The Use of Fluorescence Spectrometry to Determine the Botanical Origin of Filtered Honeys
Abstract
1. Introduction
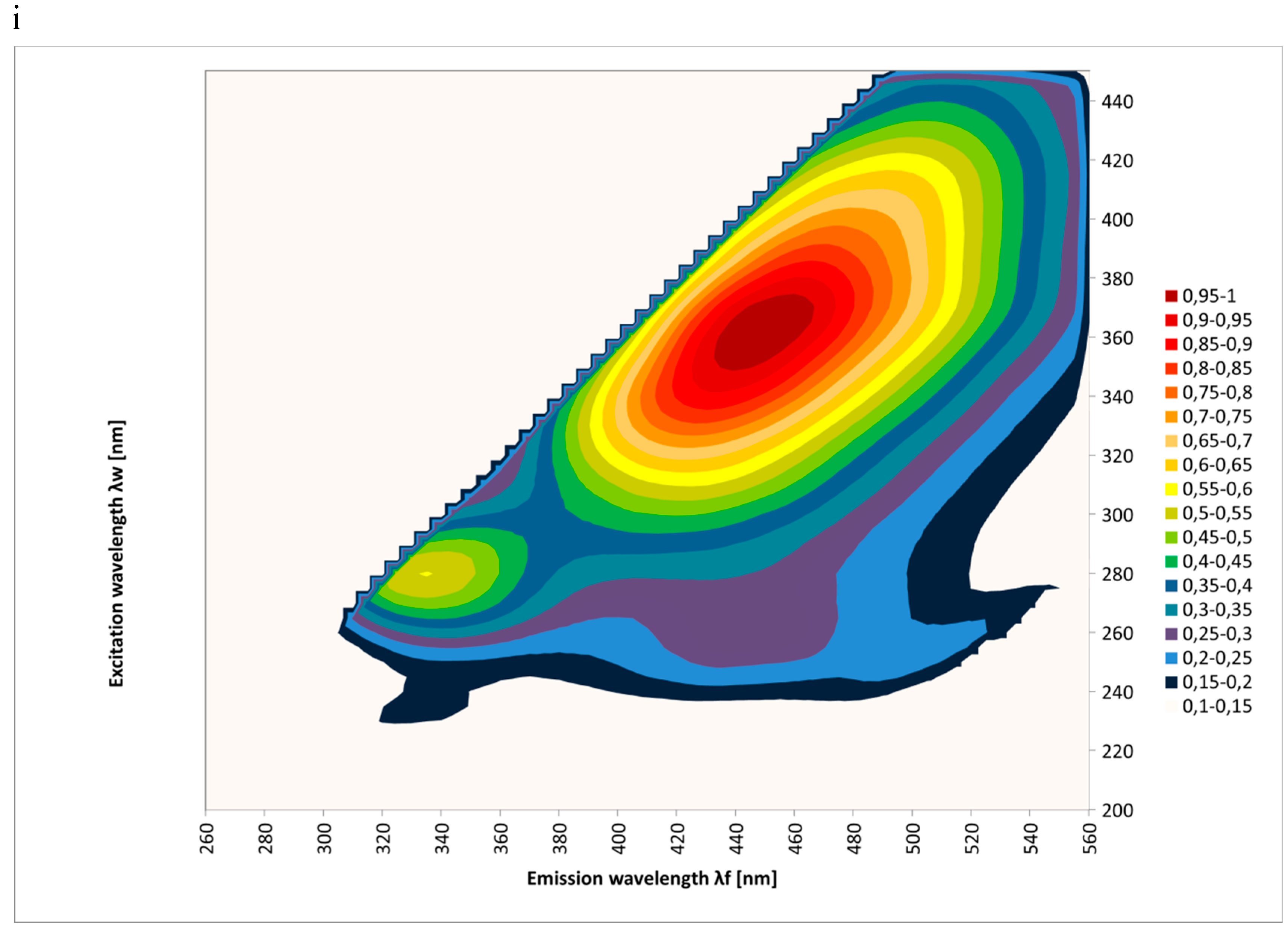
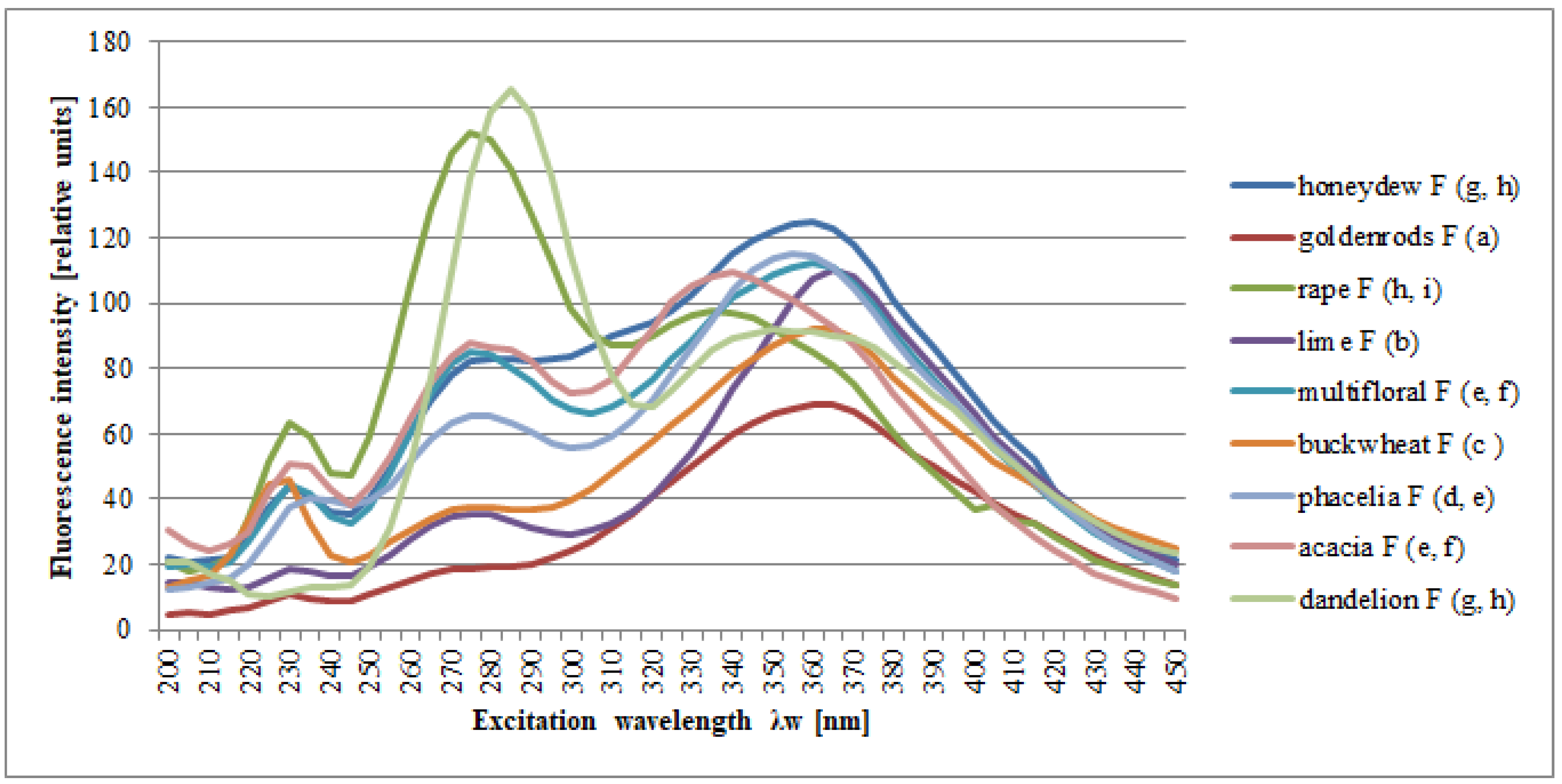
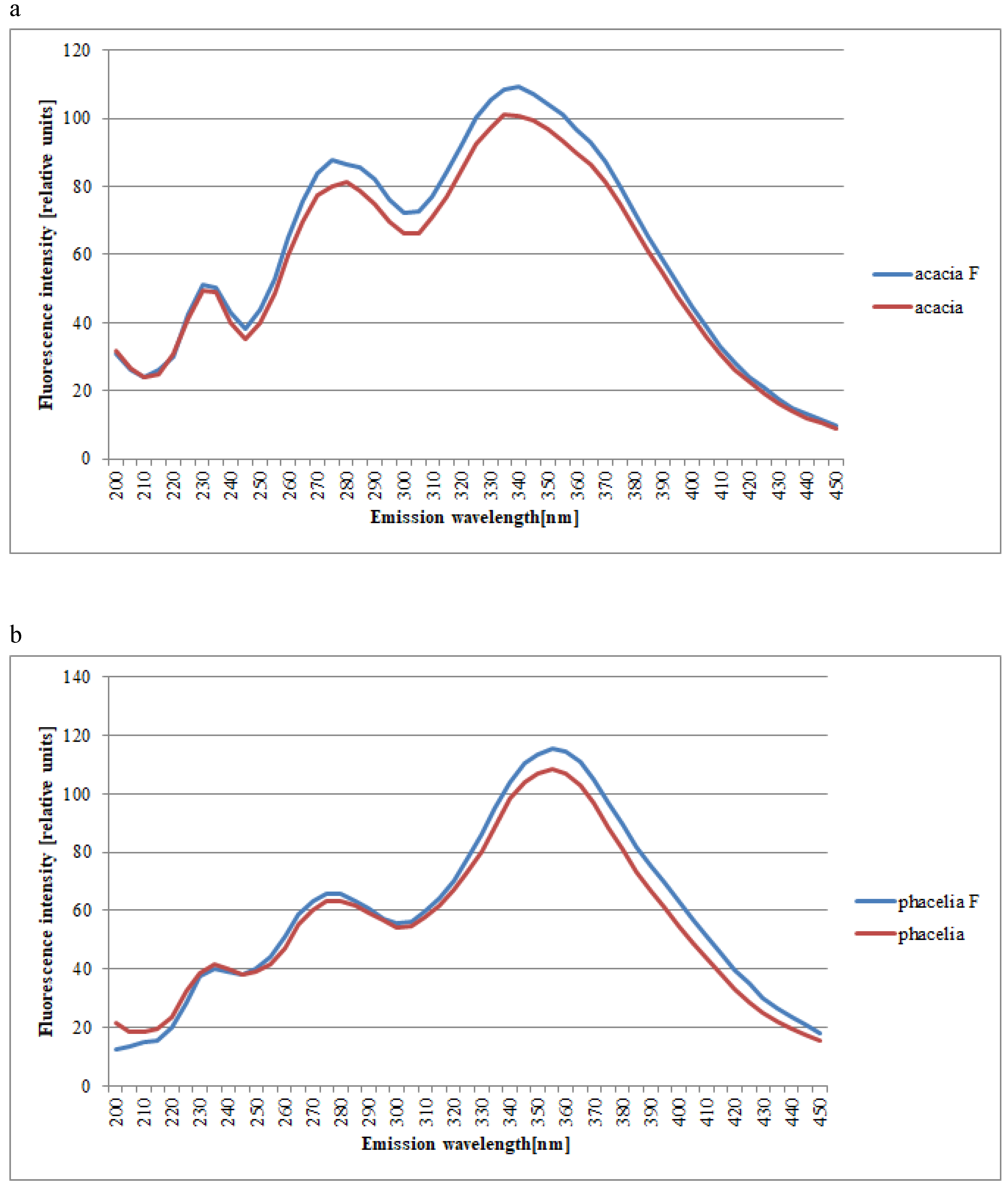
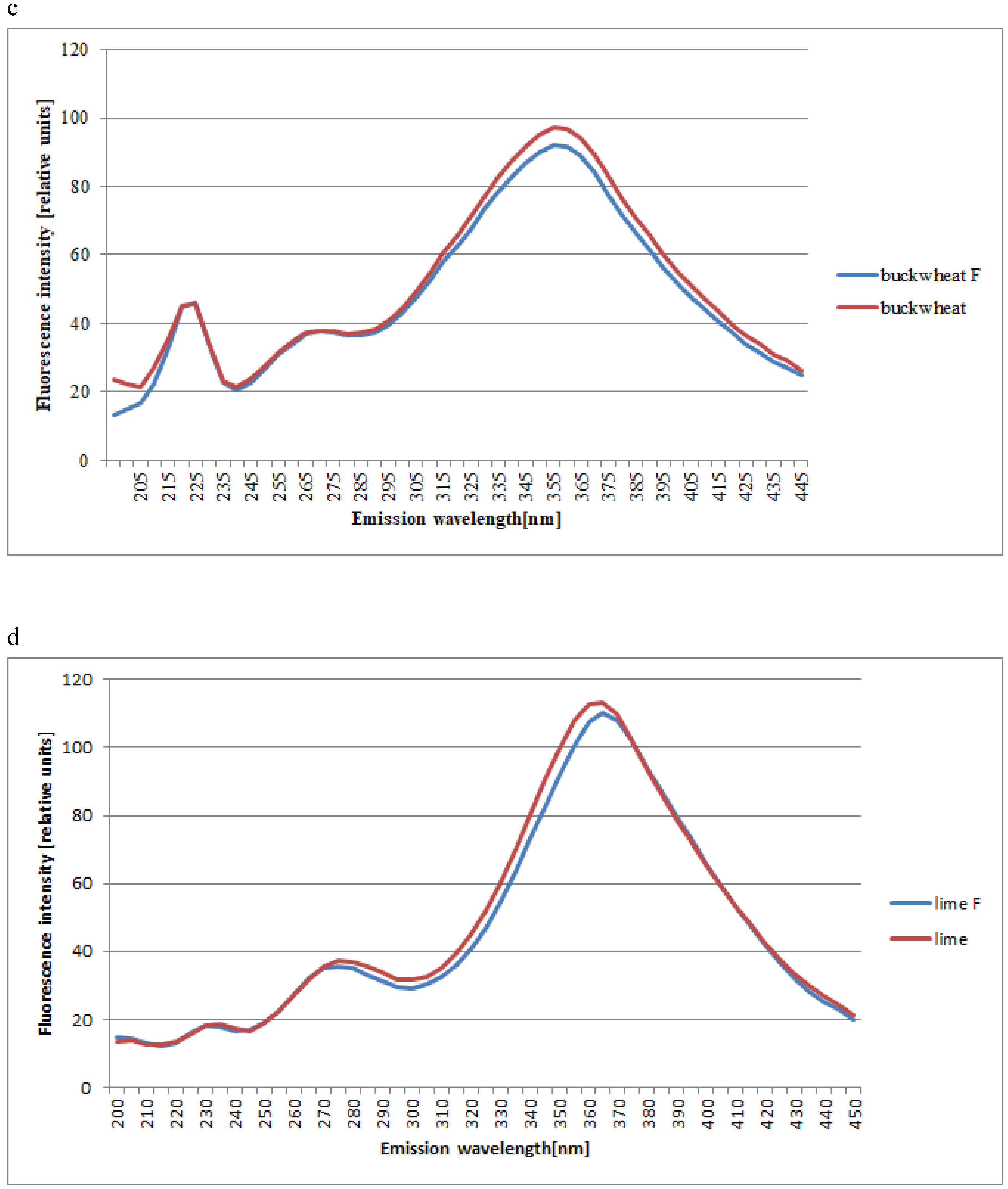
2. Results and Discussion
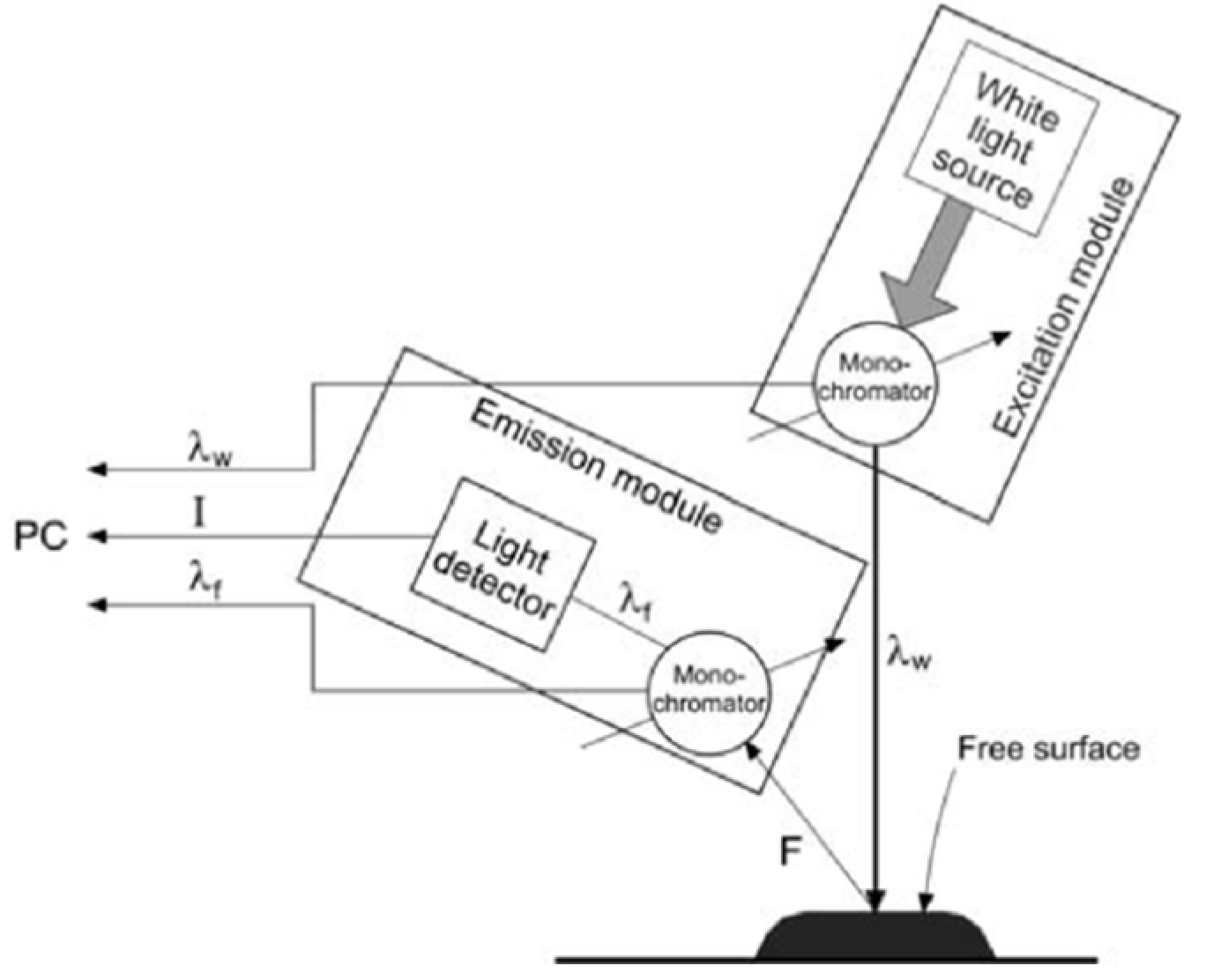
3. Materials and Methods
3.1. Samples
3.2. Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bogdanov, S.; Jurendic, T.; Sieber, R.; Gallmann, P. Honey for nutrition and health: A review. JACN 2008, 27, 677–689. [Google Scholar] [CrossRef] [PubMed]
- De Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Escuredo, O.; Dobre, I.; Fernández-González, M.; Seijo, M.C. Contribution of botanical origin and sugar composition of honeys on the crystallization phenomenon. Food Chem. 2014, 149, 84–90. [Google Scholar] [CrossRef]
- Kaskoniene, V.; Venskutonis, P.R.; Ceksterytè, V. Carbohydrate composition and electrical conductivity of different origin honeys from Lithuania. LWT-Food Sci. Technol. 2010, 43, 801–807. [Google Scholar] [CrossRef]
- Soares, S.; Amaral, J.S.; Oliveira, M.B.P.P.; Mafra, I. A comprehensive review on the main honey authentication issues: Production and origin, Comp. Rev. Food Sci. and Food Safety. 2017, 16, 1072–1100. [Google Scholar] [CrossRef]
