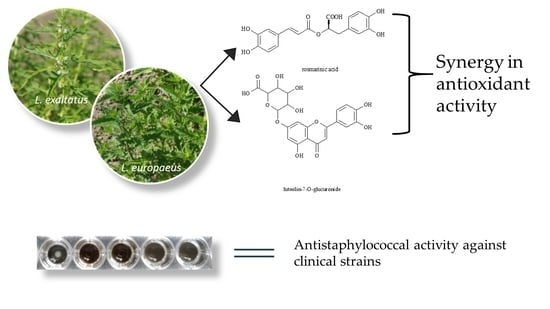
Antimicrobial and Antioxidant Properties of Four Lycopus Taxa and an Interaction Study of Their Major Compounds
Abstract
1. Introduction
2. Results
2.1. Identification and Quantification
2.2. Evaluation of Antimicrobial Activity
2.3. Antioxidant Activity by ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid), DPPH (2,2-diphenyl-1-picrylhydrazyl), and DCFH-DA (dichlorodihydrofluorescein diacetate) Assays
2.4. Interaction Analysis by ABTS, DPPH, and DCFH-DA Assays
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. The Preparation of Infusions
4.3. LC-MS-DAD Analyses: Identification and Quantification of the Constituents
4.4. Antimicrobial Activity Testing
4.5. Evaluation of Antioxidant Activity
4.5.1. DPPH Radical Scavenging Assay
4.5.2. ABTS Radical Scavenging Assay
4.5.3. Detection of Intracellular Oxidative Stress
4.6. Interaction Analysis of Antioxidant Activities
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wagner, H.; Ulrich-Merzenich, G. Synergy research: Approaching a new generation of phytopharmaceuticals. Phytomedicine 2009, 16, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E. Synergy and other interactions in phytomedicines. Phytomedicine 2001, 8, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.H.; Zheng, C.J.; Han, L.Y.; Xie, B.; Jia, J.; Cao, Z.W.; Li, Y.X.; Chen, Y.Z. Synergistic therapeutic actions of herbal ingredients and their mechanisms from molecular interaction and network perspectives. Drug Discov. Today 2009, 14, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Kurin, E.; Nagy, M. Theoretical Models for Analysis of Syergy and Antagonism of Drugs (Teoretické modely analýzy synergie a antagonizmu liečiv). Chem. Listy 2012, 106, 653–659. [Google Scholar]
- Lehár, J.; Krueger, A.; Zimmermann, G.; Borisy, A. High-order combination effects and biological robustness. Mol. Syst. Biol. 2008, 4, 215. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.-K.; Hong, S.-P. Nutlet morphology and anatomy of the genus Lycopus (Lamiaceae: Mentheae). J. Plant. Res. 2006, 119, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Yin, Z.; Yang, B.; Kang, W. Research Progress on the Chemical Constituents of Lycopus Genus. Curr. Opin. Complement. Alternat. Med. 2014, 1, 28–33. [Google Scholar]
- Moon, H.-K.; Hong, S.-P. Pollen morphology of the genus Lycopus (Lamiaceae). Ann. Bot. Fenn. 2003, 40, 191–198. [Google Scholar]
- Hussein, A.A.; Rodríguez, B.; de la Paz Martí-nez-Alcázar, M.; Cano, F.H. Diterpenoids from Lycopus europaeus and Nepeta septemcrenata: Revised Structures and New Isopimarane Derivatives. Tetrahedron 1999, 55, 7375–7388. [Google Scholar] [CrossRef]
- Aziz, A.; Khan, I.A.; Perveen, A.; Agha, S.; Munawar, S.H.; Manzoor, Z. Evaluation of antitussive activity of Lycopus europaeus on cough reflex induced by different cough induced models in mice. Int. J. Pharma. Sci. 2013, 3, 381–385. [Google Scholar]
- Aziz, A.; Khan, I.A.; Hussain, M.; Afzal, A. Antinociceptive and anti-inflammatory activity of the extract of Lycopus europaes on laboratory animals. Int. J. Res. Dev. Pharm. Life Sci. 2014, 3, 896–904. [Google Scholar]
