Antibacterial and Antioxidant Activity of Extracts from Rose Fruits (Rosa rugosa)
Abstract
1. Introduction
2. Results and Discussion
2.1. Antibacterial Activity of Extracts
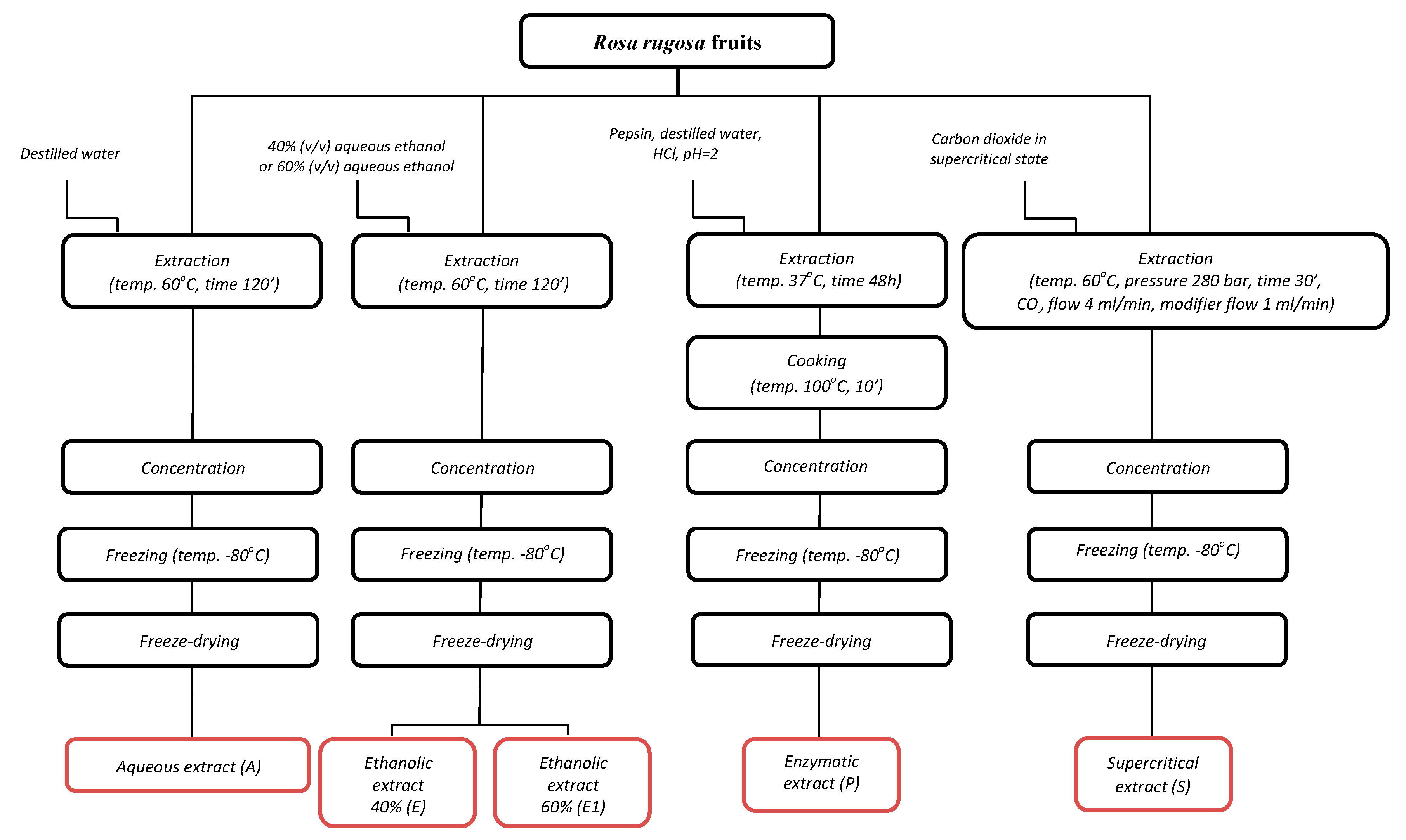
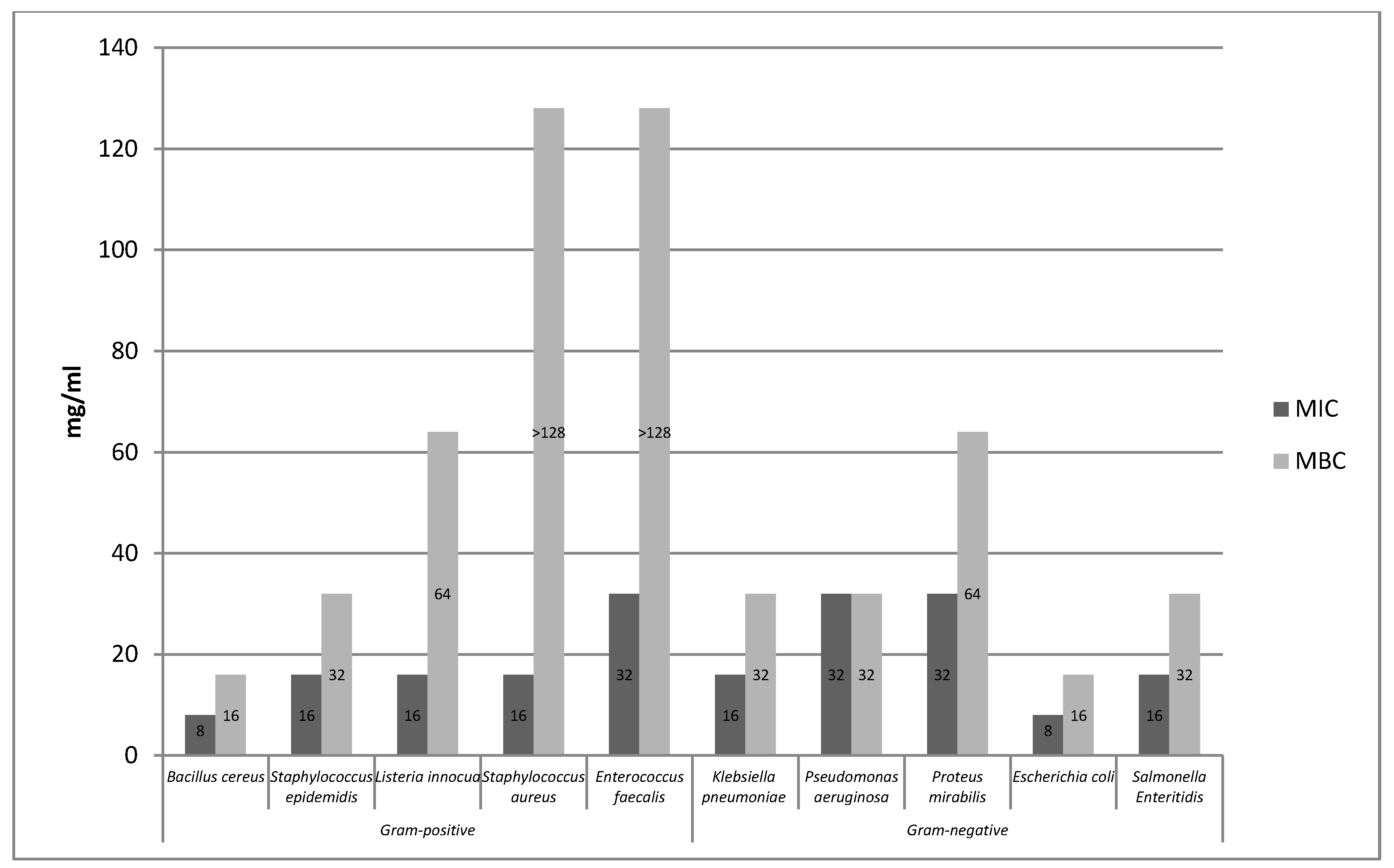
2.1.1. Determination of the Minimum Inhibitory Concentration (MIC) and the Minimum Bactericidal Concentration (MBC) of Rose Fruits Extracts
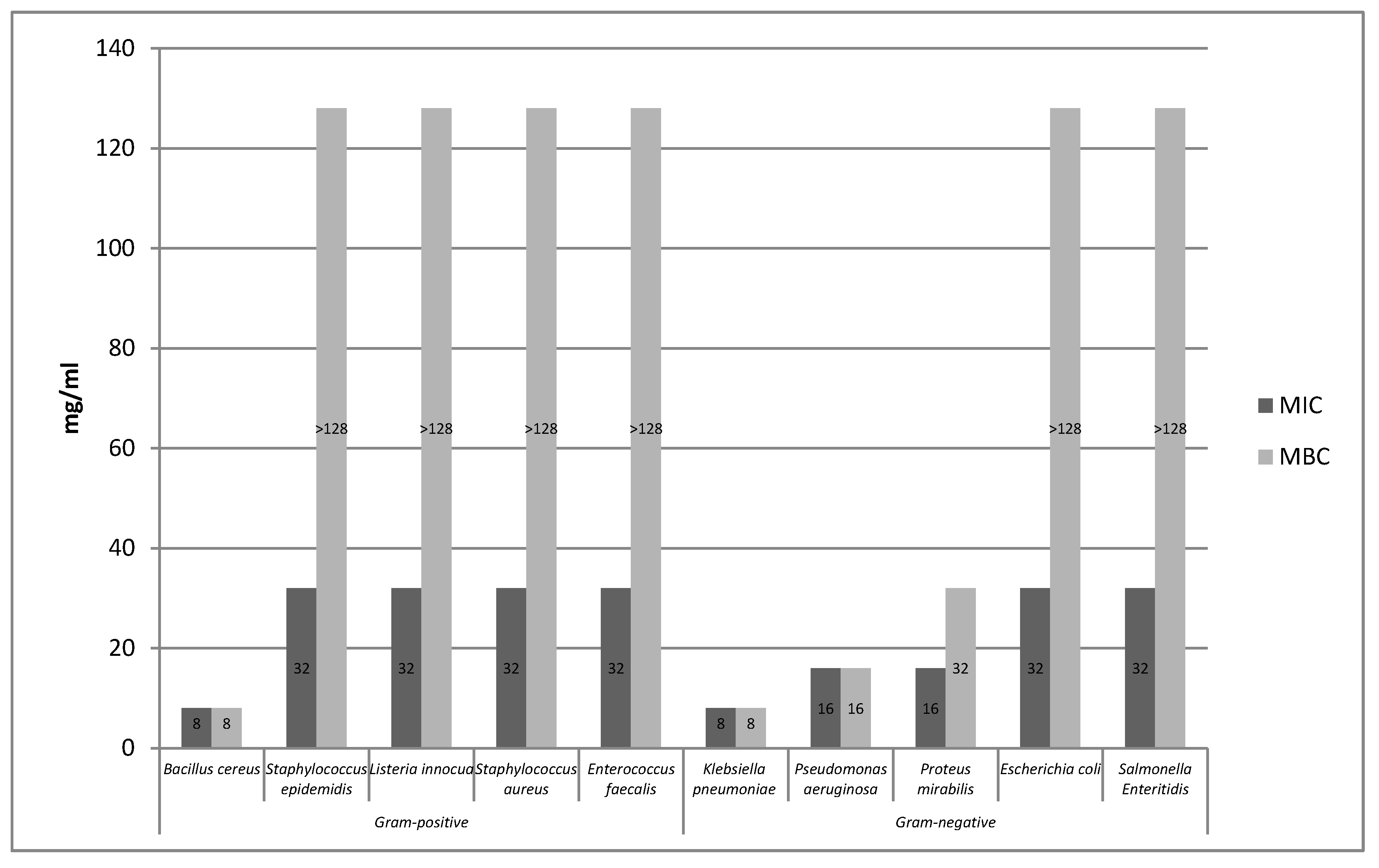
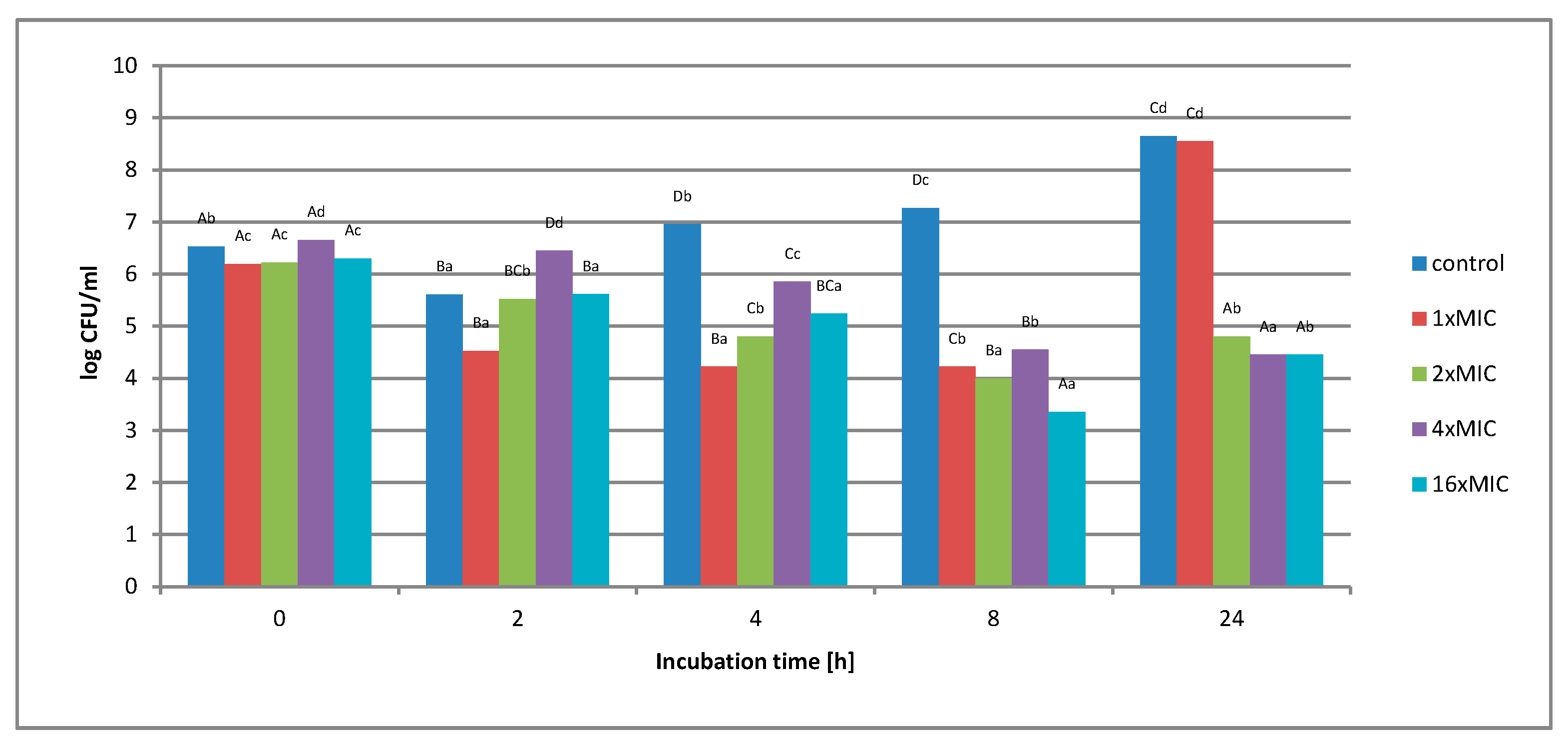
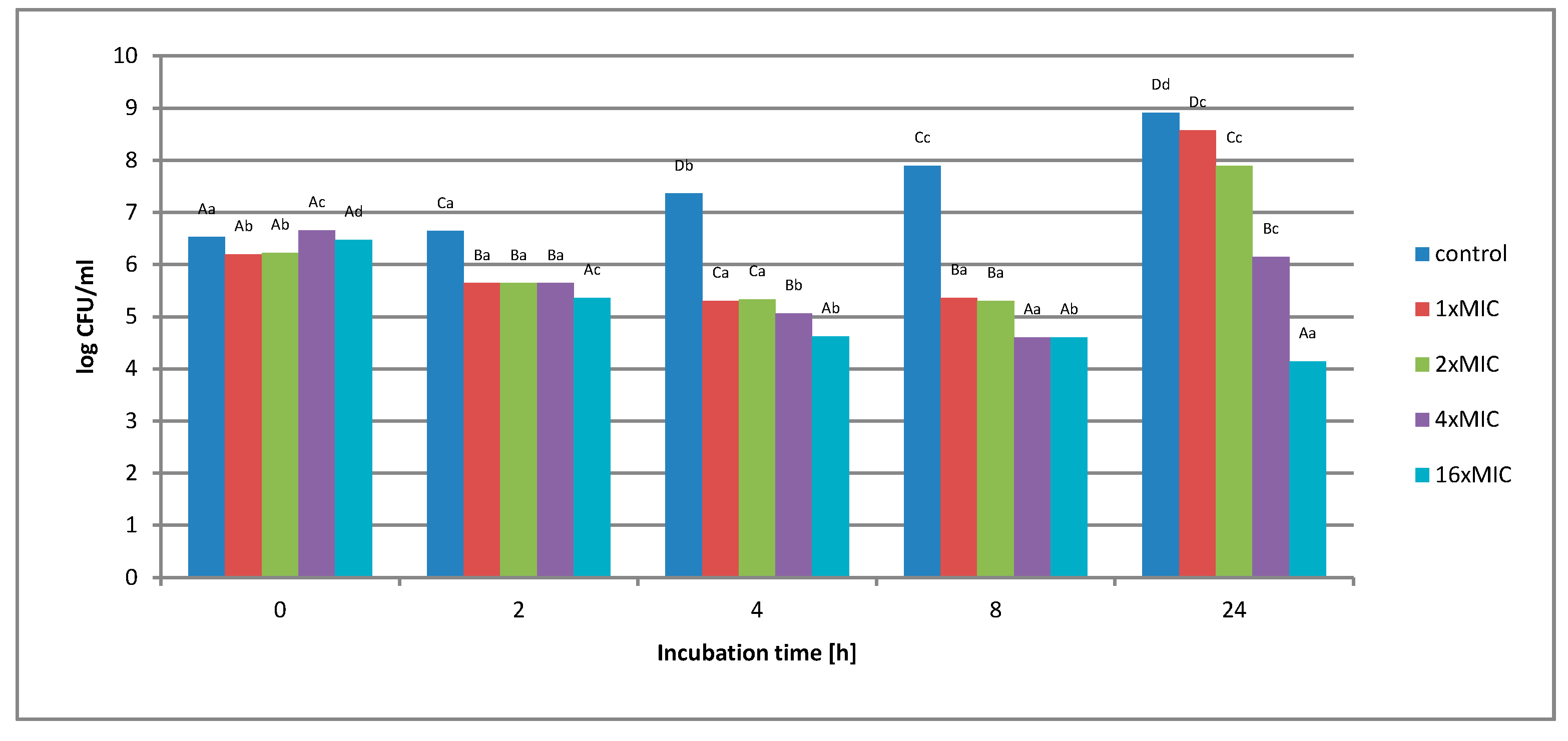
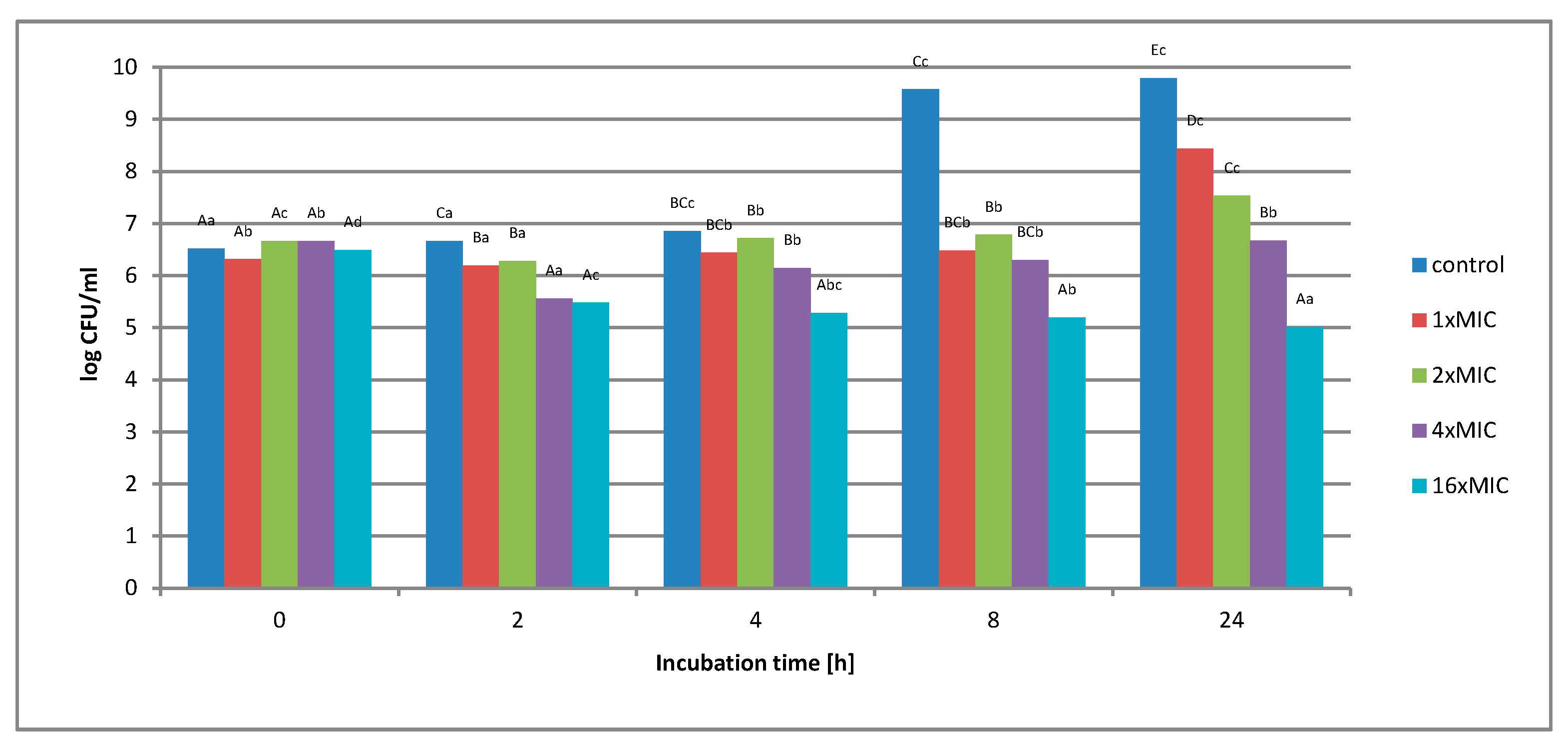
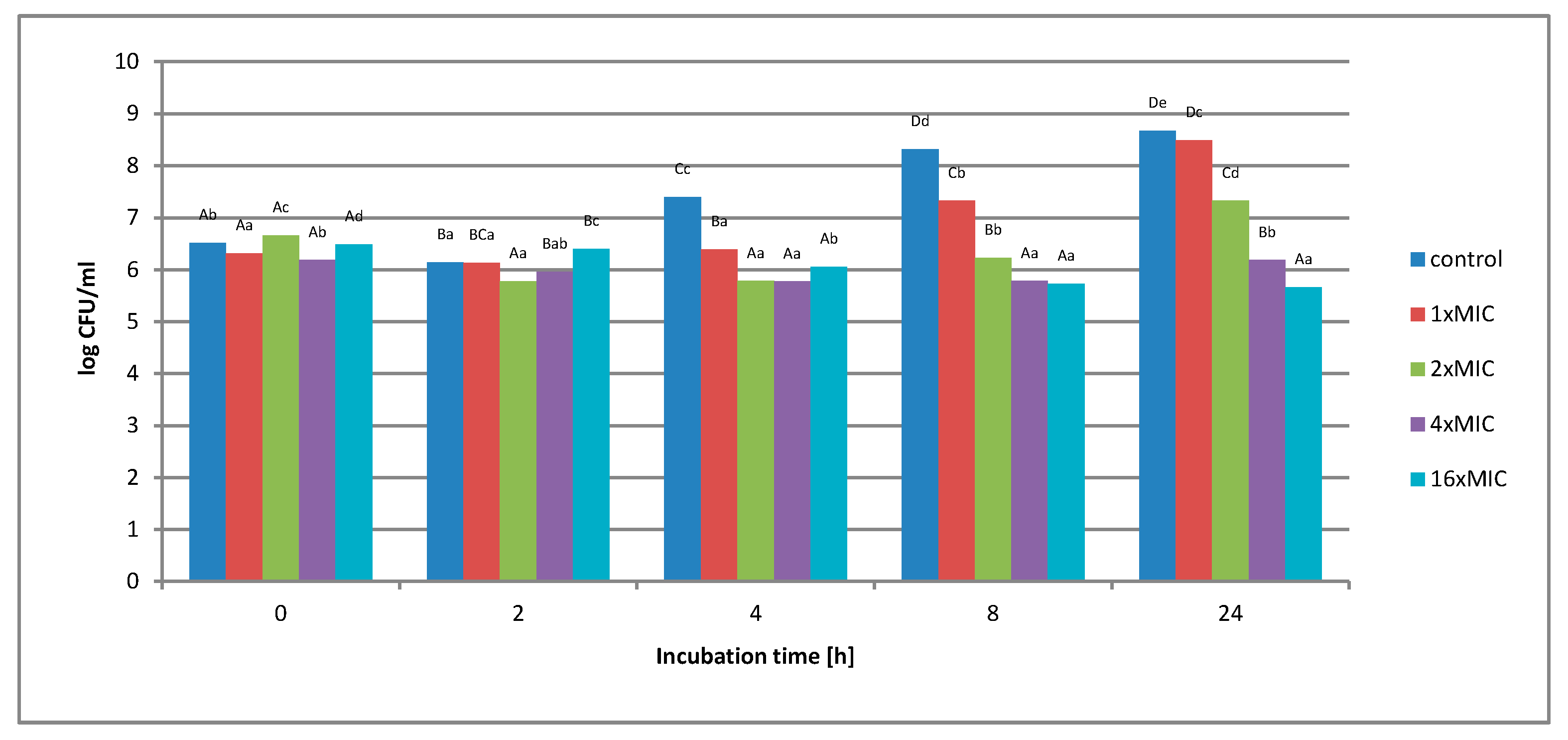
2.1.2. Survival of Gram-Negative and Gram-Positive Bacteria by the Time-Kill Method in Model Fluids Imitating Protein Food
Study of the Survival of Salmonella Enteritidis in Model Fluids Imitating Protein Food
Study of the Survival of Listeria innocua in Model Fluids Imitating Protein Food
2.2. Antioxidant Activity of Extracts
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals
3.3. Preparation of Extracts
3.3.1. Aqueous Extract (A)
3.3.2. Ethanolic Extract (E-, E1)
3.3.3. Supercritical Extract (S)
3.3.4. Enzymatic Extract (P)
3.4. Antibacterial Activity
3.4.1. Test Microorganisms and Preparation of Inoculum
3.4.2. The Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
3.4.3. Preparation of Model Fluids Imitating Protein Food
3.4.4. Survival of Selected Bacterial Strains Using the Time-Kill Method
3.5. Antioxidant Activity
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A

References
- Yilmaz, S.O.; Ercisli, S. Antibacterial and antioxidant activity of fruits of some rose species from Turkey. Rom. Biotechnol. Lett. 2011, 16, 6407–6411. [Google Scholar]
