1. Introduction
pH is routinely measured in the laboratory using a glass electrode [
1]. The necessity for continued recalibration and the dependence of the liquid junction potential on solution composition and concentration have limited the application of glass electrodes in clinical, biomedical, and biotechnological analyses. Although there has been considerable interest in the in-vivo pH sensing of blood during surgery for patients who suffer from tissue ischemia [
2], the glass electrode is too bulky for invasive tissue analysis. Glass pH electrodes are also not suitable to the study of gastroesophageal reflux disease [
3] because of a lack of stability of the calibration over an extended period of time. As pH is a ubiquitous indicator of cell growth and metabolism, monitoring and controlling pH in fermentation baths [
4,
5,
6] is crucial. Although conventional glass pH electrodes have been adapted to fermentation reactions and bioreactors, they suffer from several drawbacks, including the necessity for pressurized compensation of the electrolyte and interference by other components (e.g., proteinaceous materials) in the medium.
For these reasons, optical pH sensing implemented through fiber optics has attracted considerable attention. The term optrode is often used to describe these sensors. pH optrodes usually consist of a pH indicator bound onto a solid support or immobilized in a polymer matrix at the distal end of an optical fiber [
7]. Interaction of the indicator at the end of the fiber with the sample leads to changes in the optical properties of the indicator which is detected through the optical fiber by absorbance or fluorescence from the sensing material [
8,
9]. Optrodes have several advantages over glass electrodes including low cost, small size and the robustness of the optical fiber. However, the use of chromophores (e.g., pH sensitive dyes as indicators) also has its drawbacks. The reagent may leach out over time and chromophores can photodegrade, limiting the lifetime of the sensor. The chromophores are wavelength-specific, imposing restrictions on the instrumentation that can be used. Furthermore, optrodes based on the fluorescence of chromophores typically generate smaller responses.
To overcome these disadvantages, optrodes utilizing swellable polymers functionalized to respond to pH have been developed [
10,
11,
12,
13,
14,
15,
16]. Polymer swelling has several advantages over detection methods involving chromophores. The analyte signal is based on turbidity and is wavelength-independent. The problem of photodegradation is obviated, and near infrared (NIR) wavelengths, which can be used to monitor changes in the turbidity of the swellable polymer, can be transmitted through an optical fiber without appreciable attenuation, unlike the ultraviolet or visible electromagnetic radiation used in absorption or fluorescence.
Several studies [
13,
14,
15,
16] which have been undertaken on polymer swelling for optrodes have focused on the development of formulations to prepare derivatized polystyrene that can undergo a large number of swelling and shrinking cycles without cracking. The optical properties of the derivatized polystyrene changes with swelling in aqueous solution. In the unswollen state, the polymer is turbid, whereas the polymer is more transparent in the swollen state. The reason for this is that derivatized polystyrene contains large pores filled with water. Since water has a lower refractive index than the polymer, these pores will both reflect and scatter light. When the polystyrene particles swell, the amount of water in the pores of the polymer increases and the refractive index decreases. As the refractive index of the polymer particles approaches that of water, the polymer both reflects and scatters less light. As this is the reason for the change in the optical properties of the polymer that accompanies swelling, a better approach would involve reversing the phases, i.e., preparing microparticles and embedding them in a hydrogel membrane [
17] instead of synthesizing a polymer with water-filled pores.
There are several advantages in preparing microparticles embedded in a hydrogel membrane. The weight fraction of the microparticles in the membrane is only on the order of a few percent. This means that less analyte has to react with the membrane to cause an optical change. The polymer is also free to swell in all three directions (x, y, and z) when the polymer particles are embedded in a hydrogel membrane. For chemical sensing, derivatized polystyrene particles with water-filled pores have been attached to a glass substrate in an optical reflectance device [
15] or at the distal end of an optical fiber [
13,
14]. This prevents them from swelling parallel to the surface as they can only swell perpendicular to the substrate. Since the volume change is equal to the cube of the swelling ratio in one dimension when the polymer is free to swell in all directions, there is a large increase in sensitivity when the microparticles are embedded in a hydrogel membrane. Furthermore, the microparticles are protected from direct contact with the sample by the hydrogel membrane, which also serves as a “filter” to reject proteinaceous materials or other large macromolecular compounds present in the medium. The polymer particles will not leach out of the membrane, and the membranes have been shown to be stable for several years [
17].
Previously published studies on swellable polymers for chemical sensing have primarily focused on charged polymers. The swelling of a charged polymer can be viewed as an osmotic pressure effect that results from differences between the charge density of the bulk polymer and the surrounding solution [
18]. Low ionic strength solutions promote ionic swelling because of an absence of shielding of the charges by the solvent ions. In these solutions, large differences exist between the charge density of the bulk polymer and the surrounding solution. In high ionic strength solutions, however, shielding of the charges on the polymer backbone occurs. In these solutions, the differences between the charge density of the bulk polymer and the surrounding solution are smaller, which causes a reduction in swelling.
By comparison, the swelling of pH sensitive
N-isopropylacrylamide (NIPA) polymers (which are investigated in this study), is not suppressed by the ionic strength of the solution in contact with the polymer, as swelling is both nonionic and controlled by the polymer solvent interaction parameter [
19,
20]. This is a distinct advantage of
N-isopropylacrylamide (NIPA) polymers over other pH-sensitive swellable polymers previously investigated for sensor applications whose use is restricted to low ionic strength media. Another advantage is that copolymers of NIPA sensitized to pH using the appropriate functional comonomer undergo significant swelling and shrinking as a function of the pH of the solution in contact with the polymer. Furthermore, swelling is accompanied by a change in the refractive index of the polyNIPA particles from a transparent state (where the refractive index of the particles and the solvent are equal) to a milky state, due to a large amount of light scattered when the polymer is in its shrunken state.
In this study, swellable polymers that respond to pH have been prepared from NIPA copolymerized with acrylic acid (AA), methacrylic acid (MAA), ethacrylic acid (EAA), or propacrylic acid (PAA) to develop materials that respond over a large pH range, including a portion of the physiological pH range. When these NIPA polymer particles are embedded in a polyvinyl alcohol (PVA) hydrogel membrane, large changes in the turbidity of the membrane (measured by an absorbance spectrometer) occur as the pH of the buffer solution in contact with the hydrogel membrane is varied. Changes of approximately 0.3 absorbance units are observed in the swelling and shrinking of the pH-sensitive NIPA polymer particles dispersed in the membrane. Swelling of the NIPA polymer particles is nonionic as the ionic strength of the buffer solution in contact with the PVA membrane was increased from 0.1 to 1.0 M without a decrease in swelling. For many of these NIPA copolymer particles, swelling is also reversible in both low- and high ionic strength pH buffered media and at ambient and physiological temperatures.
It is the goal of this study to gain an understanding of how changes in the composition of the formulation influence the response of the functionalized polyNIPA particles to changes in the pH of the buffered media in contact with them. In particular, we seek to better understand the relationship between monomer content and type, and how changes in the composition of the formulation impact the pH response and allows the response to be tuned. The incorporation of a pH-sensitive functional co-monomer in the polymer formulation imparts unique properties to polyNIPA. For this reason, the characterization of several NIPA copolymers of acrylic acid (AA), methacrylic acid (MAA), ethacrylic acid (EAA), and propacrylic acid (PAA) was undertaken in this study. Unlike previous studies on pH-initiated swelling of acrylamide gels, which were limited to a specific pH-sensitive comonomer, a wide range of alkyl acrylic acids were investigated. Furthermore, the temperature-activated pH response of these gels has been examined in far greater detail than in previous studies, as sufficient data were available to compute the change in both the enthalpy and entropy associated with pH-induced polymer swelling.