- Radulović, N.; Denić, M.; Stojanović-Radić, Z.; Skropeta, D. Fatty and Volatile Oils of the Gypsywort Lycopus europaeus L. and the Gaussian-Like Distribution of its Wax Alkanes. J. Am. Oil Chem. Soc. 2012, 89, 2165–2185. [Google Scholar] [CrossRef]
- Fialová, S.; Slobodníková, L.; Veizerová, L.; Grančai, D. Lycopus europaeus: Phenolic fingerprint, antioxidant activity and antimicrobial effect on clinical Staphylococcus aureus strains. Nat. Prod. Res. 2015, 29, 2271–2274. [Google Scholar] [CrossRef]
- Gibbons, S.; Oluwatuyi, M.; Veitch, N.C.; Gray, A.I. Bacterial resistance modifying agents from Lycopus europaeus. Phytochemistry 2003, 62, 83–87. [Google Scholar] [CrossRef]
- Radulović, N.; Denić, M.; Stojanović-Radić, Z. Antimicrobial phenolic abietane diterpene from Lycopus europaeus L. (Lamiaceae). Bioorg. Med. Chem. Lett. 2010, 20, 4988–4991. [Google Scholar] [CrossRef] [PubMed]
- Bär, B.; Roesel, R.; Bollbach, D.; Hagels, H.; Langer, E. Wolfstrapp. DAZ 2000, 140, 67–76. [Google Scholar]
- Beer, A.-M.; Wiebelitz, K.R.; Schmidt-Gayk, H. Lycopus europaeus (Gypsywort): Effects on the thyroidal parameters and symptoms associated with thyroid function. Phytomedicine 2008, 15, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Eiling, R.; Wieland, V.; Niestroj, M. Besserung der Symptome einer leichten Schilddrüsenüberfunktion mit einem Trockenextrakt aus Wolfstrappkraut (Thyreogutt® mono). Wien. Med. Wochenschr. 2013, 163, 95–101. [Google Scholar] [CrossRef]
- Vonhoff, C.; Baumgartner, A.; Hegger, M.; Korte, B.; Biller, A.; Winterhoff, H. Extract of Lycopus europaeus L. reduces cardiac signs of hyperthyroidism in rats. Life Sci. 2006, 78, 1063–1070. [Google Scholar] [CrossRef]
- Nikpay, A.; Soltani, M. In vitro anti-parasitic activities of Pulicaria dysenterica and Lycopus europaeus methanolic extracts against Trichomonas gallinae. J. Herbmed. Pharmacol. 2018, 7, 112–118. [Google Scholar] [CrossRef]
- Henderson, N.C. A Taxonomic Revision of the Genus Lycopus (Labiatae). Am. Midl. Nat. 1962, 68, 95. [Google Scholar] [CrossRef]
- Bucar, F.; Kartnig, T. Flavone Glucuronides of Lycopus virginicus. Planta Med. 1995, 61, 378–380. [Google Scholar] [CrossRef] [PubMed]
- Miron, T.L.; Herrero, M.; Ibáñez, E. Enrichment of antioxidant compounds from lemon balm (Melissa officinalis) by pressurized liquid extraction and enzyme-assisted extraction. J. Chromatogr. A. 2013, 1288, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-C. Theoretical Basis, Experimental Design, and Computerized Simulation of Synergism and Antagonism in Drug Combination Studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Fialova, S.; Rendekova, K.; Mucaji, P.; Slobodnikova, L. Plant Natural Agents: Polyphenols, Alkaloids and Essential Oils as Perspective Solution of Microbial Resistance. Curr. Org. Chem. 2017, 21. [Google Scholar] [CrossRef]
- Lu, L.; Hu, W.; Tian, Z.; Yuan, D.; Yi, G.; Zhou, Y.; Cheng, Q.; Zhu, J.; Li, M. Developing natural products as potential anti-biofilm agents. Chin. Med. 2019, 14, 11. [Google Scholar] [CrossRef] [PubMed]
