form amyloid channels. Metals such as Al2+, Zn2+, Cu2+ and Mn2+ enhance the oligomerization of AβP and facilitate the process. Thereafter, disruption of Ca2+ homeostasis by these amyloid channels triggers various apoptotic pathways, and causes synaptotoxicity and neurotoxicity. These changes contribute to the pathogenesis of AD. Colored circles represent Zn, Cu, Ca, Mn, and Al.
4. Conclusions
Our working hypothesis, shown in Figure 1 and Figure 3, may explain the two aspects of neurodegeneration—loss of the normal metal regulatory function of amyloidogenic proteins, and gain of the toxic function of amyloidogenic proteins. As discussed here, the crosstalk between neurometals and amyloidogenic proteins at the synapse is highly complex and vulnerable to perturbation. The loss of normal functions of amyloidogenic proteins causes the disruption of metal homeostasis, resulting in neurodegeneration. Furthermore, the conformational change in amyloidogenic proteins and the formation of unregulated Ca2+ channels is a gain of toxic function that contributes to the pathogenesis of these neurodegenerative diseases. These dual facets of neurotoxicity highlight the major role of trace elements in the pathogenesis of amyloid-associated neurodegenerative diseases. Recently, La Rosa et al. exhibited the similarities of oligomerization patterns and structures among hIAPP, AβP and other amyloidogenic proteins using the theoretical model [166]. These similarities in the conformations of various amyloidogenic proteins may strengthen our hypothesis about the common toxic mechanism in amyloidosis.
Our hypothesis also has important implications for the development of drugs for neurodegenerative diseases. It is possible that compounds that regulate metal homeostasis and block amyloid channels may have potential for the treatment of neurodegenerative diseases. Indeed, we found that carnosine attenuates Zn2+-induced neurotoxicity [167]. Given that carnosine inhibits oligomerization and attenuates AβP-induced neurotoxicity [168] and PrP106–126-induced neurotoxicity [159], it may have therapeutic potential for amyloid diseases. Further research is needed to test this working hypothesis, particularly about the link between lipids and metals.
Author Contributions
Wrote or contributed to the writing of the manuscript: M.K. and M.K.-N; Participated in research design: M.K., K.-i.T., and M.K.-N.; Conducted experiments: K.-i.T. and M.K.-N. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by a Grant-in Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. (JSPS Kakennhi Grant numbers. JP26460177 and JP 17H03197).
Acknowledgments
We thank Barry Patel, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Conflicts of Interest
All of the authors declare there is no conflicts of interests.
Abbreviations
AD: Alzheimer’s disease, AβP; Alzheimer’s β-amyloid protein, AFM; atomic force microscopy, AMPA; α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid, CSF; cerebrospinal fluid, D-APV; 2-amino-5-phosphonovalerate, DMT1; divalent metal transporter-1, ER; endoplasmic reticulum, GABA; γ-aminobutyric acid, [Ca2+]i; intracellular calcium levels, NMDA; N-methyl-d-aspartate, ROS; reactive oxygen species, VD; vascular dementia.
References
- Carrell, R.W.; Lomas, D.A. Conformational disease. Lancet 1997, 350, 134–138. [Google Scholar] [CrossRef]
- Birol, M.; Melo, A.M. Untangling the conformational polymorphism of disordered proteins associated with neurodegeneration at the single-molecule level. Front. Mol. Neurosci. 2020, 12, 309. [Google Scholar] [CrossRef] [PubMed]
- Dugger, B.N.; Dickson, D.W. Pathology of neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, pii:a028035. [Google Scholar] [CrossRef] [PubMed]
- Sipe, J.D.; Cohen, A.S. Review: History of the amyloid fibril. J. Struct. Biol. 2000, 130, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Akter, R.; Cao, P.; Noor, H.; Ridgway, Z.; Tu, L.H.; Wang, H.; Wong, A.G.; Zhang, X.; Abedini, A.; Schmidt, A.M.; et al. Islet amyloid polypeptide: Structure, function, and pathophysiology. J. Diabetes Res. 2016, 2016, 2798269. [Google Scholar] [CrossRef] [PubMed]
- Jaikaran, E.T.; Clark, A. Islet amyloid and type 2 diabetes: From molecular misfolding to islet pathophysiology. Biochim. Biophys. Acta 2001, 29, 179–203. [Google Scholar] [CrossRef]
- Aftabizadeh, M.; Tatarek-Nossol, M.; Andreetto, E.; Bounkari, O.; Kipp, M.; Beyer, C.; Latz, E.; Bernhagen, J.; Kapurniotu, A. Blocking inflammasome activation caused by β-amyloid peptide (Aβ) and islet amyloid polypeptide (IAPP) through an IAPP mimic. ACS Chem. Neurosci. 2019, 10, 3703–3717. [Google Scholar] [CrossRef]
- Alghrably, M.; Czaban, I.; Jaremko, Ł.; Jaremko, M. Interaction of amylin species with transition metals and membranes. J. Inorg. Biochem. 2019, 191, 69–76. [Google Scholar] [CrossRef]
- Ward, B.; Walker, K.; Exley, C. Copper(II) inhibits the formation of amylin amyloid in vitro. J. Inorg. Biochem. 2008, 102, 371–375. [Google Scholar] [CrossRef]
