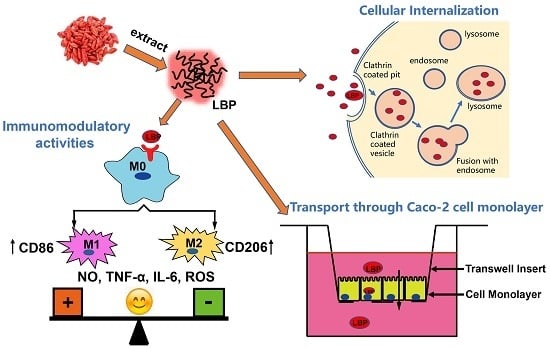
Immunomodulatory Effects of Lycium barbarum Polysaccharide Extract and Its Uptake Behaviors at the Cellular Level
Abstract
1. Introduction
2. Results and Discussion
2.1. Preparation and Characterization of LBP
2.2. Fluorescent Labeling of LBP
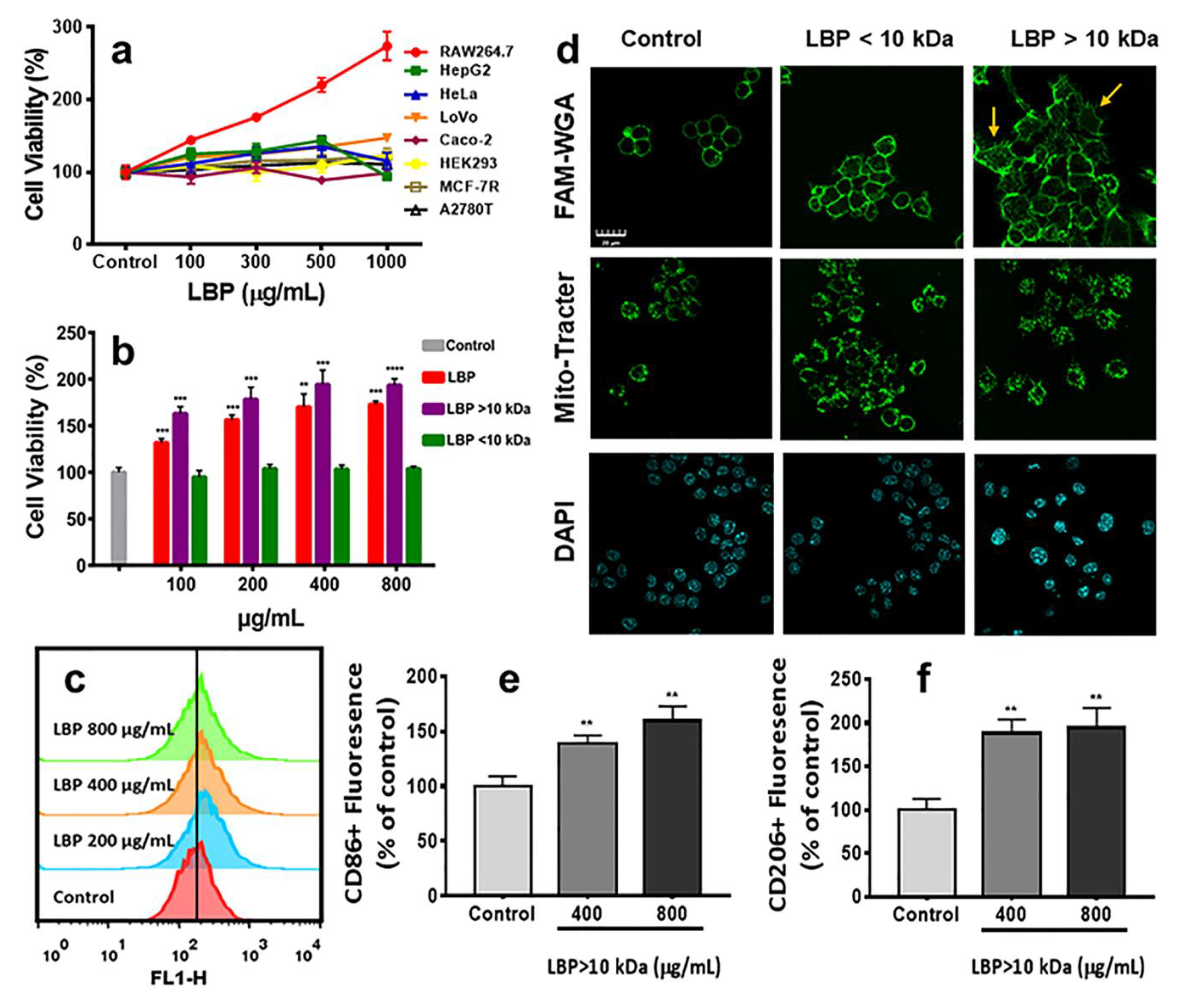
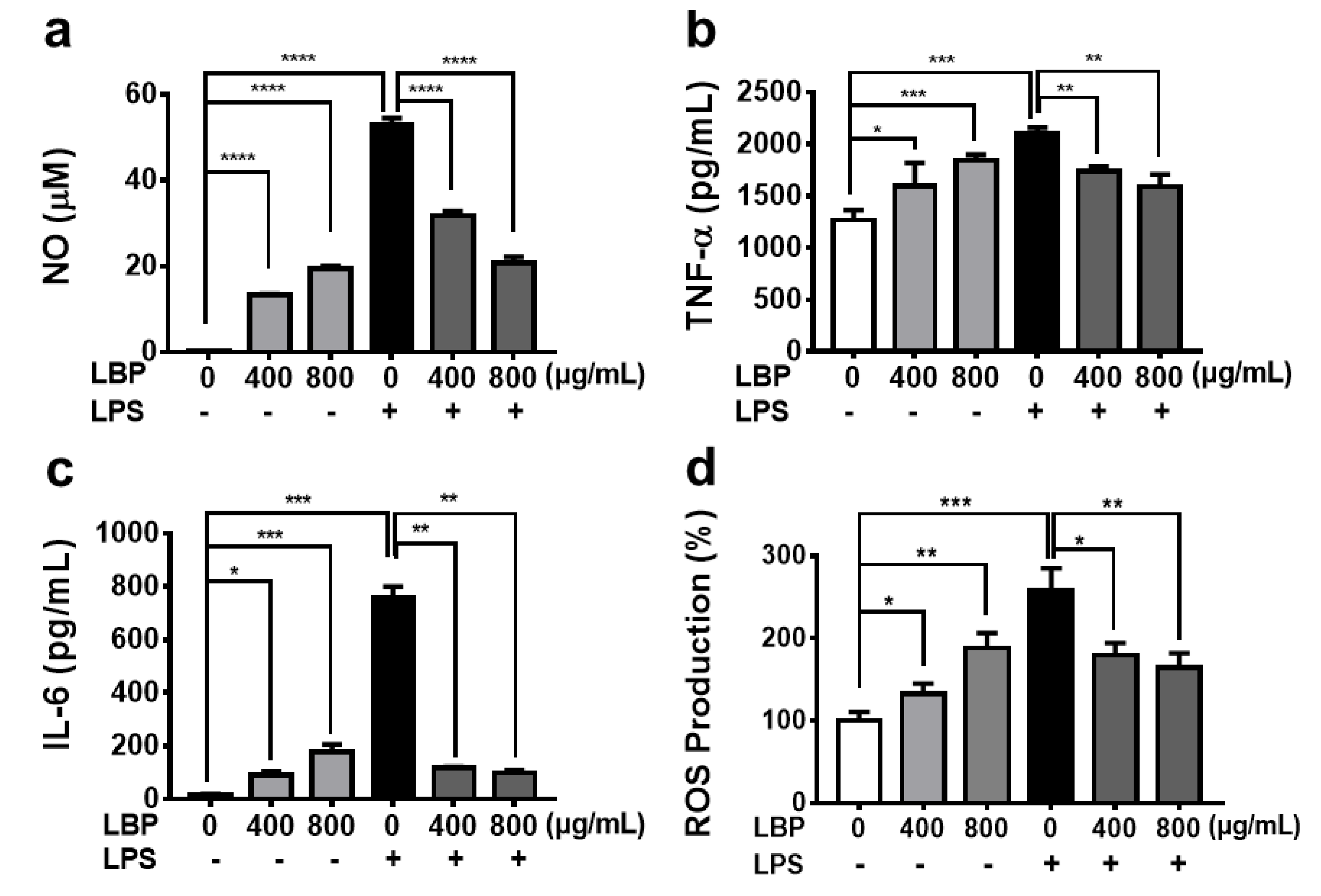
2.3. Immunomodulatory Activities of LBP In Vitro
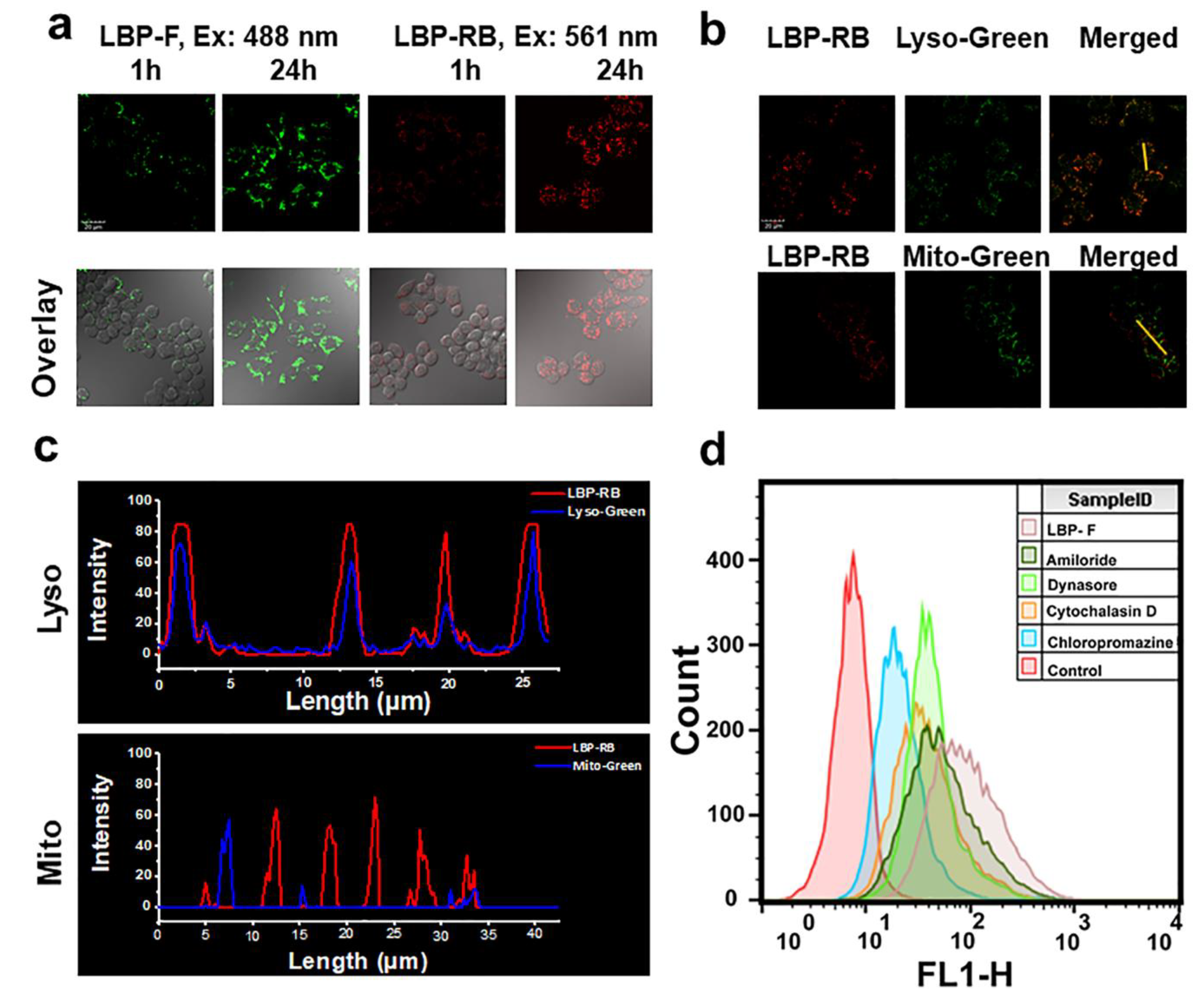
2.4. The Uptake of Dye Labeled LBP by Macrophages
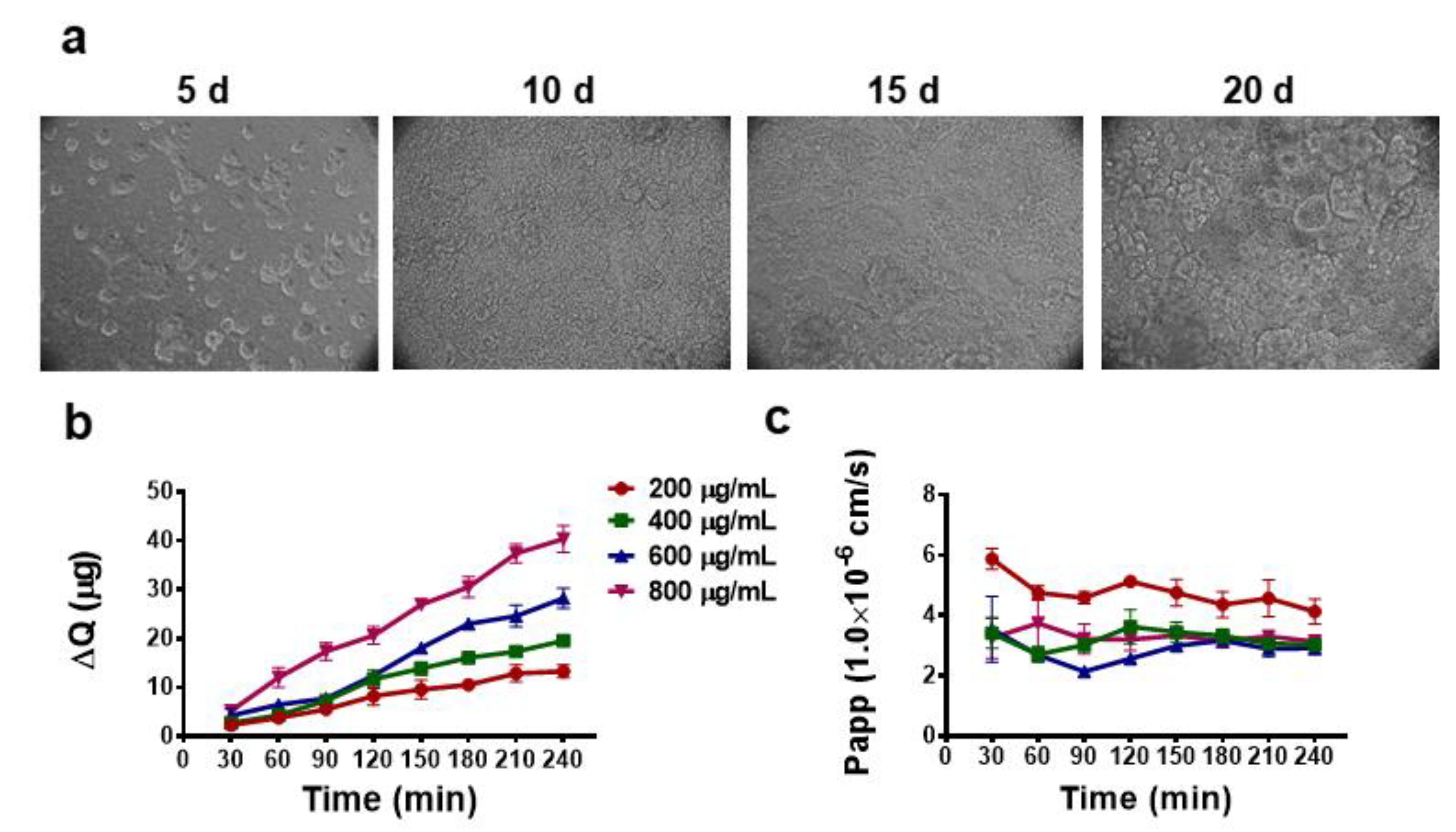
2.5. Transport of Dye Labeled LBP through Caco-2 Cell Monolayer
3. Materials and Methods
3.1. Materials and Reagents
3.2. Preparation, Purification and Fractionation of LBP
3.3. Structural Characterization of Polysaccharides
3.3.1. Infrared Spectral Analysis of the Polysaccharides
3.3.2. Determination of Molecular Weight
3.3.3. Monosaccharide Composition Analysis
3.3.4. Analysis of Chemical Composition
3.3.5. Elemental Analysis
3.4. Synthesis and Characterization of LBP-F/LBP-RB
3.5. Cell Culture and Cell Analysis
3.5.1. Cell Viability Assay
3.5.2. Cell Proliferation Assay
3.5.3. Detection of Morphological Changes of Macrophages
3.5.4. Induction Polarization of RAW264.7 Macrophages
3.5.5. Measurement of Nitric Oxide (NO) and Cytokines Production
3.5.6. Determination of ROS Production
3.5.7. Cellular Uptake and Localization of LBP
3.5.8. Investigation of Endocytosis Pathways in RAW264.7 Cells
3.6. Transportation of LBP-F
3.6.1. Establishment of Cellular Intestinal Model
3.6.2. Cell Monolayer Integrity Testing
3.6.3. Transport of the LBP-F through Caco-2 Cells Monolayer
3.6.4. Effects of Temperature and Clathrin Inhibitor on the Absorption
3.7. Data Handling and Presentation/Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yang, J.; Wei, Y.Q.; Ding, J.-b.; Li, Y.-l.; Ma, J.-l.; Liu, J.-l. Research and application of Lycii Fructus in medicinal field. Chin. Herb. Med. 2018, 10, 339–352. [Google Scholar] [CrossRef]
