Inhibitory Effects of Aucklandia lappa Decne. Extract on Inflammatory and Oxidative Responses in LPS-Treated Macrophages
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction of ALDE
2.2. HPLC Chromatographic Analysis
2.3. Cell Culture
2.4. Cell Viability Assay
2.5. Measurement of NO Production
2.6. RNA Preparation and cDNA Synthesis
2.7. Semiquantitative Reverse Transcription (RT)-PCR
2.8. Western Blotting
2.9. ELISA
2.10. Subcellular Fractionation
2.11. DPPH Free Radical Scavenge Activity
2.12. Statistical Analysis
3. Results
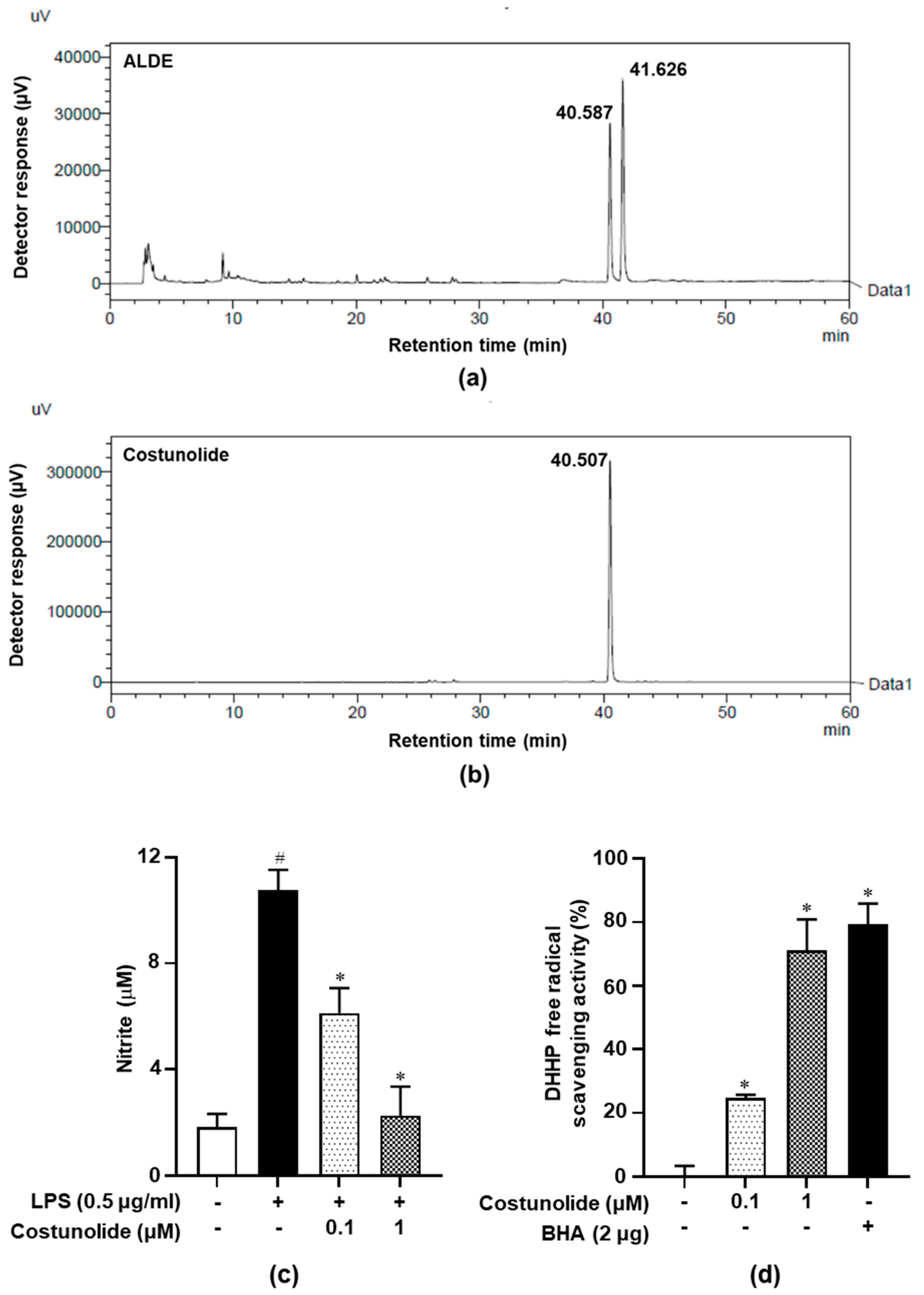
3.1. HPLC and Costunolide-Related Results
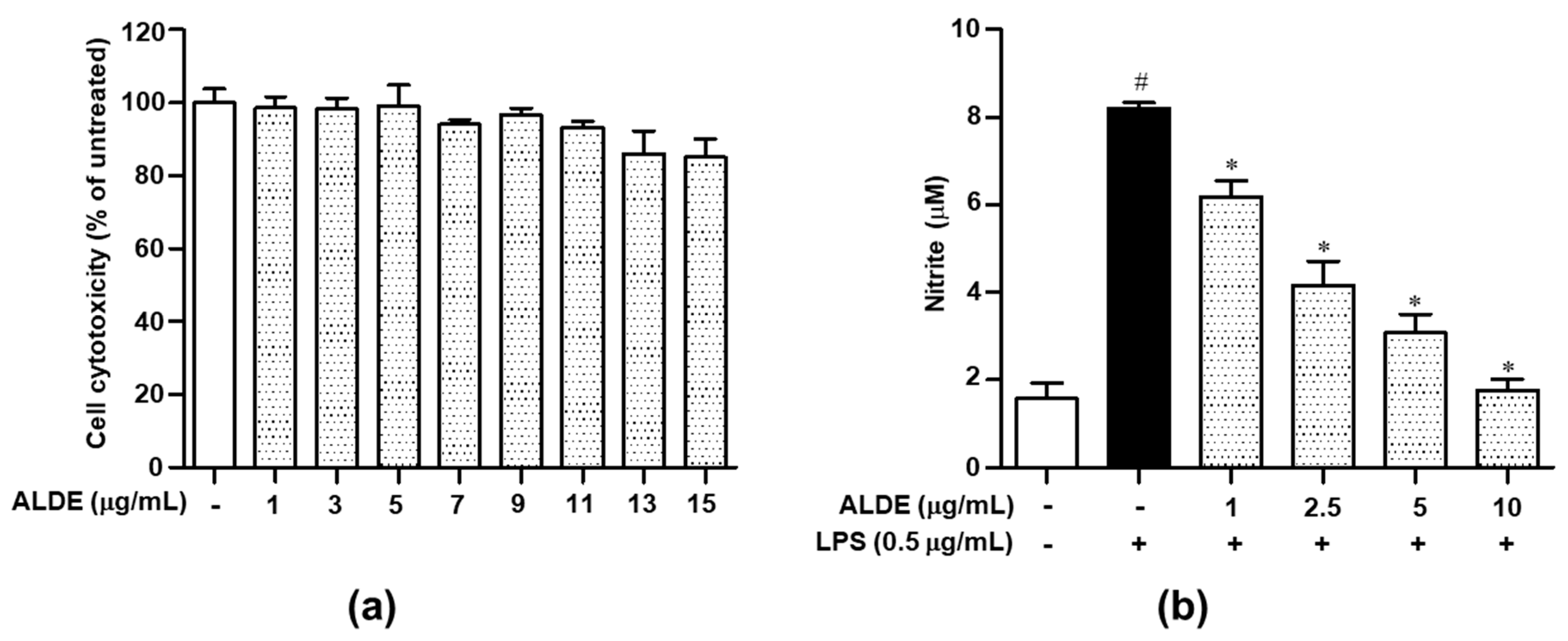
3.2. ALDE Suppressed the Release of NO in LPS-Stimulated RAW 264.7 Cells
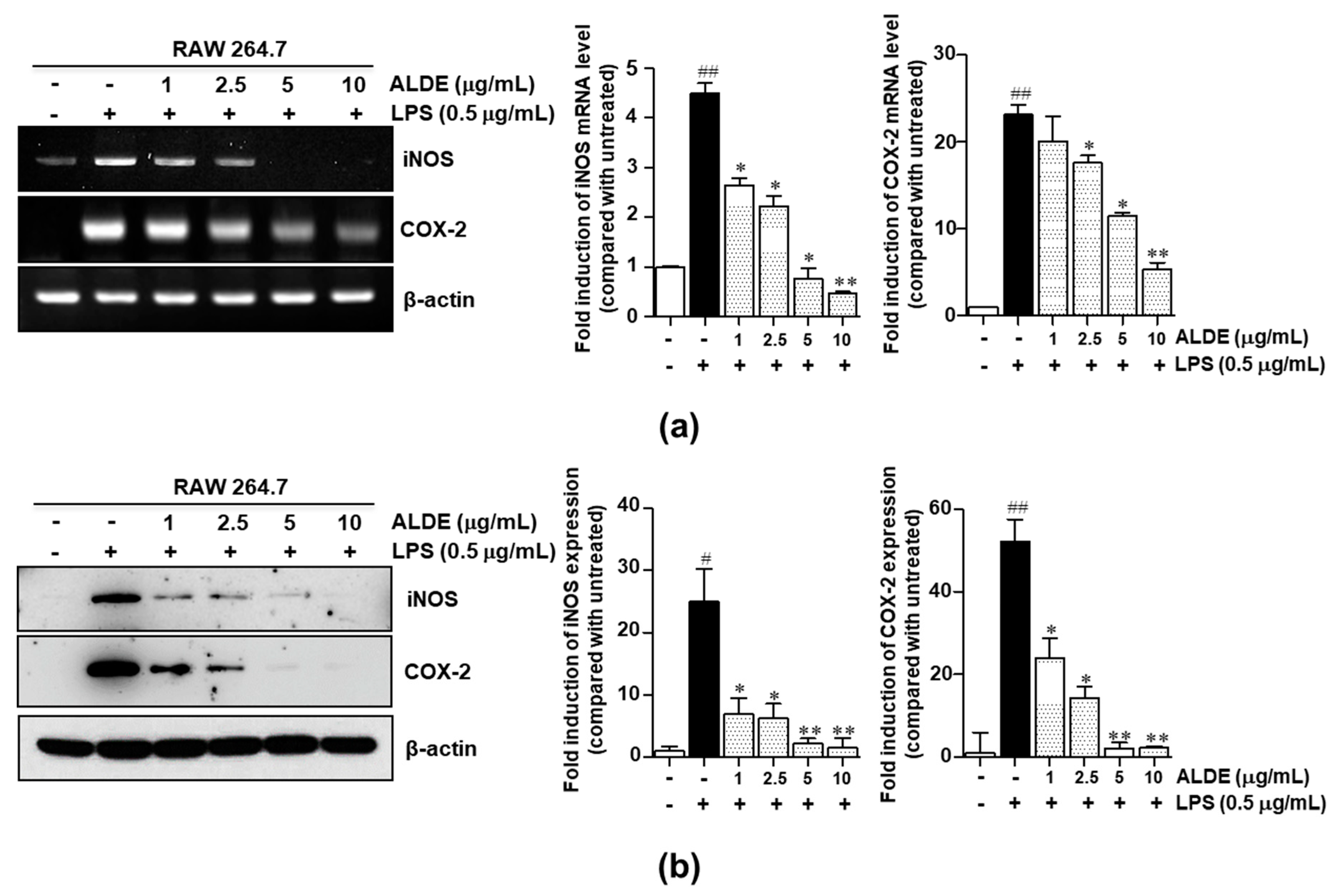
3.3. ALDE Inhibited the Expression of Proinflammatory Enzymes, iNOS and COX-2, in LPS-Stimulated RAW 264.7 Cells
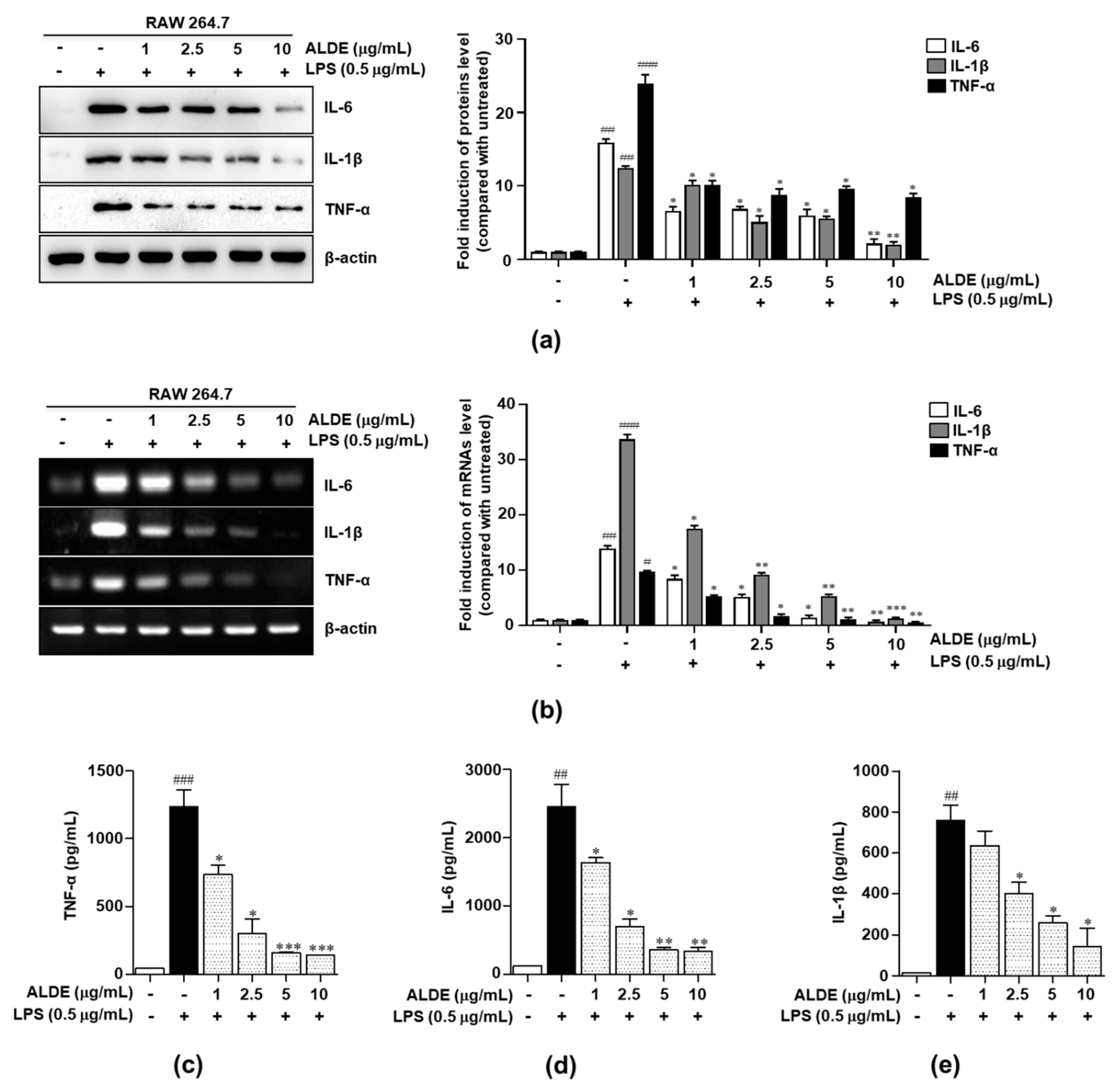
3.4. ALDE Inhibited the Production of Proinflammatory Cytokines in LPS-Stimulated Macrophages
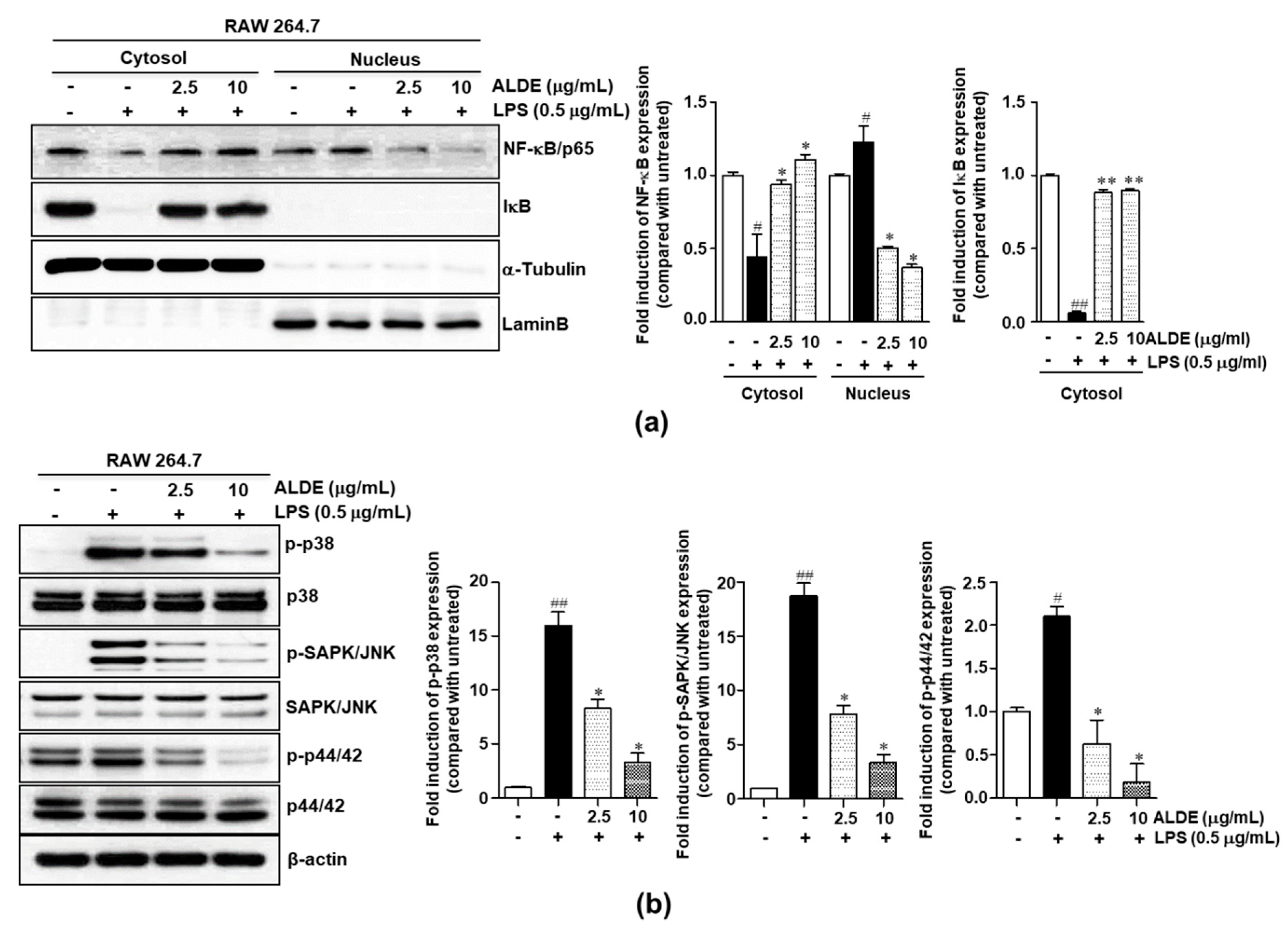
3.5. ALDE Suppressed Both NF-κB Activation and MAPK Phosphorylation in LPS-Stimulated Macrophages
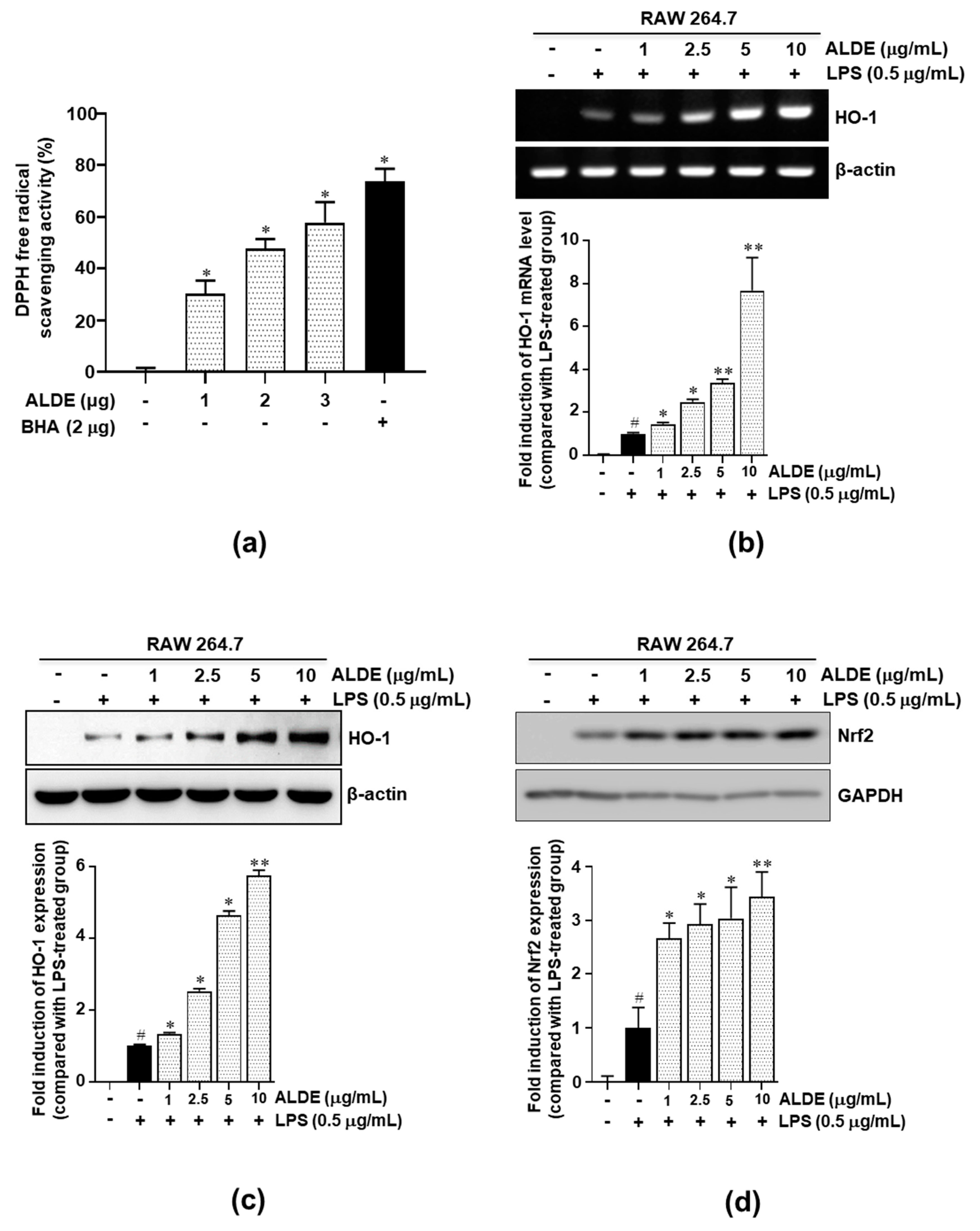
3.6. ALDE Increased the Expression of HO-1 and the Nuclear Translocation of Nrf2 in LPS-Stimulated Macrophages
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Hanada, T.; Yoshimura, A. Regulation of cytokine signaling and inflammation. Cytokine Growth Factor Rev. 2002, 13, 413–421. [Google Scholar] [CrossRef]