2. Results
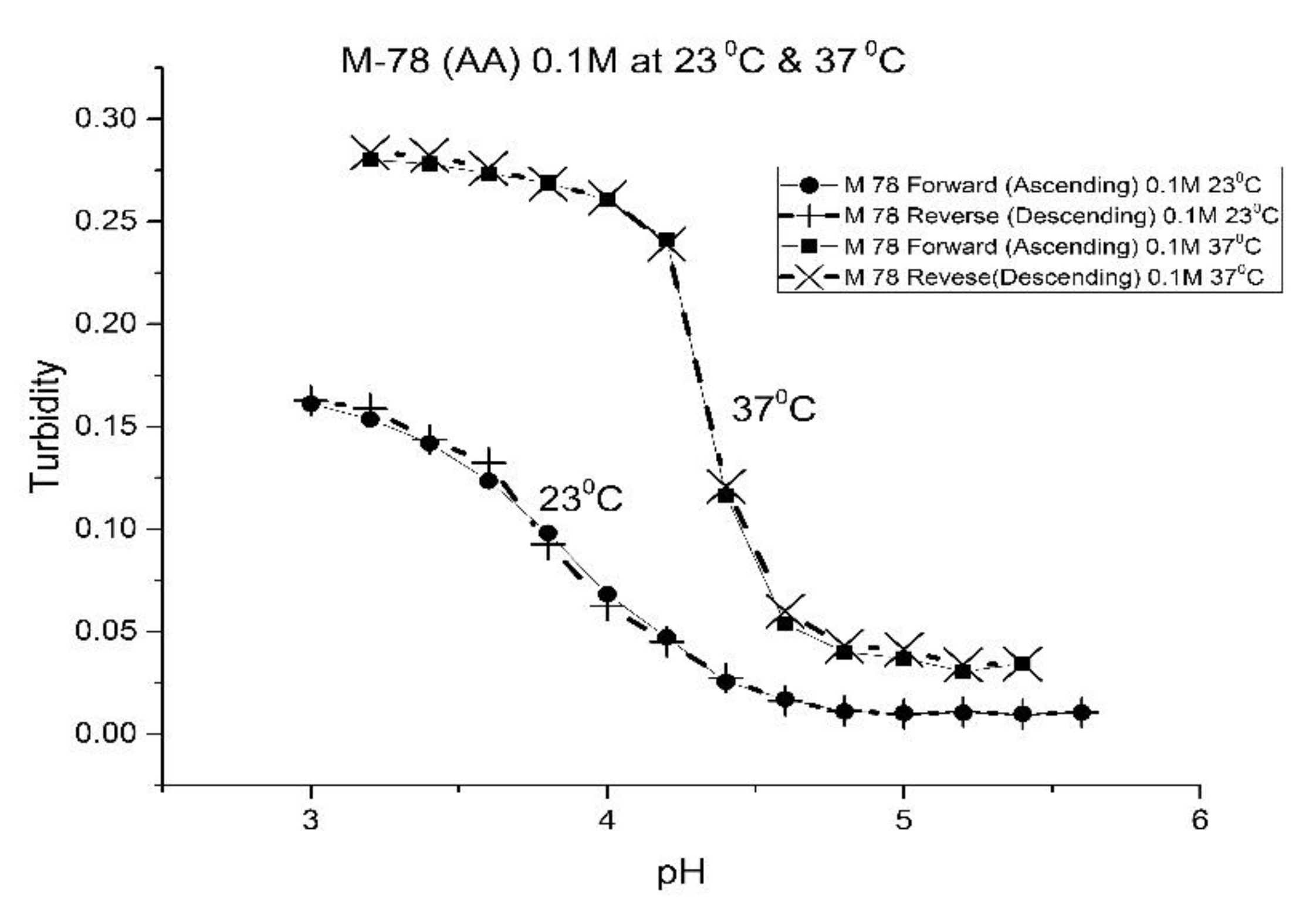
Figure 1,
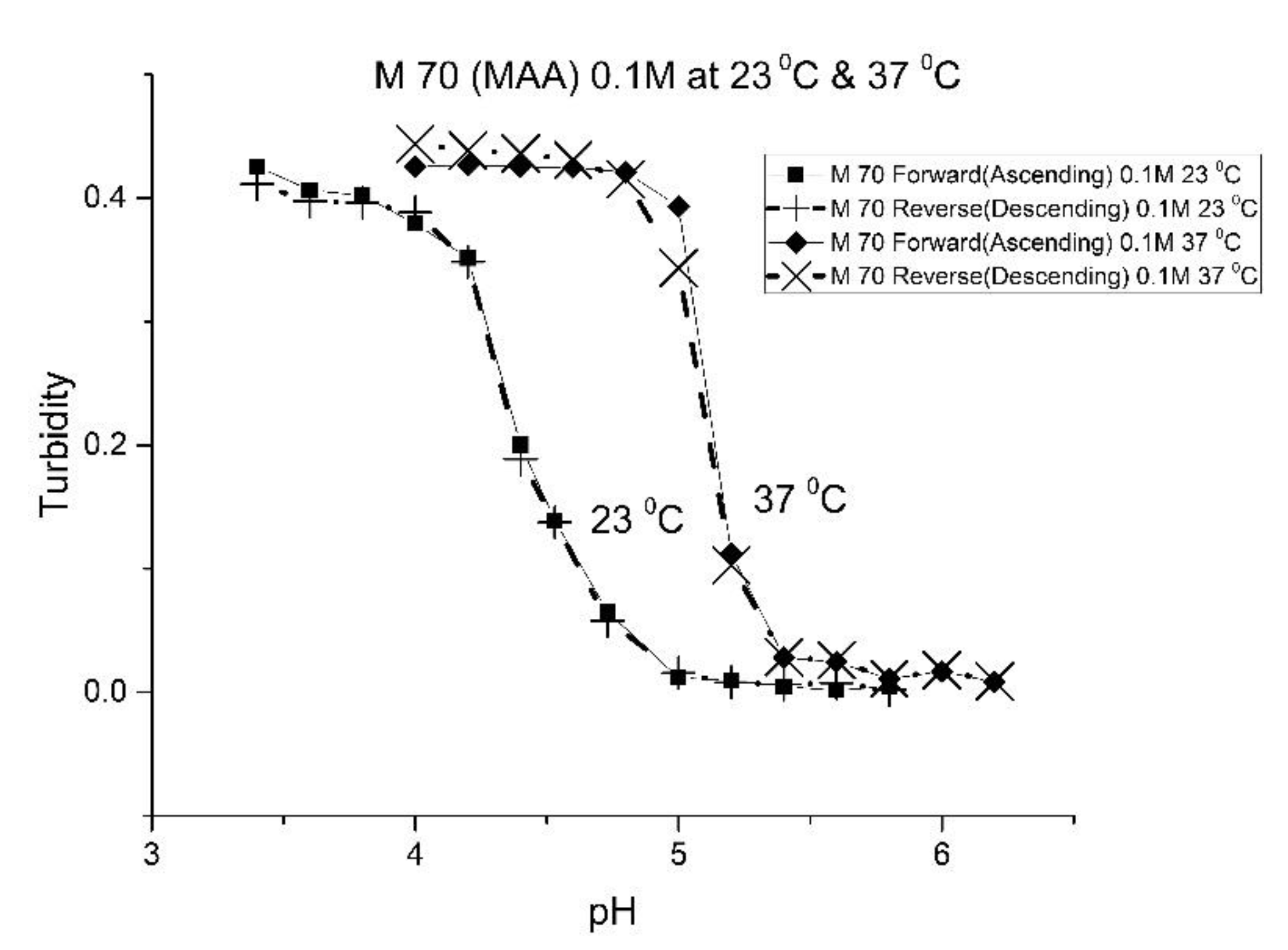
Figure 2,
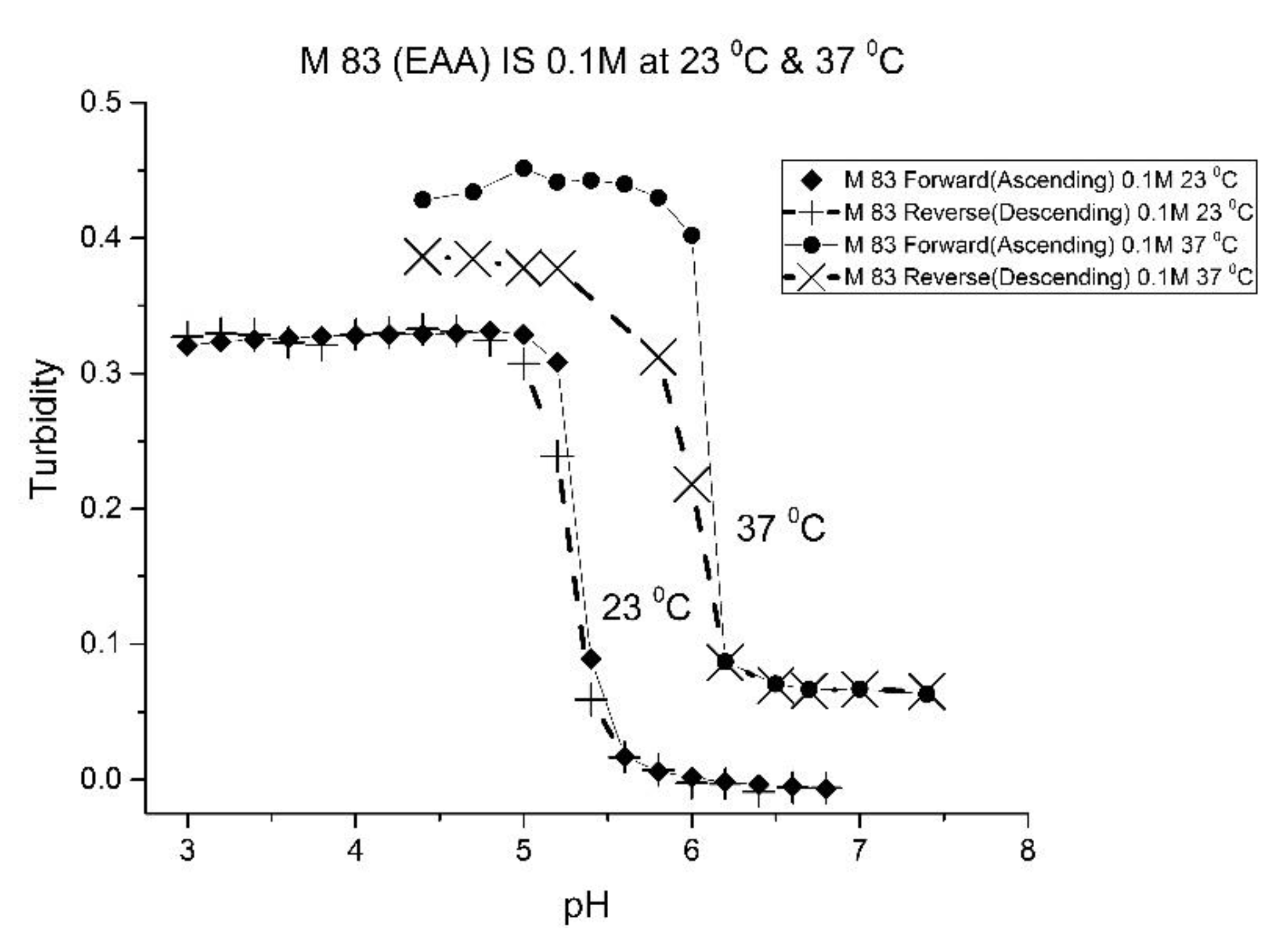
Figure 3 and
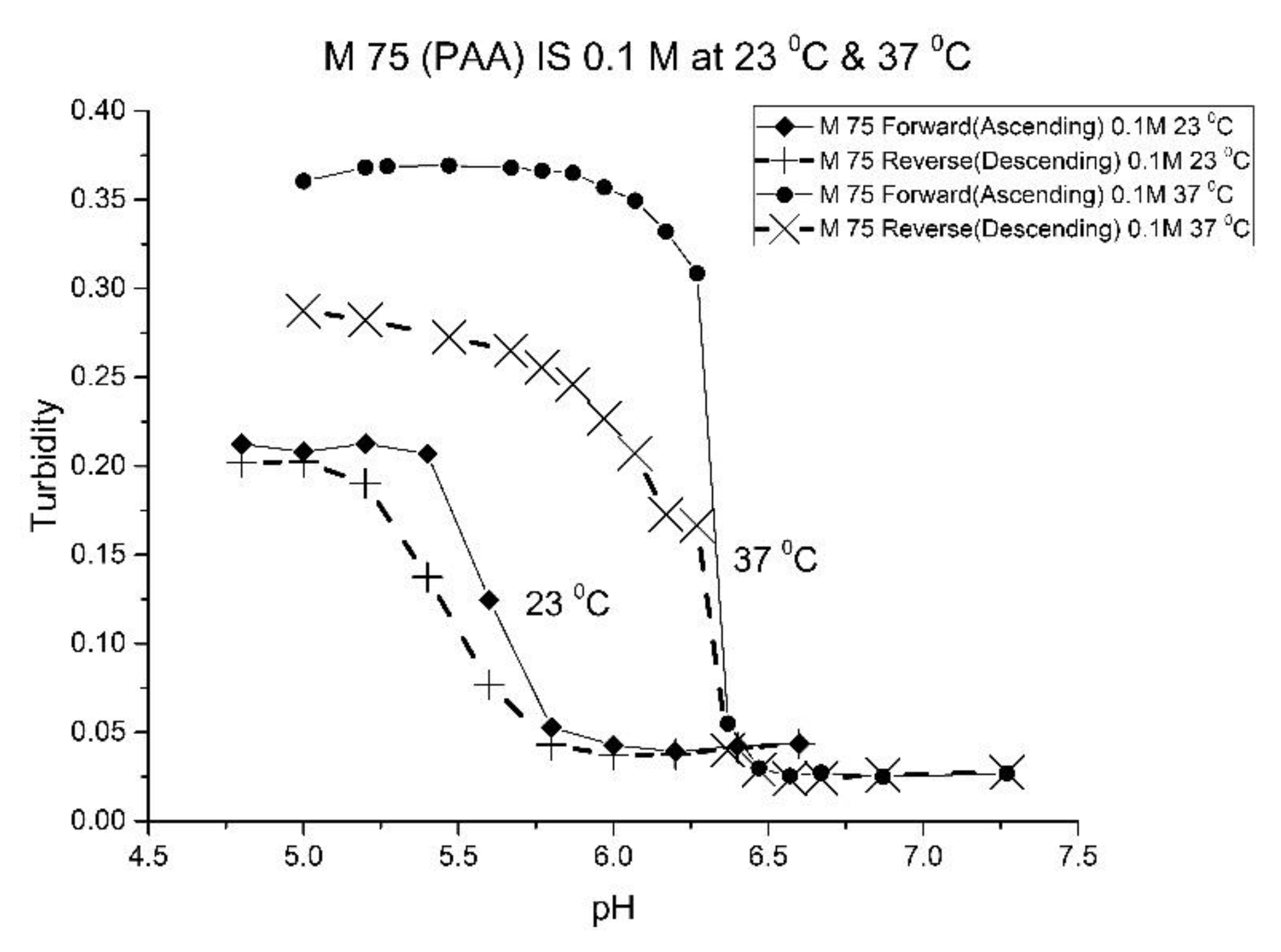
Figure 4 show the pH response profile of four polymers prepared by the copolymerization of NIPA with AA, MAA, EAA, and PAA. The turbidity (absorbance value) collected at 700 nm is plotted against the pH of the buffer solutions in contact with the pH-sensitive hydrogel. Each polymer, whose formulation is summarized in
Table 1, was embedded in a PVA hydrogel membrane. A unique aspect of this study is the use of light crosslinking in the preparation of these NIPA polymers, as many of the formulations used limited the amount of crosslinker,
N,
N-methylene bisacrylamide (MBA), to 5%. For each datapoint, the membrane is rinsed three times with a buffer solution of the proscribed pH and is allowed to equilibrate for 15 min prior to analysis by turbidimetry. Although the response time of the pH polymer particles is dependent on both the buffer capacity of the solution in contact with the membrane and the percentage of the alkyl acrylic acid in the copolymer, we chose to wait 15 min for each measurement to ensure that a full-scale response was always obtained. Lowering the percentage of the alkyl acrylic acid in the formulation can lead to even faster response times. For some membranes, a full-scale response to the change in pH was obtained in as little as five minutes.
