State-of-the-Art Production Chains for Peas, Beans and Chickpeas—Valorization of Agro-Industrial Residues and Applications of Derived Extracts
Abstract
1. Introduction
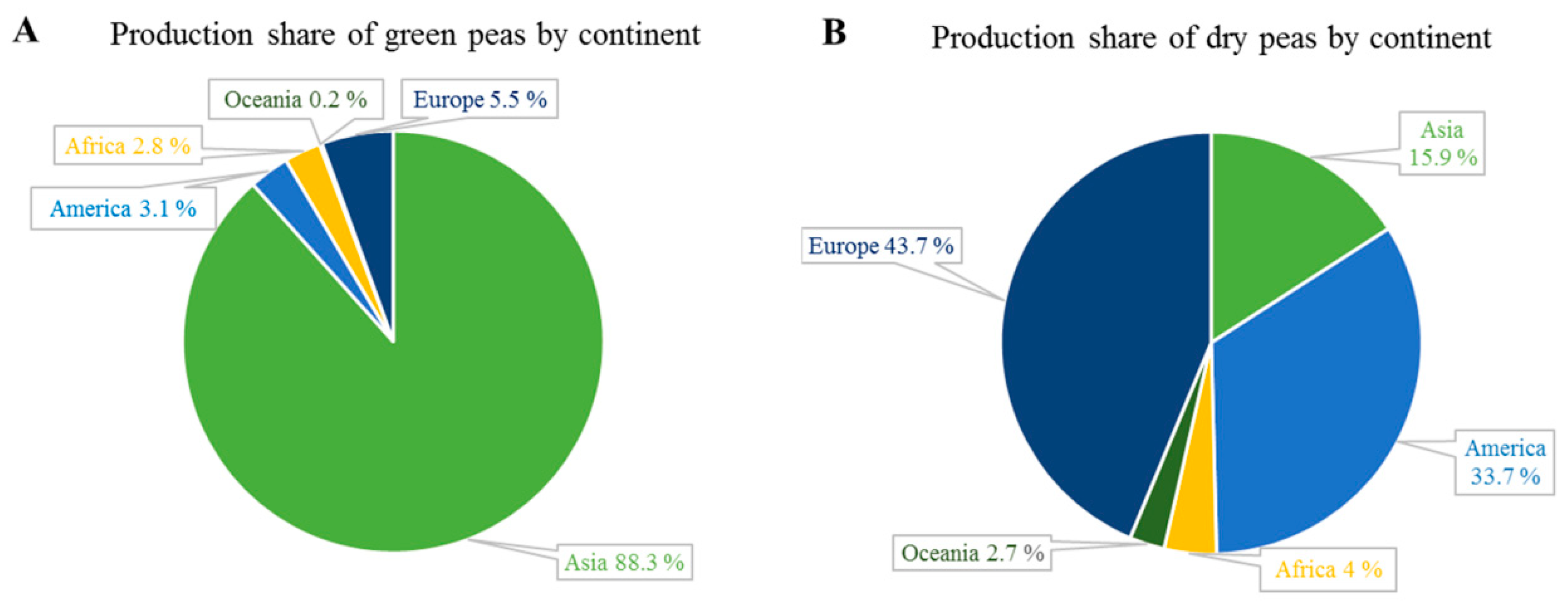
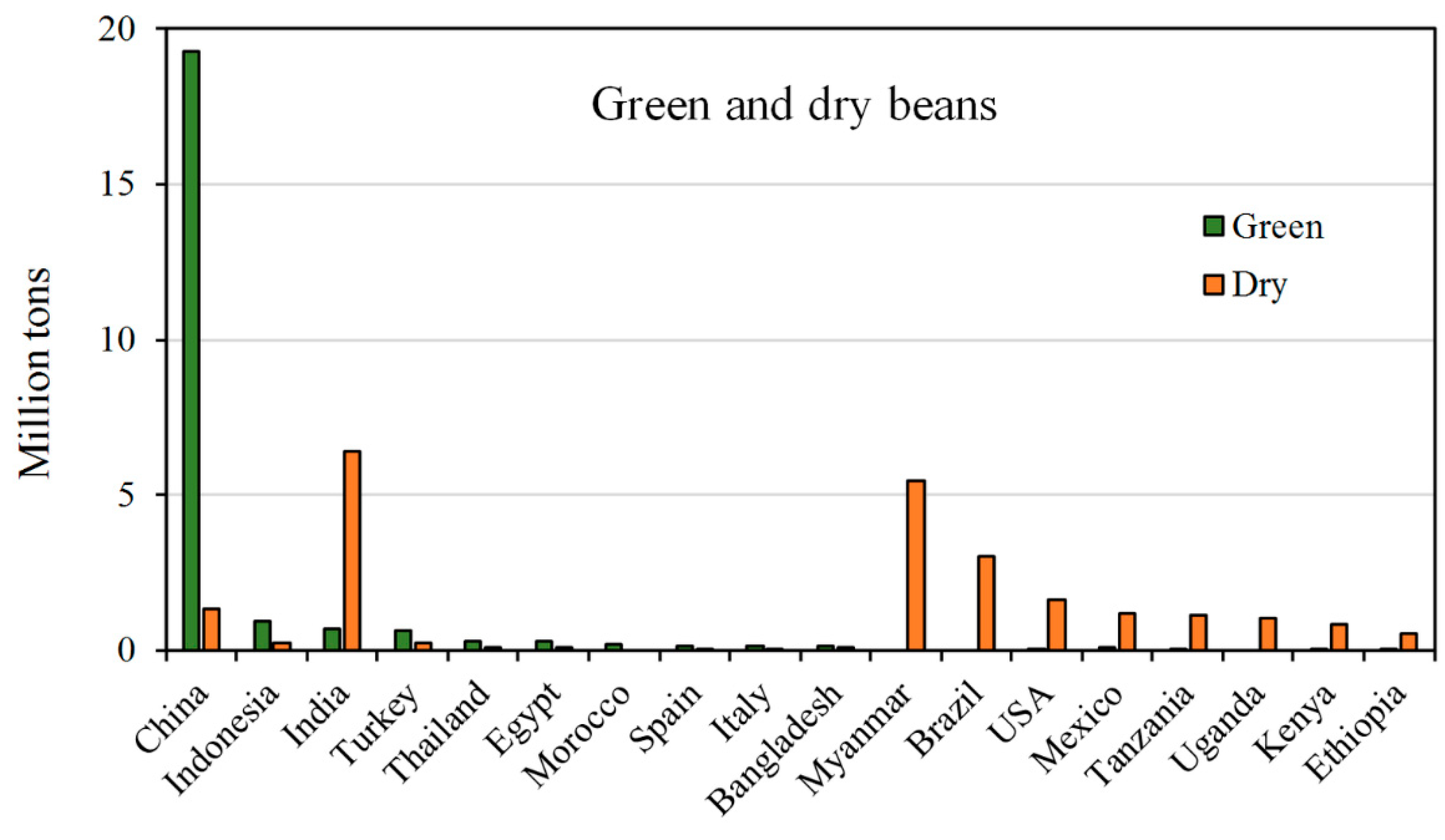
2. World and European Legume Production
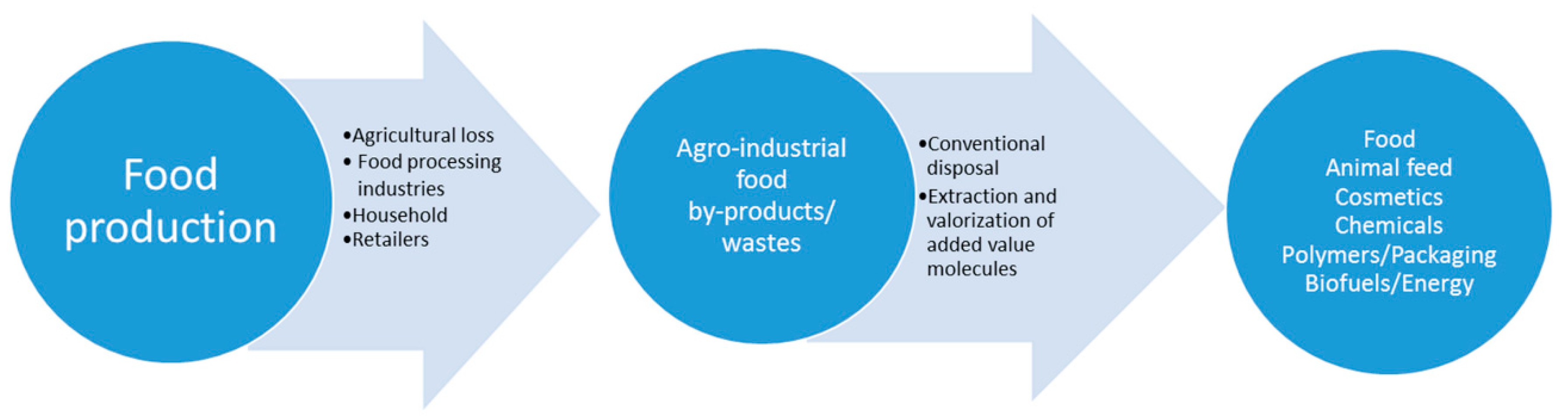
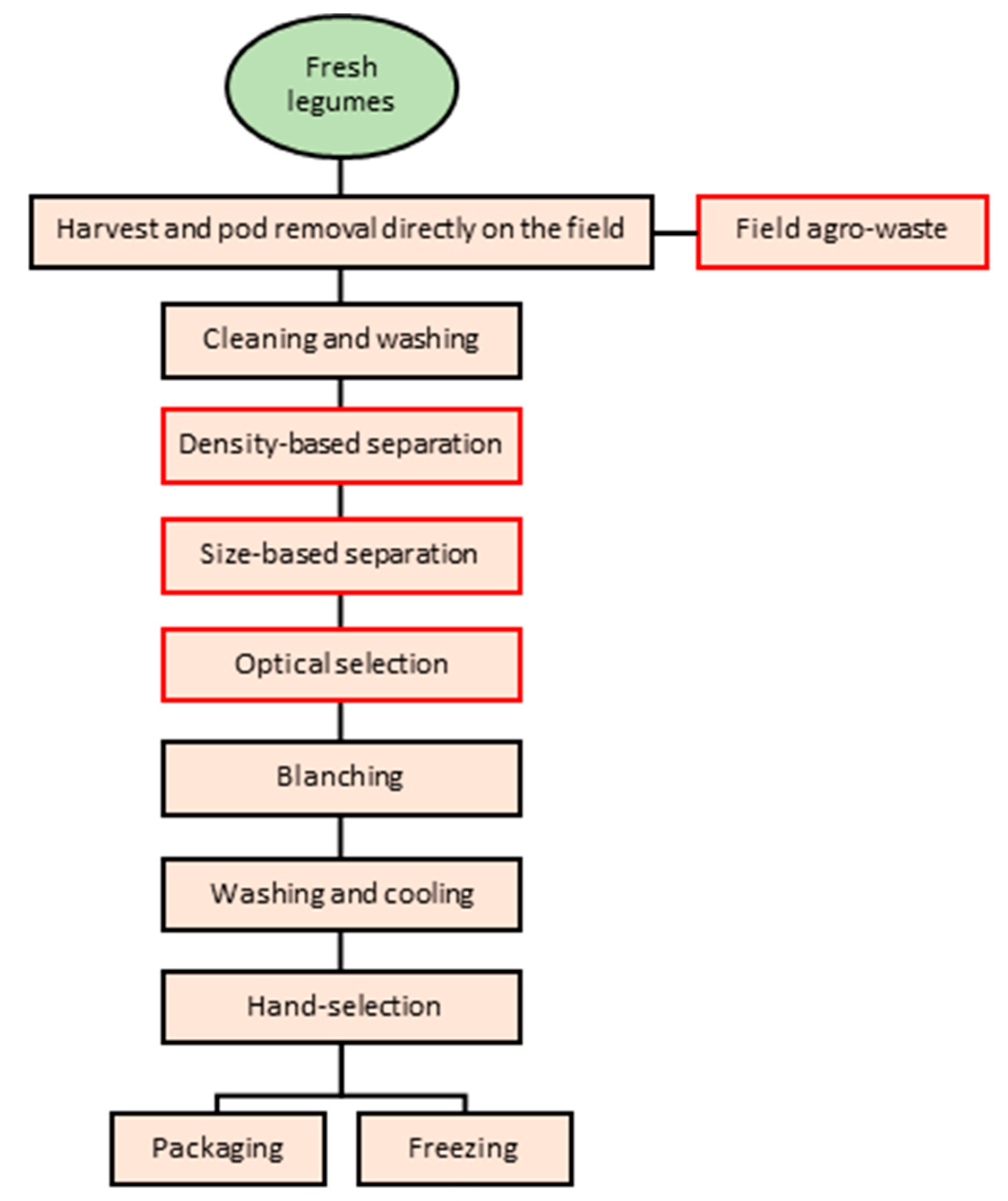
3. Legume By-Products/Wastes Generation During the Processing Chain
4. Legume Extraction Technologies
5. Applications of Peas, Beans and Chickpeas By-Products and Wastes
5.1. Feed
5.2. Food
5.3. Cosmetics
5.4. Packaging
5.5. Other Uses
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Helkar, P.B.; Sahoo, A.K.; Patil, N.J. Review: Food industry by-products used as a functional food ingredients. Int. J. Waste Resour. 2016, 6, 248. [Google Scholar]
- Nora, S.M.S.; Ashutosh, S.; Vijaya, R. Potential utilization of fruit and vegetable wastes for food through drying or extraction techniques. Nov. Tech. Nutr. Food Sci. 2017, 1, NTNF.000506. [Google Scholar]
- Vis, M.; van den Berg, D. Harmonisation of Biomass Resource Assessments, Volume 1, Best Practices and Methods Handbook, public Deliverable n. 5.3 of the FP7 project BEE Biomass Energy Europe. 2010. [CrossRef]
- Camia, A.; Robert, N.; Jonsson, R.; Pilli, R.; García-Condado, S.; López-Lozano, R.; van der Velde, M.; Ronzon, T.; Gurría, P.; M’Barek, R.; et al. Biomass production, supply, uses and flows in the European Union. JRC Sci. Policy Rep. 2018. Available online: https://ec.europa.eu/jrc/en/publication/eur-scientific-and-technical-research-reports/biomass-production-supply-uses-and-flows-european-union-first-results-integrated-assessment (accessed on 10 January 2020).
- Ronzon, T.; Piotrowski, S. Are primary agricultural residues promising feedstock for the European bioeconomy? Ind. Biotech. 2017, 13, 113–127. [Google Scholar] [CrossRef]
- Kaltschmitt, M.; Hartmann, H.; Hofbauer, H. Energie aus Biomasse: Grundlagen, Techniken und Verfahren, 2nd ed.; Springer: Heidelberg, Germany, 2009. [Google Scholar]
- European Commission. European Commission Decision of 18 December 2014 Amending Decision 2000/532/EC on the list of Waste Pursuant to Directive 2008/98/EC of the European Parliament and of the Council Text with EEA Relevance. 2014. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:OJ.L_.2014.370.01.0044.01.ENG (accessed on 9 December 2019).
- Sárvári Horváth, I.; Tabatabaei, M.; Karimi, K.; Kumar, R. Recent updates on biogas production-a review. Biofuel. Res. J. 2016, 10, 394–402. [Google Scholar] [CrossRef]
- Martínez, E.J.; Raghavan, V.; González-Andrés, F.; Gómez, X. New biofuel alternatives: integrating waste management and single cell oil production. Int. J. Mol. Sci. 2015, 16, 9385–9405. [Google Scholar] [CrossRef]