- Matysiak-Żurowska, D.; Borowicz, A. A comparison of spectrophotometric Winkler method and HPLC technique for determination of 5-hydroxymethylfurfural in natural honey. Chem. Anal. 2009, 54, 939–947. [Google Scholar]
- Beckmann, K.; Beckh, G.; Luellmann, C.; Speer, K. Characterization of filtered honey by electrophoresis of enzyme fractions. Apidologie 2010, 42, 59. [Google Scholar] [CrossRef]
- Wilczyńska, A. Effect of filtration on colour, antioxidant activity and total phenolics of honey. LWT-Food Sci. Technol. 2014, 57, 767–774. [Google Scholar] [CrossRef]
- Juan-Borrás, M.; Domenech, E.; Hellebrandova, M.; Escriche, I. Effect of country origin on physicochemical, sugar and volatile composition of acacia, sunflower and tilia honeys. Food Res. Int. 2014, 60, 86–94. [Google Scholar] [CrossRef]
- Escriche, I.; Kadar, M.; Juan-Borrás, M.; Domenech, E. Using flavonoids, phenolic compounds and headspace volatile profile for botanical authentication of lemon and orange honeys. Food Res. Int. 2011, 44, 1504–1513. [Google Scholar] [CrossRef]
- Persano-Oddo, L.; Bogdanov, S. Determination of honey botanical origin: Problems and issues. Apidologie 2004, 35, 2–3. [Google Scholar] [CrossRef]
