The Influence of COD Fraction Forms and Molecules Size on Hydrolysis Process Developed by Comparative OUR Studies in Activated Sludge Modelling
Abstract
1. Introduction
2. Results and Discussion
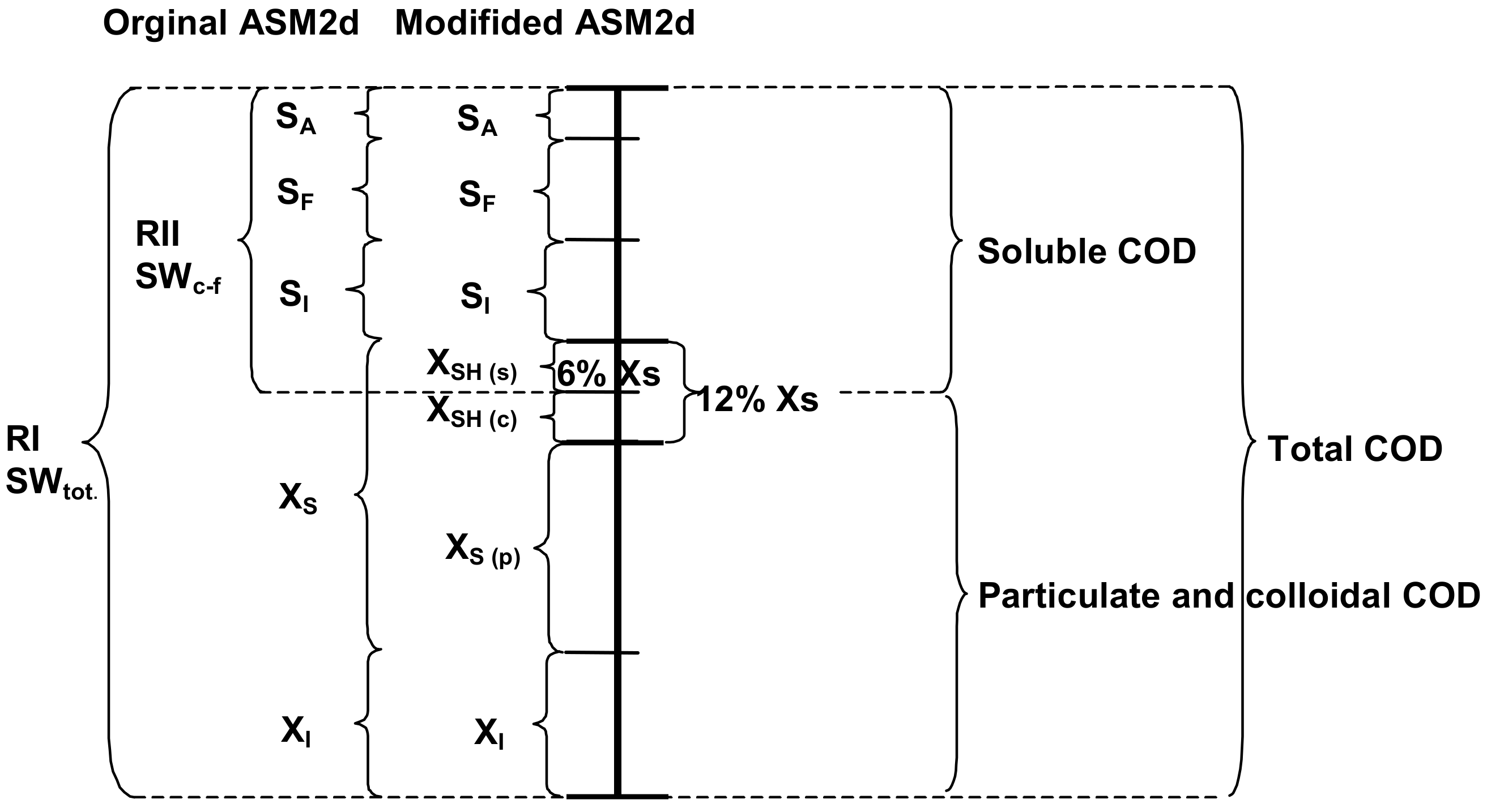
2.1. Wastewater and Biomass Characterization, Including New XSH Fraction