- Cutolo, M. Macrophages as effectors of the immunoendocrinologic interactions in autoimmune rheumatic diseases. Ann. N. Y. Acad. Sci. 1999, 876, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Valledor, A.F.; Comalada, M.; Santamaría-Babi, L.F.; Lloberas, J.; Celada, A. Macrophage Proinflammatory Activation and Deactivation. A Question of Balance. Adv. Immunol. 2010, 108, 1–20. [Google Scholar] [PubMed]
- Wedge, D.E.; Galindo, J.C.G.; Macías, F.A. Fungicidal activity of natural and synthetic sesquiterpene lactone analogs. Phytochemistry 2000, 53, 747–757. [Google Scholar] [CrossRef]
- Kassuya, C.A.L.; Cremoneze, A.; Barros, L.F.L.; Simas, A.S.; da Rocha Lapa, F.; Mello-Silva, R.; Stefanello, M.É.A.; Zampronio, A.R. Antipyretic and anti-inflammatory properties of the ethanolic extract, dichloromethane fraction and costunolide from Magnolia ovata (Magnoliaceae). J. Ethnopharmacol. 2009, 124, 369–376. [Google Scholar] [CrossRef]
- Jeong, S.J.; Itokawa, T.; Shibuya, M.; Kuwano, M.; Ono, M.; Higuchi, R.; Miyamoto, T. Costunolide, a sesquiterpene lactone from Saussurea lappa, inhibits the VEGFR KDR/Flk-1 signaling pathway. Cancer Lett. 2002, 187, 129–133. [Google Scholar] [CrossRef]
- Choi, J.Y.; Na, M.; Hwang, I.H.; Lee, S.H.; Bae, E.Y.; Kim, B.Y.; Ahn, J.S. Isolation of betulinic acid, its methyl ester and guaiane sesquiterpenoids with protein tyrosine phosphatase 1B inhibitory activity from the roots of Saussurea lappa C.B.Clarke. Molecules 2009, 14, 266–272. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Hatakeyama, S.; Inoue, Y.; Yamahara, J. Saussureamines A, B, C, D, and E, New Anti-Ulcer Principles from Chinese Saussureae Radix. Chem. Pharm. Bull. 1993, 41, 214–216. [Google Scholar] [CrossRef]