- Yao, R.; Heinrich, M.; Weckerle, C.S. The genus Lycium as food and medicine: A botanical, ethnobotanical and historical review. J. Ethnopharmacol. 2018, 212, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Protti, M.; Gualandi, I.; Mandrioli, R.; Zappoli, S.; Tonelli, D.; Mercolini, L. Analytical profiling of selected antioxidants and total antioxidant capacity of goji (Lycium spp.) berries. J. Pharm. Biomed. Anal. 2017, 143, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Huang, C.; Chen, X.; Yin, Z.; Fu, Y.; Li, L.; Feng, B.; Song, X.; He, C.; Yue, G.; et al. Two complement fixing pectic polysaccharides from pedicel of Lycium barbarum L. promote cellular antioxidant defense. Int. J. Biol. Macromol. 2018, 112, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.H.; Fu, X.; Zhang, R.; Wang, Z.; Guo, M. Neuroprotective effects of plant polysaccharides: A review of the mechanisms. Int. J. Biol. Macromol. 2018, 106, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.C.; Chang, R.C.-C.; Ip, A.K.-C.; Chiu, K.; Yuen, W.-H.; Zee, S.-Y.; So, K.-F. Neuroprotective effects of Lycium barbarum Lynn on protecting retinal ganglion cells in an ocular hypertension model of glaucoma. Exp. Neurol. 2007, 203, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Qiong, L.; Xiaoyan, C.; Jun, Y.; Mingliang, Y.; Jun, L.; Yuanhai, J.; Jingjing, L.; Yinzhu, Z. Antagonistic effects of Lycium barbarum polysaccharides on the impaired reproductive system of male rats induced by local subchronic exposure to 60Co-γ irradiation. Phytother. Res. 2011, 25, 694–701. [Google Scholar]
- Liu, Y.; Cao, L.; Du, J.; Jia, R.; Wang, J.; Xu, P.; Yin, G. Protective effects of Lycium barbarum polysaccharides against carbon tetrachloride-induced hepatotoxicity in precision-cut liver slices in vitro and in vivo in common carp ( Cyprinus carpio L.). Comp. Biochem. Physiol. Toxicol. Pharm. Cbp. 2015, 169, 65–72. [Google Scholar] [CrossRef]
- Puiyan, L.; Fan, C.; Hor, T.; Ning, W.; Man, Y.; Yibin, F. Hepatoprotective Effects of Chinese Medicinal Herbs: A Focus on Anti-Inflammatory and Anti-Oxidative Activities. Int. J. Mol. Sci. 2016, 17, 465–472. [Google Scholar]