2.2. Influence of COD Fractions on Modelling OUR
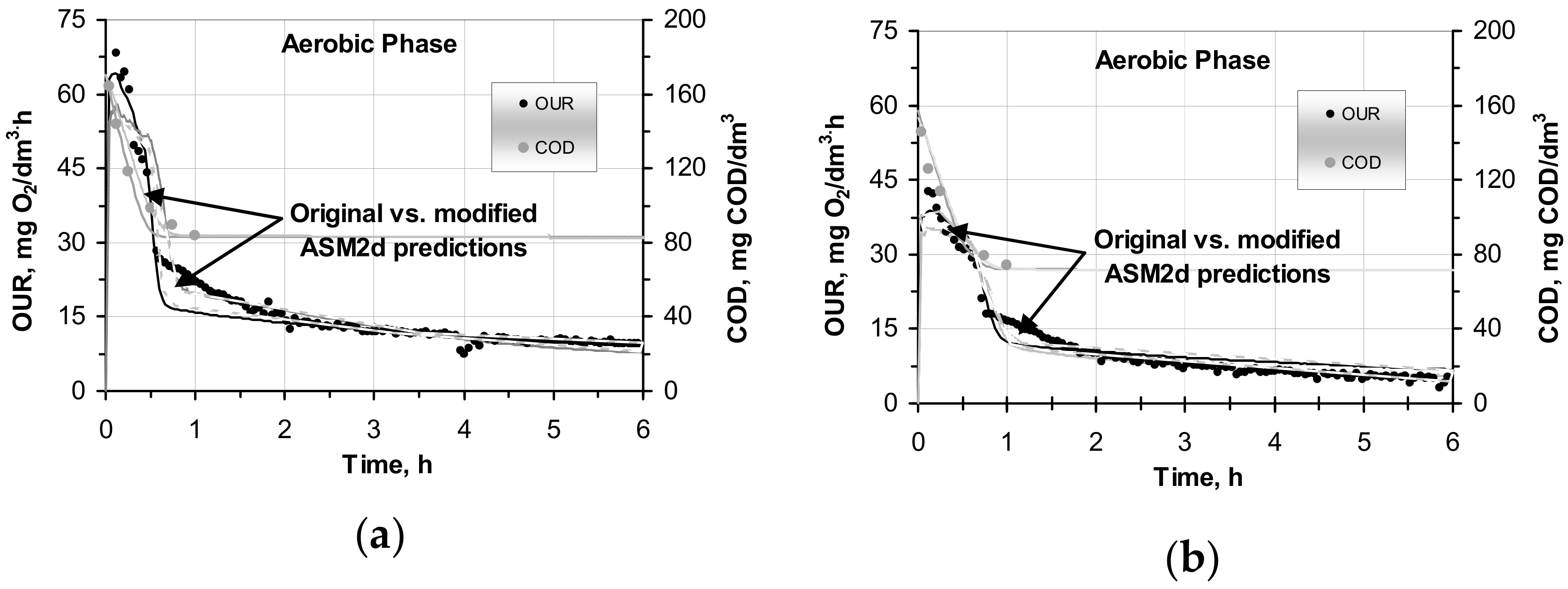
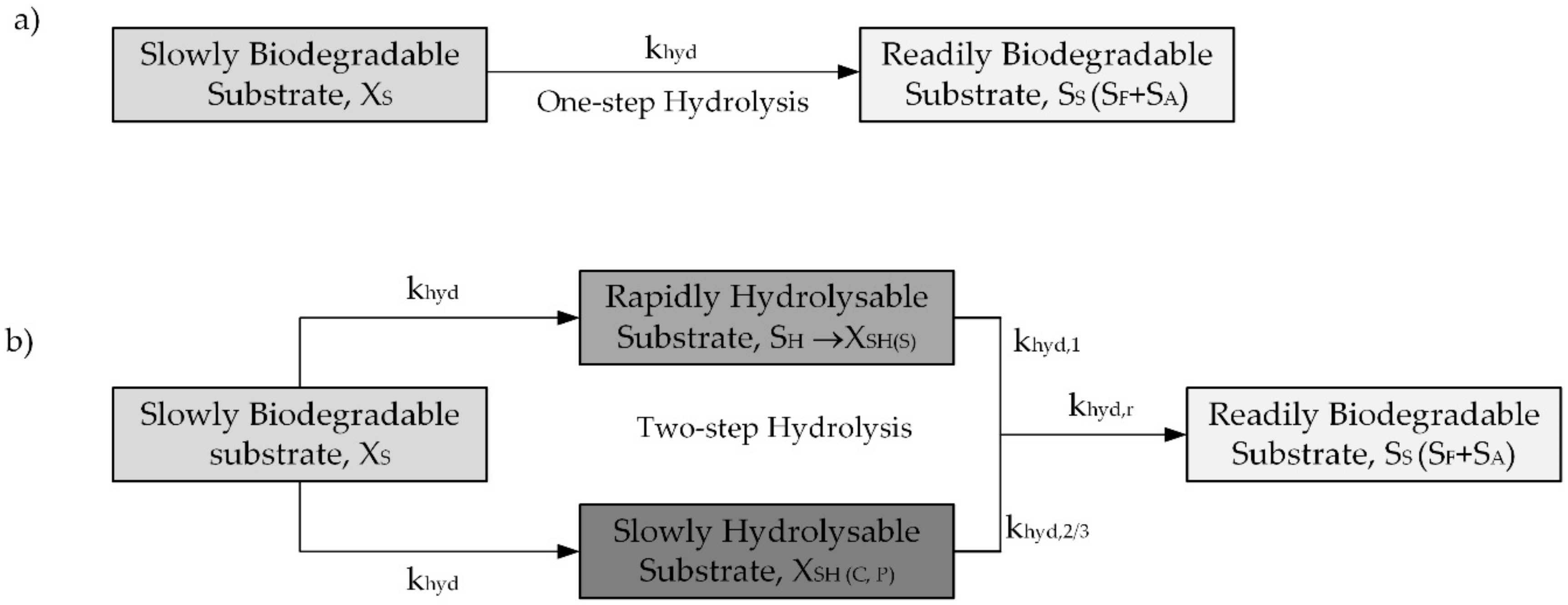
2.3. Modelling Hydrolysis Process under OUR Batch Tests in the Original and Modified ASM2d
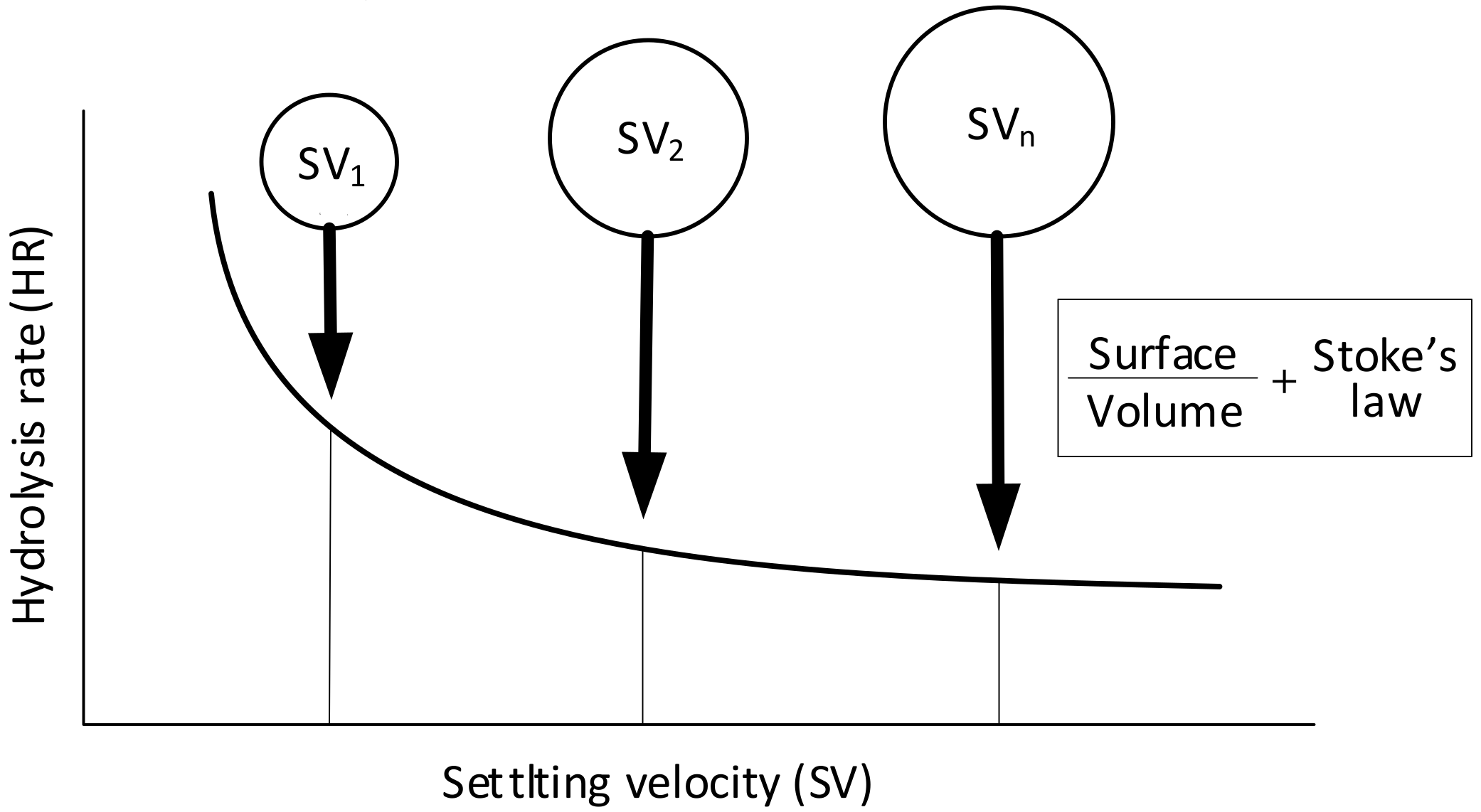
2.4. Modelling Hydrolysis Rate vs Settling Velocity of the COD Forms and Particle Size of Molecules