- Lin, C.S.K.; Koutinas, A.A.; Stamatelatou, K.; Mubofu, E.B.; Matharu, A.S.; Kopsahelis, N.; Pfaltzgraff, L.A.; Clark, J.H.; Papanikolaou, S.; Kwan, T.H.; et al. Current and future trends in food waste valorization for the production of chemicals, materials and fuels: A global perspective. Biofuel Bioprod. Bior. 2014, 8, 686–715. [Google Scholar]
- Voisin, A.-S.; Guéguen, J.; Huyghe, C.; Jeuffroy, M.-H.; Magrini, M.-B.; Meynard, J.-M.; Mougel, C.; Pellerin, S.; Pelzer, E. Legumes for feed, food, biomaterials and bioenergy in Europe: A review. Agron. Sustain. Dev. 2014, 34, 361–380. [Google Scholar]
- Maity, S.K. Opportunities, recent trends and challenges of integrated biorefinery: Part II. Renew. Sustain. Energy Rev. 2015, 43, 1446–1466. [Google Scholar] [CrossRef]
- RedCorn, R.; Fatemi, S.; Engelberth, A.S. Comparing end-use potential for industrial food-waste sources. Eng. Prc. 2018, 4, 371–380. [Google Scholar] [CrossRef]
- Jin, Q.; Yang, L.C.; Poe, N.; Huang, H.B. Integrated processing of plant-derived waste to produce value-added products based on the biorefinery concept. Trends Food Sci. Tech. 2018, 74, 119–131. [Google Scholar] [CrossRef]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: a review. Bioresour. Bioprocess 2018, 5. [Google Scholar] [CrossRef]
- Cecilia, J.A.; García-Sancho, C.; Maireles-Torres, P.J.; Luque, R. Industrial food waste valorization: a general overview. In Biorefinery, Integrated Sustainable Processes for Biomass Conversion to Biomaterials, Biofuels, and Fertilizers; Bastidas-Oyanedel, J., Schmidt, J., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 253–277. [Google Scholar]
- Galanakis, C.M. Recovery of high added-value components from food wastes: conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Galanakis, C.M. Separation of functional macromolecules and micromolecules: From ultrafiltration to the border of nanofiltration. Trends Food Sci. Tech. 2015, 42, 44–63. [Google Scholar] [CrossRef]
- del Mar Contreras, M.; Lama-Munoz, A.; Gutierrez-Perez, J.M.; Espinola, F.; Moya, M.; Castro, E. Protein extraction from agri-food residues for integration in biorefinery: potential techniques and current status. Bioresour. Technol. 2019, 280, 459–477. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Aparicio, I.; Redondo-Cuenca, A.; Villanueva-Suarez, M.J. Broad bean and pea by-products as sources of fibre-rich ingredients: potential antioxidant activity measured in vitro. J. Sci. Food Agric. 2012, 92, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Aparicio, I.; Redondo-Cuenca, A.; Villanueva-Suarez, M.J.; Zapata-Revilla, M.A.; Tenorio-Sanz, M.D. Pea pod, broad bean pod and okara, potential sources of functional compounds. Lwt-Food Sci. Technol. 2010, 43, 1467–1470. [Google Scholar] [CrossRef]
- Mateos-Aparicio, I.; Redondo-Cuenca, A.; Villanueva-Suarez, M.J. Isolation and characterisation of cell wall polysaccharides from legume by-products: Okara (soymilk residue), pea pod and broad bean pod. Food Chem. 2010, 122, 339–345. [Google Scholar] [CrossRef]
