Removal of Zinc from Aqueous Solutions Using Lamellar Double Hydroxide Materials Impregnated with Cyanex 272: Characterization and Sorption Studies
Abstract
1. Introduction
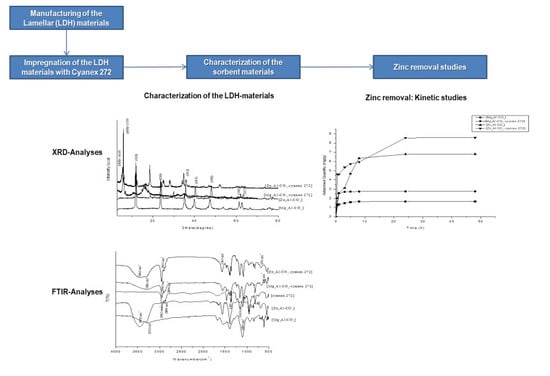
2. Experimental
2.1. Materials
2.2. Preparation of Sorbents
2.3. Preparation of Impregnated Sorbents
2.4. Characterization of Sorbents
2.5. Methods
2.5.1. Metal Sorption Procedure
2.5.2. Effect of Contact Time
2.5.3. Equilibrium Studies
3. Results and Discussion
3.1. Characterization of the Materials
3.1.1. X-Ray Diffraction Analyses (XRD)
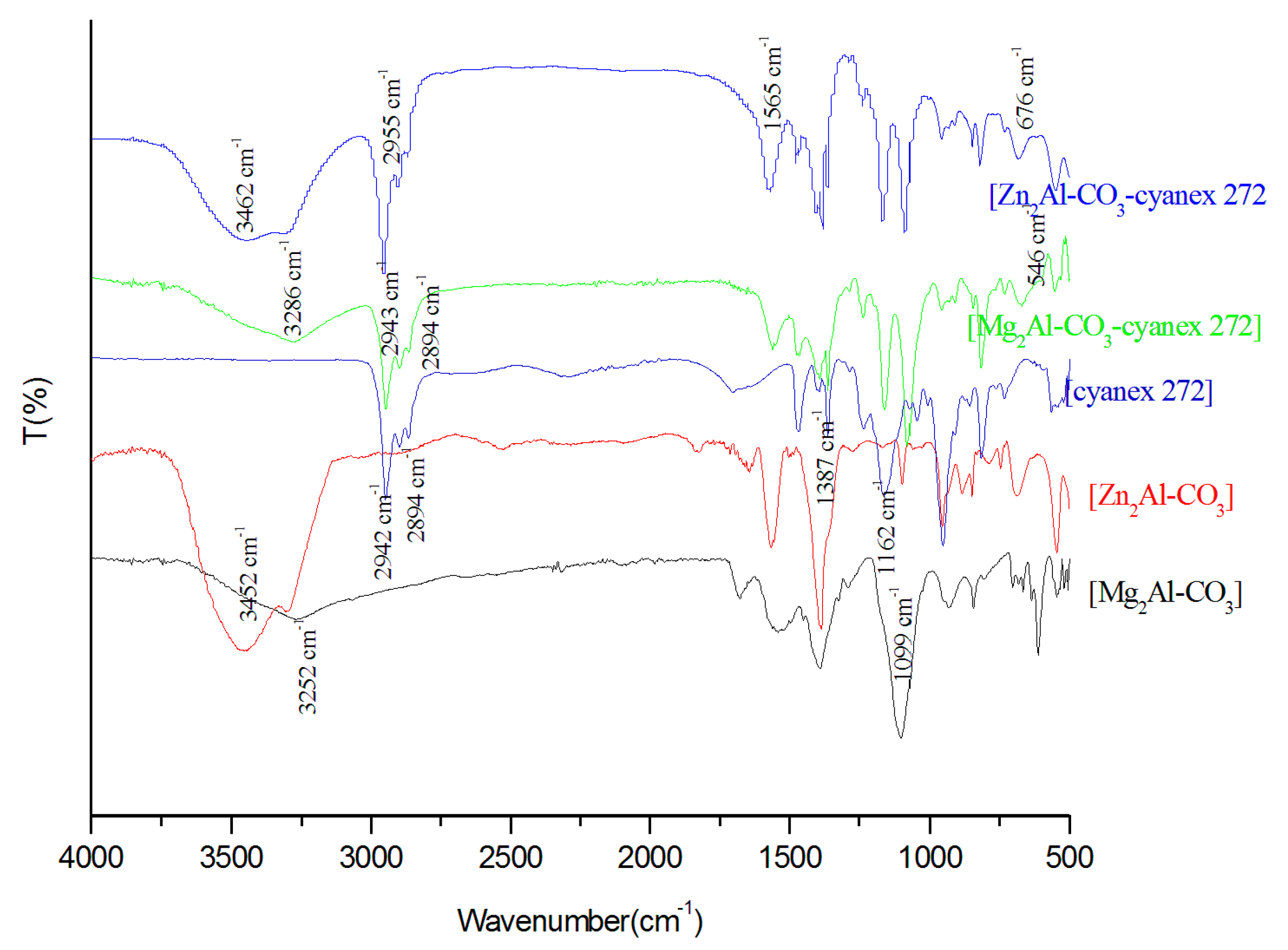
3.1.2. Infrared Spectroscopy (FTIR)
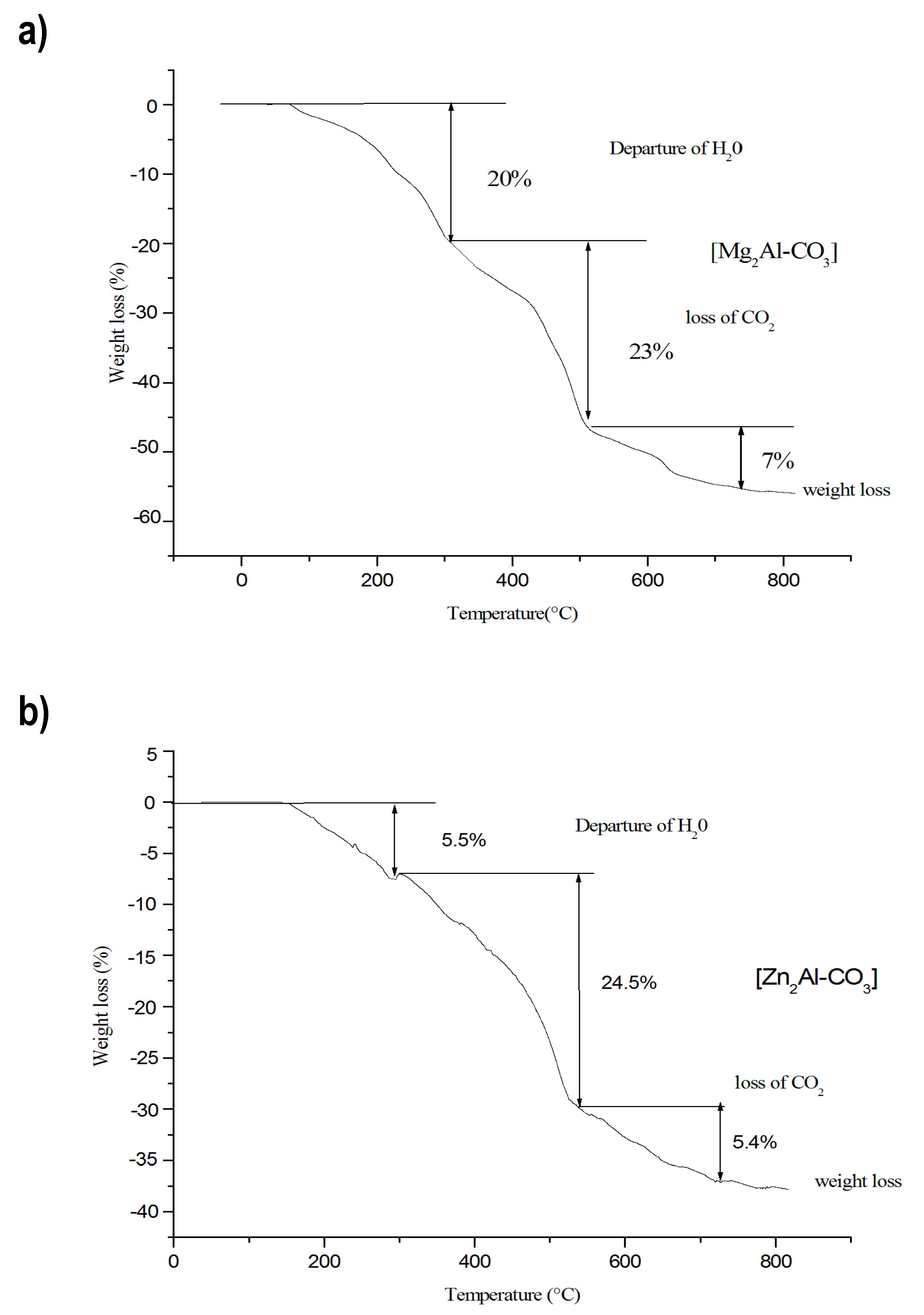
3.1.3. Thermal–Gravimetric Analyses (TGA)
3.2. Sorption Studies
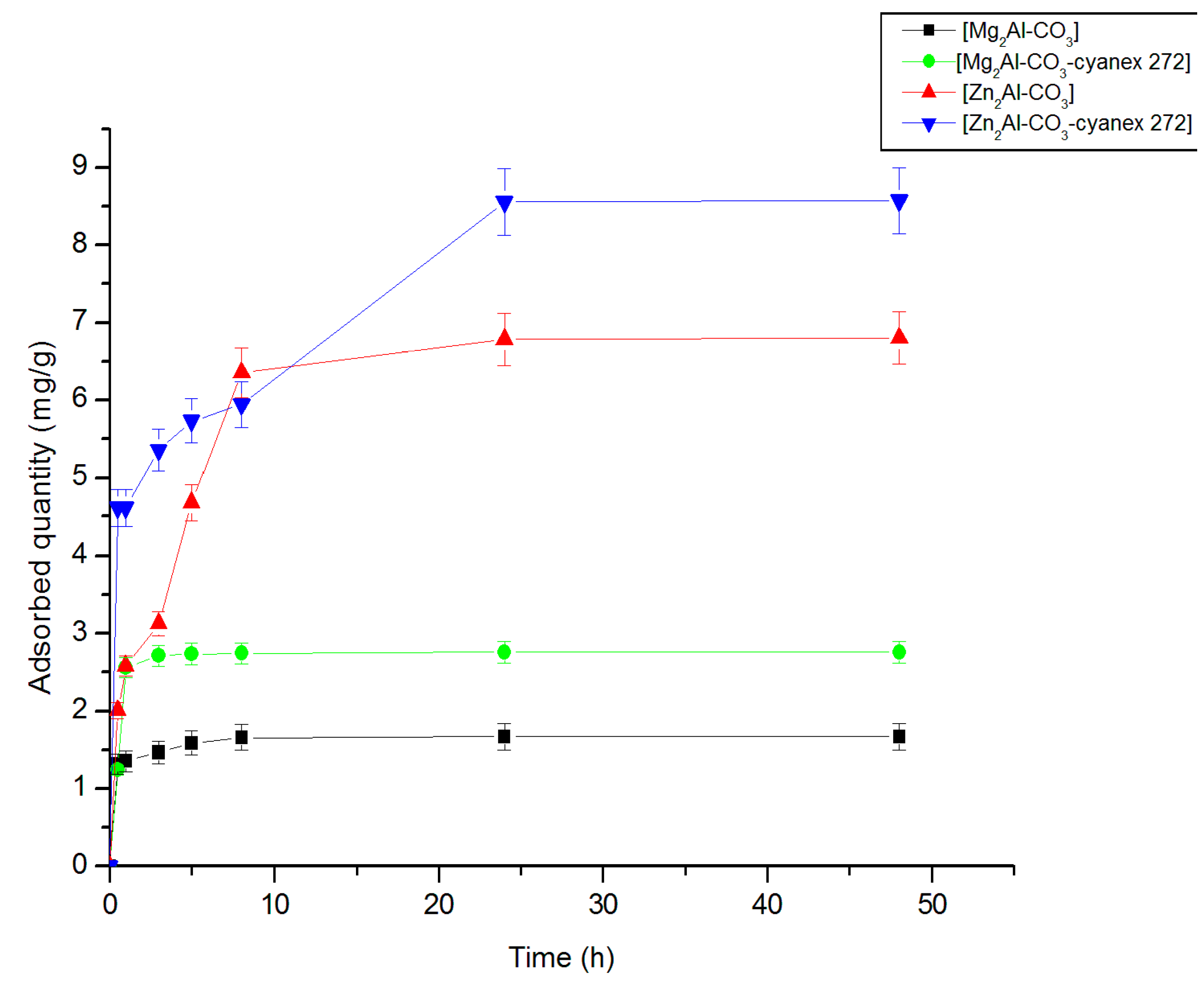
3.2.1. Effect of contact time
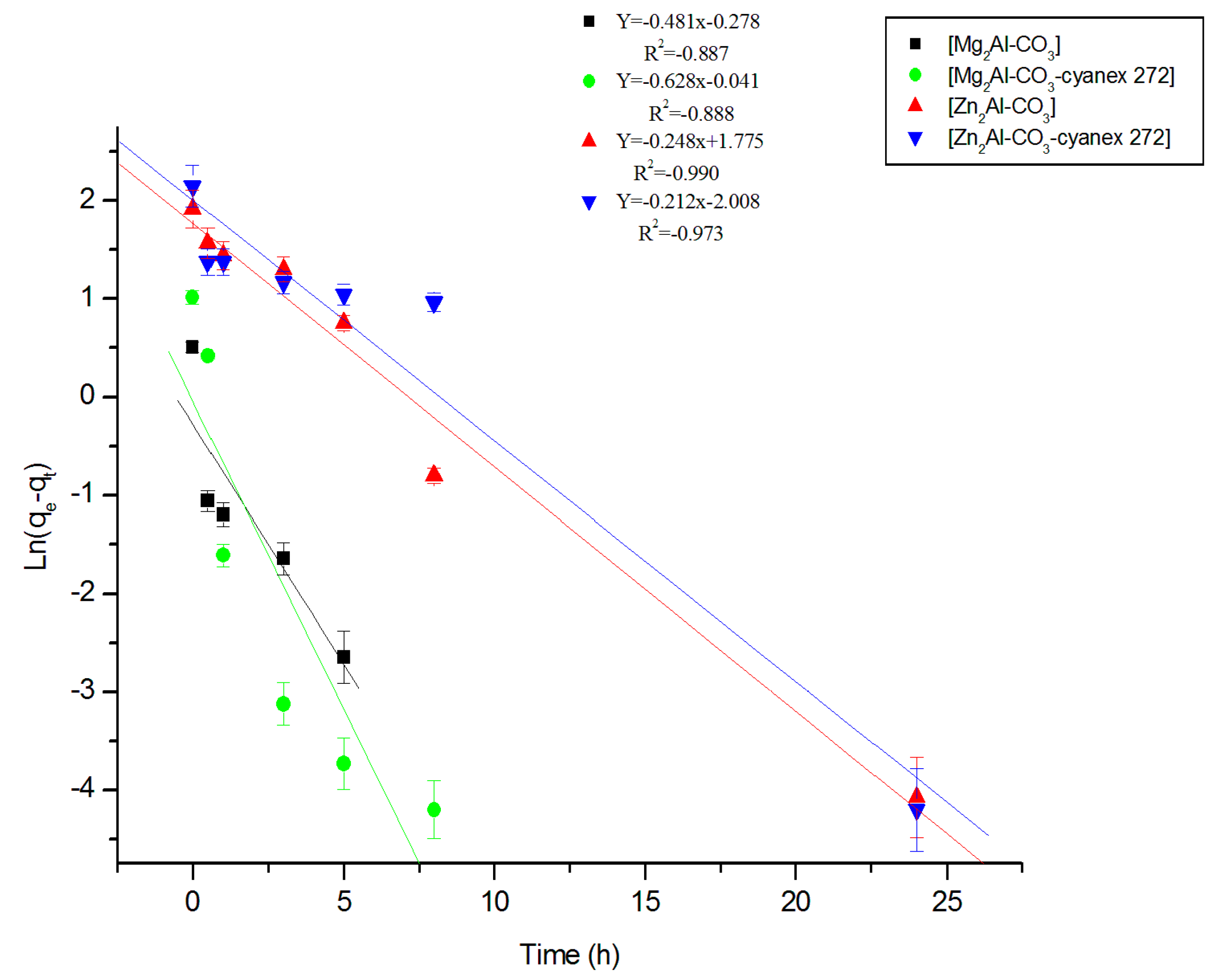
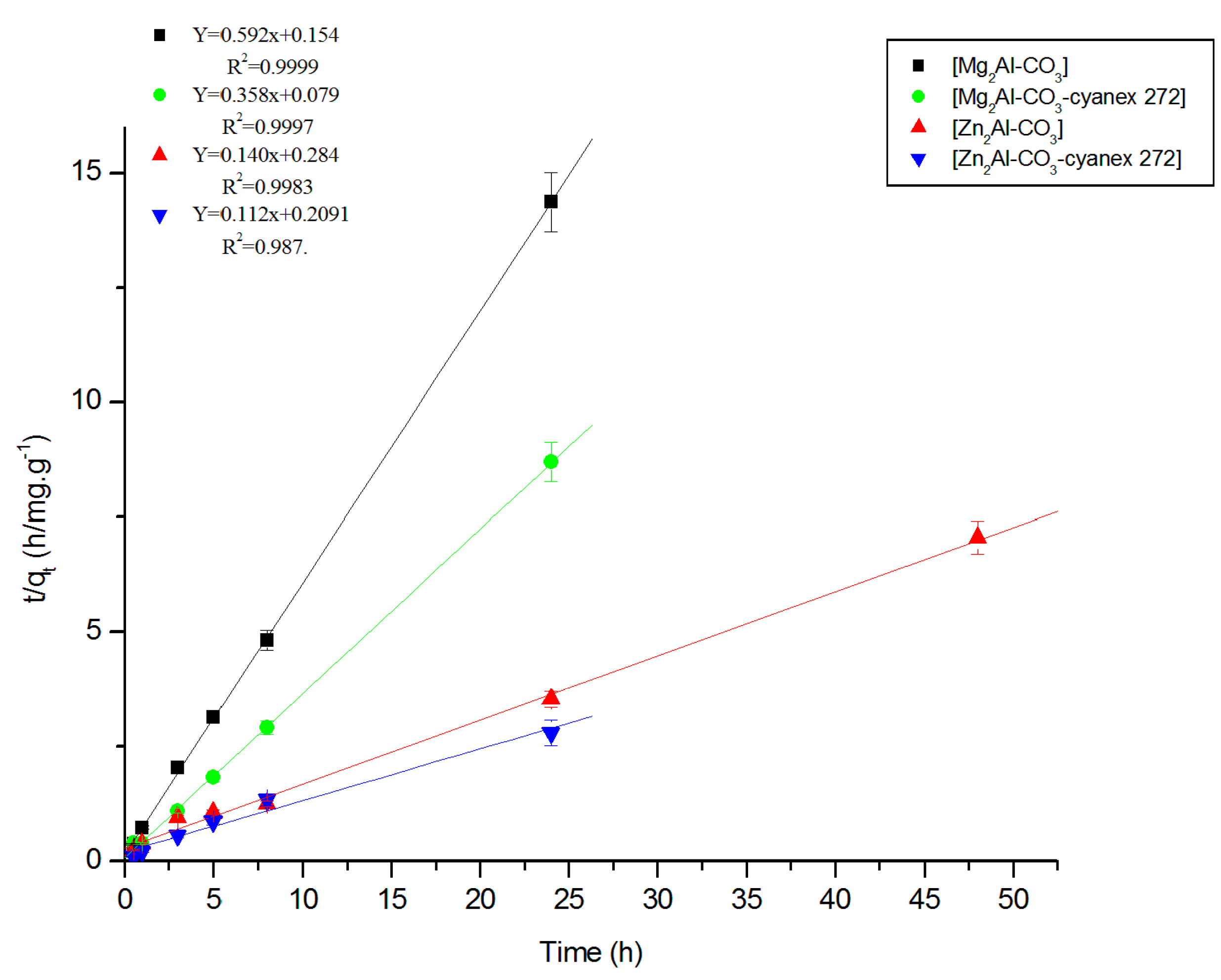
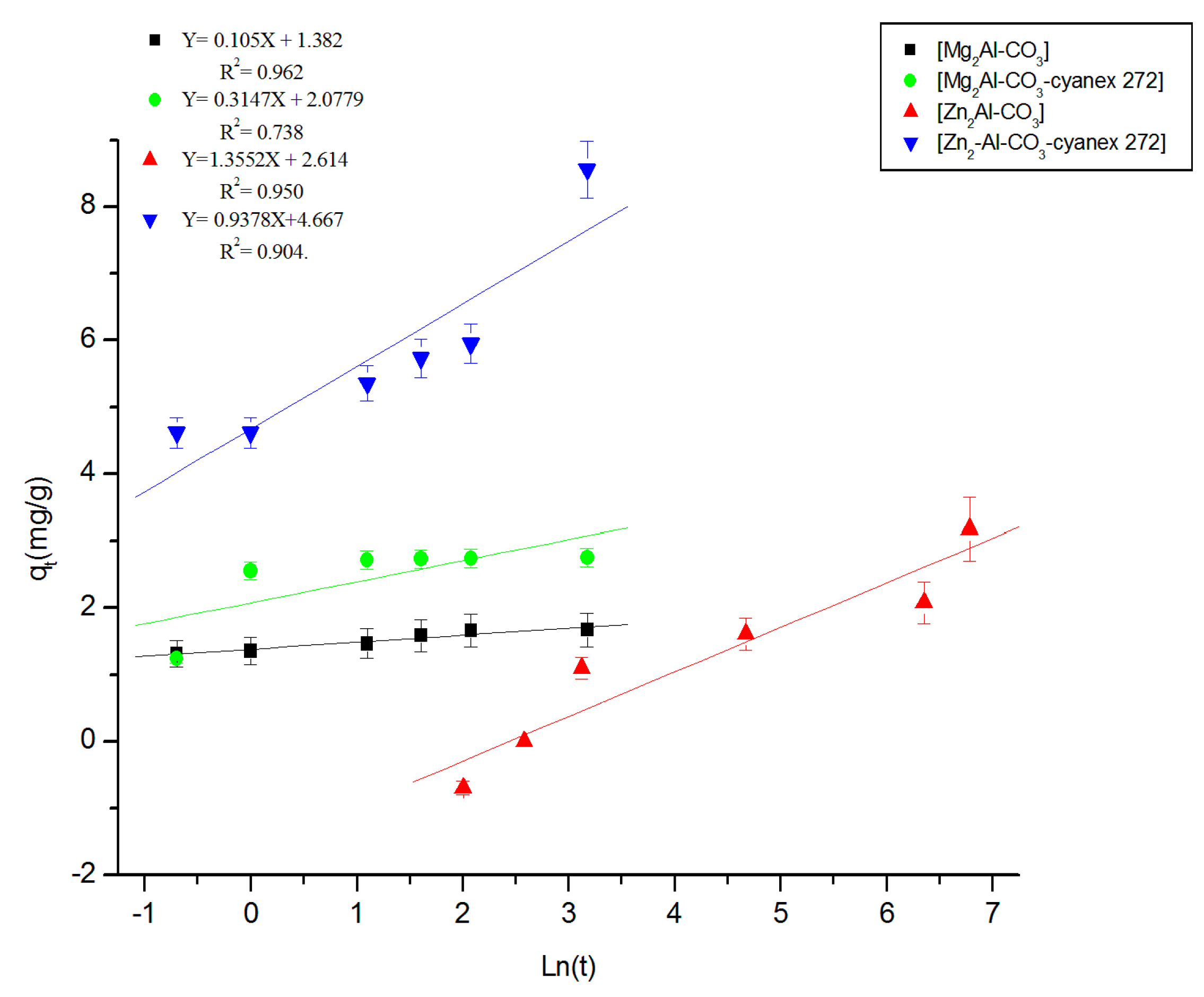
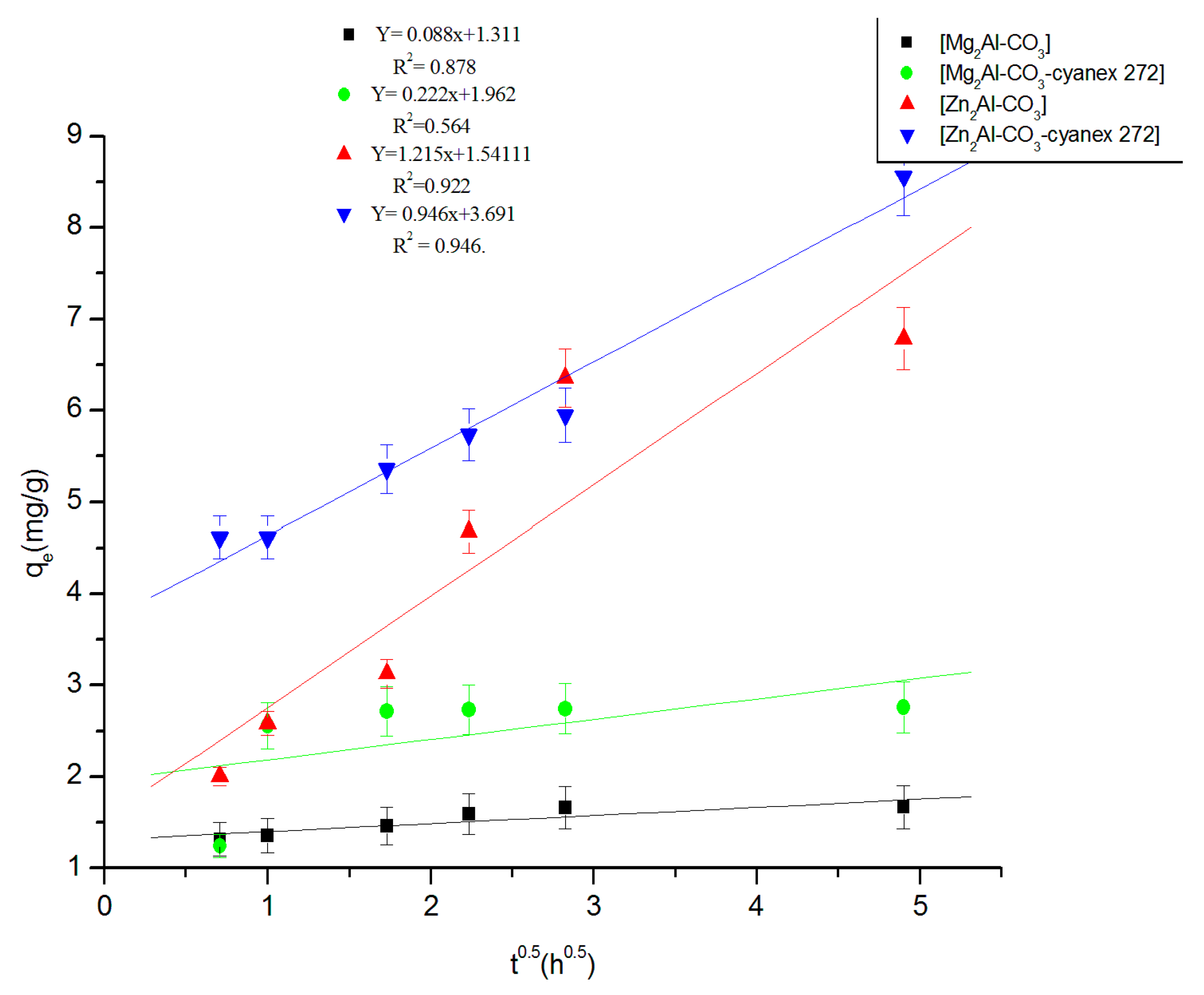
3.2.2. Fitting of the Kinetic Data
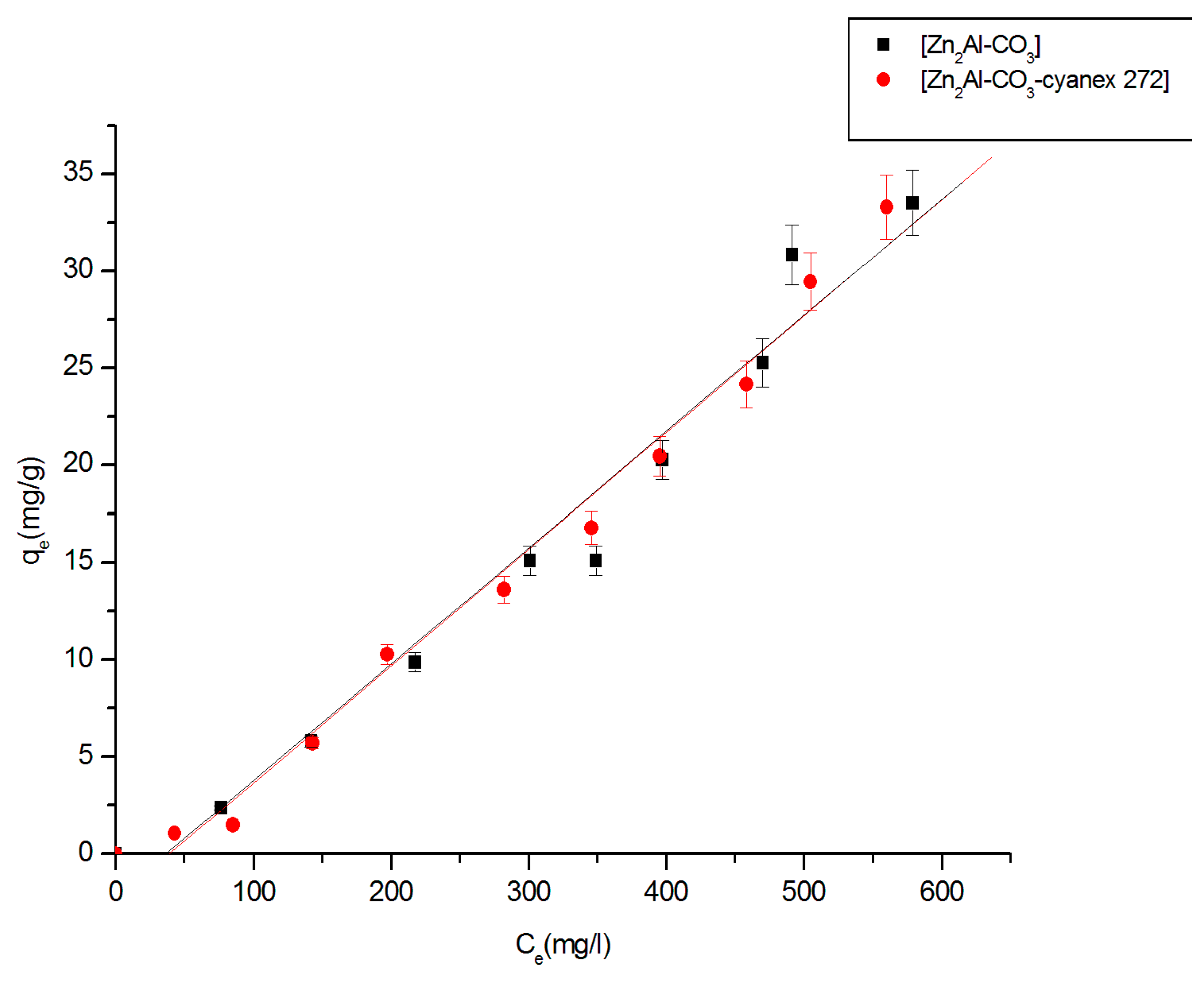
3.2.3. Equilibrium Studies
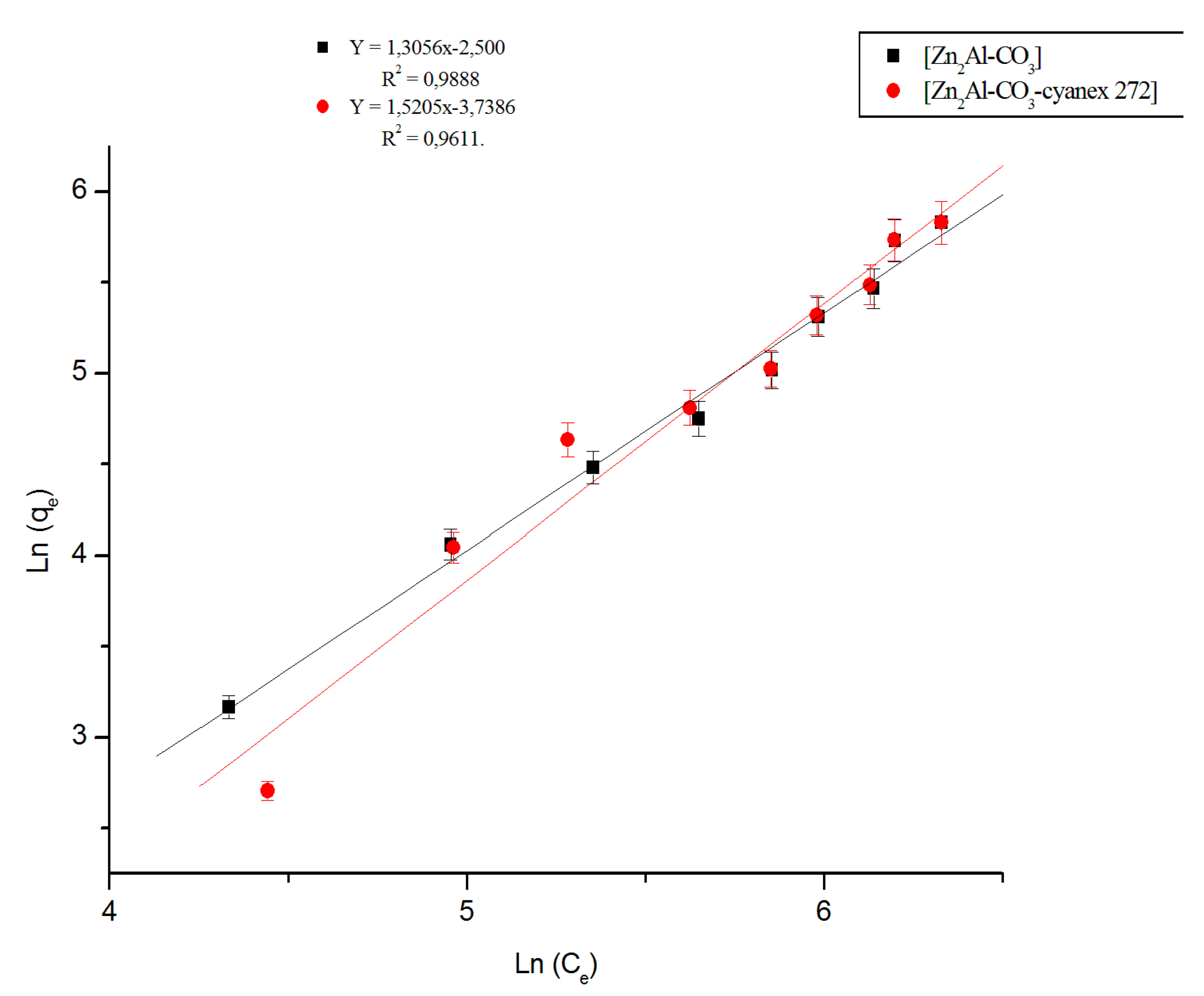
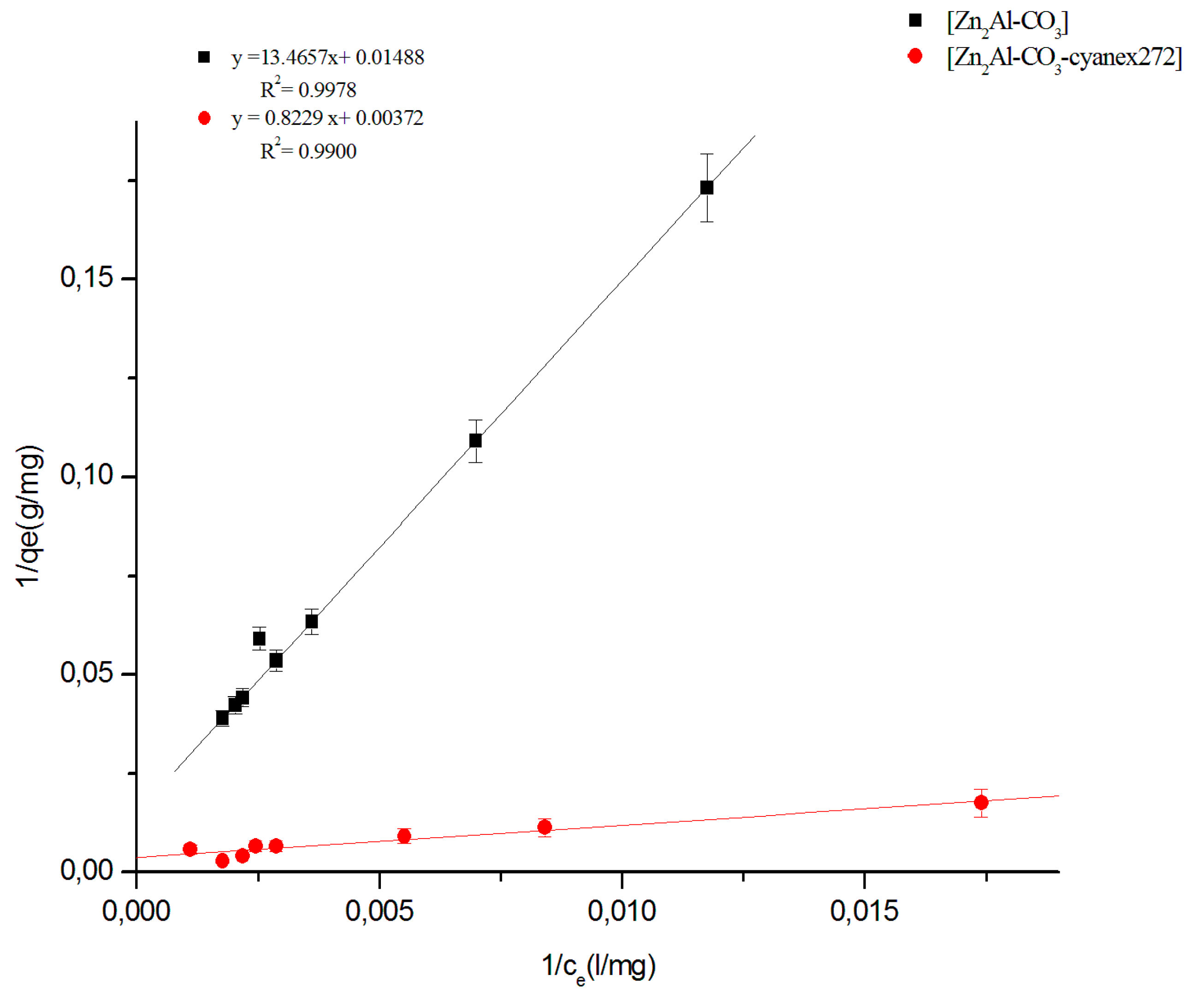
3.2.4. Fitting of the isotherm data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bradl, H. Sources and origins of heavy metals. Interface Sci. Technol. 2005, 6, 1–27. [Google Scholar]
- Liang, X.; Zang, Y.; Xu, Y.; Tan, X.; Hou, W.; Wang, L.; Sun, Y. Sorption of metal cations on layered double hydroxides. Colloids Surf A: Phys.Chem. Eng. Ass 2013, 433, 122–131. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Env. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Attar, K.; Demey, H.; Bouazza, D.; Sastre, A.M. Sorption and desorption studies of Pb (II) and Ni (II) from aqueous solutions by a new composite based on alginate and magadiite materials. Polym. J. 2019, 11, 340. [Google Scholar] [CrossRef] [PubMed]
- Lapo, B.; Demey, H.; Zapata, J.; Romero, C.; Sastre, A. Sorption of Hg (II) and Pb (II) ions on chitosan-iron (III) from aqueous solutions: Single and binary systems. Polym. J. 2018, 10, 367. [Google Scholar] [CrossRef]