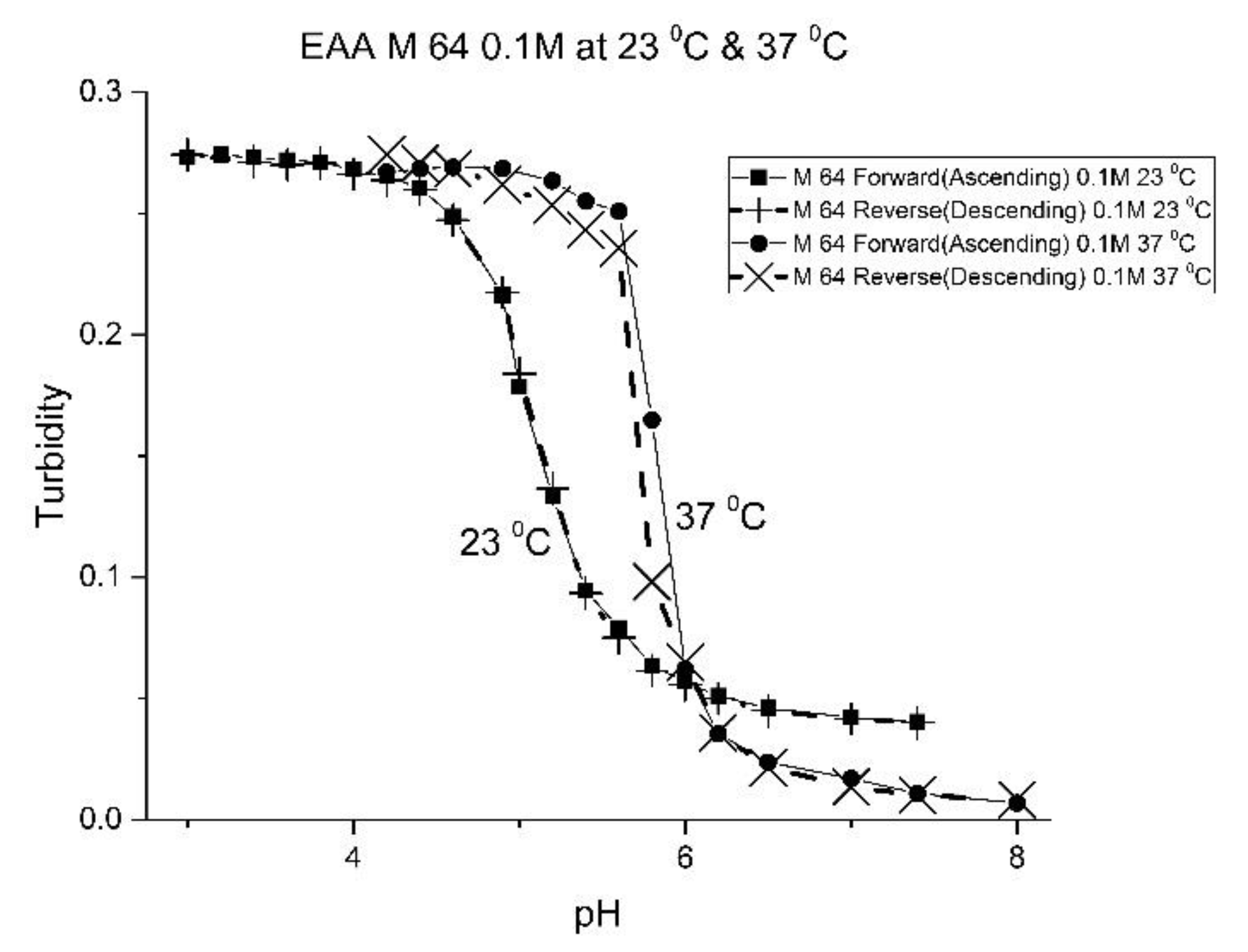
For AA and MAA, swelling is reversible at 23 and 37 °C, as both the ascending (solid line) and descending (dashed line) pH profiles are superimposable, whereas swelling for the EAA copolymer is reversible only at 23 °C, and swelling for the PAA copolymer is irreversible at both temperatures. At a low pH, the polymer exists in a shrunken state and the water content of the polyNIPA particles is low. Turbidity, as measured by the UV/visible absorbance spectrometer, is high because the refractive index of the particles is higher than the poly vinyl alcohol (PVA) hydrogel membrane. The result is a loss of transmitted light due to reflection, which translates into higher absorbance values. At higher pH values, the water content of the polymer increases, due to swelling induced by the deprotonation of the pH-sensitive alkyl acrylic acid co-monomer. The refractive index of the polyNIPA particles decreases as the particles swell and approaches the refractive index of the PVA hydrogel, which is approximately 90% water. Eventually, the turbidity reaches a limiting value that corresponds to the maximum swelling of the polymer.
The inflection point in the plot of turbidity (absorbance) versus pH (see
Figure 1,
Figure 2,
Figure 3 and
Figure 4) is the apparent pK
a of the polymer. The term “apparent pK
a” is used to refer to the point where the response is halfway between the response at a low pH and at a high pH, as turbidity versus pH curves have not yet been described by theory in a manner that would allow for calculation of the pK
a from the observed data. The change in the pH response of the membrane occurs over a narrower range than a typical pH indicator (which is plus or minus one pH unit). In all likelihood, only partial deprotonation of the carboxylic acid is necessary to generate maximum polymer swelling. Lowering the percentage of the alkyl acrylic acid in the NIPA formulation may yield polymer particles that respond over a wider pH range with a shift in their “apparent pK
a”. The pH response range of the NIPA copolymer particles has been investigated as a function of the amount of the alkyl acrylic acid present in the formulation and is the subject of a future publication [
21].
Table 2 lists the “apparent pK
a” values of the four NIPA copolymers at 23 and 37 °C. The “apparent pK
a”, which was computed for each pH response curve using the first derivative, can be tuned by increasing the alkyl chain length (i.e., hydrophobicity) of the pH-sensitive functional co-monomer. For the four copolymers listed in
Table 1, NIPA is the dominant monomer, as there is only a small amount of pH-sensitive functional co-monomer present. For AA and MAA, NIPA is polymerized into chains containing well-separated pH-sensitive functional co-monomer units, as the relative reactivity ratios of all the monomers comprising these copolymers are approximately equal. This is probably the reason why the “apparent pK
a” of M-78 and M-70 is similar to the pK
a of the AA or MAA monomers (see
Table 2). Because of the lower reactivity ratio of EAA and PAA [
22], the pH-functional co-monomer units in M-83 and M-75 are not as well separated. The net result is higher “apparent pK
a” values, analogous to the higher pK
a values obtained for the second proton of maleic acid versus that of fumaric acid. (The “apparent pK
a” value of M-83 and M-75 is substantially larger than the pK
a of the EAA or PAA monomer (see
Table 2.) The greater hydrophobicity of EAA and PAA compared to AA and MAA may also be a factor, as it can result in a decrease in the water content of the polymer, resulting in higher “apparent pK
a” values. The presence of PAA in polyNIPA also causes a decrease in the lower critical solution temperature of polyNIPA to 23 °C compared to 36.7 °C for MAA. This is attributed to the hydrophobic nature of the protonated form of PAA [
23].
The increase in pK
a with temperature of these four NIPA copolymers is the opposite to what would occur for a dye used as a pH indicator in an optical fiber, which would be a decrease of approximately 0.1–0.3 pH units for a 10 °C increase in temperature [
24]. The shift of the pK
a towards higher pH values can be attributed to a decrease in the water content of the acrylamide gel at higher temperatures. Park and Hoffman [
25], in a previous study, have shown that a decrease in the water content of the acrylamide gel occurs as the temperature is increased. The distance between adjacent alkyl acrylic acids units, the source of charge in the NIPA copolymer, decreases due to water molecules being driven out of the polyNIPA copolymer as the temperature is increased. This, in turn, causes the apparent pK
a of the NIPA copolymer to increase.
The pH response of the NIPA copolymers at higher temperatures (i.e., physiological versus ambient) appears to be enhanced. Since the water content of the NIPA copolymer decreases at higher temperatures, there is an increase in the surface area to volume ratio of the polymer network. The result is that fewer protons are required to react in order to yield a full-scale pH response. Thus, the pH response appears to be enhanced.
To better understand the relationship between the “apparent pK
a” of the pH-sensitive polyNIPA particles and temperature, pH response curves (23–40 °C) for each of these four copolymers were obtained. The “apparent pK
a” for each response curve was computed, with the relationship between pK
a and temperature modeled using the van’t Hoff relationship, see Equation (1), where
Ka is the apparent acid dissociation constant of the NIPA copolymer, T is the temperature of the copolymer, ΔH° is the change in enthalpy associated with pH-induced swelling of the polymer, ΔS° is the change in entropy associated with the pH-induced swelling of the polymer, and R is the gas constant.
Table 3 lists the changes in the enthalpy and entropy that occur due to pH-induced polymer swelling. The linearity of these plots over the temperature range investigated was excellent. The uncertainties in the enthalpies and entropies of swelling determined from the least squares fitting of the data shown in
Table 3 are small, which is indicative of a good fit of the data. For AA, MAA, and EAA, the increase in the hydrophobicity of the co-monomer in polyNIPA can be correlated to an increase in the enthalpy and entropy of swelling for these copolymers. However, PAA does not follow this trend. The anomalous behavior of PAA may be due to the absence of
N-tert-butyl acrylamide (NTBA) in the formulation used to prepare this NIPA copolymer.
As one of the motivations for undertaking this study is physiological pH sensing (pH 5–pH 7.4), we chose to focus our investigation on EAA and PAA co-polymers of NIPA.