- Zander, P.; Amjath-Babu, T.S.; Preissel, S.; Reckling, M.; Bues, A.; Schlafke, N.; Kuhlman, T.; Bachinger, J.; Uthes, S.; Stoddard, F.; et al. Grain legume decline and potential recovery in European agriculture: A review. Agron. Sustain. Dev. 2016, 36, 26. [Google Scholar] [CrossRef]
- Sirtori, C.R.; Galli, C.; Anderson, J.W.; Arnoldi, A. Nutritional and nutraceutical approaches to dyslipidemia and atherosclerosis prevention: Focus on dietary proteins. Atherosclerosis 2009, 203, 8–17. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture data. 2017. Available online: http://www.fao.org/faostat/en/#home (accessed on 8 January 2020).
- Klupšaitė, D.; Juodeikienė, G. Legume: Composition, protein extraction and functional properties. A review. Chem. Technol. 2015, 66. [Google Scholar] [CrossRef]
- Chickpea Production Guide. 2004. Available online: https://catalog.extension.oregonstate.edu/sites/catalog/files/project/pdf/em8791.pdf (accessed on 8 January 2020).
- Merga, B.; Haji, J. Economic importance of chickpea: production, value, and world trade. Cogent. Food Agric. 2019, 5, 1615718. [Google Scholar] [CrossRef]
- Westhoek, H.; Rood, T.; van den Berg, M.; Janse, J.; Nijdam, D.; Reudink, M.; Stehfest, E. The Protein Puzzle. The Consumption and Production of Meat, Dairy and Fish in the European Union; PBL Netherlands Environmental Assessment Agency: The Hague, The Netherlands, 2011. [Google Scholar]
- Report EIP-AGRI Focus Group on protein crops. 2014. Available online: https://ec.europa.eu/eip/agriculture/en/publications/eip-agri-focus-group-protein-crops-final-report (accessed on 8 January 2020).
- Andreotti, R. La Fabbricazione Delle Conserve di Piselli; Stazione Sperimentale per l’Industria delle Conserve Alimentari: Parma, Italy, 1983. [Google Scholar]
- Tiwari, B.K.; Gowen, A.; McKenna, B. Pulse Foods. Processing, Quality and Nutraceutical Applications; Academic Press: Cambridge, MA, USA; Elsevier: London, UK, 2011. [Google Scholar]
- Annor, G.A.; Zhen, M.; Boye, J.I. Crops – Legumes. In Food Processing. Principles and Applications, 2nd ed.; Clark, S., Jung, S., Lamsal, B., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 305–337. [Google Scholar]
- Schutyser, M.; van der Goot, A.J. The potential of dry fractionation processes for sustainable plant protein production. Trends Food Sci. Tech. 2011, 22, 154–164. [Google Scholar] [CrossRef]
- Kamani, M.H.; Martin, A.; Meera, M.S. Valorization of by-products derived from milled moth bean: evaluation of chemical composition, nutritional profile and functional characteristics. Waste Biomass Valor. 2019. [Google Scholar] [CrossRef]
- Gençdağ, E.; Görgüç, A.; Yılmaz, F. Recent advances in the recovery techniques of plant-based proteins from agro-industrial by-products. Food Rev. Int. 2020. [Google Scholar] [CrossRef]