- Adlard, P.A.; Bush, A.I. Metals and Alzheimer’s disease: How far have we come in the clinic? J. Alzheimers Dis. 2018, 62, 1369–1379. [Google Scholar] [CrossRef]
- Brown, D.R. Metalloproteins and neuronal death. Metallomics 2010, 2, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Song, N.; Jiao, Q.; Shi, L.; Du, X. Iron pathophysiology in parkinson diseases. Adv. Exp. Med. Biol. 2019, 1173, 45–66. [Google Scholar] [PubMed]
- Kawahara, M.; Kato-Negishi, M.; Tanaka, K. Cross talk between neurometals and amyloidogenic proteins at the synapse and the pathogenesis of neurodegenerative diseases. Metallomics 2017, 9, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, M. Role of calcium dyshomeostasis via amyloid channels in the pathogenesis of Alzheimer’s disease. Curr. Pharm. Design 2010, 16, 2779–2789. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, M.; Ohtsuka, I.; Yokoyama, S.; Kato-Negishi, M.; Sadakane, Y. Membrane incorporation, channel formation, and disruption of calcium homeostasis by Alzheimer’s β-amyloid protein. Int. J. Alzheimer Dis. 2011, 2011. [Google Scholar] [CrossRef]
- Becker, J.S.S.; Matusch, A.; Palm, C.; Salber, D.; Morton, K.; Becker, S. Bioimaging of metals in brain tissue by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) and metallomics. Metallomics 2010, 2, 104–111. [Google Scholar] [CrossRef]
- Thirupathi, A.; Chang, Y.Z. Brain iron metabolism and CNS diseases. Adv. Exp. Med. Biol. 2019, 1173, 1–19. [Google Scholar]
- Wang, Y.; Wu, Y.; Li, T.; Wang, X.; Zhu, C. Iron metabolism and brain development in premature infants. Front. Physiol. 2019, 10, 463. [Google Scholar] [CrossRef]
- Frederickson, C.J.; Suh, S.W.; Silva, D.; Frederickson, C.J.; Thompson, R.B. Importance of zinc in the central nervous system: The zinc-containing neuron. J. Nutr. 2000, 130, 1471S–1483S. [Google Scholar] [CrossRef]
- Takeda, A.; Tamano, H. The impact of synaptic Zn2+ dynamics on cognition and its decline. Int. J. Mol. Sci. 2017, 18, 2411. [Google Scholar] [CrossRef]
- Prasad, A.S. Impact of the discovery of human zinc deficiency on health. J. Am. Coll. Nutr. 2009, 28, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Hambidge, M. Human zinc deficiency. J. Nutr. 2000, 130, 1344S–1349S. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Tamano, H. Subclinical zinc deficiency impairs human brain function. J. Trace Elem. Med. Biol. 2012, 26, 70–73. [Google Scholar]
- Fukada, T.; Kambe, T. Molecular and genetic features of zinc transporters in physiology and pathogenesis. Metallomics 2011, 3, 662–674. [Google Scholar] [CrossRef] [PubMed]
- Fukunaka, A.; Fujitani, Y. Role of zinc homeostasis in the pathogenesis of diabetes and obesity. Int. J. Mol. Sci. 2018, 19, 476. [Google Scholar] [CrossRef]
- Bolognin, S.; Cozzi, B.; Zambenedetti, P.; Zatta, P. Metallothioneins and the central nervous system: From a deregulation in neurodegenerative diseases to the development of new therapeutic approaches. J. Alzheimers Dis. 2014, 41, 29–42. [Google Scholar] [CrossRef]
- Uchida, Y.; Takio, K.; Titani, K.; Ihara, Y.; Tomonaga, M. The growth inhibitory factor that is deficient in the Alzheimer’s disease brain is a 68 amino acid metallothionein-like protein. Neuron 1991, 7, 337–347. [Google Scholar] [CrossRef]
- Vašák, M.; Meloni, G. Mammalian metallothionein-3: New functional and structural insights. Int. J. Mol. Sci. 2017, 18, 1117. [Google Scholar] [CrossRef]
- D’Ambrosi, N.; Rossi, L. Copper at synapse: Release, binding and modulation of neurotransmission. Neurochem. Int. 2015, 90, 36–45. [Google Scholar] [CrossRef]
- Kodama, H.; Fujisawa, C.; Bhadhprasit, W. Inherited copper transport disorders: Biochemical mechanisms, diagnosis, and treatment. Curr. Drug Metab. 2012, 13, 237–250. [Google Scholar] [CrossRef]
- Szabo, S.T.; Harry, G.J.; Hayden, K.M.; Szabo, D.T.; Birnbaum, L. Comparison of metal levels between postmortem brain and ventricular fluid in Alzheimer’s disease and nondemented elderly controls. Toxicol. Sci. 2016, 150, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Roos, P.M.; Vesterberg, O.; Syversen, T.; Flaten, T.P.; Nordberg, M. Metal concentrations in cerebrospinal fluid and blood plasma from patients with amyotrophic lateral sclerosis. Biol. Trace Elem. Res. 2013, 151, 159–170. [Google Scholar] [CrossRef]
- Schikorski, T.; Stevens, C.F. Quantitative ultrastructural analysis of hippocampal excitatory synapses. J. Neurosci. 1997, 17, 5858–5867. [Google Scholar] [CrossRef] [PubMed]
- Vogt, K.; Mellor, J.; Tong, G.; Nicoll, R. The actions of synaptically released zinc at hippocampal mossy fiber synapses. Neuron 2000, 26, 187–196. [Google Scholar] [CrossRef]
- Hopt, A.; Korte, S.; Fink, H.; Panne, U.; Niessner, R.; Jahn, R.; Herms, J. Methods for studying synaptosomal copper release. J. Neurosci. Methods 2003, 128, 159–172. [Google Scholar] [CrossRef]