- Demey, H.; Barron-Zambrano, J.; Mhadhbi, T.; Miloudi, H.; Yang, Z.; Ruiz, M.; Sastre, A.M. Boron removal from aqueous solutions by using a novel alginate-based sorbent: Comparison with Al2O3 particles. Polym. J. 2019, 11, 1509. [Google Scholar] [CrossRef]
- Cavani, F.; Trifiro, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Carpani, I.; Berrettoni, M.; Ballarin, B.; Giorgetti, M.; Scavetta, E.; Tonelli, D. Study on the intercalation of hexacyanoferrate (II) in a Ni, Al based hydrotalcite. Solid State Ion. 2004, 168, 167–175. [Google Scholar] [CrossRef]
- Terry, P.A. Characterization of Cr ion exchange with hydrotalcite. Chemosphere 2004, 57, 541–546. [Google Scholar] [CrossRef]
- Vaccari, A. Clays and catalysis: A promising future. Appl. Clay Sci. 1999, 14, 161–198. [Google Scholar] [CrossRef]
- Malherbe, F.; Depege, C.; Forano, C.; Besse, J.; Atkins, M.; Sharma, B.; Wade, S. Alkoxylation reaction catalysed by layered double hydroxides. Appl. Clay Sci. 1998, 13, 451–466. [Google Scholar] [CrossRef]
- He, L.; Huang, Y.; Wang, A.; Wang, X.; Chen, X.; Delgado, J.J.; Zhang, T. A noble-metal-free catalyst derived from Ni-Al hydrotalcite for hydrogen generation from N2H4 ⋅ H2O decomposition. Angew. Chem. Int. Ed. 2012, 51, 6191–6194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cui, X.; Shi, F.; Deng, Y. Nano-gold catalysis in fine chemical synthesis. Chem. Rev. 2011, 112, 2467–2505. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-J.; Choy, J.-H. Layered double hydroxide nanoparticles as target-specific delivery carriers: Uptake mechanism and toxicity. Nanomediae 2011, 6, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Barick, K.; Bahadur, D. Oxide and hybrid nanostructures for therapeutic applications. Adv. Drug Deliv.Rev. 2011, 63, 1267–1281. [Google Scholar] [CrossRef] [PubMed]