- Pelgrom, P.J.M.; Vissers, A.M.; Boom, R.M.; Schutyser, M.A.I. Dry fractionation for production of functional pea protein concentrates. Food Res. Int. 2013, 53, 232–239. [Google Scholar] [CrossRef]
- Brummer, Y.; Kaviani, M.; Tosh, S.M. Structural and functional characteristics of dietary fibre in beans, lentils, peas and chickpeas. Food Res. Int. 2015, 67, 117–125. [Google Scholar] [CrossRef]
- Roy, F.; Boye, J.I.; Simpson, B.K. Bioactive proteins and peptides in pulse crops: Pea, chickpea and lentil. Food Res. Int. 2010, 43, 432–442. [Google Scholar] [CrossRef]
- Tosh, S.M.; Yada, S. Dietary fibres in pulse seeds and fractions: Characterization, functional attributes, and applications. Food Res. Int. 2010, 43, 450–460. [Google Scholar] [CrossRef]
- Boye, J.; Zare, F.; Pletch, A. Pulse proteins: Processing, characterization, functional properties and applications in food and feed. Food Res. Int. 2010, 43, 414–431. [Google Scholar] [CrossRef]
- Makri, E.; Papalamprou, E.; Doxastakis, G. Study of functional properties of seed storage proteins from indigenous European legume crops (lupin, pea, broad bean) in admixture with polysaccharides. Food Hydrocoll. 2005, 19, 583–594. [Google Scholar] [CrossRef]
- Gong, A.; Aguirre, A.M.; Bassi, A. Technical issues related to characterization, extraction, recovery, and purification of proteins from different waste sources. In Protein Byproducts; Dhillon, G., Ed.; Academic Press; Elsevier: Cambridge, MA, USA, 2016; pp. 89–106. [Google Scholar]
- Des Marchais, L.-P.; Foisy, M.; Mercier, S.; Villeneuve, S.; Mondor, M. Bread-making potential of pea protein isolate produced by a novel ultrafiltration/diafiltration process. Procedia Food Sci. 2011, 1, 1425–1430. [Google Scholar] [CrossRef]
- Mondor, M.; Aksay, S.; Drolet, H.; Roufik, S.; Farnworth, E.; Boye, J.I. Influence of processing on composition and antinutritional factors of chickpea protein concentrates produced by isoelectric precipitation and ultrafiltration. Innov. Food Sci. Emerg. Technol. 2009, 10, 342–347. [Google Scholar] [CrossRef]
- Sarethy, I.P. Plant peptides: bioactivity, opportunities and challenges. Protein Pept. Lett. 2017, 24, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Ribotta, P.D.; Rosell, C.M. Effects of enzymatic modification of soybean protein on the pasting and rheological profile of starch-protein systems. Starch 2010, 62, 373–383. [Google Scholar] [CrossRef]
- Barac, M.B.; Jovanovic, S.T.; Stanojevic, S.P.; Pesic, M.B. Effect of limited hydrolysis on traditional soy protein concentrate. Sensors 2006, 6, 1087–1101. [Google Scholar] [CrossRef]
- Pownall, T.L.; Udenigwe, C.C.; Aluko, R.E. Amino acid composition and antioxidant properties of pea seed (Pisum sativum L.) enzymatic protein hydrolysate fractions. J. Agric. Food Chem. 2010, 58, 4712–4718. [Google Scholar] [CrossRef]