- Jin, M.; Huang, Q.; Shang, P.; Zhao, K. Biological activities and potential health benefit effects of;polysaccharides isolated from Lycium barbarum L. Int. J. Biol. Macromol. 2013, 54, 16–23. [Google Scholar] [CrossRef]
- Yao, L.Q.; Li, F.L. Lycium barbarum polysaccharides ameliorates physical fatigue. Afr. J. Agric. Res. 2010, 5, 2153–2157. [Google Scholar]
- Chen, Z.; Lu, J.; Srinivasan, N.; Tan, B.K.; Chan, S.H. Polysaccharide-protein complex from Lycium barbarum L. is a novel stimulus of dendritic cell immunogenicity. J. Immunol. 2009, 182, 3503–3509. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Dang, T.; Deng, Y.; Han, J.; Zou, Z.; Jing, S.; Zhang, Y.; Liu, Q.; Huang, L.; Wang, Z. Physicochemical properties and biological activities of polysaccharides from Lycium barbarum prepared by fractional precipitation. Int. J. Biol. Macromol. 2018, 109, 611–618. [Google Scholar] [CrossRef]
- Deng, X.; Liu, Q.; Fu, Y.; Luo, X.; Hu, M.; Ma, F.; Wang, Q.; Lai, X.; Zhou, L. Effects of Lycium barbarum polysaccharides with different molecular weights on function of RAW264.7 macrophages. Food Agric. Immunol. 2018, 29, 808–820. [Google Scholar] [CrossRef]
- Wan, L.Q.; Tan, Y.; Jiang, M.; Hua, Q. The prognostic impact of traditional Chinese medicine monomers on tumor-associated macrophages in non-small cell lung cancer. Chin. J. Nat. Med. 2019, 17, 729–737. [Google Scholar] [CrossRef]
- Zhang, X.R.; Qi, C.H.; Cheng, J.P.; Liu, G.; Huang, L.J.; Wang, Z.F.; Zhou, W.X.; Zhang, Y.X. Lycium barbarum polysaccharide LBPF4-OL may be a new Toll-like receptor 4/MD2-MAPK signaling pathway activator and inducer. Int. Immunopharmacol. 2014, 19, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Redgwell, R.J.; Curti, D.; Wang, J.; Dobruchowska, J.M.; Gerwig, G.J.; Kamerling, J.P.; Bucheli, P. Cell wall polysaccharides of Chinese Wolfberry (Lycium barbarum): Part 2. Characterisation of arabinogalactan-proteins. Carbohydr. Polym. 2011, 84, 1075–1083. [Google Scholar] [CrossRef]