- Aliferis, K.A.; Ttarantilis, P.A.; Harizanis, P.C.; Alissandrakis, E. Botanical discrimination and classification of honey samples applying gas chromatography/mass spectrometry fingerprinting of headspace volatile compounds. Food Chem. 2010, 121, 856–862. [Google Scholar] [CrossRef]
- Ruoff, K.; Luginbuehl, W.; Bogdanov, S.; Bosset, J.O.; Esterman, B.; Ziorko, T.; Kheradmandan, S.; Amad, R. Quantitative determination of physical and chemical measurands in honey by near-infrared spectrometry. Eur. Food Res. Technol. 2007, 225, 415–423. [Google Scholar] [CrossRef]
- Terrab, A.; Gustavo-González, A.; Díez, M.J.; Heredia, F.J. Characterization of Moroccan unifloral honeys using multivariate analysis. Eur. Food Res. Technol. 2003, 218, 88–95. [Google Scholar] [CrossRef]
- Oroian, M.; Ropciuc, S.; Buculei, A. Romanian honey authentication based on physico-chemical parameters and chemometrics. J. Food Measur. Charac. 2017, 11, 719–725. [Google Scholar] [CrossRef]
- Popek, S.; Halagarda, M.; Kursa, K. A new model to identify botanical origin of Polish honeys based on the physicochemical parameters and chemometric analysis. LWT–Food Sci. Technol. 2017, 77, 482–487. [Google Scholar] [CrossRef]
- Kuś, P.M.; van Ruth, S. Discrimination of Polish unifloral honeys using overall PTR-MS and HPLC fingerprints combined with chemometrics. LWT–Food Sci. Technol. 2015, 62, 69–75. [Google Scholar] [CrossRef]