- Aluko, R.E. Determination of nutritional and bioactive properties of peptides in enzymatic pea, chickpea, and mung bean protein hydrolysates. AOAC Int. 2008, 91, 947–956. [Google Scholar] [CrossRef]
- Rojas, M.J.; Siqueira, P.F.; Miranda, L.C.; Tardioli, P.W.; Giordano, R.L.C. Sequential proteolysis and cellulolytic hydrolysis of soybean hulls for oligopeptides and ethanol production. Ind. Crop. Prod. 2014, 61, 202–210. [Google Scholar] [CrossRef]
- Sawada, M.M.; Venancio, L.L.; Toda, T.A.; Rodrigues, C.E.C. Effects of different alcoholic extraction conditions on soybean oil yield, fatty acid composition and protein solubility of defatted meal. Food Res. Int. 2014, 62, 662–670. [Google Scholar] [CrossRef]
- Xu, B.J.; Chang, S.K.C. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J. Food Sci. 2007, 72, S159–S166. [Google Scholar] [CrossRef] [PubMed]
- Girish, T.K.; Pratape, V.M.; Rao, U.J.S.P. Nutrient distribution, phenolic acid composition, antioxidant and alpha-glucosidase inhibitory potentials of black gram (Vigna mungo L.) and its milled by-products. Food Res. Int. 2012, 46, 370–377. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Preecea, K.E.; Hooshyar, N.; Krijgsman, A.; Fryer, P.J.; Zuidam, N.J. Intensified soy protein extraction by ultrasound. Chem. Eng. Process. 2017, 113, 94–101. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, J.; Gan, R.Y.; Zhou, T.; Xu, D.P.; Li, H.B. Optimization of ultrasound-assisted extraction of antioxidants from the mung bean coat. Molecules 2017, 22, 638. [Google Scholar] [CrossRef]
- Lafarga, T.; Alvarez, C.; Bobo, G.; Aguilo-Aguayo, I. Characterization of functional properties of proteins from Ganxet beans (Phaseolus vulgaris L. var. Ganxet) isolated using an ultrasound-assisted methodology. Lwt-Food Sci. Technol. 2018, 98, 106–112. [Google Scholar] [CrossRef]
- Ochoa-Rivas, A.; Nava-Valdez, Y.; Serna-Saldivar, S.O.; Chuck-Hernandez, C. Microwave and ultrasound to enhance protein extraction from peanut flour under alkaline conditions: effects in yield and functional properties of protein isolates. Food Bioprocess Tech. 2017, 10, 543–555. [Google Scholar] [CrossRef]
- Choi, I.; Choi, S.J.; Chun, J.K.; Moon, T.W. Extraction yield of soluble protein and microstructure of soybean affected by microwave heating. J. Food. Process. Pres. 2006, 30, 407–419. [Google Scholar] [CrossRef]
- de Boer, J.; Helms, M.; Aiking, H. Protein consumption and sustainability: Diet diversity in EU-15. Ecol. Econ. 2006, 59, 267–274. [Google Scholar] [CrossRef]
- The International Year of Pulses-Final Report; FAO: Rome, Italy, 2019; Available online: http://www.fao.org/3/CA2853EN/ca2853en.pdf (accessed on 20 December 2019).
- Sharasia, P.L.; Garg, M.R.; Bhanderi, B.M. Pulses and Their by-Products as Animal Feed; FAO: Rome, Italy, 2017; Available online: http://www.fao.org/3/a-i7779e.pdf (accessed on 20 December 2019).