- Shuttleworth, C.W.; Weiss, J.H. Zinc: New clues to diverse roles in brain ischemia. Trends Pharm. Sci. 2011, 32, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. The molecular pathology of Alzheimer’s disease. Neuron 1991, 6, 487–498. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Goate, A.; Chartier-Harlin, M.C.; Mullan, M.; Brown, J.; Crawford, F.; Fidani, L.; Giuffra, L.; Haynes, A.; Irving, N.; James, L.; et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature 1991, 349, 704–706. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Wolfe, M.S. Presenilin: Running with scissors in the membrane. Cell 2007, 131, 215–221. [Google Scholar] [CrossRef]
- Sherrington, R.; Rogaev, E.I.; Liang, Y.; Rogaeva, E.A.; Levesque, G.; Ikeda, M.; Chi, H.; Lin, C.; Li, G.; Holman, K.; et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature 1995, 375, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Yankner, B.A.; Duffy, L.K.; Kirschner, D.A. Neurotropic and neurotoxic effects of amyloid β protein: Reversal by tachykinin neuropeptides. Nature 1990, 250, 279–282. [Google Scholar]
- Pike, C.J.; Walencewicz, A.J.; Glabe, C.G.; Cotman, C.W. In vitro aging of β-amyloid protein causes peptide aggregation and neurotoxicity. Brain Res. 1991, 563, 311–314. [Google Scholar] [CrossRef]
- Simmons, L.K.; May, P.C.; Tomaselli, K.J.; Rydel, R.E.; Fuson, K.S.; Brigham, E.F.; Wright, S.; Lieberburg, I.; Becker, G.W.; Brems, D.N.; et al. Secondary structure of amyloid β peptide correlates with neurotoxic activity in vitro. Mol. Pharm. 1994, 45, 373–379. [Google Scholar]
- Jarrett, J.T.; Lansbury, P.T., Jr. Seeding “one-dimensional crystallization” of amyloid: A pathogenic mechanism in Alzheimer’s disease and scrapie? Cell 1993, 73, 1055–1058. [Google Scholar] [CrossRef]
- Wirths, O.; Multhaup, G.; Bayer, T.A. A modified β-amyloid hypothesis: Intraneuronal accumulation of the beta-amyloid peptide—The first step of a fatal cascade. J. Neurochem. 2004, 91, 513–520. [Google Scholar] [CrossRef]
- Walsh, D.M.; Selkoe, D.J. Aβ oligomers—A decade of discovery. J. Neurochem. 2007, 101, 1172–1184. [Google Scholar] [CrossRef]
- Fukuyama, R.; Mizuno, T.; Mori, S.; Nakajima, K.; Fushiki, S.; Yanagisawa, K. Age-dependent change in the levels of Aβ40 and Aβ42 in cerebrospinal fluid from control subjects, and a decrease in the ratio of Aβ40 to Aβ42 level in cerebrospinal fluid from Alzheimer’s disease patients. Eur. Neurol. 2000, 43, 155–160. [Google Scholar] [CrossRef]
- Choi, M.L.; Gandhi, S. Crucial role of protein oligomerization in the pathogenesis of Alzheimer’s and Parkinson’s diseases. FEBS J. 2018, 285, 3631–3644. [Google Scholar] [CrossRef]
- Sadakane, Y.; Kawahara, M. Implications of metal binding and asparagine deamidation for amyloid formation. Int. J. Mol. Sci. 2018, 19, 2449. [Google Scholar] [CrossRef]
- Kawahara, M.; Muramoto, K.; Kobayashi, K.; Mori, H.; Kuroda, Y. Aluminum promotes the aggregation of Alzheimer’s amyroid β-protein in vitro. Biochem. Biophys. Res. Commun. 1994, 198, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, M.; Kato, M.; Kuroda, Y. Effects of aluminum on the neurotoxicity of primary cultured neurons and on the aggregation of β-amyloid protein. Brain Res. Bull. 2001, 55, 211–217. [Google Scholar] [CrossRef]
- Bush, A.I.; Pettingell, W.H.; Multhaup, G.; D Paradis, M.; Vonsattel, J.P.; Gusella, J.F.; Beyreuther, K.; Masters, C.L.; Tanzi, R.E. Rapid induction of Alzheimer Aβ amyloid formation by zinc. Science 1994, 265, 1464–1467. [Google Scholar] [CrossRef] [PubMed]
- Atwood, C.S.; Moir, R.D.; Huang, X.; Scarpa, R.C.; Bacarra, N.M.; Romano, D.M.; Hartshorn, M.A.; Tanzi, R.E.; Bush, A.I. Dramatic aggregation of Alzheimer Aβ by Cu(II) is induced by conditions representing physiological acidosis. J. Biol. Chem. 1998, 273, 12817–12826. [Google Scholar] [CrossRef]
- Wallin, C.; Kulkarni, Y.S.; Abelein, A.; Jarvet, J.; Liao, Q.; Strodel, B.; Olsson, L.; Luo, J.; Abrahams, J.P.; Sholts, S.B.; et al. Characterization of Mn(II) ion binding to the amyloid-β peptide in Alzheimer’s disease. J. Trace Elem. Med. Biol. 2016, 38, 183–193. [Google Scholar] [CrossRef]
- Dyrks, T.; Dyrks, E.; Masters, C.L.; Beyreuther, K. Amyloidogenicity of rodent and human beta A4 sequences. FEBS Lett. 1993, 324, 231–236. [Google Scholar] [CrossRef]
- White, A.R.; Multhaup, G.; Maher, F.; Bellingham, S.; Camakaris, J.; Zheng, H.; Bush, A.I.; Beyreuther, K.; Masters, C.L.; Cappai, R. The Alzheimer’s disease amyloid precursor protein modulates copper-induced toxicity and oxidative stress in primary neuronal cultures. J. Neurosci. 1999, 19, 9170–9179. [Google Scholar] [CrossRef]
- Baumkötter, F.; Schmidt, N.; Vargas, C.; Schilling, S.; Weber, R.; Wagner, K.; Fiedler, S.; Klug, W.; Radzimanowski, J.; Nickolaus, S.; et al. Amyloid precursor protein dimerization and synaptogenic function depend on copper binding to the growth factor-like domain. J. Neurosci. 2014, 34, 11159–11172. [Google Scholar] [CrossRef]
- Gerber, H.; Wu, F.; Dimitrov, M.; Garcia Osuna, G.M.; Fraering, P.C. Zinc and copper differentially modulate amyloid precursor protein processing by γ-secretase and amyloid-β peptide production. J. Biol. Chem. 2017, 292, 3751–3767. [Google Scholar] [CrossRef]