- Wereńska, M. Natural antioxidants used to meat. Eng. Sci. Technol. 2013, 1, 79–90. [Google Scholar]
- Giampieri, F.; Alvarezsuarez, J.M.; Cordero, M.D.; Gasparrini, M.; Forbeshernandez, T.Y.; Afrin, S.; Battino, M. Strawberry consumption improves aging-associated impairments, mitochondrial biogenesis and functionality through the amp activated protein kinase signaling cascade. Food Chem. 2017, 234, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Santhakumar, A.B.; Battino, M.; Alvarez-Suarez, J.M. Dietary polyphenols: Structures, bioavailability and protective effects against atherosclerosis. Food Chem. Toxicol. 2018, 113, 49–65. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. J. Oxid. Med. Cell Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Kaur, C.; Kapoor, H.C. Antioxidants in fruits and vegetables-the millennium’s health. Int. J. Food Sci. Technol. 2001, 36, 703–725. [Google Scholar] [CrossRef]
- Sebranek, J.G.; Sewalt, V.J.H.; Robbins, K.L.; Houser, T.A. Comparison of natural rosemary extract and BHA/BHT for relative antioxidant effectiveness in pork sausage. Meat Sci. 2005, 69, 289–296. [Google Scholar] [CrossRef]
- Georgantelis, D.; Blekas, G.; Katikou, P.; Ambrosiadis, I.; Fletouris, D.J. Effect of rosemary extract, chitosan and a-tocopherol on lipid oxidation and colour stability during frozen storage of beef burgers. Meat Sci. 2007, 75, 266–274. [Google Scholar] [CrossRef]
- Li, H.B.; Wong, C.C.; Cheng, K.W.; Chen, F. Antioxidant properties in vitro and total phenolic contents in methanol extracts from medicinal plants. LWT Food Sci. Technol. 2008, 41, 385–390. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, S.; Samaraweera, H.; Lee, E.J.; Ahn, D.U. Improving functional value of meat products. Meat Sci. 2010, 86, 15–31. [Google Scholar] [CrossRef]