- Soetan, K.O.; Oyewole, O.E. The need for adequate processing to reduce the antinutritional factors in plants used as human foods and animal feeds: a review. Afr. J. Food Sci. 2009, 3, 223–232. [Google Scholar]
- Naila, A.; Flint, S.; Fletcher, G.; Bremer, P.; Meerdink, G. Control of biogenic amines in food-existing and emerging approaches. J. Food Sci. 2010, 75, R139–R150. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, M.; Kaushal, S.; Bakshi, M.P.S. Nutritive evaluation of vegetable wastes as complete feed for goat bucks. Small Ruminant Res. 2006, 64, 279–284. [Google Scholar] [CrossRef]
- Dhillon, G.S. Protein Byproducts–Transformation from Environmental Burden Into Value-Added Products; Academic Press: London, UK, 2016. [Google Scholar]
- Tiwari, B.K.; Brennan, C.S.; Jaganmohan, R.; Surabi, A.; Alagusundaram, K. Utilisation of pigeon pea (Cajanus cajan L) byproducts in biscuit manufacture. Lwt-Food Sci. Technol. 2011, 44, 1533–1537. [Google Scholar] [CrossRef]
- Nimbalkar, P.R.; Khedkar, M.A.; Chavan, P.V.; Bankar, S.B. Biobutanol production using pea pod waste as substrate: Impact of drying on saccharification and fermentation. Renew. Energy 2018, 117, 520–529. [Google Scholar] [CrossRef]
- Verma, N.; Bansal, M.C.; Kumar, V. Pea peel waste: A lignocellulosic waste and its utility in cellulase production by Trichoderma reesei under solid state cultivation. Bioresources 2011, 6, 1505–1519. [Google Scholar]
- Tiwari, U.; Gunasekaran, M.; Jaganmohan, R.; Alagusundaram, K.; Tiwari, B.K. Quality characteristic and shelf life studies of deep-fried snack prepared from rice brokens and legumes by-product. Food Bioprocess. Tech. 2011, 4, 1172–1178. [Google Scholar] [CrossRef]
- Aigbodion, V.S. Bean pod ash nanoparticles a promising reinforcement for aluminium matrix biocomposites. J. Mater. Res. Technol. 2019, 8, 6011–6020. [Google Scholar] [CrossRef]
- ANSA. Arriva Carta Eco-Sostenibile da Scarti di Fagioli. Available online: http://www.ansa.it/canale_ambiente/notizie/rifiuti_e_riciclo/2015/09/18/arriva-carta-eco-sostenibile-da-scarti-di-fagioli_1ed81931-73db-11e5-9836-00505695d1bc.html (accessed on 31 January 2020).
- Zhang, L.; Sun, X.Y. Effects of bean dregs and crab shell powder additives on the composting of green waste. Bioresour. Technol. 2018, 260, 283–293. [Google Scholar] [CrossRef]
- Zhu, G.Y.; Zhu, X.; Fan, Q.; Wan, X.L. Production of reducing sugars from bean dregs waste by hydrolysis in subcritical water. J. Anal. Appl. Pyrol. 2011, 90, 182–186. [Google Scholar] [CrossRef]
- Taylor, W.J.; Ford, R. Chickpea. In Pulses, Sugar and Tuber Crops; Springer-Verlag: Berlin/Heidelberg, Germany, 2007; pp. 109–122. [Google Scholar]
- Bampidis, V.A.; Christodoulou, V. Chickpeas (Cicer arietinum L.) in animal nutrition: A review. Anim. Feed Sci. Tech. 2011, 168, 1–20. [Google Scholar] [CrossRef]
- Kumar, Y.; Yadav, D.N.; Ahmad, T.; Narsaiah, K. Recent Trends in the Use of Natural Antioxidants for Meat and Meat Products. Compr. Rev. Food Sci. Food Saf. 2015, 14, 796–812. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Arjun, K.; Sharma, A. Antioxidant and antimicrobial activity of legume hulls. Food Res. Int. 2011, 44, 3182–3187. [Google Scholar] [CrossRef]
- Niño-Medina, G.; Muy-Rangel, D.; de la Garza, A.L.; Rubio-Carrasco, W.; Perez-Meza, B.; Araujo-Chapa, A.P.; Gutierrez-Alvarez, K.A.; Urias-Orona, V. Dietary fiber from chickpea (Cicer arietinum) and soybean (Glycine max) husk byproducts as baking additives: functional and nutritional properties. Molecules 2019, 24, 991. [Google Scholar] [CrossRef] [PubMed]
- Jose, S.; Pandit, P.; Pandey, R. Chickpea husk-A potential agro waste for coloration and functional finishing of textiles. Ind. Crop. Prod. 2019, 142, 111833. [Google Scholar] [CrossRef]
- Niño-Medina, G.; Muy-Rangel, D.; Urías-Orona, V. Chickpea (Cicer arietinum) and soybean (Glycine max) hulls: byproducts with potential use as a source of high value-added food products. Waste Biomass. Valor. 2017, 8, 1199–1203. [Google Scholar] [CrossRef]
- Buhl, T.F.; Christensen, C.H.; Hammershoj, M. Aquafaba as an egg white substitute in food foams and emulsions: protein composition and functional behavior. Food Hydrocoll. 2019, 96, 354–364. [Google Scholar] [CrossRef]
- Mustafa, R.; He, Y.; Shim, Y.Y.; Reaney, M.J.T. Aquafaba, wastewater from chickpea canning, functions as an egg replacer in sponge cake. Int. J. Food. Sci. Tech. 2018, 53, 2247–2255. [Google Scholar] [CrossRef]
- Material District. Bioplastic made from Aquafaba from Chickpeas. 2019. Available online: https://materialdistrict.com/article/bioplastic-aquafaba-chickpeas/ (accessed on 31 January 2020).