- Tichit, D.; Fajula, F. Layered double hydroxides as solid base catalysts and catalyst precursors. Surf. Sci. Catal. 1999, 125, 329–340. [Google Scholar]
- Bouraada, M.; Lafjah, M.; Ouali, M.S.; De Menorval, L.C. Basic dye removal from aqueous solutions by dodecylsulfate-and dodecyl benzene sulfonate-intercalated hydrotalcite. J. Hazards Mater. 2008, 153, 911–918. [Google Scholar] [CrossRef]
- Ferreira, O.P.; Alves, O.L.; Gouveia, D.X.; Souza Filho, A.G.; de Paiva, J.A.; Mendes Filho, J. Thermal decomposition and structural reconstruction effect on Mg–Fe-based hydrotalcite compounds. J. Solid State Chem. 2004, 177, 3058–3069. [Google Scholar] [CrossRef]
- Borda, M.; Sparks, D. Kinetics and mechanisms of sorption-desorption in soils: A multiscale assessment. Chem Process Heavy. Met. Met. Soil Environ. 2008, 97–124. [Google Scholar]
- Scheidegger, A.M.; Lamble, G.M.; Sparks, D.L. Spectroscopic evidence for the formation of mixed-cation hydroxide phases upon metal sorption on clays and aluminum oxides. J.Colloid Interface Sci. 1997, 186, 118–128. [Google Scholar] [CrossRef]
- Khaokaew, S.; Landrot, G.; Chaney, R.L.; Pandya, K.; Sparks, D.L. Speciation and release kinetics of zinc in contaminated paddy soils. Env. Sci. Technol. 2012, 46, 3957–3963. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.; Pavlovic, I.; Barriga, C.; Cornejo, J.; Hermosín, M.; Ulibarri, M. Uptake of Cu2+, Cd2+ and Pb2+ on Zn–Al layered double hydroxide intercalated with edta. Appl. Clay Sci. 2006, 32, 245–251. [Google Scholar] [CrossRef]
- Tsyganok, A.I.; Suzuki, K.; Hamakawa, S.; Takehira, K.; Hayakawa, T. Mg–Al layered double hydroxide intercalated with [Ni (edta)] 2− chelate as a precursor for an efficient catalyst of methane reforming with carbon dioxide. T. Catal. Lett. 2001, 77, 75–86. [Google Scholar] [CrossRef]
- Kaneyoshi, M.; Jones, W. Formation of Mg-Al layered double hydroxides intercalated with nitrilotriacetate anions. J. Mater. Chem. 1999, 9, 805–811. [Google Scholar] [CrossRef]