- Kaczmarek, A.; Muzolf-Panek, M.; Tomaszewska-Gras, J.; Konieczny, P. Predicting the botanical origin of honeys with chemometric analysis according to their antioxidant and physicochemical properties. Pol. J. Food Nutr. Sci. 2019, 69, 191–201. [Google Scholar] [CrossRef]
- Dżugan, M.; Tomczyk, M.; Sowa, P.; Grabek-Lejko, D. Antioxidant Activity as Biomarker of Honey Variety. Molecules 2018, 23, 2069. [Google Scholar] [CrossRef]
- Kropf, U.; Korosec, M.; Bertoncelj, J.; Ogrinc, N.; Necemer, M.; Kump, P.; Golob, T. Determination of the geographical origin of Slovenian black locust, lime and chestnut honey. Food Chem. 2010, 121, 839–846. [Google Scholar] [CrossRef]
- Etzold, E.; Lichtenberg-Kraag, B. Determination of the botanical origin of honey by Fourier-transformed infrared spectroscopy: An approach for routine analysis. Eur. Food Res. Technol. 2007, 227, 579–586. [Google Scholar] [CrossRef]
- Lenhardt, L.; Zekovic, I.; Dramicanin, T.; Dramicanin, M.D.; Bro, R. Determination of the botanical origin of honey by front-face synchronous fluorescence spectroscopy. Appl. Spectroscopy. 2014, 68, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Ruoff, K. Authentication of the Botanical Origin of Honey. Doctoral Thesis, ETH Zurich, Zurich, Switzerland, 2006. [Google Scholar] [CrossRef]