- Ekambaram, S.; Perumal, S.; Balakrishnan, A.; Marappan, N.; Gajendran, S.; Viswanathan, V. Antibacterial synergy between Rosmarinic acid and antibiotics against Methicillin resistant Staphylococcus aureus. J. Intercult. Ethnopharmacol. 2016, 5, 358. [Google Scholar] [CrossRef]
- Klančnik, A.; Guzej, B.; Kolar, M.H.; Abramovič, H.; Možina, S.S. In Vitro Antimicrobial and Antioxidant Activity of Commercial Rosemary Extract Formulations. J. Food Prot. 2009, 72, 1744–1752. [Google Scholar] [CrossRef]
- Slobodníková, L.; Fialová, S.; Hupková, H.; Grančai, D. Rosmarinic Acid Interaction with Planktonic and Biofilm Staphylococcus aureus. Nat. Prod. Commun. 2013, 8. [Google Scholar] [CrossRef]
- Bittner Fialová, S.; Kello, M.; Čoma, M.; Slobodníková, L.; Drobná, E.; Holková, I.; Garajová, M.; Mrva, M.; Zachar, V.; Lukáč, M. Derivatization of Rosmarinic Acid Enhances its in vitro Antitumor, Antimicrobial and Antiprotozoal Properties. Molecules 2019, 24, 1078. [Google Scholar] [CrossRef]
- Goszcz, K.; Deakin, S.J.; Duthie, G.G.; Stewart, D.; Leslie, S.J.; Megson, I.L. Antioxidants in Cardiovascular Therapy: Panacea or False Hope? Front. Cardiovasc. Med. 2015, 2, 29. [Google Scholar] [CrossRef]
- Khan, M.F.; Wang, G. Environmental agents, oxidative stress and autoimmunity. Curr.Opin. Toxicol. 2018, 7, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Leopold, J.A. Antioxidants and coronary artery disease: From pathophysiology to preventive therapy. Coron. Artery Dis. 2015, 26, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Ghosh, K.K. Recent Advances in the Antioxidant Therapies for Alzheimer’s Disease: Emphasis on Natural Antioxidants. In Pathology, Prevention and Therapeutics of Neurodegenerative Disease; Singh, S., Joshi, N., Eds.; Springer: Singapore, 2019; pp. 253–263. [Google Scholar]
- Croft, K.D. Dietary polyphenols: Antioxidants or not? Arch. Biochem. Biophys. 2016, 595, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant Polyphenols as Antioxidant and Antibacterial Agents for Shelf-Life Extension of Meat and Meat Products: Classification, Structures, Sources, and Action Mechanisms. Compr. Rev. Food Sci. F. 2017, 16, 1243–1268. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, J.; Li, Y.; Ma, T.; Sang, P.; Wang, W.; Gao, C. Variation in nutritional compositions, antioxidant activity and microstructure of Lycopus lucidus Turcz. root at different harvest times. Food Chem. 2015, 183, 91–100. [Google Scholar] [CrossRef]
- Ślusarczyk, S.; Hajnos, M.; Skalicka-Woźniak, K.; Matkowski, A. Antioxidant activity of polyphenols from Lycopus lucidus Turcz. Food Chem. 2009, 113, 134–138. [Google Scholar] [CrossRef]
- Kurin, E.; Mučaji, P.; Nagy, M. In Vitro Antioxidant Activities of Three Red Wine Polyphenols and Their Mixtures: An Interaction Study. Molecules 2012, 17, 14336–14348. [Google Scholar] [CrossRef]
- Miláčková, I.; Meščanová, M.; Ševčíková, V.; Mučaji, P. Water leaves extracts of Cornus mas and Cornus kousa as aldose reductase inhibitors: The potential therapeutic agents. Chem. Pap. 2017, 71, 2335–2341. [Google Scholar] [CrossRef]