3. Materials and Methods
3.1. Wastewater and Biomass Characterization for Lab Tests and Modelling
3.2. Conventional OUR Batch Test Measurement
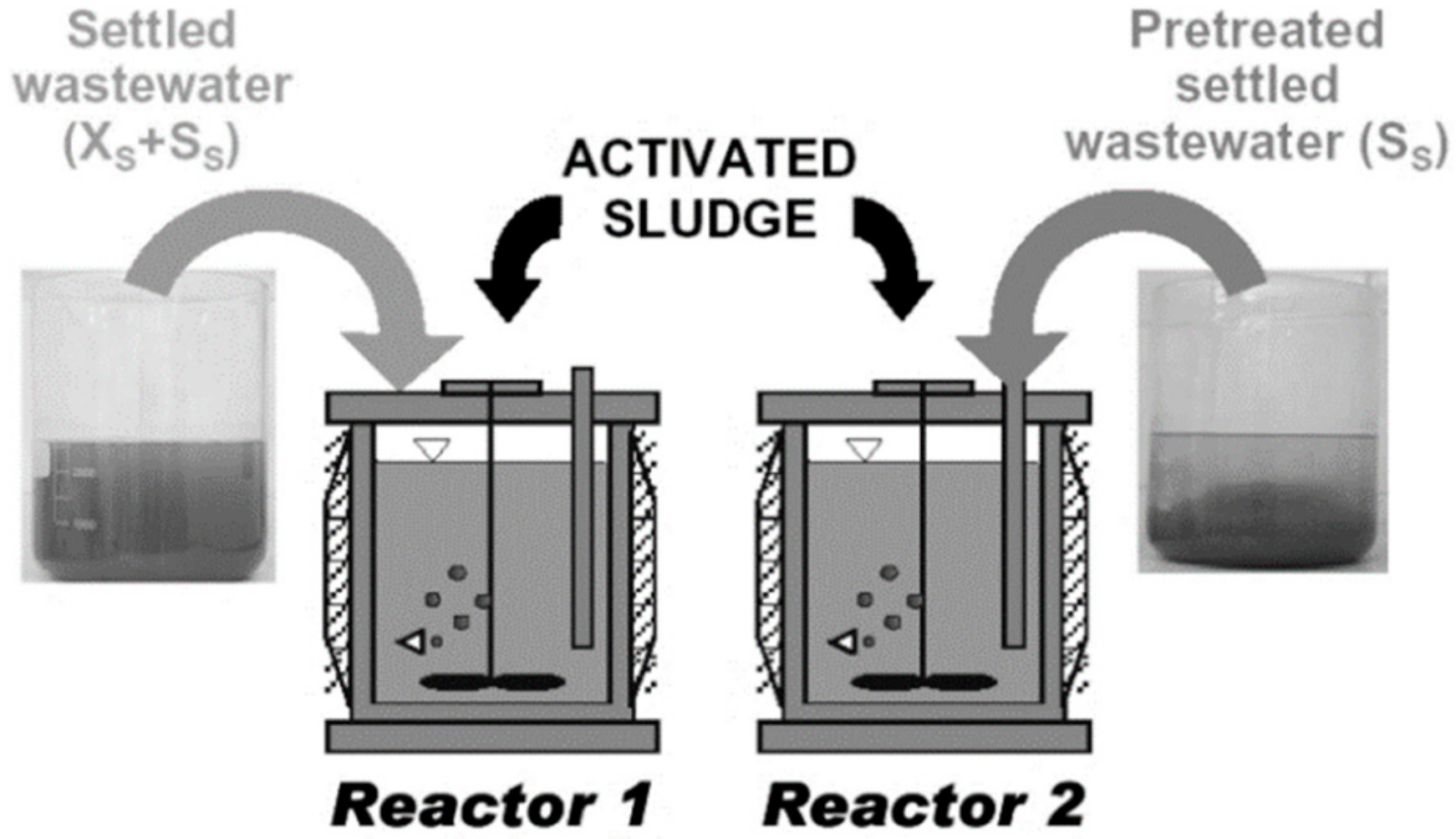
3.3. Organization of the Modelling Study
3.4. Analytical Methods
4. Conclusions
- OUR measurements, together with COD consumption, are a versatile tool for characterizing the biodegradability of organic matter and could be used for evaluations of conceptual models as well as the modelling and optimization of biochemical processes (e.g., denitrification and EBPR) in modern, cost-effective BNR-activated sludge systems.