- Karoui, R.; Dufour, E.; Bosset, J.-O.; De Baerdemaeker, J. The use of front face fluorescence spectroscopy to classify the botanical origin of honey samples produced in Switzerland. Food Chem. 2007, 101, 314–323. [Google Scholar] [CrossRef]
- Lenhardt, L.; Bro, R.; Zekovic, I.; Dramicanin, T.; Dramicanin, M.D. Fluorescence spectroscopy coupled with PARAFAC and PLS DA for characterization and classification of honey. Food Chem. 2015, 175, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Ruoff, K.; Karoui, R.; Dufour, E.; Luginbuhl, W.; Bosset, J.O.; Bogdanov, S.; Amadò, R. Authentication of the botanical origin of honey by front-face fluorescence spectroscopy, a preliminary study. J. Agric. Food Chem. 2005, 53, 1343–1347. [Google Scholar] [CrossRef]
- Ruoff, K.; Luginbühl, W.; Künzli, R.; Bogdanov, S.; Bosset, J.O.; von der Ohe, K.; von der Ohe, W.; Amadò, R. Authentication of the botanical and geographical origin of honey by front-face fluorescence spectroscopy. J. Agric. Food Chem. 2006, 54, 6858–6866. [Google Scholar] [CrossRef]
- Drami´canin, T.; Lenhardt Ackovic, L.; Zekovic, I.; Dramicanin, M.D. Detection of Adulterated Honey by Fluorescence Excitation-Emission Matrices. J. Spectrosc. 2018, 8395212. [Google Scholar] [CrossRef]