- López, S.; Davies, D.R.; Giraldez, F.J.; Dhanoa, M.S.; Dijkstra, J.; France, J. Assessment of nutritive value of cereal and legume straws based on chemical composition and in vitro digestibility. J. Sci. Food Agric. 2005, 85, 1550–1557. [Google Scholar] [CrossRef]
- Kumar, P.; Chatli, M.K.; Mehta, N.; Singh, P.; Malav, O.P.; Verma, A.K. Meat analogues: health promising sustainable meat substitutes. Crit. Rev. Food Sci. Nutr. 2017, 57, 923–932. [Google Scholar] [CrossRef]
- Singhal, A.; Karaca, A.C.; Tyler, R.; Nickerson, M. Pulse proteins: from processing to structure-function relationships. In Grain Legumes; Goyal, A., Ed.; IntechOpen Limited: London, UK, 2016. [Google Scholar]
- Jahreis, G.; Brese, M.; Leiterer, M.; Schafer, U.; Bohm, V. Legume flours: nutritionally important sources of protein and dietary fiber. Ernahrungs Umschau 2016, 63, M82–M88. [Google Scholar]
- Raikos, V.; Neacsu, M.; Russell, W.; Duthie, G. Comparative study of the functional properties of lupin, green pea, fava bean, hemp, and buckwheat flours as affected by pH. Food Sci. Nutr. 2014, 2, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Burger, T.G.; Zhang, Y. Recent progress in the utilization of pea protein as an emulsifier for food applications. Trends Food Sci. Tech. 2019, 86, 25–33. [Google Scholar] [CrossRef]
- Liu, F.; Chen, Z.; Tang, C.H. Microencapsulation properties of protein isolates from three selected Phaseolus legumes in comparison with soy protein isolate. Lwt-Food Sci. Technol. 2014, 55, 74–82. [Google Scholar] [CrossRef]
- Boschin, G.; Scigliuolo, G.M.; Resta, D.; Arnoldi, A. ACE-inhibitory activity of enzymatic protein hydrolysates from lupin and other legumes. Food Chem. 2014, 145, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Oreopoulou, V.; Tzia, C. Utilization of plant by-products for the recovery of proteins, dietary fibers, antioxidants, and colorants. In Utilization of By-Products and Treatment of Waste in the Food Industry; Oreopoulou, V., Russ, W., Eds.; Springer: Boston, MA, USA, 2007; pp. 209–232. [Google Scholar]
- Pina-Pérez, M.C.; Ferrús Pérez, M.A. Antimicrobial potential of legume extracts against foodborne pathogens: a review. Trends Food Sci. Tech. 2018, 72, 114–124. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Santos, M.J.M.C.; Silva, L.K.R.; Pereira, L.C.L.; Santo, I.A.; Lannes, S.C.D.; da Silva, M.V. Natural antioxidants used in meat products: A brief review. Meat Sci. 2019, 148, 181–188. [Google Scholar] [CrossRef]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; Ghissassi, F.E.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K. International Agency for Research on Cancer Monograph Working, G., Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.R.; Zhou, K.Q. Chelating and radical scavenging activities of soy protein hydrolysates prepared from microbial proteases and their effect on meat lipid peroxidation. Bioresour. Technol. 2010, 101, 2084–2089. [Google Scholar] [CrossRef]
- Brewer, M.S. Reducing the fat content in ground beef without sacrificing quality: A review. Meat Sci. 2012, 91, 385–395. [Google Scholar] [CrossRef]
- Voegeli, R.; Stocker, K.; Mueller, C. Protein fraction for cosmetic and dermatology care of the skin. US Patent US 94384892 A, 21 June 1994. [Google Scholar]
- Dal Farra, C.; Domloge, N.; Botto, J.M. Use of A Peptide Hydrolysate of pea as Moisturizing Active Agent. US Patent US201113640827 A, 1 December 2015. [Google Scholar]
- Banowski, B.; Evers, S. Antiperspirant Cosmetics Comprising Specific Proteins from Legumes of the Genus Pisum and/or Phaseolus and/or Vigna and/or Macrotyloma or from Cruciferous Plants of the Genus Brassica and Including no Aluminum and/or Zirconium Halides and/or Hydroxy Halides. US Patent US 201715400773 A, 13 November 2018. [Google Scholar]
- Moussou, P.; Danoux, L.; Bailly, L.; Gillon, V. Cosmetic Composition Comprising a Combination of a Sugar Fatty Acid Ester with a Plant Extract of Waltheria indica or Pisum sativum for Skin Whitening. US Patent US 29420307 A, 24 April 2018. [Google Scholar]
- Galanakis, C.M.; Tsatalas, P.; Galanakis, I.M. Implementation of phenols recovered from olive mill wastewater as UV booster in cosmetics. Ind. Crops Prod. 2018, 111, 30–37. [Google Scholar] [CrossRef]
- Kumar, V.; Longhurst, P. Recycling of food waste into chemical building blocks. Curr. Opin. Green. Sustain. Chem. 2018, 13, 118–122. [Google Scholar] [CrossRef]
- Tuck, C.O.; Perez, E.; Horvath, I.T.; Sheldon, R.A.; Poliakoff, M. Valorization of biomass: Deriving more value from waste. Science 2012, 337, 695–699. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Packaging-Requirements for Packaging Recoverable through Composting and Biodegradation-Test Scheme and Evaluation Criteria for the Final Acceptance of Packaging, CEN EN 13432:2000. 2000. Available online: http://ec.europa.eu/environment/waste/packaging/standards.htm (accessed on 9 December 2019).
- Totaro, G.; Sisti, L.; Vannini, M.; Marchese, P.; Tassoni, A.; Lenucci, M.S.; Lamborghini, M.; Kalia, S.; Celli, A. A new route of valorization of rice endosperm by-product: production of polymeric biocomposites. Compos. Part B-Eng. 2018, 139, 195–202. [Google Scholar] [CrossRef]
- Saccani, A.; Sisti, L.; Manzi, S.; Fiorini, M. PLA composites formulated recycling residues of the winery industry. Polym. Compos. 2019, 40, 1378–1383. [Google Scholar] [CrossRef]
- Cinelli, P.; Mallegni, N.; Gigante, V.; Montanari, A.; Seggiani, M.; Coltelli, M.B.; Bronco, S.; Lazzeri, A. Biocomposites based on polyhydroxyalkanoates and natural fibres from renewable by-products. Appl. Food Biotech. 2019, 6, 35–42. [Google Scholar]
- Marek, A.A.; Verney, V.; Taviot-Gueho, C.; Totaro, G.; Sisti, L.; Celli, A.; Leroux, L. Oustanding chain-extension effect and high UV resistance of polybutylene succinate containing amino-acid-modified layered double hydroxides. Beilstein J. Nanotechnol. 2019, 10, 684–695. [Google Scholar] [CrossRef]