- Chen, H.C.; Chou, C.K.; Lee, S.D.; Wang, J.C.; Yeh, S.F. Active compounds from Saussurea lappa Clarks that suppress hepatitis B virus surface antigen gene expression in human hepatoma cells. Antiviral Res. 1995, 27, 99–109. [Google Scholar]
- Kim, H.R.; Kim, J.M.; Kim, M.S.; Hwang, J.K.; Park, Y.J.; Yang, S.H.; Kim, H.J.; Ryu, D.G.; Lee, D.S.; Oh, H.; et al. Saussurea lappa extract suppresses TPA-induced cell invasion via inhibition of NF-κB-dependent MMP-9 expression in MCF-7 breast cancer cells. BMC Complement. Altern. Med. 2014, 14, 170. [Google Scholar] [CrossRef]
- Seo, C.S.; Lim, H.S.; Jeong, S.J.; Shin, H.K. Anti-allergic effects of sesquiterpene lactones from the root of Aucklandia lappa Decne. Mol. Med. Rep. 2015, 12, 7789–7795. [Google Scholar] [PubMed]
- Kim, M.S.; Kim, N.S.; Kwon, J.; Kim, H.R.; Lee, D.Y.; Oh, M.J.; Kim, H.J.; Lee, C.H.; Oh, C.H. Anti-inflammatory and Immune Regulatory Effects of Aucklandia lappa Decne 70% Ethanol Extract. Korean J. Med. Crop Sci. 2018, 26, 8–18. [Google Scholar]
- Suzuki, K.; Bose, P.; Leong-Quong, R.Y.; Fujita, D.J.; Riabowol, K. REAP: A two minute cell fractionation method. BMC Res. Notes 2010, 3, 294. [Google Scholar]