- Gebala, S.; Przybylowski, P.; Borawska, M.H.; Piekut, J. Klasyfikacja naturalnych miodów pszczelich na podstawie analizy ksztaltu widm fluorescencyjnych (Classification of natural honeys based on the analysis of surface spectrofluorimetry shapes). Brom. Chem. Toksykol. 2005, 627–631. [Google Scholar]
- Gębala, S. Measurements of solution fluorescence–a new concept. Opt. Appl. 2009, 39, 391–399. [Google Scholar]
- Gębala, S.; Przybyłowski, P. Sposób Identyfikacji Odmian Miodu. Polska Patent nr 214784, 15 March 2010. [Google Scholar]
Sample Availability: Samples of the honeys are available from the authors. |
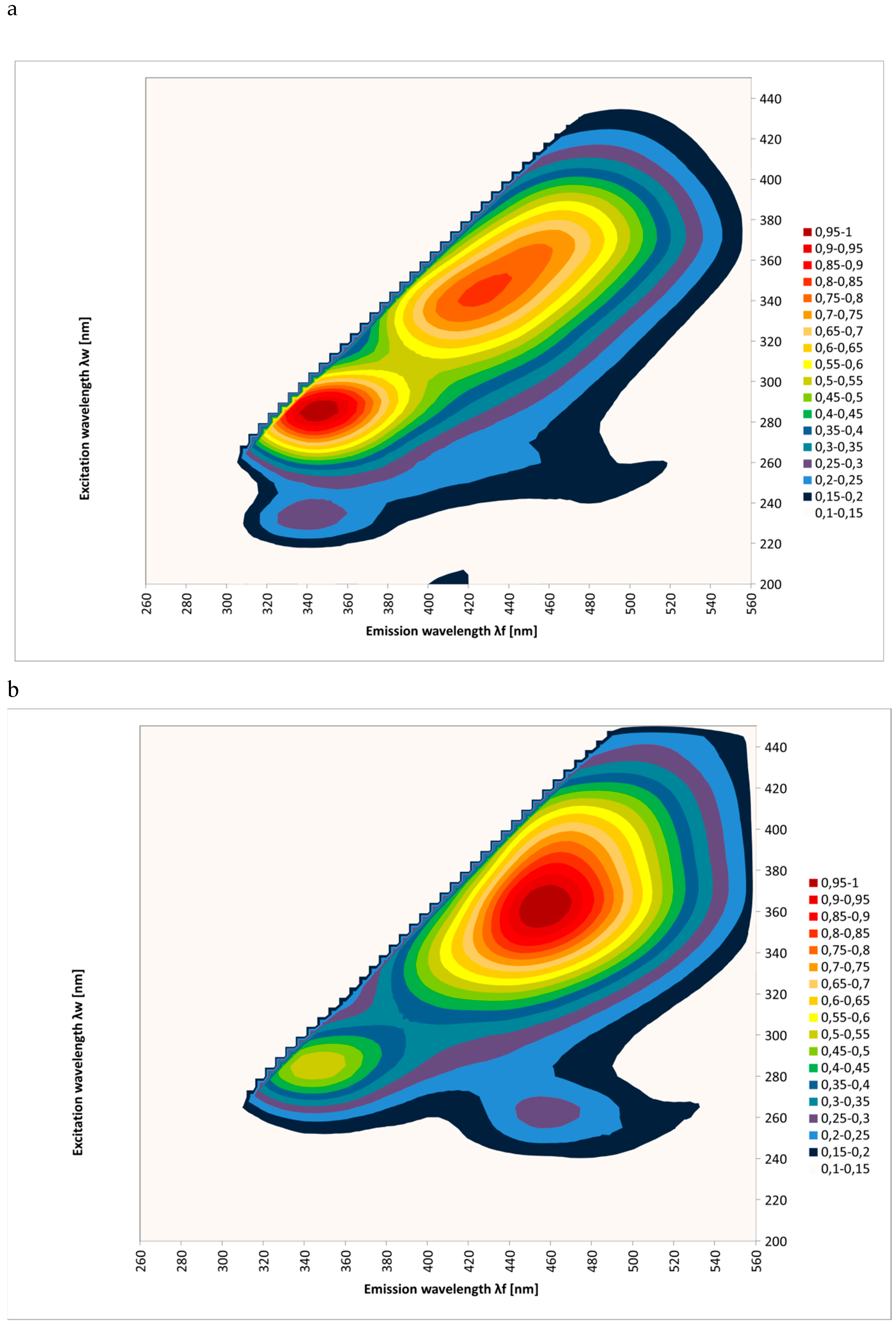
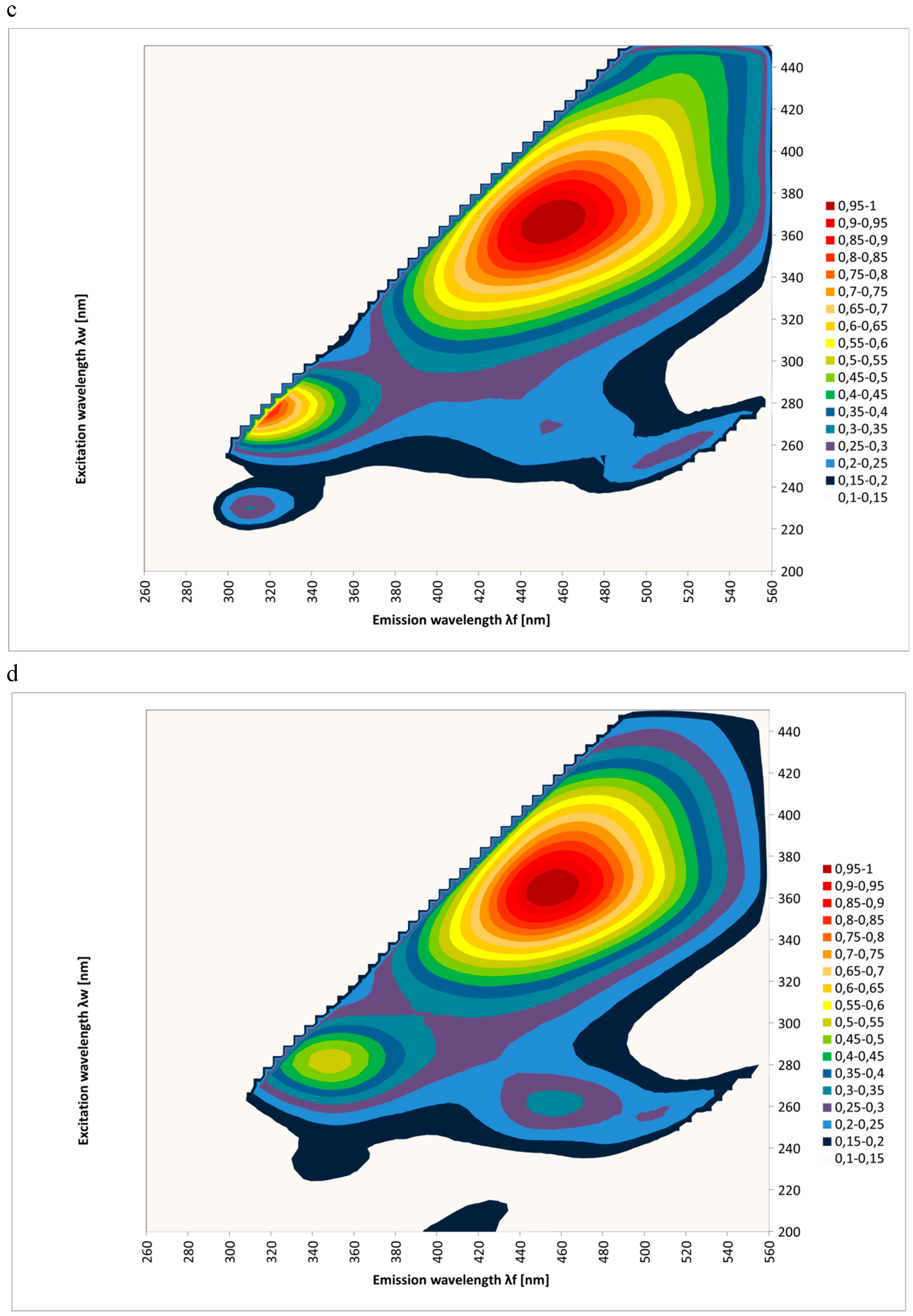

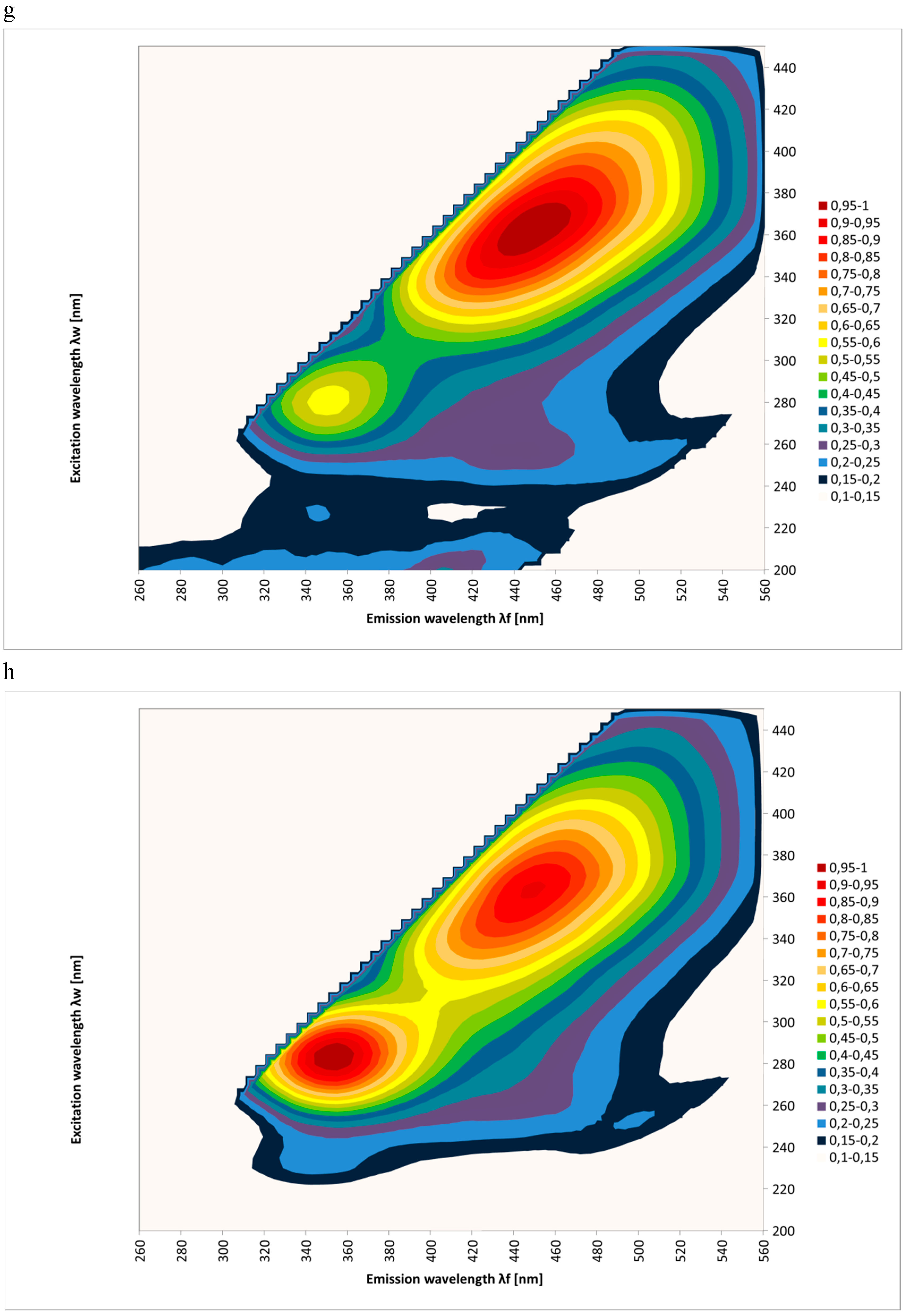

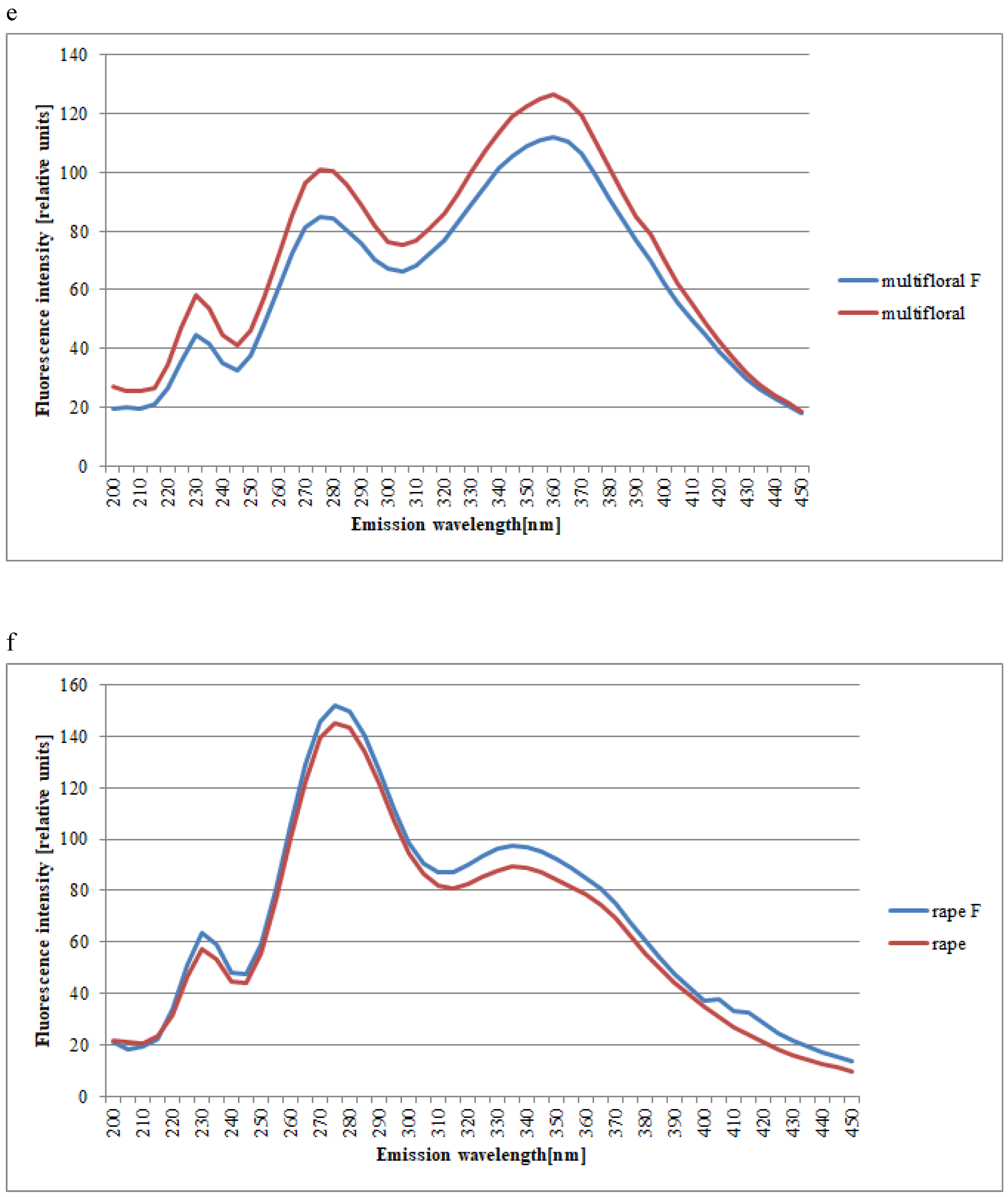
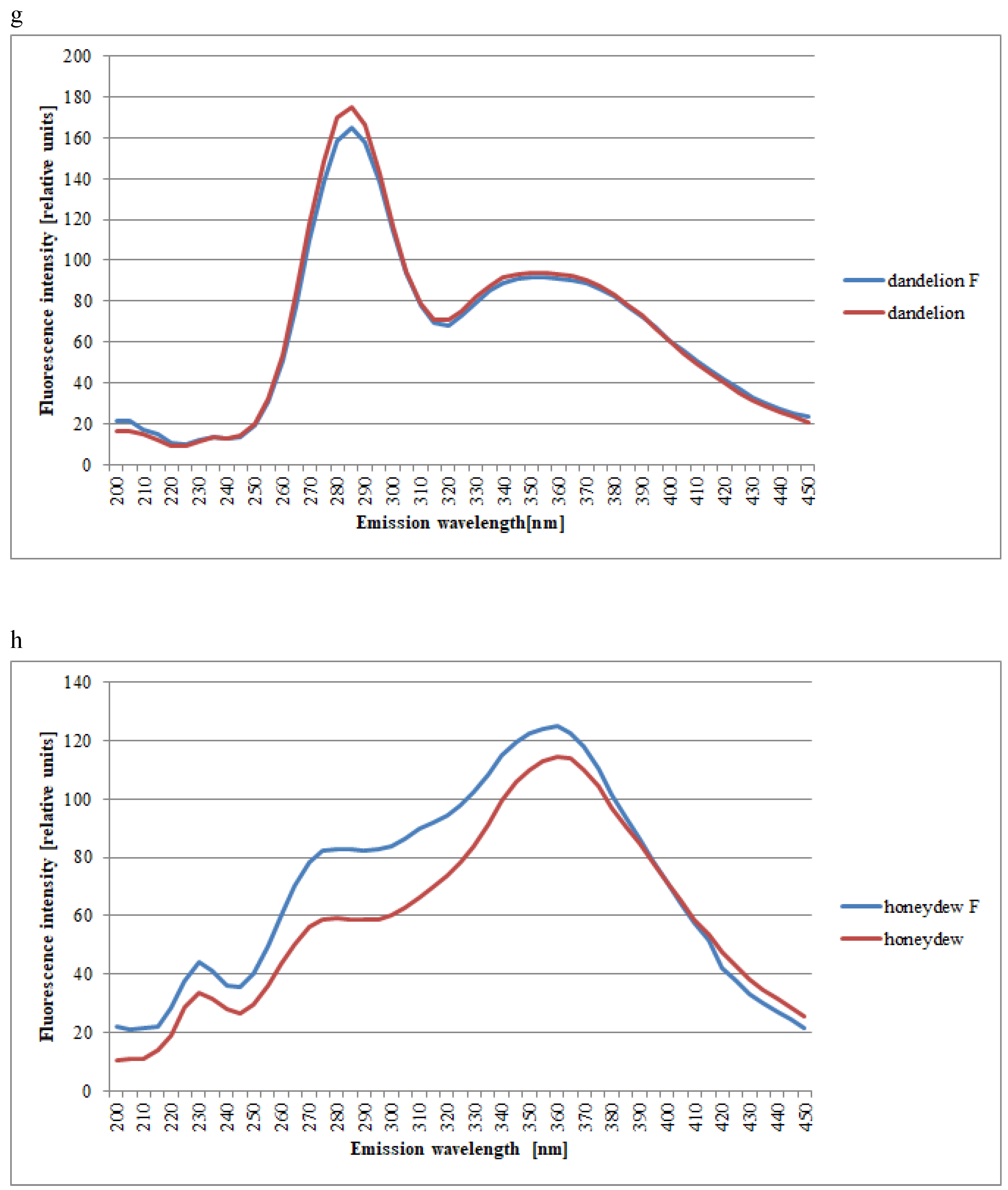
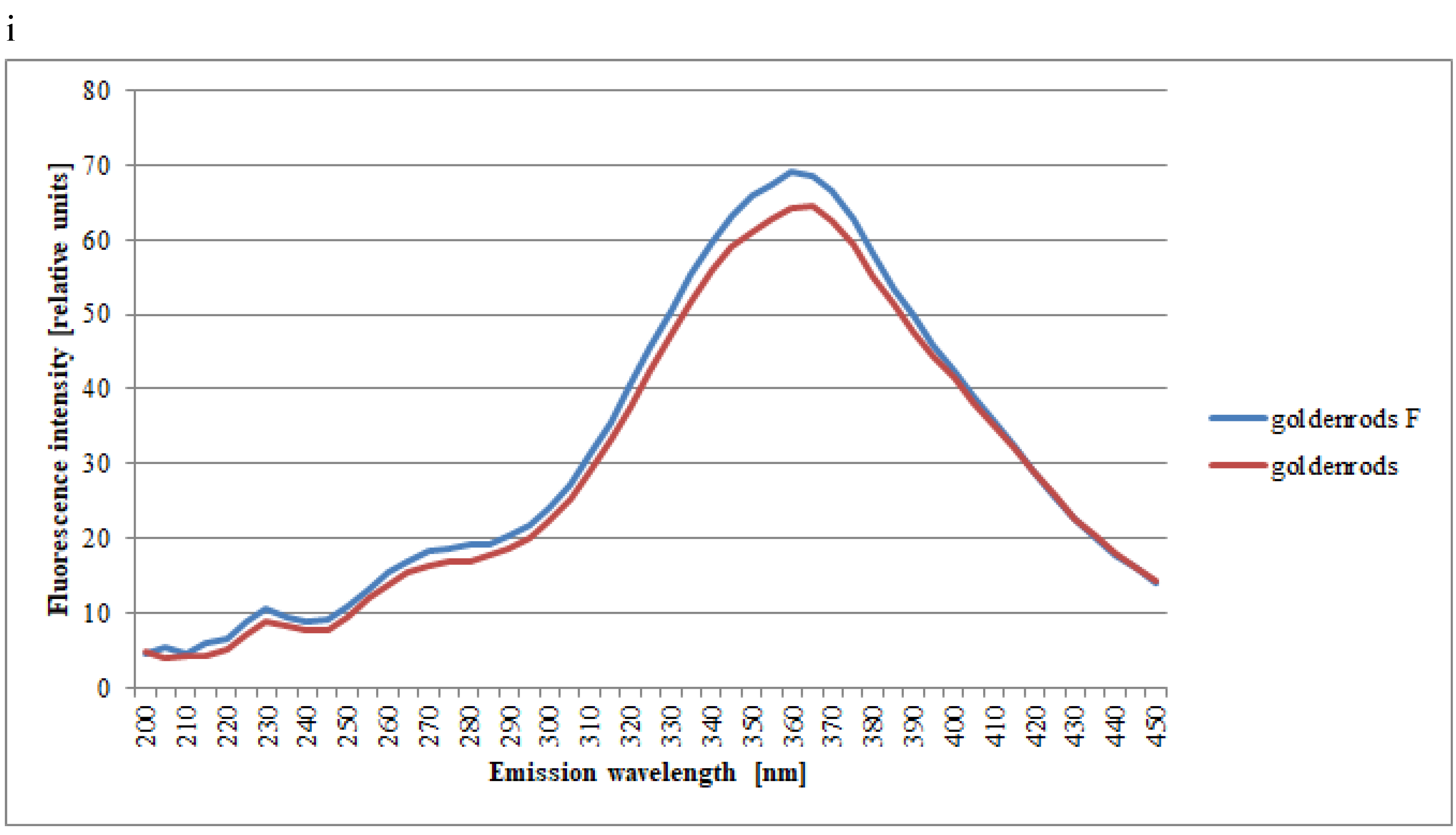
| Spectral Region | Excitation | Type of Honey | Kind of Processing | Mean Intensities of Fluorescence | SD |
|---|---|---|---|---|---|
| 1st | 200 | dandelion | unfiltered | 21.19 | 1.69 |
| 1st | 230 | honeydew | 33.34 | 3.43 | |
| 1st | 230 | goldenrods | 8.96 | 2.62 | |
| 1st | 230 | rape | 45.78 | 2.82 | |
| 1st | 230 | multifloral | 58.15 | 3.98 | |
| 1st | 230 | acacia | 51.04 | 3.50 | |
| 1st | 235 | phacelia | 41.46 | 3.62 | |