- Ortega-Ramirez, L.A.; Rodriguez-Garcia, I.; Leyva, J.M.; Cruz-Valenzuela, M.R.; Silva-Espinoza, B.A.; Gonzalez-Aguilar, G.A.; Siddiqui, W.; Ayala-Zavala, J.F. Potential of medicinal plants as antimicrobial and antioxidant agents in food industry: A hypothesis. J Food Sci. 2014, 79, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Henie, E.F.P.; Zaiton, H.; Suhaila, M. Bacterial membrane disruption in food pathogens by Psidium guajava leaf extracts. Int. Food Res. J. 2009, 16, 297–311. [Google Scholar]
- Ultee, A.; Bennik, M.H.J.; Moezelaar, R. The Phenolic Hydroxyl Group of Carvacrol is Essential for Action against the Food-Borne Pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods- a review. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Sikkema, J.; Bont, J.A.; Poolman, B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 1995, 59, 201–222. [Google Scholar] [CrossRef]
- Silva, N.C.C.; Fernandes, J.A. Biological properties of medicinal plants: A review of their antimicrobial activity. J. Venom. Anim. Toxins Incl. Trop. Dis. 2010, 16, 402. [Google Scholar] [CrossRef]
- Tiwari, B.K. Ultrasound: A clean, green extraction technology. Trac Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, C.; Quirantes-Piné, R.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Optimization of extraction method to obtain a phenolic compounds-rich extract from Moringa oleifera Lam leaves. Ind. Crops Prod. 2015, 66, 246–254. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Fan, H.; Zhu, F.; Zhang, C. Chemical and nutritional constituents of fruit and concentrated fruit juice of Rosa davurica Pall. Yingyang Xuebao 1994, 16, 226–228. [Google Scholar]