- Bittner Fialová, S.; Kurin, E.; Trajčíková, E.; Jánošová, L.; Šušaníková, I.; Tekeľová, D.; Nagy, M.; Mučaji, P. Mentha Rhizomes as an Alternative Source of Natural Antioxidants. Molecules 2020, 25, 200. [Google Scholar] [CrossRef] [PubMed]
- Adomako-Bonsu, A.G.; Chan, S.L.; Pratten, M.; Fry, J.R. Antioxidant activity of rosmarinic acid and its principal metabolites in chemical and cellular systems: Importance of physico-chemical characteristics. Toxicol. in Vitro 2017, 40, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Alagawany, M.; Abd El-Hack, M.E.; Farag, M.R.; Gopi, M.; Karthik, K.; Malik, Y.S.; Dhama, K. Rosmarinic acid: Modes of action, medicinal values and health benefits. Anim. Health. Res. Rev. 2017, 18, 167–176. [Google Scholar] [CrossRef]
- Erkan, N.; Ayranci, G.; Ayranci, E. Antioxidant activities of rosemary (Rosmarinus officinalis L.) extract, blackseed (Nigella sativa L.) essential oil, carnosic acid, rosmarinic acid and sesamol. Food Chem. 2008, 110, 76–82. [Google Scholar] [CrossRef]
- Nadeem, M.; Imran, M.; Aslam Gondal, T.; Imran, A.; Shahbaz, M.; Muhammad Amir, R.; Wasim Sajid, M.; Batool Qaisrani, T.; Atif, M.; Hussain, G.; et al. Therapeutic Potential of Rosmarinic Acid: A Comprehensive Review. Appl. Sci. 2019, 9, 3139. [Google Scholar] [CrossRef]
- De Martino, L.; Mencherini, T.; Mancini, E.; Aquino, R.P.; De Almeida, L.F.R.; De Feo, V. In Vitro Phytotoxicity and Antioxidant Activity of Selected Flavonoids. Int. J. Mol. Sci. 2012, 13, 5406–5419. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Özgen, U.; Mavi, A.; Terzi, Z.; Kazaz, C.; Aşçı, A.; Kaya, Y.; Seçen, H. Relationship Between Chemical Structure and Antioxidant Activity of Luteolin and Its Glycosides Isolated from Thymus sipyleus subsp. sipyleus var. sipyleus. Rec. Nat. Pod. 2011, 5, 12–21. [Google Scholar] [CrossRef]
- Cho, B.O.; Yin, H.H.; Fang, C.Z.; Ha, H.O.; Kim, S.J.; Jeong, S.I.; Jang, S.I. Synergistic Anti-inflammatory Effect of Rosmarinic Acid and Luteolin in Lipopolysaccharide-Stimulated RAW264.7 Macrophage Cells. Korean J. Food Sci. Technol. 2015, 47, 119–125. [Google Scholar] [CrossRef]
- PhBs, I.V. Pharmacopoea Bohemoslovaca; Avicenum: Praha, Czech Republic, 1987; pp. 43–44. [Google Scholar]
- EUCAST Documents. Available online: http://www.eucast.org/ast_of_bacteria/ (accessed on 20 April 2019).
- Martineau, F.; Picard, F.J.; Ke, D.; Paradis, S.; Roy, P.H.; Ouellette, M.; Bergeron, M.G. Development of a PCR Assay for Identification of Staphylococci at Genus and Species Levels. J. Clin. Microbiol. 2001, 39, 2541–2547. [Google Scholar] [CrossRef]
- DNA Sequencing of the spa Gene; Document Version 1.1, June 2004. Ridom Bioinformatics. Available online: http://www.ridom.de/doc/Ridom_spa_sequencing.pdf (accessed on 20 November 2019).
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard-Third Edition. In CLSI Document M27-A3; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008; Available online: https://clsi.org/media/1461/m27a3_sample.pdf (accessed on 20 November 2019).