- Gutmann, N.H.; Spiccia, L.; Turney, T.W. Complexation of Cu (II) and Ni (II) by nitrilotriacetate intercalated in Zn–Cr layered double hydroxides. J. Mater. Chem. 2000, 10, 1219–1224. [Google Scholar] [CrossRef]
- Tarasov, K.A.; O’Hare, D.; Isupov, V.P. Solid-state chelation of metal ions by ethylenediaminetetraacetate intercalated in a layered double hydroxide. Inorg. Chem. 2003, 42, 1919–1927. [Google Scholar] [CrossRef]
- Li, C.; Wang, G.; Evans, D.G.; Duan, X. Incorporation of rare-earth ions in Mg–Al layered double hydroxides: Intercalation with an [Eu (EDTA)]− chelate. J. Solid State Chem. 2004, 177, 4569–4575. [Google Scholar] [CrossRef]
- Heidarpour, A.; Aliasgharzad, N.; Khoshmanzar, E.; Lajayer, B.A. Bio-removal of Zn from contaminated water by using green algae isolates. Env. Technol. Innova. 2019, 16, 100464. [Google Scholar] [CrossRef]
- Franus, M.; Bandura, L.; Madej, J. Mono and Poly-Cationic Adsorption of Heavy Metals Using Natural Glauconite. J. Min. 2019, 9, 470. [Google Scholar] [CrossRef]
- Bouazza, D.; Miloudi, H.; Adjdir, M.; Tayeb, A.; Boos, A. Competitive adsorption of Cu (II) and Zn (II) on impregnate raw Algerian bentonite and efficiency of extraction. Appl Clay Sci. 2018, 151, 118–123. [Google Scholar] [CrossRef]
- Cortina, J. Developments in solid-liquid extraction by solvent-impregnated resins. J. Ion Exch. Solvent Extrac 1997, 15, 1067. [Google Scholar] [CrossRef]
- Bouazza, D.; Miloudi, H.; Sassi, M.; Boos, A.; Goetz, G.; Tayeb, A.; Bengueddach, A. Preparation of montmorillonite clays containing DTMPPA for Zinc extraction. J. Phys. Chem Solids 2006, 67, 1032–1036. [Google Scholar] [CrossRef]
- Carriazo, D.; Del Arco, M.; Martín, C.; Rives, V. A comparative study between chloride and calcined carbonate hydrotalcites as adsorbents for Cr (VI). Appl. Clay Sci. 2007, 37, 231–239. [Google Scholar] [CrossRef]
- Triantafyllidis, K.S.; Peleka, E.N.; Komvokis, V.G.; Mavros, P.P. Iron-modified hydrotalcite-like materials as highly efficient phosphate sorbents. J. Colloid Interface Sci. 2010, 342, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Solin, S.; Hines, D.; Seidler, G.; Treacy, M. Novel structural properties of Ni1− x Alx layer double hydroxides. J. Phys. Chem. Solids 1996, 57, 1043–1048. [Google Scholar] [CrossRef]