- Hashidoko, Y. The phytochemistry of Rosa rugosa. Phytochemistry 1996, 43, 535–549. [Google Scholar] [CrossRef]
- Ercisli, S. Chemical composition of fruits in some rose (Rosa spp.) species. Food Chem. 2007, 104, 1379–1384. [Google Scholar] [CrossRef]
- Fecka, I. Qualitative and quantitative determination of hydrolysable tannins and other polyphenols in herbal products from meadowsweet and dog rose. Phytochem. Anal. 2009, 20, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Kazaz, S.; Baydar, H.; Erbas, S. Variations in chemical compositions of Rosa damascena Mill. and Rosa canina L. fruits. Czech J. Food Sci. 2009, 27, 178–184. [Google Scholar] [CrossRef]
- Türkben, C.; Uylaser, V.; Incedayi, B. Influence of traditional processing on some compounds of Rose Hip (Rosa canina L.) fruits collected from habitat in Bursa, Turkey. Asian J. Chem. 2010, 22, 2309–2318. [Google Scholar]
- Koczka, N.; Stefanovits-Bányai, É.; Ombódi, A. Total polyphenol content and antioxidant capacity of rosehips of some rosa species. Medicines 2018, 5, 84. [Google Scholar] [CrossRef] [PubMed]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 565–568. [Google Scholar] [CrossRef]
- Król, S.K.; Skalicka-Woźniak, K.; Kandefer-Szerszeń, M.; Stepulak, A. The biological and pharmacological activity of essential oils in the treatment and prevention of infectious diseases. Postęp. Hig. Med. Dośw. 2013, 67, 1000–1007. [Google Scholar] [CrossRef]