- Totaro, G.; Sisti, L.; Celli, A.; Aloisio, I.; Di Gioia, D.; Marek, A.A.; Verney, V.; Leroux, F. Dual chain extension effect and antibacterial properties of biomolecules interleaved within LDH dispersed into PBS by in situ polymerization. Dalton Trans. 2018, 47, 3155–3165. [Google Scholar] [CrossRef]
- Shi, W.; Dumont, M.J. Review: bio-based films from zein, keratin, pea, and rapeseed protein feedstocks. J. Mater. Sci. 2014, 49, 1915–1930. [Google Scholar] [CrossRef]



| Legume Type | World (MT) | Europe (MT) |
|---|---|---|
| Beans (dry) | 31.41 | 0.62 |
| Beans (green) | 24.22 | 0.77 |
| Broad Beans (dry) | 4.84 | 0.97 |
| Caw Peas | 7.41 | 0.002 |
| Chickpeas | 14.78 | 0.13 |
| Lentils | 7.59 | 0.07 |
| Lupins | 1.61 | 0.25 |
| Peas (dry) | 16.21 | 2.60 |
| Peas (green) | 20.70 | 0.93 |
| Soybeans | 35.26 | 2.67 |
| Legume Feedstock | Field of Application | Application | Bioactive Compounds | Outcome | Reference |
|---|---|---|---|---|---|
| Pea pods Pulses by-products | Feed | Monogastric and polygastric animal feed | Proteins, fibers, minerals | Biochemical and nutritional characterization. Impact on animal performance. | [62,63,66,67] |
| Pigeon pea by-products | Food | High protein biscuits | Proteins | Chemical composition; physical and sensory parameters | [68] |
| Pea and broad bean pods | Food | Food ingredients | Fibers, soluble sugars, minerals, linoleic acid | Biochemical and nutritional characterization; antioxidant activity | [20,21] |
| Pea pod waste | Bio-resources | Bio-butanol production | Cellulose/hemicellulose | Potential carbon source for bio-butanol production | [69] |
| Pea peel waste | Bio-resources | Cellulase enzyme production | Cellulose | Potential source for cellulose production | [70] |
| Moth bean milling residues | Food | Food ingredients | High essential amino acids, fatty acids, minerals. | Water and oil absorption capacities, foaming and emulsification properties. | [35] |
| Black gram (Vigna mungo) milling by-products | Food | Food ingredients | Phenolic acids like gallic, protocatechuic, gentisic, vanillic, syringic, caffeic and ferulic acids | Biochemical and nutritional characterization; α-glucosidase inhibitory activities correlated to potential antioxidant and anti-diabetic properties. | [54] |
| Red, green and black gram by-products | Food | Deep-fried snacks | Proteins | Sensory results and shelf life studies | [71] |
| Bean pod ash nanoparticles | Automobile application | Composites with bioreinforcements | Nano-fibers, cellulose | Increased tensile strength and hardness values, reduced weight and energy impact | [72] |
| Process bean waste | Packaging | Ecopaper for food packaging | Fibers, cellulose | 100% recyclable packaging paper obtained by an eco-sustainable process and certified for application in direct contact with food | [73] |
| Bean dregs | Compost | Compost product of high-quality | Cellulose, hemicellulose | Improved composting conditions and compost quality | [74]. |
| Bean dregs | Bio-resources | Production of reducing sugar | Sugars | Efficient method for biomass wastes liquefaction. | [75] |
| Chickpea straw | Feed | Alternative forage in ruminant diet | Proteins, fibers | High nutritional value, dry matter digestibility, rumen degradability | [76,77] |
| Chickpea, mung bean, pigeon pea hulls | Food | Meat additives | Phenolics, flavonoids | Antioxidant, antimicrobial, antinitrosant activities | [78,79] |
| Chickpea husk | Food | Baking additives | Fibers, polyphenols | Calcium content, antioxidant activity and phenolic compounds content slightly improved; increase in shelf life, rheological, physical and sensory parameters. | [80] |
| Chickpea husk | Textile | Textile grade dye | Flavonoids, tannins, terpenoids | Functional finishing features of textiles, good ultraviolet protection, excellent resistance against bacteria. | [81] |
| Chickpeas hulls | Food | Food additives | Fibers, polyphenols | Source of dietary fiber and phenolics with antioxidant capacity | [82] |
| Aquafaba | Food | Egg-white substitute in food foam and emulsions. | Proteins, carbohydrates | Foaming and emulsification properties | [83,84] |
| Aquafaba | Packaging | Bioplastic | Proteins, carbohydrates | Biodegradable bioplastic | [85] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tassoni, A.; Tedeschi, T.; Zurlini, C.; Cigognini, I.M.; Petrusan, J.-I.; Rodríguez, Ó.; Neri, S.; Celli, A.; Sisti, L.; Cinelli, P.; et al. State-of-the-Art Production Chains for Peas, Beans and Chickpeas—Valorization of Agro-Industrial Residues and Applications of Derived Extracts. Molecules 2020, 25, 1383. https://doi.org/10.3390/molecules25061383
Tassoni A, Tedeschi T, Zurlini C, Cigognini IM, Petrusan J-I, Rodríguez Ó, Neri S, Celli A, Sisti L, Cinelli P, et al. State-of-the-Art Production Chains for Peas, Beans and Chickpeas—Valorization of Agro-Industrial Residues and Applications of Derived Extracts. Molecules. 2020; 25(6):1383. https://doi.org/10.3390/molecules25061383
Chicago/Turabian StyleTassoni, Annalisa, Tullia Tedeschi, Chiara Zurlini, Ilaria Maria Cigognini, Janos-Istvan Petrusan, Óscar Rodríguez, Simona Neri, Annamaria Celli, Laura Sisti, Patrizia Cinelli, and et al. 2020. "State-of-the-Art Production Chains for Peas, Beans and Chickpeas—Valorization of Agro-Industrial Residues and Applications of Derived Extracts" Molecules 25, no. 6: 1383. https://doi.org/10.3390/molecules25061383
APA StyleTassoni, A., Tedeschi, T., Zurlini, C., Cigognini, I. M., Petrusan, J.-I., Rodríguez, Ó., Neri, S., Celli, A., Sisti, L., Cinelli, P., Signori, F., Tsatsos, G., Bondi, M., Verstringe, S., Bruggerman, G., & Corvini, P. F. X. (2020). State-of-the-Art Production Chains for Peas, Beans and Chickpeas—Valorization of Agro-Industrial Residues and Applications of Derived Extracts. Molecules, 25(6), 1383. https://doi.org/10.3390/molecules25061383