- Inacio, J.; Taviot-Gueho, C.; Forano, C.; Besse, J. Adsorption of MCPA pesticide by MgAl-layered double hydroxides. J. Appl Clay Sci. 2001, 18, 255–264. [Google Scholar] [CrossRef]
- Pope, M.T. Heteropoly and Isopoly Oxometalates; Springer: Berlin/Heidelberg, Germany, 1983. [Google Scholar]
- Bottero, J.; Axelos, M.; Tchoubar, D.; Cases, J.; Fripiat, J.; Fiessinger, F. Mechanism of formation of aluminum trihydroxide from Keggin Al13 polymers. J. Colloid Interface Sci. 1987, 117, 47–57. [Google Scholar] [CrossRef]
- Benito, P.; Guinea, I.; Labajos, F.; Rives, V. Microwave-assisted reconstruction of Ni, Al hydrotalcite-like compounds. J. Solid State Chem. 2008, 181, 987–996. [Google Scholar] [CrossRef]
- Venugopal, B.; Shivakumara, C.; Rajamathi, M. A composite of layered double hydroxides obtained through random costacking of layers from Mg–Al and Co–Al LDHs by delamination–restacking: Thermal decomposition and reconstruction behavior. Solid State Sci. 2007, 9, 287–294. [Google Scholar] [CrossRef]
- Xu, Z.; Zeng, H. Ionic interactions in crystallite growth of CoMgAl-hydrotalcite-like compounds. Chem. Mater. 2001, 13, 4555–4563. [Google Scholar] [CrossRef]
- Kagunya, W.; Chibwe, M.; Jones, W. Synthesis and structural characterisation of LDH-organic intercalates. Mol. Cryst. Liq Cryst. Sci. Technol. 1994, 244, 155–160. [Google Scholar] [CrossRef]
- Hibino, T.; Kosuge, K.; Tsunashima, A. Synthesis of carbon-hydrotalcite complex and its thermal degradation behavior. Clays Clay Min. 1996, 44, 151–154. [Google Scholar] [CrossRef]
- Bellotto, M.; Rebours, B.; Clause, O.; Lynch, J.; Bazin, D.; Elkaïm, E. Hydrotalcite decomposition mechanism: A clue to the structure and reactivity of spinel-like mixed oxides. J. Phys. Chem. 1996, 100, 8535–8542. [Google Scholar] [CrossRef]