- Wong, B.X.; Tsatsanis, A.; Lim, L.Q.; Adlard, P.A.; Bush, A.I.; Duce, J.A. β-Amyloid precursor protein does not possess ferroxidase activity but does stabilize the cell surface ferrous iron exporter ferroportin. PLoS ONE 2014, 9, e114174. [Google Scholar] [CrossRef] [PubMed]
- Namekata, K.; Imagawa, M.; Terashi, A.; Ohta, S.; Oyama, F.; Ihara, Y. Association of transferrin C2 allele with late-onset Alzheimer’s disease. Hum. Genet. 1997, 101, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Imagawa, M.; Naruse, S.; Tsuji, S.; Fujioka, A.; Yamaguchi, H. Coenzyme Q10, iron, and vitamin B6 in genetically-confirmed Alzheimer’s disease. Lancet 1992, 340, 671. [Google Scholar] [CrossRef]
- Zhou, Z.D.; Tan, E.K. Iron regulatory protein (IRP)-iron responsive element (IRE) signaling pathway in human neurodegenerative diseases. Mol. Neurodegener. 2017, 12, 75. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.T.; Randall, J.D.; Cahill, C.M.; Eder, P.S.; Huang, X.; Gunshin, H.; Leiter, L.; McPhee, J.; Sarang, S.S.; Utsuki, T.; et al. An iron-responsive element type II in the 5′-untranslated region of the Alzheimer’s amyloid precursor protein transcript. J. Biol. Chem. 2002, 277, 45518–45528. [Google Scholar] [CrossRef] [PubMed]
- Greenough, M.A.; Volitakis, I.; Li, Q.X.; Laughton, K.; Evin, G.; Ho, M.; Dalziel, A.H.; Camakaris, J.; Bush, A.I. Presenilins promote the cellular uptake of copper and zinc and maintain copper chaperone of SOD1-dependent copper/zinc superoxide dismutase activity. J. Biol. Chem. 2011, 286, 9776–9786. [Google Scholar] [CrossRef] [PubMed]
- Prusiner, S.B. Biology and genetics of prions causing neurodegeneration. Annu. Rev. Genet. 2013, 47, 601–623. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.R.; Qin, K.; Herms, J.W.; Madlung, A.; Manson, J.; Strome, R.; Fraser, P.E.; Kruck, T.; Von Bohlen, A.; Schulz-Schaeffer, W.; et al. The cellular prion protein binds copper in vivo. Nature 1997, 390, 684–687. [Google Scholar] [CrossRef]
- Brown, D.R.; Wong, B.S.; Hafiz, F.; Clive, C.; Haswell, S.J.; Jones, I.M. Normal prion protein has an activity like that of superoxide dismutase. Biochem. J. 1999, 344, 1–5. [Google Scholar] [CrossRef]
- Jackson, G.S.; Murray, I.; Hosszu, L.L.; Gibbs, N.; Waltho, J.P.; Clarke, A.R.; Collinge, J. Location and properties of metal-binding sites on the human prion protein. Proc. Natl. Acad. Sci. USA 2001, 98, 8531–8535. [Google Scholar] [CrossRef]
- Huang, S.; Chen, L.; Bladen, C.; Stys, P.K.; Zamponi, G.W. Differential modulation of NMDA and AMPA receptors by cellular prion protein and copper ions. Mol. Brain 2018, 11, 62. [Google Scholar] [CrossRef]
- Varela-Nallar, L.; González, A.; Inestrosa, N.C. Role of copper in prion diseases: Deleterious or beneficial? Curr. Pharm. Des. 2006, 12, 2587–2595. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, X.T.A.; Tran, T.H.; Cojoc, D.; Legname, G. Copper binding regulates cellular prion protein function. Mol. Neurobiol. 2019, 56, 6121–6133. [Google Scholar] [CrossRef] [PubMed]
- Schmitt-Ulms, G.; Ehsani, S.; Watts, J.C.; Westaway, D.; Wille, H. Evolutionary descent of prion genes from the ZIP family of metal ion transporters. PLoS ONE 2009, 4, e7208. [Google Scholar] [CrossRef] [PubMed]
- Watt, N.T.; Griffiths, H.H.; Hooper, N.M. Neuronal zinc regulation and the prion protein. Prion 2013, 7, 203–208. [Google Scholar] [CrossRef]
- Singh, A.; Haldar, S.; Horback, K.; Tom, C.; Zhou, L.; Meyerson, H.; Singh, N. Prion protein regulates iron transport by functioning as a ferrireductase. J. Alzheimers Dis. 2013, 35, 541–552. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Haldar, S.; Qian, J.; Beserra, A.; Suda, S.; Singh, A.; Hopfer, U.; Chen, S.G.; Garrick, M.D.; Turner, J.R.; et al. Prion protein functions as a ferrireductase partner for ZIP14 and DMT1. Free Radic. Biol. Med. 2015, 84, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kong, Q.; Luo, X.; Petersen, R.B.; Meyerson, H.; Singh, N. Prion protein (PrP) knock-out mice show altered iron metabolism: A functional role for PrP in iron uptake and transport. PLoS ONE 2009, 4, e6115. [Google Scholar] [CrossRef]
- Haldar, S.; Beveridge, J.; Wong, J.; Singh, A.; Galimberti, D.; Borroni, B.; Zhu, X.; Blevins, J.; Greenlee, J.; Perry, G.; et al. A low-molecular-weight ferroxidase is increased in the CSF of sCJD cases: CSF ferroxidase and transferrin as diagnostic biomarkers for sCJD. Antioxid. Redox Signal. 2013, 19, 1662–1675. [Google Scholar] [CrossRef]
- Choi, C.J.; Anantharam, V.; Martin, D.P.; Nicholson, E.M.; Richt, J.A.; Kanthasamy, A.; Kanthasamy, A.G. Manganese upregulates cellular prion protein and contributes to altered stabilization and proteolysis: Relevance to role of metals in pathogenesis of prion disease. Toxicol. Sci. 2010, 115, 535–546. [Google Scholar] [CrossRef]