- Kim, M.H.; Park, D.H.; Bae, M.S.; Song, S.H.; Seo, H.J.; Han, D.G.; Oh, D.S.; Jung, S.T.; Cho, Y.C.; Park, K.M.; et al. Analysis of the Active Constituents and Evaluation of the Biological Effects of Quercus acuta Thunb. (Fagaceae) Extracts. Molecules 2018, 23, 1772. [Google Scholar]
- Pae, H.O.; Jeong, G.S.; Kim, H.S.; Woo, W.H.; Rhew, H.Y.; Kim, H.S.; Sohn, D.H.; Kim, Y.C.; Chung, H.T. Costunolide inhibits production of tumor necrosis factor-α and interleukin-6 by inducing heme oxygenase-1 in RAW264.7 macrophages. Inflamm. Res. 2007, 56, 520–526. [Google Scholar]
- Suh, G.Y.; Jin, Y.; Yi, A.K.; Wang, X.M.; Choi, A.M.K. CCAAT/enhancer-binding protein mediates carbon monoxide-induced suppression of cyclooxygenase-2. Am. J. Respir. Cell Mol. Biol. 2006, 35, 220–226. [Google Scholar]
- Oh, G.S.; Pae, H.O.; Lee, B.S.; Kim, B.N.; Kim, J.M.; Kim, H.R.; Jeon, S.B.; Jeon, W.K.; Chae, H.J.; Chung, H.T. Hydrogen sulfide inhibits nitric oxide production and nuclear factor-κB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free Radic. Biol. Med. 2006, 41, 106–119. [Google Scholar]
- Guha, M.; Mackman, N. LPS induction of gene expression in human monocytes. Cell. Signal. 2001, 13, 85–94. [Google Scholar]
- Lawrence, T.; Willoughby, D.A.; Gilroy, D.W. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat. Rev. Immunol. 2002, 2, 787–795. [Google Scholar]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar]
- Yuan, G.; Wahlqvist, M.L.; He, G.; Yang, M.; Li, D. Natural products and anti-inflammatory activity. Asia Pac. J. Clin. Nutr. 2006, 15, 143–152. [Google Scholar] [PubMed]