- Inglezakis, V.; Fyrillas, M.; Park, J. Variable diffusivity homogeneous surface diffusion model and analysis of merits and fallacies of simplified adsorption kinetics equations. J. Hazard. Mater. 2019, 367, 224–245. [Google Scholar] [CrossRef] [PubMed]
- Lagergren, S.K. About the theory of so-called adsorption of soluble substances. Sven. Vetensk. Handingarl 1898, 24, 1–39. [Google Scholar]
- Ho, Y.; McKay, G. Kinetic model for lead (II) sorption on to peat. Adsorpt. Sci. Technol. 1998, 16, 243–255. [Google Scholar] [CrossRef]
- Ho, Y.-S.; McKay, G. Pseudo-second order model for sorption processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Ho, Y.-S.; McKay, G. The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res. 2000, 34, 735–742. [Google Scholar] [CrossRef]
- Ho, Y.-S.; McKay, G. Application of kinetic models to the sorption of copper (II) on to peat. Adsorpt Sci. Technolo. 2002, 20, 797–815. [Google Scholar] [CrossRef]
- Ho, Y.-S. Second-order kinetic model for the sorption of cadmium onto tree fern: A comparison of linear and non-linear methods. Water Res. 2006, 40, 119–125. [Google Scholar] [CrossRef]
- Azizian, S. Kinetic models of sorption: A theoretical analysis. J. Colloid Interface Sci. 2004, 276, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.; Clayton, W. Application of Elovich equation to the kinetics of phosphate release and sorption in soils. Soil Sci. Jacs 1980, 44, 265–268. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit Eng. Div. 1963, 89, 31–60. [Google Scholar]
- Mall, I.D.; Srivastava, V.C.; Agarwal, N.K. Removal of Orange-G and Methyl Violet dyes by adsorption onto bagasse fly ash—kinetic study and equilibrium isotherm analyses. Dye. Pigm. 2006, 69, 210–223. [Google Scholar] [CrossRef]