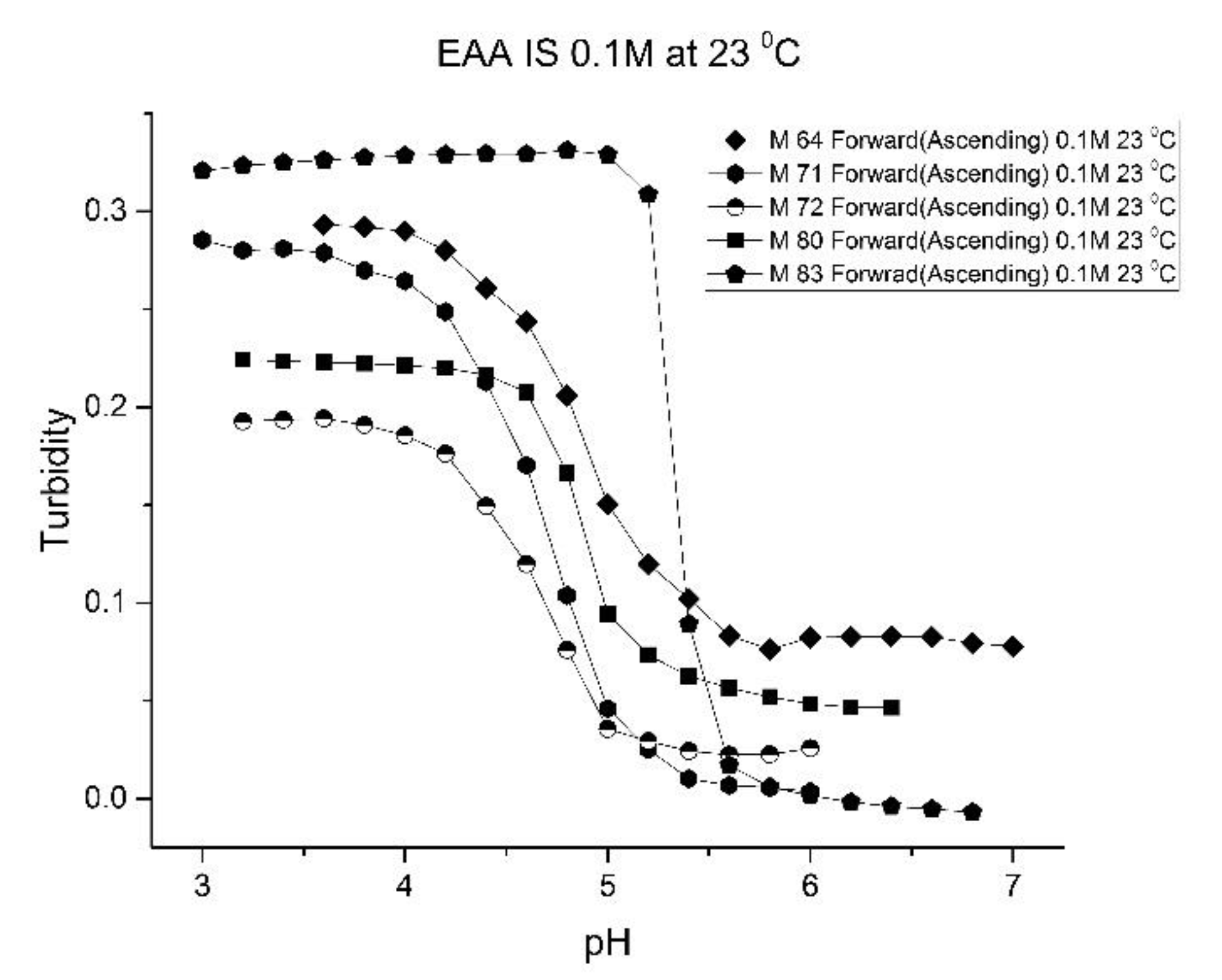
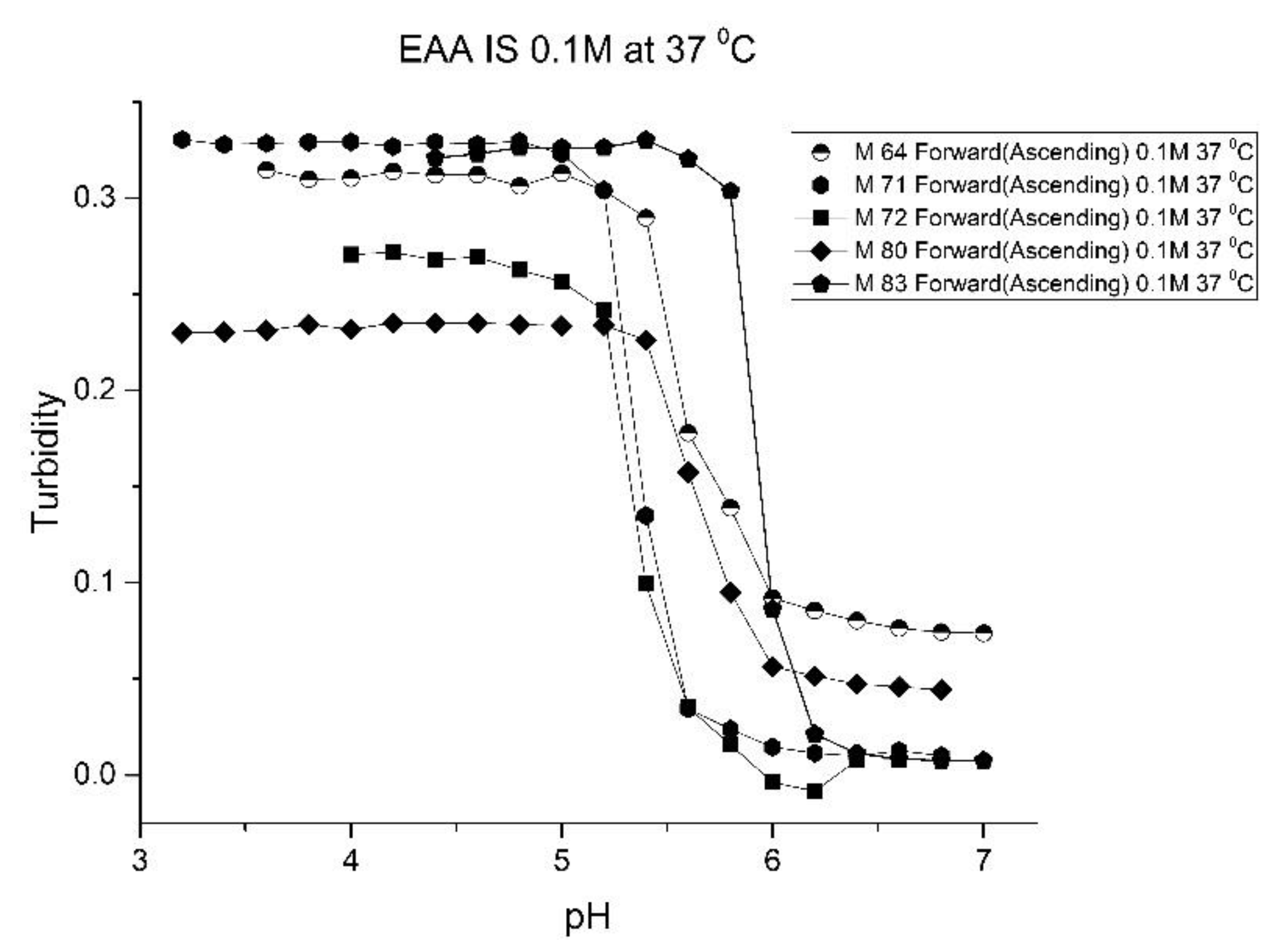
Figure 5;
Figure 6 show pH response curves at 23 and 37 °C for several EAA formulations (see
Table 4). From these response curves, the range of “apparent pK
a” values spanned by the EAA co-polymers is 4.7 to 5.3 pH units at 23 °C and 5.3 to 5.9 pH units at 37 °C. The larger pK
a value of M-83 are attributed to the presence of NTBA in the formulation, which increases the hydrophobicity of the polymer. By varying the formulation used for these five EAA co-polymers (through adjusting the amount of MBA and NTBA in the formulation), the “apparent pK
a” of these polyNIPA particles can be tuned (see
Table 5) and the reversibility of their swelling enhanced (see
Figure 7 versus
Figure 3).