- Azab, A.; Nassar, A.; Azab, A.N. Anti-inflammatory activity of natural products. Molecules 2016, 21, 1321. [Google Scholar] [CrossRef] [PubMed]
- Demain, A.L.; Vaishnav, P. Natural products for cancer chemotherapy. Microb. Biotechnol. 2011, 4, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L. Natural products in drug discovery. Drug Discov. Today 2008, 13, 894–901. [Google Scholar] [CrossRef]
- Yu, Y.; Li, X.; Qu, L.; Chen, Y.; Dai, Y.; Wang, M.; Zou, W. DXXK exerts anti-inflammatory effects by inhibiting the lipopolysaccharide-induced NF-κB/COX-2 signalling pathway and the expression of inflammatory mediators. J. Ethnopharmacol. 2016, 178, 199–208. [Google Scholar] [CrossRef]
- Makchuchit, S.; Rattarom, R.; Itharat, A. The anti-allergic and anti-inflammatory effects of Benjakul extract (a Thai traditional medicine), its constituent plants and its some pure constituents using in vitro experiments. Biomed. Pharmacother. 2017, 89, 1018–1026. [Google Scholar] [CrossRef]
- Noh, H.J.; Hwang, D.; Lee, E.S.; Hyun, J.W.; Yi, P.H.; Kim, G.S.; Lee, S.E.; Pang, C.; Park, Y.J.; Chung, K.H.; et al. Anti-inflammatory activity of a new cyclic peptide, citrusin XI, isolated from the fruits of Citrus unshiu. J. Ethnopharmacol. 2015, 163, 106–112. [Google Scholar] [CrossRef]
- Hwang, K.A.; Hwang, Y.J.; Song, J. Aster yomena extract ameliorates pro-inflammatory immune response by suppressing NF-κB activation in RAW 264.7 cells. J. Chin. Med. Assoc. 2018, 81, 102–110. [Google Scholar] [CrossRef]