- Mana, M.; Ouali, M.-S.; De Menorval, L.-C. Removal of basic dyes from aqueous solutions with a treated spent bleaching earth. J. Colloid Interface Sci. 2007, 307, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, N.B.-H.; Bentouami, A.; Derriche, Z.; Bettahar, N.; De Menorval, L.-C. Synthesis and characterization of Mg–Fe layer double hydroxides and its application on adsorption of Orange G from aqueous solution. J. Chem. Eng. 2011, 169, 231–238. [Google Scholar] [CrossRef]
- Rutherford, D.W.; Chiou, C.T. Effect of water saturation in soil organic matter on the partition of organic compounds. Env. Sci Technol. 1992, 26, 965–970. [Google Scholar] [CrossRef]
- Sheng, G.; Xu, S.; Boyd, S.A. Mechanism (s) controlling sorption of neutral organic contaminants by surfactant-derived and natural organic matter. Env. Sci. Technol. 1996, 30, 1553–1557. [Google Scholar] [CrossRef]
- Zhao, H.; Nagy, K.L. Dodecyl sulfate–hydrotalcite nanocomposites for trapping chlorinated organic pollutants in water. J. Colloid Interface Sci. 2004, 274, 613–624. [Google Scholar] [CrossRef]
- Alley, E.R. Water Quality Control Handbook; McGraw-Hill: New York, NY, USA, 2007; p. 2. [Google Scholar]
- Freundlich, H. Über die adsorption in lösungen. Z. Für Phys. Chem 1907, 57, 385–470. [Google Scholar] [CrossRef]
Sample Availability: Samples of the sorbents: Zn2Al-CO3 and Zn2Al-CO3-cyanex272 are available from the authors. |
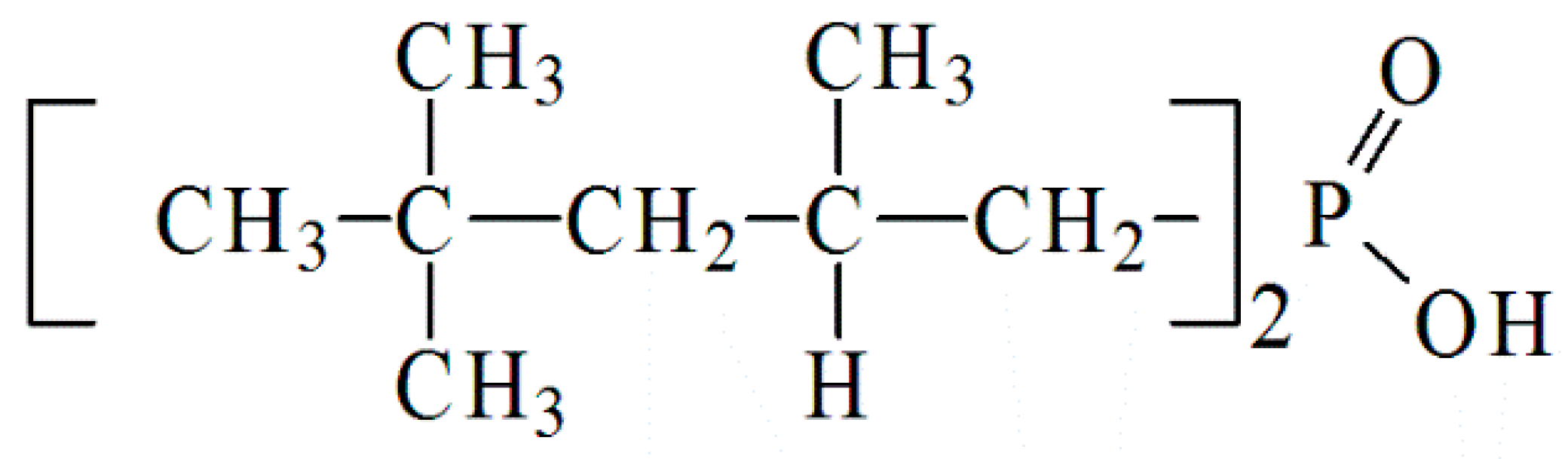
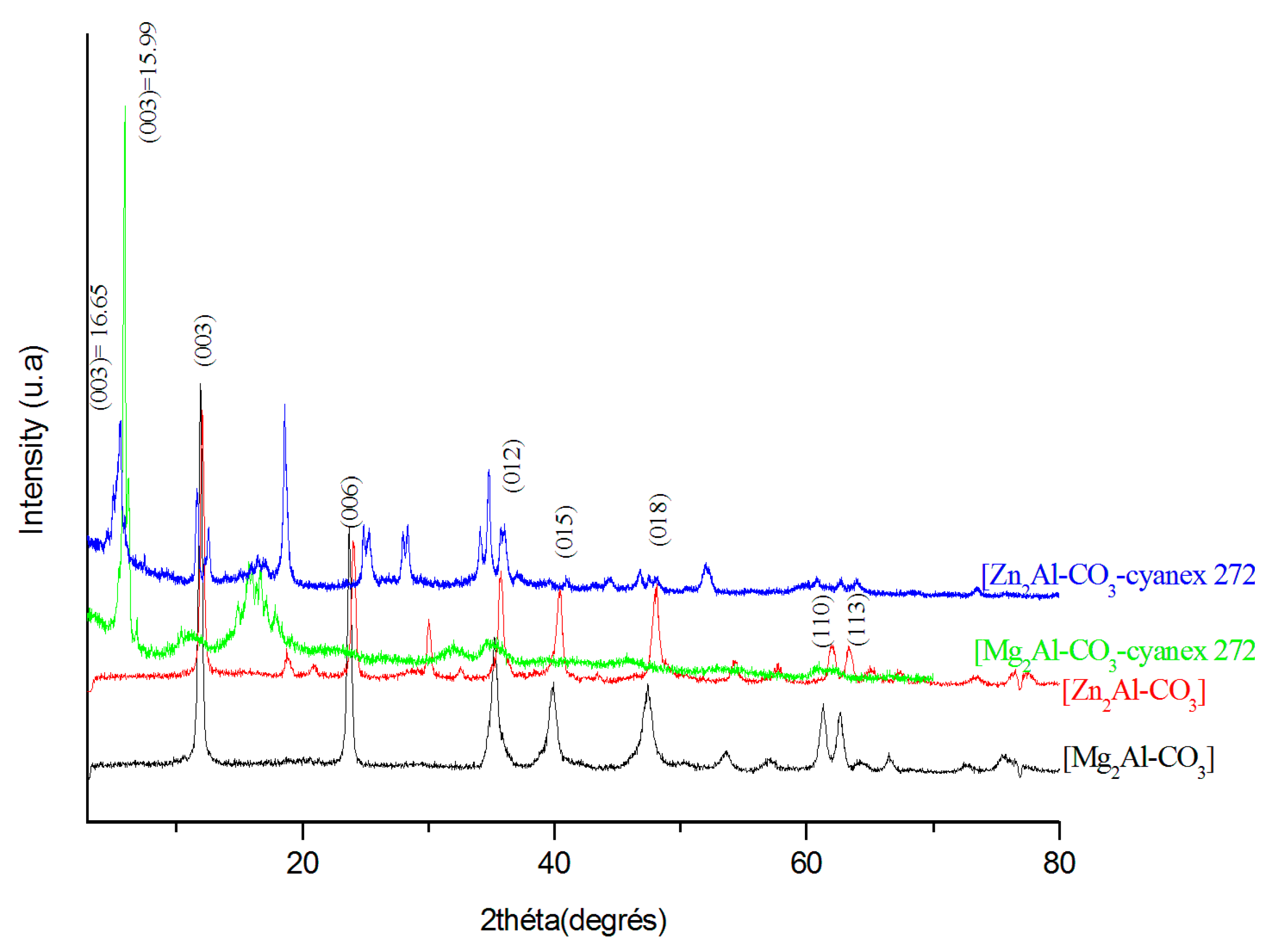
| Material | a (Å) | c (Å) | d003 (Å) |
|---|---|---|---|
| Mg2Al-CO3 | 3.030 | 23.106 | 7.702 |
| Zn2Al-CO3 | 3.043 | 23.517 | 7.839 |
| Mg2Al-CO3-cyanex 272 | 3.004 | 48.000 | 15.99 |
| Zn2Al-CO3-cyanex 272 | 3.055 | 49.950 | 16.65 |
| Experimental | Pseudo-First Order | Pseudo-Second Order | Elovich | Intraparticular Diffusion | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Materials | qexp mg/g | k1 h−1 | qtheo1 mg/g | R2 | k2g/mg.h | qtheo2 mg/g | R2 | Β g/mg | Α mg/g.h | R2 | C mg/g | Kid mg/g.h1/2 | R2 |
| Mg2Al-CO3 | 1.6 | 0.48 | 0.76 | 0.787 | 2.28 | 1.69 | 0.999 | 0.11 | 4.83 × 106 | 0.962 | 1.31 | 0.09 | 0.878 |
| Mg2Al-CO3-cyanex 272 | 2.8 | 0.63 | 0.96 | 0.789 | 1.62 | 2.79 | 0.997 | 0.32 | 2343.68 | 0.737 | 1.96 | 0.22 | 0.564 |