- Negi, P.S. Plant extracts for the control of bacterial growth: Efficacy, stability and safety issues for food application. Int. J. Food Microbiol. 2012, 156, 7–17. [Google Scholar] [CrossRef]
- Tarahovsky, Y.S.; Kim, Y.A.; Yagolnik, E.A.; Mazafarov, E.N. Flavonoid–membrane interactions: Involvement of flavonoid–metal complexes in raft signaling. Biochim. Biophys. Acta BBA Biomembr. 2014, 1835, 1235–1246. [Google Scholar] [CrossRef]
- Halawani, E.M. Antimicrobioal activity of Rosa damascena petals extracts and chemical composition by gas chromatography-mass spectrometry (GC-MS) analysis. Afr. J. Microbiol. Res. 2014, 8, 2360–2366. [Google Scholar]
- Ulusoy, S.; Gulgun, B. Tocopherol, C. Phenolic Contents and Antibacterial Properties of Rose Essential Oil, Hydrosol and Absolute. Curr. Microbiol. 2009, 59, 554. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, G.; Sagdic, O.; Baydar, N.G.; Baydar, H. Antioxidant and Antibacterial Activities of Rosa Damascena Flower Extracts. Int. J. Food Sci. Technol. 2004, 10, 277–281. [Google Scholar] [CrossRef]
- Abu-Shanab, B.; Adwin, G.M.; Jarrar, N.; Abu-Hijlen, A.; Adwin, K. Antibacterial activity of four plant extracts used in palestine in folkloric medicine against methicillin-resistant staphylococcus aureus. Turk. J. Biol. 2006, 30, 196–198. [Google Scholar]
- Nurzyńska-Wierdak, R. Therapeutic properties of essential oils. Ann. UMCS Lublin 2015, 25, 2–5. [Google Scholar]
- Diao, W.R.; Hu, Q.P.; Zhang, H.; Xu, J.G. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel. Food Control 2014, 35, 109–116. [Google Scholar] [CrossRef]
- Park, Y.J.; Biswas, R.; Phillips, R.D.; Chen, J. Antibacterial activities of blueberry and muscadine phenolic extracts. J. Food Sci. 2011, 76, 101. [Google Scholar] [CrossRef]