- De Oliveira, R.G.; Mahon, C.P.A.N.; Ascêncio, P.G.M.; Ascêncio, S.D.; Balogun, S.O.; De Oliveira Martins, D.T. Evaluation of anti-inflammatory activity of hydroethanolic extract of Dilodendron bipinnatum Radlk. J. Ethnopharmacol. 2014, 155, 387–395. [Google Scholar] [CrossRef]
- Kang, H.J.; Hong, S.H.; Kang, K.H.; Park, C.; Choi, Y.H. Anti-inflammatory effects of Hwang-Heuk-San, a traditional Korean herbal formulation, on lipopolysaccharide-stimulated murine macrophages. BMC Complement. Altern. Med. 2015, 15, 447. [Google Scholar] [CrossRef]
- Kang, J.S.; Yoon, Y.D.; Lee, K.H.; Park, S.K.; Kim, H.M. Costunolide inhibits interleukin-1β expression by down-regulation of AP-1 and MAPK activity in LPS-stimulated RAW 264.7 cells. Biochem. Biophys. Res. Commun. 2004, 313, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.H.; Lee, J.H.; Park, Y.J.; Hong, Y.S.; Kim, H.S.; Kim, K.W.; Lee, J.J. A sesquiterpene lactone, costunolide, from Magnolia grandiflora inhibits NF-κB by targeting IκB phosphorylation. Planta Med. 2001, 67, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Angeles Muñoz-Fernández, M.; Fresno, M. The role of tumour necrosis factor, interleukin 6, interferon-γ and inducible nitric oxide synthase in the development and pathology of the nervous system. Prog. Neurobiol. 1998, 56, 307–340. [Google Scholar] [CrossRef]