- Li, W.; Song, K.; Wang, S.; Zhang, C.; Zhuang, M.; Wang, Y.; Liu, T. Anti-tumor potential of astragalus polysaccharides on breast cancer cell line mediated by macrophage activation. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 685–695. [Google Scholar] [CrossRef]
- Melo-Silveira, R.F.; Viana, R.L.S.; Sabry, D.A.; da Silva, R.A.; Machado, D.; Nascimento, A.K.L.; Scortecci, K.C.; Ferreira-Halder, C.V.; Sassaki, G.L.; Rocha, H.A.O. Antiproliferative xylan from corn cobs induces apoptosis in tumor cells. Carbohydr. Polym. 2019, 210, 245–253. [Google Scholar] [CrossRef]
- Olatunji, O.J.; Chen, H.; Zhou, Y. Lycium chinensis Mill attenuates glutamate induced oxidative toxicity in PC12 cells by increasing antioxidant defense enzymes and down regulating ROS and Ca(2+) generation. Neurosci. Lett. 2016, 616, 111–118. [Google Scholar] [CrossRef]
- Xie, J.; Wu, D.T.; Li, W.Z.; Ning, C.G.; Tang, Y.P.; Jing, Z.; Li, S.P. Effects of Polysaccharides in Lycium Barbarum Berries from Different Regions of China on Macrophages Function and their Correlation to the Glycosidic Linkages. J. Food Sci. 2017, 82, 2411–2420. [Google Scholar] [CrossRef]
- Bi, D.; Yu, B.; Han, Q.; Lu, J.; White, W.L.; Lai, Q.; Cai, N.; Luo, W.; Gu, L.; Li, S.; et al. Immune Activation of RAW264.7 Macrophages by Low Molecular Weight Fucoidan Extracted from New Zealand Undaria pinnatifida. J. Agric. Food Chem. 2018, 66, 10721–10728. [Google Scholar] [CrossRef]
- Zhou, L.; Huang, L.; Yue, H.; Ding, K. Structure analysis of a heteropolysaccharide from fruits of Lycium barbarum L. and anti-angiogenic activity of its sulfated derivative. Int. J. Biol. Macromol. 2018, 108, 47–55. [Google Scholar] [CrossRef]
- Wang, K.; Cheng, F.; Pan, X.; Zhou, T.; Liu, X.; Zheng, Z.; Luo, L.; Zhang, Y. Investigation of the transport and absorption of Angelica sinensis polysaccharide through gastrointestinal tract both in vitro and in vivo. Drug. Deliv. 2017, 24, 1360–1371. [Google Scholar] [CrossRef]