| 2nd | 275 | rape | 37.59 | 2.26 | |
| 2nd | 275 | lime | 77.52 | 9.15 | |
| 2nd | 275 | multifloral | 100.96 | 6.17 | |
| 2nd | 275 | buckwheat | 37.59 | 2.21 | |
| 2nd | 275 | phacelia | 63.43 | 4.26 | |
| 2nd | 280 | honeydew | 59.42 | 6.32 | |
| 2nd | 280 | acacia | 86.65 | 5.83 | |
| 2nd | 285 | dandelion | 110.27 | 6.28 | |
| 3rd | 335 | rape | 67.70 | 2.46 | |
| 3rd | 335 | acacia | 108.37 | 4.63 | |
| 3rd | 355 | dandelion | 106.82 | 2.46 | |
| 3rd | 355 | phacelia | 108.45 | 3.96 | |
| 3rd | 360 | honeydew | 114.42 | 4.69 | |
| 3rd | 360 | multifloral | 126.41 | 4.19 | |
| 3rd | 360 | buckwheat | 91.98 | 2.90 | |
| 3rd | 365 | goldenrods | 64.49 | 4.13 | |
| 3rd | 365 | lime | 107.44 | 4.13 | |
| 1st | 200 | dandelion | filtered | 25.41 | 1.69 |
| 1st | 230 | honeydew | 43.98 | 2.96 | |
| 1st | 230 | goldenrods | 10.66 | 4.94 | |
| 1st | 230 | rape | 45.59 | 3.06 | |
| 1st | 230 | multifloral | 44.56 | 3.79 | |
| 1st | 230 | acacia | 37.89 | 3.78 | |
| 1st | 235 | phacelia | 40.16 | 3.05 | |
| 2nd | 275 | rape | 38.65 | 2.21 | |
| 2nd | 275 | lime | 35.35 | 1.74 | |
| 2nd | 275 | multifloral | 84.70 | 6.76 | |
| 2nd | 275 | buckwheat | 37.91 | 2.20 | |
| 2nd | 275 | phacelia | 65.57 | 3.96 | |
| 2nd | 275 | acacia | 80.11 | 5.39 | |