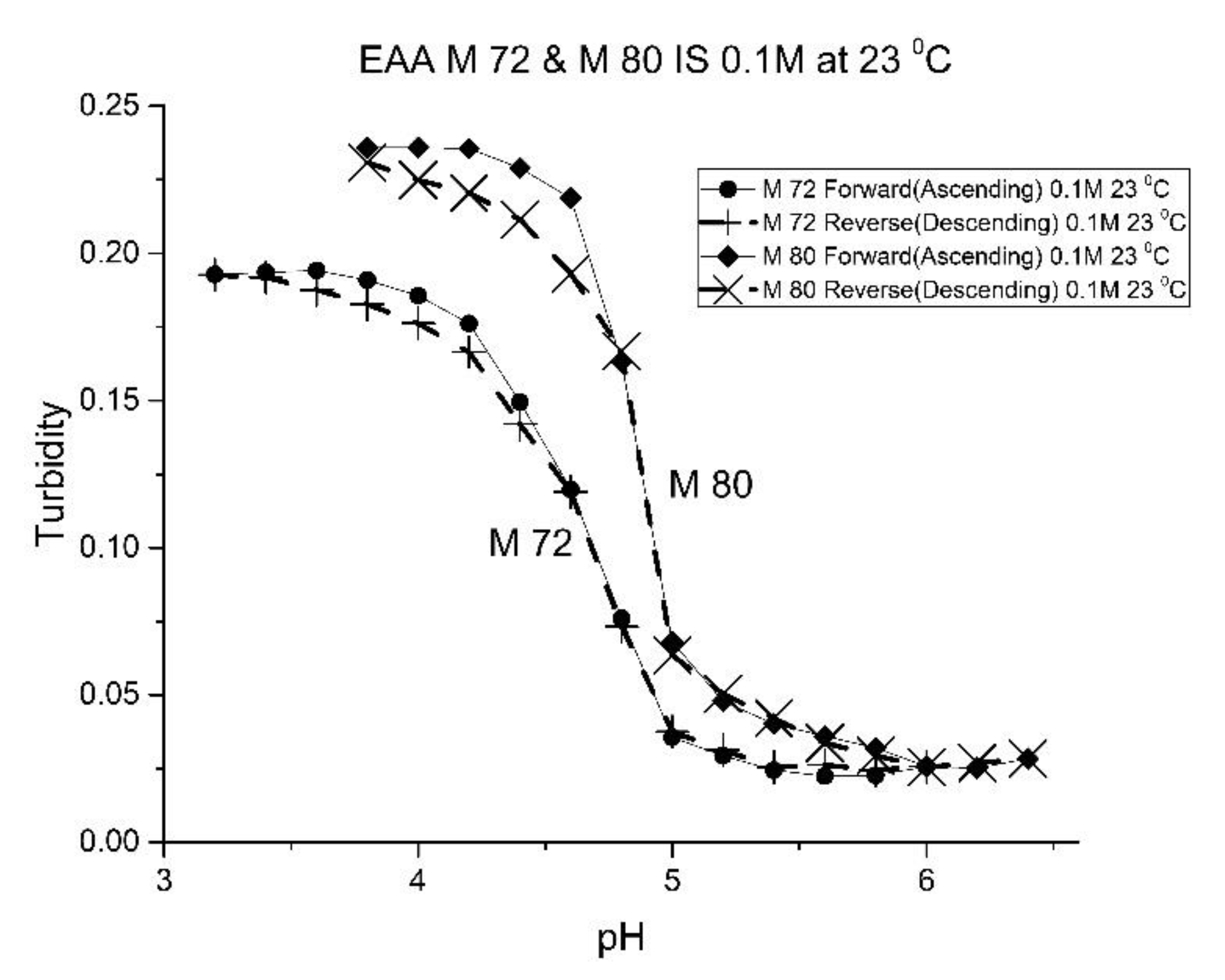
Although the formulation for M-72 and M-80 is the same, differences in their pH response curves (see
Figure 8) and the “apparent pK
a” values (see
Table 5) obtained for these two polymers suggests that variability between batches of the same formulation is a concern in the preparation of swellable pH-sensitive polyNIPA particles. For M-72 and M-80, we attribute batch variability to the aging of some of the components used to prepare these NIPA-based copolymers. M-80 was prepared using relatively fresh components (less than one year), whereas M-72 was prepared using aged components (that were stored in a refrigerator for a few years). For acrylamide gels, the properties of the crosslinked polymers can vary between batches. However, our experience in the preparation of these NIPA copolymers is that batch variability can be mitigated by using relatively fresh components to prepare these NIPA copolymers.
The change in enthalpy and entropy of the NIPA-EAA polymer particles is summarized in
Table 6. M-64, M-71, and M-72 have similar values for the entropy and enthalpy of swelling, as do M-80 and M-83. We attribute this clustering to the use of relatively fresh reagents (NIPA, NTBA, and MBA) for M-80 and M-83, whereas the formulation components used to prepare M-64, M-71, and M-72 were several years old.
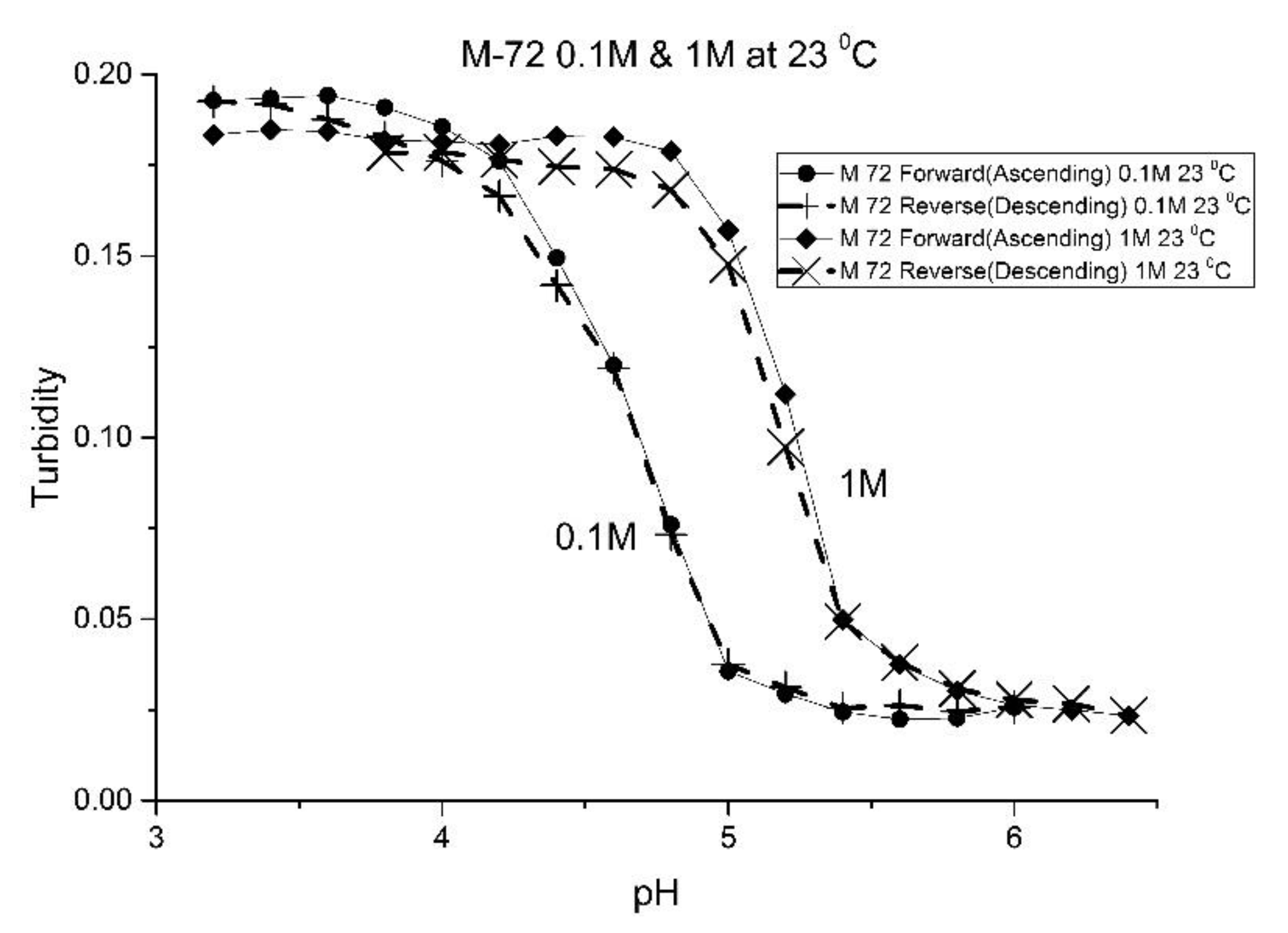
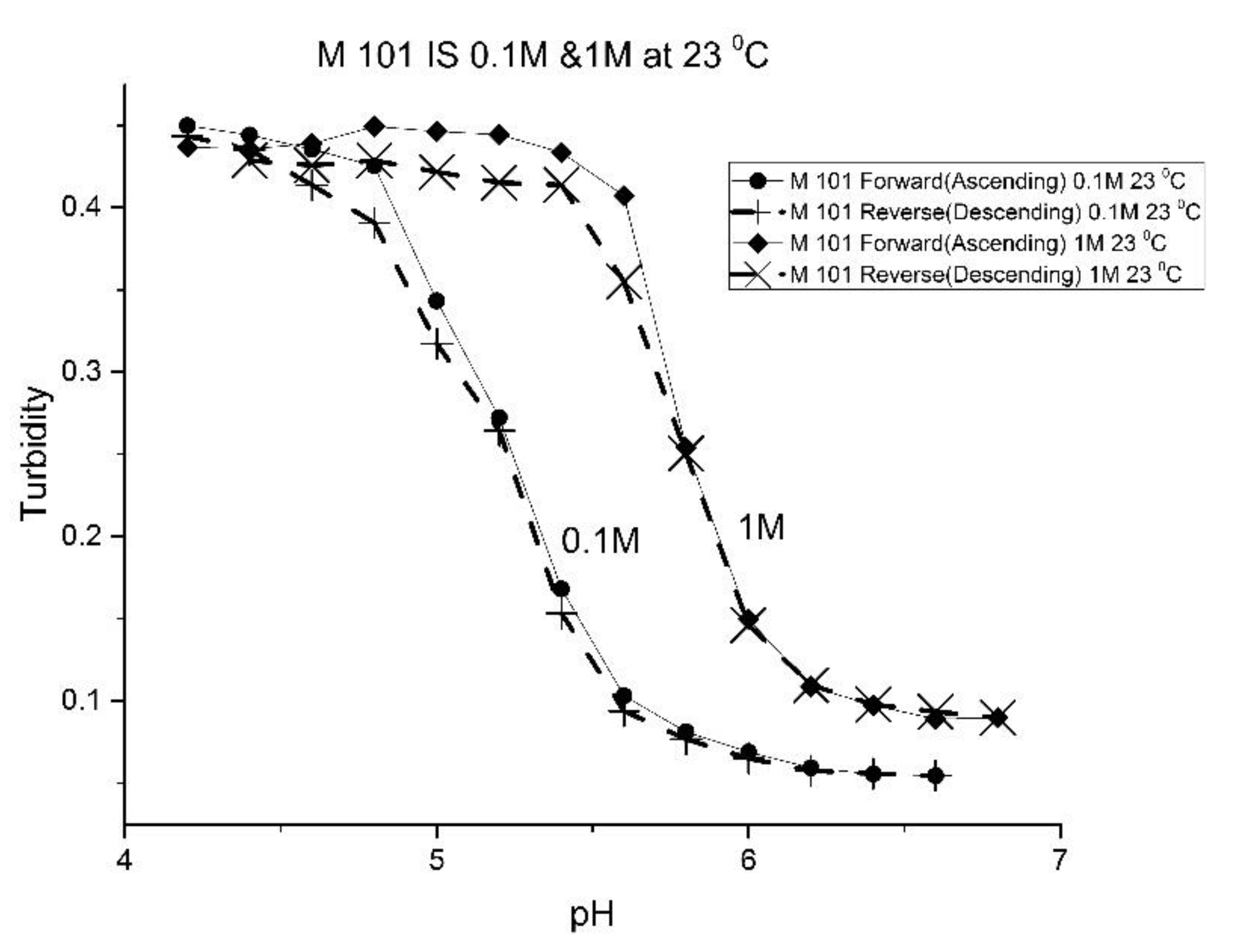
The effect of the ionic strength of the buffer solution in contact with the EAA co-polymers was also investigated at ambient temperature. pH response curves were obtained at 0.1 M and 1.0 M ionic strength with sodium chloride used to control the ionic strength of the solution.