- Slivarichová, D.; Mitrová, E.; Ursínyová, M.; Uhnáková, I.; Koscová, S.; Wsólová, L. Geographic accumulation of Creutzfeldt-Jakob disease in Slovakia—Environmental metal imbalance as a possible cofactor. Cent. Eur. J. Public Health 2011, 19, 158–164. [Google Scholar]
- Bernal-Conde, L.D.; Ramos-Acevedo, R.; Reyes-Hernández, M.A.; Balbuena-Olvera, A.J.; Morales-Moreno, I.D.; Argüero-Sánchez, R.; Schüle, B.; Guerra-Crespo, M. Alpha-synuclein physiology and pathology: A perspective on cellular structures and organelles. Front. Neurosci. 2020, 13, 1399. [Google Scholar] [CrossRef] [PubMed]
- Brookes, A.J.; St Clair, D. Synuclein proteins and Alzheimer’s disease. Trends Neurosci. 1994, 14, 404–405. [Google Scholar] [CrossRef]
- Okita, Y.; Rcom-H’cheo-Gauthier, A.N.; Goulding, M.; Chung, R.S.; Faller, P.; Pountney, D.L. Metallothionein, copper and alpha-synuclein in alpha-synucleinopathies. Front. Neurosci. 2017, 11, 114. [Google Scholar] [CrossRef] [PubMed]
- Miotto, M.C.; Binolfi, A.; Zweckstetter, M.; Griesinger, C.; Fernández, C.O. Bioinorganic chemistry of synucleinopathies: Deciphering the binding features of Met motifs and His-50 in AS-Cu(I) interactions. J. Inorg. Biochem. 2014, 141, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N.; Li, J.; Fink, A.L. Metal-triggered structural transformations, aggregation, and fibrillation of human alpha-synuclein. A possible molecular NK between Parkinson’s disease and heavy metal exposure. J. Biol. Chem. 2001, 276, 44284–44296. [Google Scholar] [CrossRef]
- Davies, P.; Moualla, D.; Brown, D.R. Alpha-synuclein is a cellular ferrireductase. PLoS ONE 2011, 6, e15814. [Google Scholar] [CrossRef]
- Wang, J.Y.; Zhuang, Q.Q.; Zhu, L.B.; Zhu, H.; Li, T.; Li, R.; Chen, S.F.; Huang, C.P.; Zhang, X.; Zhu, J.H. Meta-analysis of brain iron levels of Parkinson’s disease patients determined by postmortem and MRI measurements. Sci. Rep. 2016, 6, 36669. [Google Scholar] [CrossRef]
- Cahill, C.M.; Lahiri, D.K.; Huang, X.; Rogers, J.T. Amyloid precursor protein and alpha synuclein translation, implications for iron and inflammation in neurodegenerative diseases. Biochim. Biophys. Acta 2009, 1790, 615–628. [Google Scholar] [CrossRef]
- Wang, T.Y.; Ma, Z.; Wang, C.; Liu, C.; Yan, D.Y.; Deng, Y.; Liu, W.; Xu, Z.F.; Xu, B. Manganese-induced alpha-synuclein overexpression impairs synaptic vesicle fusion by disrupting the Rab3 cycle in primary cultured neurons. Toxicol. Lett. 2018, 285, 34–42. [Google Scholar] [CrossRef]
- Montagna, E.; Dorostkar, M.M.; Herms, J. The role of APP in structural spine plasticity. Front. Mol. Neurosci. 2017, 10, 136. [Google Scholar] [CrossRef]
- Del Prete, D.; Lombino, F.; Liu, X.; D’Adamio, L. APP is cleaved by Bace1 in pre-synaptic vesicles and establishes a pre-synaptic interactome, via its intracellular domain, with molecular complexes that regulate pre-synaptic vesicles functions. PLoS ONE 2014, 9, e108576. [Google Scholar] [CrossRef]
- Adle-Biassette, H.; Verney, C.; Peoc’h, K.; Dauge, M.C.; Razavi, F.; Choudat, L.; Gressens, P.; Budka, H.; Henin, D. Immunohistochemical expression of prion protein (PrPC) in the human forebrain during development. J. Neuropathol. Exp. Neurol. 2006, 65, 698–706. [Google Scholar] [CrossRef]
- Mellone, M.; Pelucchi, S.; Alberti, L.; Genazzani, A.A.; Di Luca, M.; Gardoni, F. Zinc transporter-1: A novel NMDA receptor-binding protein at the postsynaptic density. J. Neurochem. 2015, 132, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Purro, S.A.; Nicoll, A.J.; Collinge, J. Prion protein as a toxic acceptor of amyloid β oligomers. Biol. Psychiatry 2018, 83, 358–368. [Google Scholar] [CrossRef]
- Roberts, H.L.; Schneider, B.L.; Brown, D.R. α-Synuclein increases β-amyloid secretion by promoting β-/γ-secretase processing of APP. PLoS ONE 2017, 12, e0171925. [Google Scholar] [CrossRef]
- Bassil, F.; Brown, H.J.; Pattabhiraman, S.; Iwasyk, J.E.; Maghames, C.M.; Meymand, E.S.; Cox, T.O.; Riddle, D.M.; Zhang, B.; Trojanowski, J.Q.; et al. Amyloid-beta (Aβ) plaques promote seeding and spreading of alpha-synuclein and tau in a mouse model of Lewy body disorders with Aβ pathology. Neuron 2020, 105, 260–275. [Google Scholar] [CrossRef] [PubMed]
- Bakardjiev, A. Carnosine and β-alanine release is stimulated by glutamatergic receptors in cultured rat oligodendrocytes. Glia 1998, 24, 346–351. [Google Scholar] [CrossRef]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and pathophysiology of carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef]
- Venkataramani, V.; Doeppner, T.R.; Willkommen, D.; Cahill, C.M.; Xin, Y.; Ye, G.; Liu, Y.; Southon, A.; Aron, A.; Au-Yeung, H.Y.; et al. Manganese causes neurotoxic iron accumulation via translational repression of amyloid precursor protein and H-Ferritin. J. Neurochem. 2018, 147, 831–848. [Google Scholar] [CrossRef]
- Selkoe, D.J. Soluble oligomers of the amyloid β-protein impair synaptic plasticity and behavior. Behav. Brain Res. 2008, 192, 106–113. [Google Scholar] [CrossRef]