- Chahardehi, A.M.; Ibrahim, D.; Sulaiman, S.F.; Mousav, L. Time-kill Study of Ethyl Acetate Extract of Stinging Nettle on Bacillus subtilis subsp. spizizenii ATCC CRM-6633 Strain NRS 231. Annu. Res. Rev. Biol. 2015, 6, 33–40. [Google Scholar] [CrossRef]
- Muniandy, K.; Hassan, Z.; Mohd, M.H. Effect of heat and filter sterilization on the efficiency of coleus aromaticus as an antibacterial agent against diabetic wound pathogens. Int. J. Pharm. Pharm. Sci. 2014, 6, 439–442. [Google Scholar]
- Fan, G.J.; Ndolo, V.U.; Katundu, M.; Kerr, R.B.; Arntfield, S.; Beta, T. Comparison of phytochemicals and antioxidant capacity in three bean varieties grown in central Malawi. Plant Foods Hum. Nutr. 2016, 71, 1–7. [Google Scholar] [CrossRef]
- Ge, Q.; Ma, X. Composition and antioxidant activity of anthocyanins isolated from yunnan edible rose (an ning). J. Antimicrob. Chemother. 2013, 2, 68–74. [Google Scholar] [CrossRef]
- Machmudah, S.; Kawahito, Y.; Sasaki, M.; Goto, M. Process optimization and extraction rate analysis of carotenoids extraction from rosehip fruit using supercritical CO2. J. Supercrit. Fluids 2008, 44, 308–314. [Google Scholar] [CrossRef]
- Taneva, I.; Petkova, N.; Dimov, I.; Ivanov, I.; Denev, P. Characterization of rose hip (Rosa canina L.) fruits extracts and evaluation of their in vitro antioxidant activity. J. Pharmacogn. Phytochem. 2016, 5, 35–38. [Google Scholar]
- Franco, D.; Pinelo, M.; Sineiro, J.; Núñez, M.J. Processing of Rosa rubiginosa: Extraction of oil and antioxidant substances. Bioresour. Technol. 2007, 98, 3506–3512. [Google Scholar] [CrossRef] [PubMed]