Figure 9 shows the pH response curve at 0.1 M and 1.0 M for M-72 which is representative of the set of EAA copolymers investigated in this study.
Swelling is both significant and reversible at both 0.1 and 1.0 M ionic strength. The pH response curves at both 0.1 and 1.0 M ionic strength indicate that swelling for the EAA copolymers is nonionic. Furthermore, pH response curves of similar NIPA copolymers investigated in previous studies were unchanged by the ionic strength of the buffer solution in contact with the membrane when the ionic strength of the buffer is less than 0.5 M [
17,
21]. As for the increase in the apparent pK
a of the polyNIPA particle when the ionic strength of the buffer solution in contact with the particles is 1.0 M, the clear shift in the pH response to higher pH values can be attributed to the decrease in the water content of the acrylamide gel at high (approximately 1M NaCl) concentrations. The effect of the NaCl concentration on the water content of polyNIPA was previously reported by Park and Hoffman [
25]. At high NaCl concentrations, the distance between alkyl acrylic acid monomer units (which is responsible for the source of charge in the NIPA copolymer) decreases as a result of the loss of water from the polymer. Hence, the increase in the “apparent pK
a” of the polymer can be attributed to a decrease in the distance between pH-functional comonomer units in the three dimensional polymer network due to a decrease in the volume of the polymer, as a result of the water molecules being driven out of the NIPA copolymer at high NaCl concentrations. The fact that significant swelling is observed at high NaCl concentrations makes suitably functionalized NIPA copolymers well-suited to perform optical pH sensing in ocean and sea water.
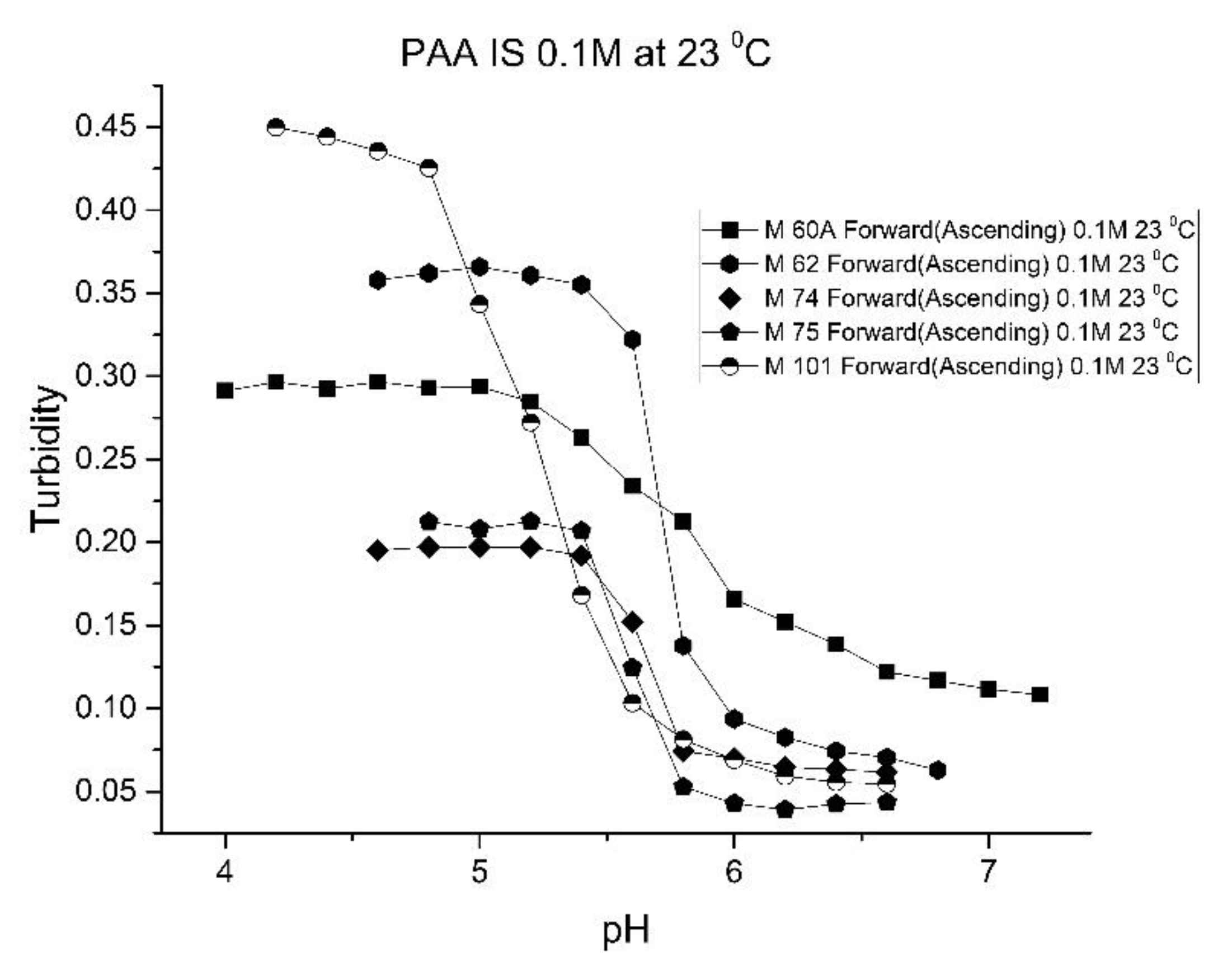
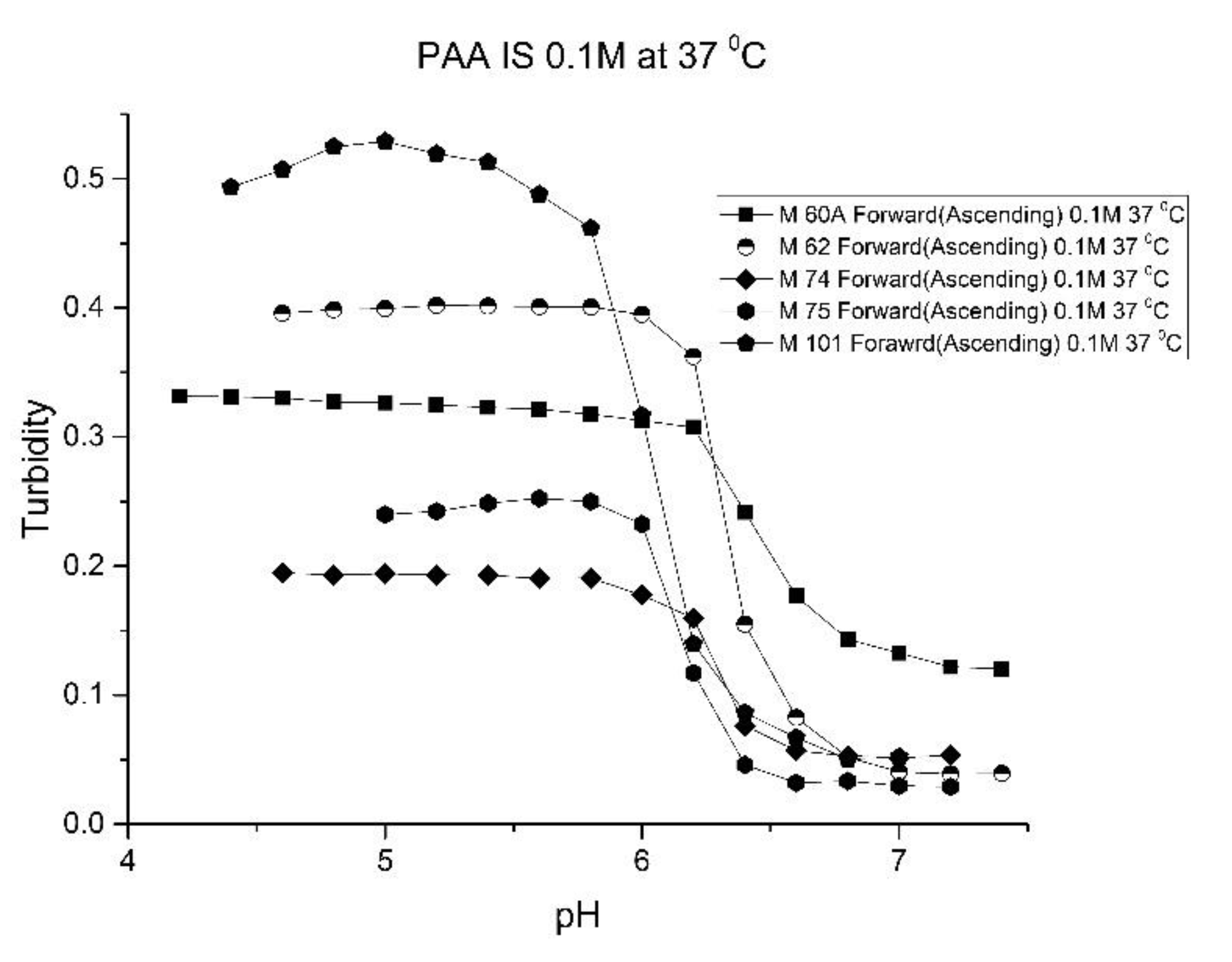
Figure 10,
Figure 11 show ascending pH response curves at 23 and 37 °C for a variety of PAA-NIPA co-polymer formulations (see
Table 7). From this set of pH response curves, the range of pK