| Zn2Al-CO3 | 6.2 | 0.25 | 5.90 | 0.970 | 0.07 | 7.14 | 0.998 | 1.36 | 5.07 | 0.951 | 1.54 | 1.22 | 0.922 |
| Zn2Al-CO3-cyanex 272 | 8.8 | 0.21 | 7.45 | 0.946 | 3 × 10−3 | 8.92 | 0.987 | 0.94 | 154.58 | 0.904 | 3.69 | 0.95 | 0.986 |
| Materials | Linear Regression | Langmuir Model | Freundlich Model | |||||
|---|---|---|---|---|---|---|---|---|
| Kd | R2 | qmax(mg/g) | Kl (L.g−1) | R2 | n | KF (mg1−1/n .g−1.L1/n) | R2 | |
| Zn2Al-CO3 | 0.277 | 0.998 | 67.17 | 0.0011 | 0.9978 | 1.3056 | 2.0848 | 0.9887 |
| Zn2Al-CO3- cyanex272 | 0.318 | 0.990 | 268.82 | 0.0045 | 0.9900 | 1.5205 | 3.3229 | 0.9611 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boudaoud, N.; Miloudi, H.; Bouazza, D.; Adjdir, M.; Tayeb, A.; Fortuny, A.; Demey, H.; Sastre, A.M. Removal of Zinc from Aqueous Solutions Using Lamellar Double Hydroxide Materials Impregnated with Cyanex 272: Characterization and Sorption Studies. Molecules 2020, 25, 1263. https://doi.org/10.3390/molecules25061263
Boudaoud N, Miloudi H, Bouazza D, Adjdir M, Tayeb A, Fortuny A, Demey H, Sastre AM. Removal of Zinc from Aqueous Solutions Using Lamellar Double Hydroxide Materials Impregnated with Cyanex 272: Characterization and Sorption Studies. Molecules. 2020; 25(6):1263. https://doi.org/10.3390/molecules25061263
Chicago/Turabian StyleBoudaoud, Nacera, Hafida Miloudi, Djamila Bouazza, Mehdi Adjdir, Abdelkader Tayeb, Agustin Fortuny, Hary Demey, and Ana Maria Sastre. 2020. "Removal of Zinc from Aqueous Solutions Using Lamellar Double Hydroxide Materials Impregnated with Cyanex 272: Characterization and Sorption Studies" Molecules 25, no. 6: 1263. https://doi.org/10.3390/molecules25061263
APA StyleBoudaoud, N., Miloudi, H., Bouazza, D., Adjdir, M., Tayeb, A., Fortuny, A., Demey, H., & Sastre, A. M. (2020). Removal of Zinc from Aqueous Solutions Using Lamellar Double Hydroxide Materials Impregnated with Cyanex 272: Characterization and Sorption Studies. Molecules, 25(6), 1263. https://doi.org/10.3390/molecules25061263