| 2nd | 280 | honeydew | 82.98 | 5.58 | |
| 2nd | 285 | dandelion | 105.81 | 6.07 | |
| 3rd | 335 | rape | 72.68 | 2.78 | |
| 3rd | 340 | acacia | 100.66 | 3.69 | |
| 3rd | 350 | dandelion | 103.96 | 2.71 | |
| 3rd | 355 | phacelia | 115.21 | 3.68 | |
| 3rd | 360 | honeydew | 124.82 | 4.69 | |
| 3rd | 360 | goldenrods | 69.06 | 6.26 | |
| 3rd | 360 | multifloral | 111.97 | 3.89 | |
| 3rd | 360 | buckwheat | 97.24 | 3.58 | |
| 3rd | 365 | lime | 110.20 | 4.75 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilczyńska, A.; Żak, N. The Use of Fluorescence Spectrometry to Determine the Botanical Origin of Filtered Honeys. Molecules 2020, 25, 1350. https://doi.org/10.3390/molecules25061350
Wilczyńska A, Żak N. The Use of Fluorescence Spectrometry to Determine the Botanical Origin of Filtered Honeys. Molecules. 2020; 25(6):1350. https://doi.org/10.3390/molecules25061350
Chicago/Turabian StyleWilczyńska, Aleksandra, and Natalia Żak. 2020. "The Use of Fluorescence Spectrometry to Determine the Botanical Origin of Filtered Honeys" Molecules 25, no. 6: 1350. https://doi.org/10.3390/molecules25061350
APA StyleWilczyńska, A., & Żak, N. (2020). The Use of Fluorescence Spectrometry to Determine the Botanical Origin of Filtered Honeys. Molecules, 25(6), 1350. https://doi.org/10.3390/molecules25061350