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Miranda-Rottmann, S.; Aspillaga, A.A.; Pérez, D.D.; Vasquez, L.; Martinez, A.L.F.; Leighton, F. Juice and Phenolic Fractions of the Berry Aristotelia chilensis Inhibit LDL Oxidation in Vitro and Protect Human Endothelial Cells against Oxidative Stress. J. Agric. Food Chem. 2002, 50, 7542–7547. [Google Scholar] [CrossRef] [PubMed]
- Hadrich, F.; Garcia, M.; Maalej, A.; Moldes, M.; Isoda, H.; Feve, B.; Sayadi, S. Oleuropein activated AMPK and induced insulin sensitivity in C2C12 muscle cells. Life Sci. 2016, 151, 167–173. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: The tested lyophilisates are available from the authors. |
| Compound | TR (min) | [[M − H]− | MS2 (20 eV) (m/z) | Mass Concentration (µg/mL)* ± SD | ||||
|---|---|---|---|---|---|---|---|---|
| LeuA | LeuB | LeuC | Lex | |||||
| 1 | Citric acid | 4.76 | 191.0191 | 111.0084 87.0087 85.037 | ND | LOD | LOD | LOD |
| 2 | Danshensu (trihydroxyphenyl propanoic acid) | 11.64 | 197.0449 | 179.0343 135.0443 | LOD | LOD | LOD | LOD |
| 3 | Caffeic acid | 21.59 | 179.0342 | 135.0445 | 15.7 ± 1.7 | 16.8 ± 0.2 | 16.9 ± 0.1 | 23.1 ± 0.1 |
| 4 | Quercetin glucuronide | 24.58 | 477.0656 | 301.0332 | 38.1 ± 1.4 | 21.5 ± 0.3 | ND | 84.3 ± 0.3 |
| 5 | Quercetin glucoside | 25.91 | 463.0857 | 301.0324 | 34.0 ± 7.7 | LOD | LOD | LOD |
| 6 | Lithospermic acid | 26.00 | 537.1034 | 359.0739 197.0456 179.0340 161.0235 135.0431 | LOD | LOD | LOD | 13.9 ± 0.1 |
| 7 | Rosmarinic acid hexoside | 28.42 | 521.1301 | 359.0767 179.0376 161.0223 | LOD | 17.4 ± 0.6 | 16.3 ± 0.5 | LOD |
| 8 | Luteolin-7-O-glucuronide | 28.57 | 461.0731 | 285.0393 | 315.1 ± 3.6 | 279.6 ± 1.4 | 275.1 ± 0.9 | 449.8 ± 0.4 |
| 9 | Sagerinic acid | 29.96 | 439.0318 | 359.0751 197.8040 161.0232 | 12.0 ± 2.4 | 15.4 ± 0.3 | 12.4 ± 0.2 | 11.1 ± 4.2 |
| 10 | Sulphated rosmarinic acid | 30.37 | 719.1620 | 539.1182 359.0774 161.0450 | 11.8 ± 0.6 | 18.7 ± 0.1 | 16.6 ± 0.4 | 13.0 ± 0.1 |
| 11 | Apigenin-7-O-glucuronide | 34.12 | 445.0753 | 269.0444 | 27.6 ± 0.1 | 23.0 ± 2.7 | 36.5 ± 1.2 | 27.2 ± 0.1 |
| 12 | Rosmarinic acid | 34.48 | 359.0751 | 197.0483 161.0234 | 213.7 ± 0.3 | 237.5 ± 0.8 | 202.3 ± 0.1 | 216.2 ± 0.1 |
| 13 | Caffeic acid derivative | 36.52 | 549.2332 | 359.0767 161.0295 | 19.6 ± 0.3 | LOD | 7.6 ± 0.2 | 168.0 ± 0.3 |
| Strain | LeuA | LeuB | LeuC | Lex | ||||
|---|---|---|---|---|---|---|---|---|
| MIC [mg/mL] | MBC [mg/mL] | MIC [mg/mL] | MBC [mg/mL] | MIC [mg/mL] | MBC [mg/mL] | MIC [mg/mL] | MBC [mg/mL] | |
| S. aureus CCM 4223 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 5 | 5 |
| S. aureus CCM 4750 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| E. faecalis CCM 4224 | >40 | >40 | 40 | >40 | 40 | >40 | 40 | 40 |
| P. aeruginosa CCM 3955 | 20 | 40 | 40 | >40 | 20 | 40 | 40 | 40 |
| E. coli CCM 3954 | 40 | 40 | 40 | >40 | >40 | >40 | 40 | >40 |
| K. pneumoniae CCM 4415 | >40 | >40 | >40 | >40 | >40 | >40 | 40 | >40 |
| P. mirabilis CCM 7188 | 5 | 5 | 2.5 | 2.5 | 2.5 | 5 | 5 | 10 |
| MIC [mg/mL] | Number of Strains (%) | MRSA (n) | MSSA (n) | MBC [mg/mL] | Number of Strains (%) | MRSA (n) | MSSA (n) |