- Forloni, G.; Angeretti, N.; Chiesa, R.; Monzani, E.; Salmona, M.; Bugiani, O.; Tagliavini, F. Neurotoxicity of a prion protein fragment. Nature 1993, 362, 543–546. [Google Scholar] [CrossRef]
- Fusco, G.; Chen, S.W.; Williamson, P.T.F.; Cascella, R.; Perni, M.; Jarvis, J.A.; Cecchi, C.; Vendruscolo, M.; Chiti, F.; Cremades, N.; et al. Structural basis of membrane disruption and cellular toxicity by α-synuclein oligomers. Science 2017, 358, 1440–1443. [Google Scholar] [CrossRef] [PubMed]
- Small, D.H.; Mok, S.S.; Bornstein, J.C. Alzheimer’s disease and Aβ toxicity: From top to bottom. Nat. Rev. Neurosci. 2001, 2, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Mucke, L.; Selkoe, D.J. Neurotoxicity of amyloid β-protein: Synaptic and network dysfunction. Cold Spring Harb. Perspect. Med. 2012, 2, a006338. [Google Scholar] [CrossRef] [PubMed]
- Woods, N.K.; Padmanabhan, J. Neuronal calcium signaling and Alzheimer’s disease. Adv. Exp. Med. Biol. 2012, 740, 1193–1217. [Google Scholar] [PubMed]
- Popugaeva, E.; Pchitskaya, E.; Bezprozvanny, I. Dysregulation of neuronal calcium homeostasis in Alzheimer’s disease—A therapeutic opportunity? Biochem. Biophys. Res. Commun. 2017, 483, 998–1004. [Google Scholar] [CrossRef]
- Demuro, A.; Parker, I.; Stutzmann, G.E. Calcium signaling and amyloid toxicity in Alzheimer disease. J. Biol. Chem. 2010, 285, 12463–12468. [Google Scholar] [CrossRef]
- Supnet, C.; Bezprozvanny, I. The dysregulation of intracellular calcium in Alzheimer disease. Cell Calcium 2010, 47, 183–189. [Google Scholar] [CrossRef]
- Camandola, S.; Mattson, M.P. Aberrant subcellular neuronal calcium regulation in aging and Alzheimer’s disease. Biochim. Biophys. Acta 2011, 1813, 965–973. [Google Scholar] [CrossRef]
- Arispe, N.; Diaz, J.C.; Simakova, O. Abeta ion channels. Prospects for treating Alzheimer’s disease with Aβ channel blockers. Biochim. Biophys. Acta 2007, 1768, 1952–1965. [Google Scholar] [CrossRef]
- Parodi, J.; Ormeño, D.; Ochoa-de la Paz, L.D. Amyloid pore-channel hypothesis: Effect of ethanol on aggregation state using frog oocytes for an Alzheimer’s disease study. BMB Rep. 2015, 48, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, M.; Negishi-Kato, M.; Sadakane, Y. Calcium dyshomeostasis and neurotoxicity of Alzheimer’s β-amyloid protein. Expert Rev. Neurother. 2009, 9, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Arispe, N.; Rojas, E.; Pollard, H.B. Alzheimer disease amyloid β protein forms calcium channels in bilayer membranes: Blockade by tromethamine and aluminum. Proc. Natl. Acad. Sci. USA 1993, 90, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Arispe, N.; Rojas, E.; Pollard, H.B. Giant multilevel cation channels formed by Alzheimer disease amyloid β protein [AβP-(1–40)] in bilayer membranes. Proc. Natl. Acad. Sci. USA 1993, 90, 10573–10577. [Google Scholar] [CrossRef]
- Rhee, S.K.; Quist, A.P.; Lal, R. Amyloid β protein-(1–42) forms calcium-permeable, Zn2+-sensitive channel. J. Biol. Chem. 1998, 273, 13379–13382. [Google Scholar] [CrossRef]
- Kawahara, M.; Arispe, N.; Kuroda, Y.; Rojas, E. Alzheimer’s disease amyloid β-protein forms Zn2+-sensitive, cation-selective channels across excised membrane patches from hypothalamic neurons. Biophys. J. 1997, 73, 67–75. [Google Scholar] [CrossRef]
- Arispe, N.; Pollard, H.B.; Rojas, E. Zn2+ interactions with Alzheimer’s amyloid β protein calcium channels. Proc. Natl. Acad. Sci. USA 1996, 93, 1710–1715. [Google Scholar] [CrossRef]
- Durell, S.R.; Guy, H.R.; Arispe, N.; Rojas, E.; Pollard, H.B. Theoretical models of the ion channel structure of amyloid β-protein. Biophys. J. 1994, 67, 2137–3145. [Google Scholar] [CrossRef]
- Strodel, B.; Lee, J.W.; Whittleston, C.S.; Wales, D.J. Transmembrane structures for Alzheimer’s Aβ(1–42) oligomers. J. Am. Chem. Soc. 2010, 132, 13300–13312. [Google Scholar] [CrossRef]
- Voelker, M.J.; Barz, B.; Urbanc, B. Fully atomistic Aβ40 and Aβ42 oligomers in water: Observation of porelike conformations. J. Chem. Theory Comput. 2017, 13, 4567–4583. [Google Scholar] [CrossRef]
- Jang, H.; Arce, F.T.; Ramachandran, S.; Capone, R.; Lal, R.; Nussinov, R. β-Barrel topology of Alzheimer’s β-amyloid ion channels. J. Mol. Biol. 2010, 404, 917–934. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, Y.H.T.; Arce, F.; Gillman, A.L.; Jang, H.; Kagan, B.L.; Nussinov, R.; Yang, J.; Lal, R. Amyloid β Ion channels in a membrane comprising brain total lipid extracts. ACS Chem. Neurosci. 2017, 8, 1348–1357. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S. In situ Aβ pores in AD brain are cylindrical assembly of Aβ protofilaments. Amyloid 2008, 15, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Kayed, R.; Pensalfini, A.; Margol, L.; Sokolov, Y.; Sarsoza, F.; Head, E.; Hall, J.; Glabe, C. Annular protofibrils are a structurally and functionally distinct type of amyloid oligomer. J. Biol. Chem. 2009, 284, 4230–4237. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, H.; Kayed, R.; Glabe, C.G.; Staufenbiel, M.; Saido, T.C.; Iwata, N.; Yamaguchi, H. Amyloid β annular protofibrils in cell processes and synapses accumulate with aging and Alzheimer-associated genetic modification. Int. J. Alzheimers Dis. 2009, 2009, 689285. [Google Scholar] [CrossRef]