- Aderem, A.; Ulevitch, R.J. Toll-like receptors in the induction of the innate immune response. Nature 2000, 406, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Takazoe, M.; Fukuda, Y.; Hibi, T.; Kusugami, K.; Andoh, A.; Matsumoto, T.; Yamamura, T.; Azuma, J.; Nishimoto, N.; et al. A Pilot Randomized Trial of a Human Anti-Interleukin-6 Receptor Monoclonal Antibody in Active Crohn’s Disease. Gastroenterology 2004, 126, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Choy, E.H.S.; Isenberg, D.A.; Garrood, T.; Farrow, S.; Ioannou, Y.; Bird, H.; Cheung, N.; Williams, B.; Hazleman, B.; Price, R.; et al. Therapeutic benefit of blocking interleukin-6 activity with an anti-interleukin-6 receptor monoclonal antibody in rheumatoid arthritis: A randomized, double-blind, placebo-controlled, dose-escalation trial. Arthritis Rheum. 2002, 46, 3143–3150. [Google Scholar] [CrossRef]
- Chen, H.G.; Xie, K.L.; Han, H.Z.; Wang, W.N.; Liu, D.Q.; Wang, G.L.; Yu, Y.H. Heme oxygenase-1 mediates the anti-inflammatory effect of molecular hydrogen in LPS-stimulated RAW 264.7 macrophages. Int. J. Surg. 2013, 11, 1060–1066. [Google Scholar] [CrossRef]
- Motterlini, R.; Foresti, R. Heme oxygenase-1 as a target for drug discovery. Antioxidants Redox Signal. 2014, 20, 1810–1826. [Google Scholar] [CrossRef]
- Immenschuh, S.; Ramadori, G. Gene regulation of heme oxygenase-1 as a therapeutic target. Biochem. Pharmacol. 2000, 60, 1121–1128. [Google Scholar] [CrossRef]
- Abraham, N.; Tsenovoy, P.; McClung, J.; Drummond, G. Heme Oxygenase: A Target Gene for Anti-Diabetic and Obesity. Curr. Pharm. Des. 2008, 14, 412–421. [Google Scholar] [CrossRef]