- Ilbay, Z.; Sahin, S.; Kırba slar, S.I. Investigation of polyhenolic content of rose hip (Rosa canina L.) tea extracts: A comparative study. Foods 2013, 2, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Butsat, S.; Siriamornpun, S. Effect of solvent types and extraction times on phenolic and flavonoid contents and antioxidant activity in leaf extracts of Amomum chinense C. Int. Food Res. J. 2016, 23, 180–187. [Google Scholar]
- Ghasemzadeh, A.; Jaafar, H.Z.E.; Juraimi, A.S.; Tayebi-Meigooni, A. Comparative evaluation of different extraction techniques and solvents for the assay of phytochemicals and antioxidant activity of Hashemi rice bran. Molecules 2015, 20, 10822–10838. [Google Scholar] [CrossRef]
- Clinical Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial a Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard, 8th ed.; CLSI: Wayne, PA, USA, 2009; CLSI document M0-A8. [Google Scholar]
- National Committee for Clinical Laboratory Standards. Methods for Determining Bactericidal Activity of Antimicrobial Agents: NCCLS Document M26-P; National Committee for Clinical Laboratory Standards: Villanova, PA, USA, 1987; Volume 7, No. 2. [Google Scholar]
- Sanna, D.; Delogu, G.; Mulas, M.; Schirra, M.; Fadda, A. Determination of free radical scavenging activity of plant extracts through DPPH assay: An EPR and UV–Vis study. Food Anal. Methods 2012, 5, 759–766. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radivcal cation decolorisation assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |





| Sample | DPPH ●(µmol TE/g for Trolox Equivalents/g Freeze-Dried Extract) | ORAC (µmol TE/g for Trolox Equivalents/g Freeze-Dried Extract) | FRAP (µmol TE/g for Trolox Equivalents/g Freeze-Dried Extract) | ABTS●+ (µmol TE/g for Trolox Equivalents/g Freeze-Dried Extract) |
|---|---|---|---|---|
| Aqueous extract (A) | 887.7 ± 39.0 b | 2142 ± 69.1 b | 880.6 ± 3.5 c | 567.0 ± 3.2 c |
| Ethanolic extract (E) | 1069.7 ± 2.7 d | 6801 ± 38.1 d | 1016.9 ± 1.2 e | 605.0 ± 1.6 e |
| Ethanolic extract (E 1) | 994.6 ± 32.2 c | 5019 ± 26.7 c | 980.1 ± 5.9 d | 574.8 ± 8.9 d |
| Supercritical extract (S) | 1.7 ± 0.1 a | 14 ± 0.3 a | 9.4 ± 0.3 a | 1.7 ± 0.1 a |
| Enzymatic extract (P) | 865.8 ± 13.7 b | 2098 ± 16.5 b | 865.3 ± 4.5 b | 560.3 ± 1.3 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cendrowski, A.; Kraśniewska, K.; Przybył, J.L.; Zielińska, A.; Kalisz, S. Antibacterial and Antioxidant Activity of Extracts from Rose Fruits (Rosa rugosa). Molecules 2020, 25, 1365. https://doi.org/10.3390/molecules25061365
Cendrowski A, Kraśniewska K, Przybył JL, Zielińska A, Kalisz S. Antibacterial and Antioxidant Activity of Extracts from Rose Fruits (Rosa rugosa). Molecules. 2020; 25(6):1365. https://doi.org/10.3390/molecules25061365
Chicago/Turabian StyleCendrowski, Andrzej, Karolina Kraśniewska, Jarosław L. Przybył, Agnieszka Zielińska, and Stanisław Kalisz. 2020. "Antibacterial and Antioxidant Activity of Extracts from Rose Fruits (Rosa rugosa)" Molecules 25, no. 6: 1365. https://doi.org/10.3390/molecules25061365
APA StyleCendrowski, A., Kraśniewska, K., Przybył, J. L., Zielińska, A., & Kalisz, S. (2020). Antibacterial and Antioxidant Activity of Extracts from Rose Fruits (Rosa rugosa). Molecules, 25(6), 1365. https://doi.org/10.3390/molecules25061365