- Kawahara, M.; Arispe, N.; Kuroda, Y.; Rojas, E. Alzheimer’s β-amyloid, human islet amylin and prion protein fragment evoke intracellular free-calcium elevations by a common mechanism in a hypothalamic GnRH neuronal cell-line. J. Biol. Chem. 2000, 275, 14077–14083. [Google Scholar] [CrossRef]
- Kawahara, M.; Kuroda, Y. Molecular mechanism of neurodegeneration induced by Alzheimer’s β-amyloid protein: Channel formation and disruption of calcium homeostasis. Brain Res. Bull. 2000, 53, 389–397. [Google Scholar] [CrossRef]
- Kawahara, M.; Kuroda, Y. Intracellular calcium changes in neuronal cells induced by Alzheimer’s β-amyloid protein are blocked by estradiol and cholesterol. Cell. Mol. Neurobiol. 2001, 21, 1–13. [Google Scholar] [CrossRef]
- Negishi-Kato, M.; Kawahara, M. Neurosteroids block the increase in intracellular calcium level induced by Alzheimer’s β-amyloid protein in long-term cultured rat hippocampal neurons. Neuropsychiatr. Dis. Treat. 2008, 4, 209–218. [Google Scholar]
- Liu, J.; Chang, L.; Song, Y.; Li, H.; Wu, Y. The role of NMDA receptors in Alzheimer’s disease. Front. Neurosci. 2019, 13, 43. [Google Scholar] [CrossRef]
- Lombardo, S.; Maskos, U. Role of the nicotinic acetylcholine receptor in Alzheimer’s disease pathology and treatment. Neuropharmacology 2015, 96, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Latulippe, J.; Lotito, D.; Murby, D. A mathematical model for the effects of amyloid β on intracellular calcium. PLoS ONE 2018, 13, e0202503. [Google Scholar] [CrossRef] [PubMed]
- Galla, L.; Redolfi, N.; Pozzan, T.; Pizzo, P.; Greotti, E. Intracellular calcium dysregulation by the Alzheimer’s disease-linked protein presenilin 2. Int. J. Mol. Sci. 2020, 21, 770. [Google Scholar] [CrossRef] [PubMed]
- Cribbs, D.H.; Pike, C.J.; Weinstein, S.L.; Velazquez, P.; Cotman, C.W. All-d-enantiomers of β-amyloid exhibit similar biological properties to all-l-β-amyloids. J. Biol. Chem. 1997, 272, 7431–7436. [Google Scholar] [CrossRef]
- Connelly, L.; Jang, H.; Arce, F.T.; Capone, R.; Kotler, S.A.; Ramachandran, S.; Kagan, B.L.; Nussinov, R.; Lal, R. Atomic force microscopy and MD simulations reveal pore-like structures of all-d-enantiomer of Alzheimer’s β-amyloid peptide: Relevance to the ion channel mechanism of AD pathology. J. Phys. Chem. B. 2012, 116, 1728–1735. [Google Scholar] [CrossRef]
- Rojko, N.; Anderluh, G. How lipid membranes affect pore forming toxin activity. Acc. Chem. Res. 2015, 48, 3073–3079. [Google Scholar] [CrossRef]
- Eckert, G.P.; Wood, W.G.; Müller, W.E. Membrane disordering effects of β-amyloid peptides. Subcell Biochem. 2005, 38, 319–337. [Google Scholar]
- Yanagisawa, K.; Odaka, A.; Suzuki, N.; Ihara, Y. GM1 ganglioside-bound amyloid β-protein (Aβ): A possible form of preamyloid in Alzheimer’s disease. Nat. Med. 1995, 1, 1062–1066. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Kato, K.; Yanagisawa, K. Ganglioside-mediated assembly of amyloid β-protein: Roles in Alzheimer’s disease. Prog. Mol. Biol. Transl. Sci. 2018, 156, 413–434. [Google Scholar]
- Matsuzaki, K. Aβ-ganglioside interactions in the pathogenesis of Alzheimer’s disease. Biochim. Biophys. Acta Biomembr. 2020, 183233. [Google Scholar] [CrossRef]
- Bhakdi, S.; Tranum-Jensen, J. α-Toxin of Staphylococcus aureus. Microbiol. Rev. 1991, 55, 733–751. [Google Scholar] [CrossRef] [PubMed]
- Imura, Y.; Choda, N.; Matsuzaki, K. Magainin 2 in action: Distinct modes of membrane permeabilization in living bacterial and mammalian cell. Biophys. J. 2008, 95, 5757–5765. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, F.J.; Parodi, J.; Peoples, R.W.; Opazo, C.; Aguayo, L.G. Synaptotoxicity of Alzheimer β amyloid can be explained by its membrane perforating property. PLoS ONE 2010, 27, e11820. [Google Scholar] [CrossRef] [PubMed]
- Soscia, S.J.; Kirby, J.E.; Washicosky, K.J.; Tucker, S.M.; Ingelsson, M.; Hyman, B.; Burton, M.A.; Goldstein, L.E.; Duong, S.; Tanzi, R.E.; et al. The Alzheimer’s disease-associated amyloid β-protein is an antimicrobial peptide. PLoS ONE 2010, 5, e9505. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.C.; Mirzabekov, T.; Kagan, B.L. Channel formation by a neurotoxic prion protein fragment. J. Biol. Chem. 1997, 272, 44–47. [Google Scholar] [CrossRef]
- Kourie, J.I.; Culverson, A. Prion peptide fragment PrP[106–126] forms distinct cation channel types. J. Neurosci. Res. 2000, 62, 120–133. [Google Scholar] [CrossRef]
- Lashuel, H.A.; Hartley, D.; Petre, B.M.; Walz, T.; Lansbury, P.T., Jr. Neurodegenerative disease: Amyloid pores from pathogenic mutations. Nature 2002, 418, 291. [Google Scholar] [CrossRef]
- Ghio, S.; Camilleri, A.; Caruana, M.; Ruf, V.C.; Schmidt, F.; Leonov, A.; Ryazanov, S.; Griesinger, C.; Cauchi, R.J.; Kamp, F.; et al. Cardiolipin promotes pore-forming activity of alpha-synuclein oligomers in mitochondrial membranes. ACS Chem. Neurosci. 2019, 10, 3815–3829. [Google Scholar] [CrossRef]