- Chen, M.; Yin, M. Design and development of fluorescent nanostructures for bioimaging. Prog. Polym. Sci. 2013, 39, 365–395. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Shen, Y.; Zhao, X.; Wang, X.; Wang, J.; Fan, K.; Zhan, X. Characterization of a novel polysaccharide from Ganoderma lucidum and its absorption mechanism in Caco-2 cells and mice model. Int. J. Biol. Macromol. 2018, 118, 320–326. [Google Scholar] [CrossRef]
- Hubatsch, I.; Ragnarsson, E.G.; Artursson, P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat. Protoc. 2007, 2, 2111–2119. [Google Scholar] [CrossRef]
- Sheng, J.; Sun, Y. Antioxidant properties of different molecular weight polysaccharides from Athyrium multidentatum (Doll.) Ching. Carbohydr. Polym. 2014, 108, 41–45. [Google Scholar] [CrossRef]
- Wu, D.T.; Guo, H.; Lin, S.; Lam, S.-C.; Zhao, L.; Lin, D.-R.; Qin, W. Review of the structural characterization, quality evaluation, and industrial application of Lycium barbarum polysaccharides. Trends Food Sci. Technol. 2018, 79, 171–183. [Google Scholar] [CrossRef]
- Hu, D.J.; Cheong, K.L.; Zhao, J.; Li, S.P. Chromatography in characterization of polysaccharides from medicinal plants and fungi. J. Sep. Sci. 2013, 36, 1–19. [Google Scholar] [CrossRef]
- Sun, X.; Wang, H.; Han, X.; Chen, S.; Zhu, S.; Dai, J. Fingerprint analysis of polysaccharides from different Ganoderma by HPLC combined with chemometrics methods. Carbohydr. Polym. 2014, 114, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qi, C.; Guo, Y.; Zhou, W.; Zhang, Y. Toll-like receptor 4-related immunostimulatory polysaccharides: Primary structure, activity relationships, and possible interaction models. Carbohydr. Polym. 2016, 149, 186–206. [Google Scholar] [CrossRef] [PubMed]
- Venter, G.; Oerlemans, F.T.; Wijers, M.; Willemse, M.; Fransen, J.A.; Wieringa, B. Glucose controls morphodynamics of LPS-stimulated macrophages. PLoS ONE 2014, 9, e96786. [Google Scholar] [CrossRef] [PubMed]