|---|---|---|---|---|---|---|---|
| 1.25 | 10 (36%) | 8 | 2 | 1.25 | 9 (32%) | 7 | 2 |
| 2.5 | 18 (64%) | 6 | 12 | 2.5 | 18 (64%) | 7 | 11 |
| 5 | - | - | - | 5 | 1 (4%) | - | 1 |
| Sample | ABTS | DPPH | DCFH-DA | |||
|---|---|---|---|---|---|---|
| IC50 (μg/mL) | r a | IC50 (μg/mL) | r a | IC50 (μg/mL) | r a | |
| LeuA | 9.35 | 0.99 | 8.42 | 0.99 | 2.64 | 0.98 |
| LeuB | 8.75 | 0.98 | 9.61 | 0.99 | 2.89 | 0.98 |
| LeuC | 9.61 | 0.96 | 9.02 | 0.98 | 2.92 | 0.99 |
| Lex | 10.41 | 0.97 | 8.30 | 0.98 | 2.17 | 0.97 |
| LGlr | 7.37 | 0.96 | 3.29 | 0.99 | 0.94 | 0.98 |
| RA | 2.20 | 0.97 | 1.81 | 0.99 | 0.63 | 0.99 |
| Method | Sample Combination | IC50 (μg/mL) a | r b | CI c | SDA d | Combined Effect | DRI e |
|---|---|---|---|---|---|---|---|
| ABTS | LeuB:RA | 2.32 | 0.97 | 0.66 | ±0.01 | Synergism | 7.19:1.25 |
| LeuB:LGlr | 6.64 | 0.98 | 0.83 | ±0.01 | Moderate synergism | 2.64:2.22 | |
| LGlr:RA | 2.74 | 0.98 | 0.81 | ±0.01 | Moderate synergism | 5.38:1.61 | |
| LeuB:RA:LGlr | 3.39 | 0.99 | 0.80 | ±0.01 | Moderate synergism | 7.74:1.95:6.52 | |
| DPPH | Lex:RA | 2.75 | 0.96 | 0.93 | ±0.01 | Nearly additive effect | 6.03:1.31 |
| Lex:LGlr | 2.96 | 0.97 | 0.63 | ±0.01 | Synergism | 5.60:2.22 | |
| LGlr:RA | 1.90 | 0.96 | 0.81 | ±0.03 | Moderate synergism | 3.46:1.90 | |
| Lex:RA:LGlr | 2.57 | 0.99 | 0.84 | ±0.01 | Moderate synergism | 9.70:2.12:3.85 | |
| DCFH-DA | Lex:RA | 0.73 | 0.98 | 0.74 | ±0.01 | Moderate synergism | 6.00:1.74 |
| Lex:LGlr | 0.98 | 0.98 | 0.75 | ±0.02 | Moderate synergism | 4.44:1.91 | |
| LGlr:RA | 0.54 | 0.96 | 0.72 | ±0.02 | Moderate synergism | 3.45:2.32 | |
| Lex:RA:LGlr | 0.58 | 0.99 | 0.61 | ±0.01 | Synergism | 11.16:3.23:4.80 |
| Latitude | Longitude | Elevation (m) | |
|---|---|---|---|
| LeuA | 50°52′30,8″ | 14°28′18,1″ | 380 |
| LeuB | 49°04′39.5″ | 14°20′45.9″ | 342 |
| LeuC | 46°07′51.42″ | 17°56′12.3″ | 174 |
| Lex | 47°54′27.00″ | 20°19′19.44″ | 324 |
| Bacterial Species | The CCM / ATCC No of Strain | Note |
|---|---|---|
| Staphylococcus aureus | CCM 4223 / ATCC 29213 | MSSA; ATM susceptibility QC strain |
| Staphylococcus aureus | CCM 4750 / ATCC 43300 | MRSA; methicillin susceptibility reference strain |
| Enterococcus faecalis | CCM 4224 / ATCC 29212 | ATM susceptibility QC strain |
| Pseudomonas aeruginosa | CCM 3955 / ATCC 27853 | ATM susceptibility QC strain |
| Escherichia coli | CCM 3954 / ATCC 25922 | ATM susceptibility QC strain |
| Klebsiella pneumoniae | CCM 4415 / ATCC 10031 | ATM susceptibility QC strain |
| Proteus mirabilis | CCM 7188 / ATCC 29906 | Assays of ATM preservative QC |
| Strain | Origin | Antimicrobial Susceptibility | mecA Gene | Spa-Type | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OXA | ERY | CLI | TET | COT | CIP | MUP | ||||
| 1 | Skin, soft tissue, or wound infection | S | R | R | S | S | S | S | − | t571 |
| 2 | S | R | R | S | S | S | S | − | t024 | |
| 3 | S | R | R | S | S | S | S | − | t084 | |
| 4 | S | R | R | S | S | R | R | − | t2716 | |
| 5 | S | R | R | R | S | S | S | − | t160 | |
| 6 | S | S | S | S | S | S | S | − | t1309 | |
| 7 | S | R | R | R | S | R | R | − | t2448 | |