a values spanned by the PAA co-polymers is 5.3 to 5.9 at 23 °C and 6.1 to 6.5 at 37 °C. By varying the formulation used to prepare these PAA co-polymers (through adjustments in the amount of the pH sensitive functional comonomer and crosslinker present in the formulation), the “apparent pK
a” of these NIPA copolymers can be tuned (see
Table 8), and the reversibility of the swelling can be enhanced (see
Figure 12 versus
Figure 4). The change in the enthalpy and entropy of these particles for pH-induced swelling is summarized in
Table 9. M-60A, M-62, and M-74 have similar values for enthalpy and entropy, as do M-75 and M-101. The dichotomy appears to be correlated to the degree of crosslinking for this set of NIPA copolymers. The amount of crosslinker in the formulation used to prepare M-75 and M-101 is 5%, whereas the amount of crosslinker is greater than 5% in the M-60A, M-62, and M-74 polymer formulations.
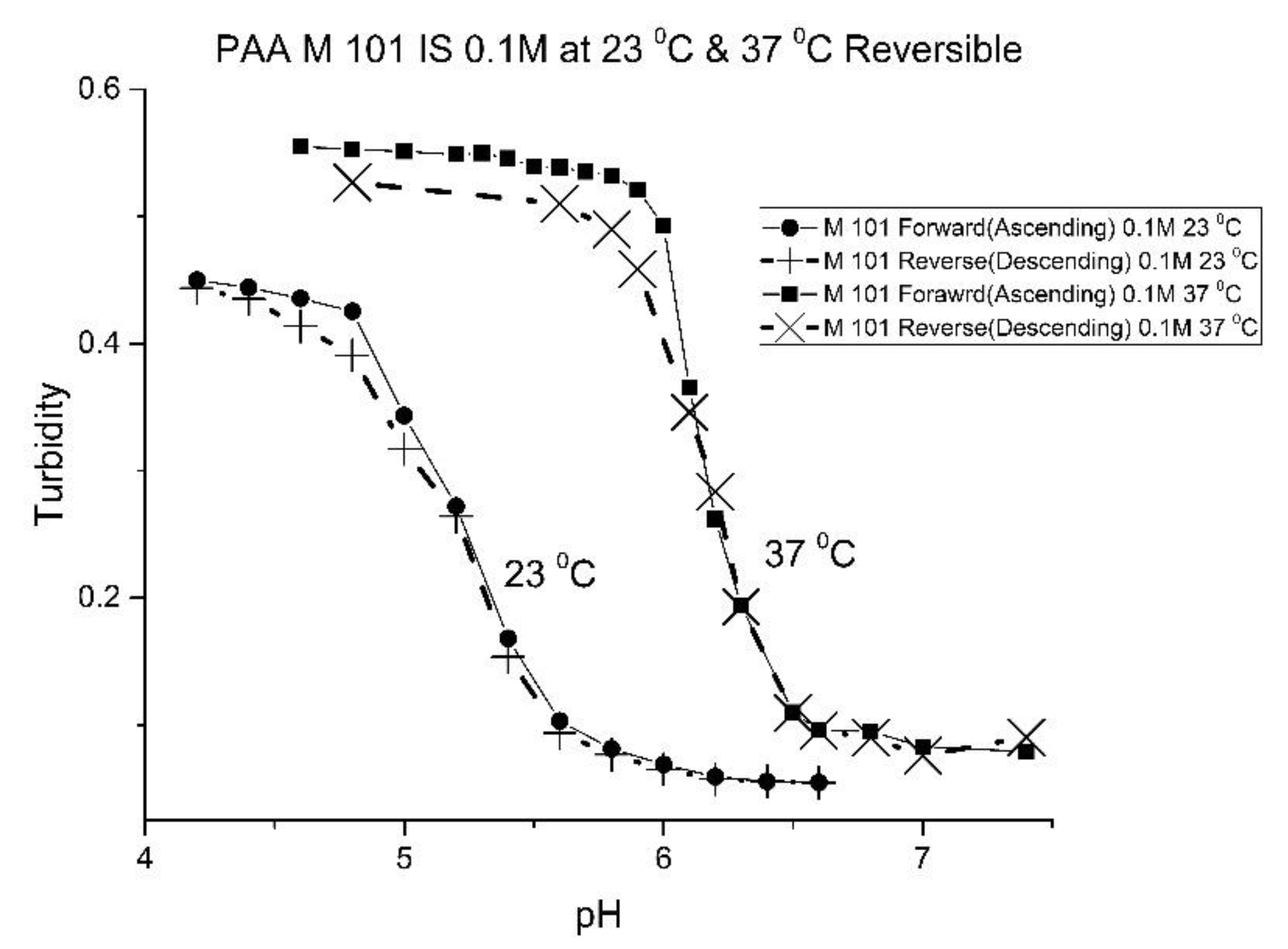
Figure 13 shows the effect of ionic strength on the pH response of M-101. The results obtained for M-101 are similar to those obtained for M-72 (see
Figure 9).
Many of the NIPA copolymers synthesized for use in this study have undergone more than one-hundred swelling and shrinking cycles without loss of functionality. These copolymers have exhibited reproducible responses for numerous swelling and shrinking cycles (e.g., see the forward and reverse titration curves for M-64, M-70, M-72, M-78, M-80, M-83, and M-101). All regions of the corresponding titration curves, including those which show relatively low changes in turbidity for a portion of the pH range, can be reproduced for the same segment of the membrane. Swelling was also reversible for many of the NIPA copolymers investigated in this study. Therefore, any calibration will only need to be performed once for copolymers that exhibit reversible swelling. When placed in a glass vial containing distilled water and stored away in a refrigerator, these polymers have retained their full operational effectiveness, even after 5 years.