- Khatua, S.; Acharya, K. Alkali treated antioxidative crude polysaccharide from Russula alatoreticula potentiates murine macrophages by tunning TLR/NF-kappaB pathway. Sci. Rep. 2019, 9, 1713–1723. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Wang, N.; Zhao, T.T.; Li, S.M.; Li, Y.H.; Wang, Y.J.; Li, D.S.; Wang, W.F. Fibroblast growth factor 21 ameliorates pancreatic fibrogenesis via regulating polarization of macrophages. Exp. Cell Res. 2019, 382, 111457–111467. [Google Scholar] [CrossRef]
- Liu, D.; Su, J.; Lin, J.; Qian, G.; Chen, X.; Song, S.; Huang, K. Activation of AMPK-dependent SIRT-1 by astragalus polysaccharide protects against ochratoxin A-induced immune stress in vitro and in vivo. Int. J. Biol. Macromol. 2018, 120, 683–692. [Google Scholar] [CrossRef]
- Graff, J.C.; Kimmel, E.M.; Freedman, B.; Schepetkin, I.A.; Holderness, J.; Quinn, M.T.; Jutila, M.A.; Hedges, J.F. Polysaccharides derived from Yamoa (Funtumia elastica) prime gammadelta T cells in vitro and enhance innate immune responses in vivo. Int. Immunopharmacol. 2009, 9, 1313–1322. [Google Scholar] [CrossRef]
- Artursson, P.; Palm, K.; Luthman, K. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv. Drug Deliver. Rev. 2012, 64, 280–289. [Google Scholar] [CrossRef]
- Kadlecova, Z.; Spielman, S.J.; Loerke, D.; Mohanakrishnan, A.; Reed, D.K.; Schmid, S.L. Regulation of clathrin-mediated endocytosis by hierarchical allosteric activation of AP2. J. Cell Biol. 2016, 216, 167–179. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Liu, X.; Wang, N.; Cao, H.; Lu, Y.; Zhou, H.; Zheng, J. Inhibition of clathrin/dynamin-dependent internalization interferes with LPS-mediated TRAM-TRIF-dependent signaling pathway. Cell Immunol. 2012, 274, 121–129. [Google Scholar] [CrossRef]
- Huerta-Garcia, E.; Ramos-Godinez, M.d.P.; Lopez-Saavedra, A.; Alfaro-Moreno, E.; Gomez-Crisostomo, N.P.; Colin-Val, Z.; López-Marure, R. Internalization of titanium dioxide nanoparticles is mediated by actin- dependent reorganization and clathrin- and dynamin-mediated endocytosis in H9c2 rat cardiomyoblasts. Chem. Res. Toxicol. 2019, 32, 578–588. [Google Scholar] [CrossRef]