- Mirzabekov, T.A.; Lin, M.C.; Kagan, B.L. Pore formation by the cytotoxic islet amyloid peptide amylin. J. Biol. Chem. 1996, 271, 1988–1992. [Google Scholar] [CrossRef]
- Sciacca, M.F.M.; Tempra, C.; Scollo, F.; Milardi, D.; La Rosa, C. Amyloid growth and membrane damage: Current themes and emerging perspectives from theory and experiments on Aβ and hIAPP. Biochim. Biophys. Acta Biomembr. 2018, 1860, 1625–1638. [Google Scholar] [CrossRef]
- Sciacca, M.F.; Lolicato, F.; Di Mauro, G.; Milardi, D.; D’Urso, L.; Satriano, C.; Ramamoorthy, A.; La Rosa, C. The role of cholesterol in driving IAPP-membrane interactions. Biophys. J. 2016, 111, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Terakawa, M.S.; Lin, Y.; Kinoshita, M.; Kanemura, S.; Itoh, D.; Sugiki, T.; Okumura, M.; Ramamoorthy, A.; Lee, Y.H. Impact of membrane curvature on amyloid aggregation. Biochim. Biophys. Acta Biomembr. 2018, 1860, 1741–1764. [Google Scholar] [CrossRef] [PubMed]
- Diociaiuti, M.; Polzi, L.Z.; Valvo, L.; Malchiodi-Albedi, F.; Bombelli, C.; Gaudiano, M.C. Calcitonin forms oligomeric pore-like structures in lipid membranes. Biophys. J. 2006, 91, 2275–2281. [Google Scholar] [CrossRef] [PubMed]
- Lal, R.; Lin, H.; Quist, A.P. Amyloid beta ion channel: 3D structure and relevance to amyloid channel paradigm. Biochim. Biophys. Acta 2007, 1768, 1966–1975. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, S.; Niu, Q. Effect of aluminum-maltolate on the content of Aβ protein and the expression of ApoER2, VLDLRs, and LRP1 in PC12-ApoE4 cells. Neurotox. Res. 2019, 35, 931–944. [Google Scholar] [CrossRef] [PubMed]
- O’Nuallain, B.; Freir, D.B.; Nicoll, A.J.; Risse, E.; Ferguson, N.; Herron, C.E.; Collinge, J.; Walsh, D.M. Amyloid beta-protein dimers rapidly form stable synaptotoxic protofibrils. J. Neurosci. 2010, 30, 14411–14419. [Google Scholar] [CrossRef]
- Chen, W.T.; Liao, Y.H.; Yu, H.M.; Cheng, I.H.; Chen, Y.R. Distinct effects of Zn2+, Cu2+, Fe3+, and Al3+ on amyloid-beta stability, oligomerization, and aggregation: Amyloid-beta destabilization promotes annular protofibril formation. J. Biol. Chem. 2011, 286, 9646–9656. [Google Scholar] [CrossRef]
- Sharma, A.K.; Pavlova, S.T.; Kim, J.; Kim, J.; Mirica, L.M. The effect of Cu(2+) and Zn(2+) on the Aβ42 peptide aggregation and cellular toxicity. Metallomics 2013, 5, 1529–1536. [Google Scholar] [CrossRef]
- Kawahara, M.; Koyama, H.; Nagata, T.; Sadakane, Y. Zinc, copper, and carnosine attenuate neurotoxicity of prion fragment PrP106-126. Metallomics 2011, 3, 726–734. [Google Scholar] [CrossRef]
- Kawahara, M.; Mizuno, D.; Koyama, H.; Konoha, K.; Ohkawara, S.; Sadakane, Y. Disruption of zinc homeostasis and the pathogenesis of senile dementia. Metallomics 2013, 6, 209–219. [Google Scholar] [CrossRef]
- Ilitchev, A.I.; Giammona, M.J.; Schwarze, J.N.; Buratto, S.K.; Bowers, M.T. Zinc-induced conformational transitions in human islet amyloid polypeptide and their role in the inhibition of amyloidosis. J. Phys. Chem. B 2018, 122, 9852–9859. [Google Scholar] [CrossRef]
- Garcia, J.J.; Martínez-Ballarín, E.; Millán-Plano, S.; Allué, J.L.; Albendea, C.; Fuentes, L.; Escanero, J.F. Effects of trace elements on membrane fluidity. J. Trace Elem. Med. Biol. 2005, 19, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhang, J.; Zhou, B.; Li, P.; Hu, X.; Zhu, Z.; Tan, Y.; Chang, C.; Lü, J.; Song, B. Anomalous behavior of membrane fluidity caused by copper-copper bond coupled phospholipids. Sci. Rep. 2018, 8, 14093. [Google Scholar] [CrossRef] [PubMed]
- Curtain, C.C.; Ali, F.E.; Smith, D.G.; Bush, A.I.; Masters, C.L.; Barnham, K.J. Metal ions, pH, and cholesterol regulate the interactions of Alzheimer’s disease amyloid-beta peptide with membrane lipid. J. Biol. Chem. 2003, 278, 2977–2982. [Google Scholar] [CrossRef] [PubMed]
- Domanska, B.; Fortea, E.; West, M.J.; Schwartz, J.L.; Crickmore, N. The role of membrane-bound metal ions in toxicity of a human cancer cell-active pore-forming toxin Cry41Aa from Bacillus thuringiensis. Toxicon 2019, 167, 123–133. [Google Scholar] [CrossRef]
- La Rosa, C.; Condorelli, M.; Compagnini, G.; Lolicato, F.; Milardi, D.; Do, T.N.; Karttunen, M.; Pannuzzo, M.; Ramamoorthy, A.; Fraternali, F.; et al. Symmetry-breaking transitions in the early steps of protein self-assembly. Eur. Biophys. J. 2020, 49, 175–191. [Google Scholar] [CrossRef]
- Mizuno, D.; Kawahara, M. Carnosine: A possible drug for vascular dementia. J. Vasc. Med. Surg. 2014, 2, 3. [Google Scholar] [CrossRef]
- Corona, C.; Frazzini, V.; Silvestri, E.; Lattanzio, R.; La Sorda, R.; Piantelli, M.; Canzoniero, L.M.; Ciavardelli, D.; Rizzarelli, E.; Sensi, S.L. Effects of dietary supplementation of carnosine on mitochondrial dysfunction, amyloid pathology, and cognitive deficits in 3xTg-AD mice. PLoS ONE 2011, 6, e17971. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).