| 8 | S | S | S | S | S | S | S | − | t1491 | |
| 9 | S | S | S | S | S | S | S | − | t065 | |
| 10 | S | R | R | S | S | R | S | − | t4559 | |
| 11 | R | R | R | R | S | R | S | + | t032 | |
| 12 | R | S | S | S | S | R | S | + | t032 | |
| 13 | R | R | R | S | S | R | S | + | t189 | |
| 14 | R | R | R | S | S | R | S | + | t003 | |
| 15 | R | R | S | S | S | R | S | + | t008 | |
| 16 | R | R | R | S | S | R | S | + | t718 | |
| 17 | R | R | R | S | S | R | S | + | t003 | |
| 18 | R | R | R | S | S | R | S | + | t1148 | |
| 19 | R | R | R | S | S | R | S | + | * | |
| 20 | CVC | R | S | S | S | S | R | S | + | t032 |
| 21 | HC | S | R | S | R | S | S | S | − | t024 |
| 22 | S | R | S | S | S | S | S | − | t036 | |
| 23 | S | R | R | S | S | S | S | − | t1451 | |
| 24 | S | R | R | S | S | R | S | − | t3723 | |
| 25 | R | R | R | I | S | R | S | + | t003 | |
| 26 | R | R | R | S | S | R | S | + | t014 | |
| 27 | R | S | S | S | S | R | S | + | t032 | |
| 28 | R | R | R | S | S | R | S | + | t4559 | |
| Antibiotic | Susceptible [mg/L] | Resistant [mg/L] |
|---|---|---|
| Oxacillin | <2 | >2 |
| Erythromycin | ≤1 | >2 |
| Clindamycin | ≤0.25 | >0.5 |
| Tetracycline | ≤1 | >2 |
| Cotrimoxazole | ≤2 | >4 |
| Ciprofloxacin | ≤0.001 | >1 |
| Mupirocin | ≤1 | NA |
| Range of Combination Index | Description |
|---|---|
| <0.1 | Very strong synergism |
| 0.1–0.3 | Strong synergism |
| 0.3–0.7 | Synergism |
| 0.7–0.85 | Moderate synergism |
| 0.85–0.90 | Slight synergism |
| 0.90–1.10 | Nearly additive |
| 1.10–1.20 | Slight antagonism |
| 1.20–1.45 | Moderate antagonism |
| 1.45–3.3 | Antagonism |
| 3.3–10 | Strong antagonism |
| >10 | Very strong antagonism |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trajčíková, E.; Kurin, E.; Slobodníková, L.; Straka, M.; Lichváriková, A.; Dokupilová, S.; Čičová, I.; Nagy, M.; Mučaji, P.; Bittner Fialová, S. Antimicrobial and Antioxidant Properties of Four Lycopus Taxa and an Interaction Study of Their Major Compounds. Molecules 2020, 25, 1422. https://doi.org/10.3390/molecules25061422
Trajčíková E, Kurin E, Slobodníková L, Straka M, Lichváriková A, Dokupilová S, Čičová I, Nagy M, Mučaji P, Bittner Fialová S. Antimicrobial and Antioxidant Properties of Four Lycopus Taxa and an Interaction Study of Their Major Compounds. Molecules. 2020; 25(6):1422. https://doi.org/10.3390/molecules25061422
Chicago/Turabian StyleTrajčíková, Eva, Elena Kurin, Lívia Slobodníková, Marek Straka, Aneta Lichváriková, Svetlana Dokupilová, Iveta Čičová, Milan Nagy, Pavel Mučaji, and Silvia Bittner Fialová. 2020. "Antimicrobial and Antioxidant Properties of Four Lycopus Taxa and an Interaction Study of Their Major Compounds" Molecules 25, no. 6: 1422. https://doi.org/10.3390/molecules25061422
APA StyleTrajčíková, E., Kurin, E., Slobodníková, L., Straka, M., Lichváriková, A., Dokupilová, S., Čičová, I., Nagy, M., Mučaji, P., & Bittner Fialová, S. (2020). Antimicrobial and Antioxidant Properties of Four Lycopus Taxa and an Interaction Study of Their Major Compounds. Molecules, 25(6), 1422. https://doi.org/10.3390/molecules25061422