- Lucero, D.; Islam, P.; Freeman, L.A.; Jin, X.; Pryor, M.; Tang, J.; Kruth, H.S.; Remaley, A.T. Interleukin 10 promotes macrophage uptake of HDL and LDL by stimulating fluid-phase endocytosis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158537–158551. [Google Scholar] [CrossRef]
- Duizer, E.; Gilde, A.J.; Versantvoort, C.H.M.; Groten, J.P. Effects of cadmium chloride on the paracellular barrier function of intestinal epithelial cell lines. Toxicol. Appl. Pharm. 1999, 155, 117–126. [Google Scholar] [CrossRef]
- Yee, S. In vitro permeability across Caco-2 cells (colonic) can predict in vivo (small intestinal) absorption in man--fact or myth. Pharm. Res. 1997, 14, 763–766. [Google Scholar] [CrossRef]
- Sun, M.; Su, F.; Yang, J.; Gao, Z.; Geng, Y. Fluorescent Labeling of Polysaccharides from Masson Pine Pollen and Its Effect on RAW264.7 Macrophages. Polymer (Basel) 2018, 10, 372. [Google Scholar] [CrossRef]
- Sun, S.; Li, K.; Xiao, L.; Lei, Z.; Zhang, Z. Characterization of polysaccharide from Helicteres angustifolia L. and its immunomodulatory activities on macrophages RAW264.7. Biomed. Pharm. 2019, 109, 262–270. [Google Scholar] [CrossRef]
- Unnikrishnan, B.S.; Preethi, G.U.; Sreeranganathan, M.; Syama, H.P.; Archana, M.G.; Sreelekha, T.T. Fabrication of fluorescein labeled galactoxyloglucan polysaccharide for tumor and macrophage tagging. J. Drug Deliv. Sci. Technol. 2019, 52, 863–869. [Google Scholar]
Sample Availability: Not available. |

| LBP | LBP < 10 kDa | LBP > 10 kDa | ||
|---|---|---|---|---|
| Peak 1 | Peak 2 | |||
| Molecular Characteristics | ||||
| Mw | 51.88 | 6.71 | 6.99 | 27.71 |
| Mn | 15.10 | 4.88 | 3.55 | 12.21 |
| Mw/Mn | 3.44 | 1.27 | 1.97 | 2.27 |
| Monosaccharide (Molar Ratio %) | ||||
| Mannose | 1.29 | 1.28 | 1.28 | |
| Glucose | 30.61 | 47.27 | 11.68 | |
| Galacturonic acid | 1.04 | 0.39 | 1.46 | |
| Galactose | 7.03 | 1.20 | 13.37 | |
| Xylose | 1.44 | 0.73 | 1.91 | |