- Oh, G.S.; Pae, H.O.; Choi, B.M.; Chae, S.C.; Lee, H.S.; Ryu, D.G.; Chung, H.T. 3-Hydroxyanthranilic acid, one of metabolites of tryptophan via indoleamine 2,3-dioxygenase pathway, suppresses inducible nitric oxide synthase expression by enhancing heme oxygenase-1 expression. Biochem. Biophys. Res. Commun. 2004, 320, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.S.; Lee, S.H.; Lee, S.R.; Lim, H.-J.; Roh, Y.-S.; Won, E.J.; Cho, N.; Chun, C.; Cho, Y.-C. Inhibitory Effects of Aucklandia lappa Decne. Extract on Inflammatory and Oxidative Responses in LPS-Treated Macrophages. Molecules 2020, 25, 1336. https://doi.org/10.3390/molecules25061336
Lim JS, Lee SH, Lee SR, Lim H-J, Roh Y-S, Won EJ, Cho N, Chun C, Cho Y-C. Inhibitory Effects of Aucklandia lappa Decne. Extract on Inflammatory and Oxidative Responses in LPS-Treated Macrophages. Molecules. 2020; 25(6):1336. https://doi.org/10.3390/molecules25061336
Chicago/Turabian StyleLim, Jae Sung, Sung Ho Lee, Sang Rok Lee, Hyung-Ju Lim, Yoon-Seok Roh, Eun Jeong Won, Namki Cho, Changju Chun, and Young-Chang Cho. 2020. "Inhibitory Effects of Aucklandia lappa Decne. Extract on Inflammatory and Oxidative Responses in LPS-Treated Macrophages" Molecules 25, no. 6: 1336. https://doi.org/10.3390/molecules25061336
APA StyleLim, J. S., Lee, S. H., Lee, S. R., Lim, H.-J., Roh, Y.-S., Won, E. J., Cho, N., Chun, C., & Cho, Y.-C. (2020). Inhibitory Effects of Aucklandia lappa Decne. Extract on Inflammatory and Oxidative Responses in LPS-Treated Macrophages. Molecules, 25(6), 1336. https://doi.org/10.3390/molecules25061336