| Arabinose | 14.81 | 0.81 | 27.76 | |
| Fucose | 0.38 | 1.81 | 0.62 | |
| Glucosamine | 0.57 | 1.37 | 0.78 | |
| Rhamnose | 0.91 | 0.82 | 1.67 | |
| Ribose | 0.11 | 0.37 | n.d. | |
| Chemical Compositions (%) | ||||
| Protein | 0.65 ± 0.14 | 0.47 ± 0.07 | 1.10 ± 0.03 * | |
| Carbohydrates | 26.58 ± 0.51 | 16.25 ± 0.24 | 29.69 ± 0.80 *** | |
| Elemental Analyses (%) | ||||
| C | 33.18 ± 0.05 | 30.08 ± 0.05 | 36.78 ± 0.01 **** | |
| N | 3.33 ± 0.02 | 3.38 ± 0.04 | 3.30 ± 0.06 | |
| H | 4.78 ± 0.03 | 4.44 ± 0.02 | 5.41 ± 0.02 **** | |
| S | 0.73 ± 0.02 | 1.09 ± 0.04 | 0.96 ± 0.02 | |
| Groups | ΔQ ug/cm2 | Papp (×10−6 cm/s) |
|---|---|---|
| 37 °C | 8.11 ± 0.95 | 2.82 ± 0.44 |
| 4 °C | 1.33 ± 0.55 *** | 0.46 ± 0.26 ** |
| Chlorpromazine hydrochloride (37 °C) | 1.74 ± 0.39 *** | 0.61 ± 0.18 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, L.; Xiao, X.; Liu, J.; Wang, J.; Zhang, N.; Bing, T.; Liu, X.; Zhang, Z.; Shangguan, D. Immunomodulatory Effects of Lycium barbarum Polysaccharide Extract and Its Uptake Behaviors at the Cellular Level. Molecules 2020, 25, 1351. https://doi.org/10.3390/molecules25061351
Feng L, Xiao X, Liu J, Wang J, Zhang N, Bing T, Liu X, Zhang Z, Shangguan D. Immunomodulatory Effects of Lycium barbarum Polysaccharide Extract and Its Uptake Behaviors at the Cellular Level. Molecules. 2020; 25(6):1351. https://doi.org/10.3390/molecules25061351
Chicago/Turabian StyleFeng, Le, Xiao Xiao, Jing Liu, Junyan Wang, Nan Zhang, Tao Bing, Xiangjun Liu, Ziping Zhang, and Dihua Shangguan. 2020. "Immunomodulatory Effects of Lycium barbarum Polysaccharide Extract and Its Uptake Behaviors at the Cellular Level" Molecules 25, no. 6: 1351. https://doi.org/10.3390/molecules25061351
APA StyleFeng, L., Xiao, X., Liu, J., Wang, J., Zhang, N., Bing, T., Liu, X., Zhang, Z., & Shangguan, D. (2020). Immunomodulatory Effects of Lycium barbarum Polysaccharide Extract and Its Uptake Behaviors at the Cellular Level. Molecules, 25(6), 1351. https://doi.org/10.3390/molecules25061351