- A new approach of particulate COD substrate modelling involving the use of OUR in activated sludge systems is necessary for adequate hydrolysis model prediction and could be recommended for monitoring the wastewater and activated sludge conditions.
- Hydrolysis constitutes a surface-limited reaction in the biochemical processes of activated sludge, and hydrolysis rates decrease along with the particle radius and, in line with Stokes’ law, with particle settling velocity.
- The definition of a new slowly biodegradable component (the XSH fraction was subdivided into distinct forms: the particulate, colloidal and soluble) and three new processes enhanced the OUR description of the activated sludge processes in the ASM2d.
- In comparison with the original ASM2d, the modified ASM2d model more accurately predicted the aerobic behavior of OUR. The deviations between both models with respect to the above-mentioned predictions were strongly correlated (r2 = 0.973–0.991) in the parallel reactors that dealt with the SWW without pretreatment and with the wastewater after C–F.
- The average MARDs were 11.3–29.5% and 18.9–45.8% (original ASM2d) vs. 9.7–15.8% and 11.8–30.3% (modified ASM2d) in the samples with the SWW without pretreatment and after C–F, respectively.
- In contrast, the MARD differences of COD concentrations, which were measured during the OUR tests, were in a narrow range (0.15–1.9%) for both the models with the SWW without pretreatment and those after C–F.
- Simulations in the GPS-X platform version from 6.5 to 7.0 showed that operating conditions in which the predictions were better appeared when the technological system lacked a primary settling tank—this is the case of typical WWTPs functioning as sequential batch reactors with activated sludge.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Drewnowski, J. The Impact of Slowly Biodegradable Organic Compounds on the Oxygen Uptake Rate in Activated Sludge Systems. Water Sci. Technol. 2014, 69, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Directive, E.U.W. Council Directive 91/271/EEC of 21 May 1991 Concerning Urban Waste-Water Treatment. J. Eur. Commun. 1991, 34, 40. [Google Scholar]
- Fernández, F.J.; Castro, M.C.; Villasenor, J.; Rodríguez, L. Agro-Food Wastewaters as External Carbon Source to Enhance Biological Phosphorus Removal. Chem. Eng. J. 2011, 166, 559–567. [Google Scholar] [CrossRef]
- Drewnowski, J.; Makinia, J. The Role of Biodegradable Particulate and Colloidal Organic Compounds in Biological Nutrient Removal Activated Sludge System. Int. J. Water Sci. Technol. 2014, 11, 1973–1988. [Google Scholar] [CrossRef]
- Suschka, J.; Grübel, K. Low intensity surplus activated sludge pretreatment before anaerobic digestion. Arch. Environ. Prot. 2017, 43, 50–57. [Google Scholar] [CrossRef][Green Version]
- Gori, R.; Jiang, L.M.; Sobhani, R.; Rosso, D. Effects of Soluble and Particulate Substrate on the Carbon and Energy Footprint of Wastewater Treatment Processes. Wat. Res. 2011, 18, 5858–5872. [Google Scholar] [CrossRef]
- Pajdak-Stós, A.; Fiałkowska, E.; Fyda, J.; Babko, R. Resistance of nitrifiers inhabiting activated sludge to ciliate grazing. Water Sci. Technol. 2010, 61, 573–580. [Google Scholar] [CrossRef]
- Babko, R.; Kuzmina, T.; Jaromin-Gleń, K.; Bieganowski, A. Bioindication assessment of activated sludge adaptation in a lab-scale experiment. Ecol. Chem. Eng. S 2014, 21, 605–616. [Google Scholar] [CrossRef]
- Bieganowski, A.; Łagód, G.; Ryżak, M.; Montusiewicz, A.; Chomczyńska, M.; Sochan, A. Measurement of activated sludge particle diameters using laser diffraction method. Ecol. Chem. Eng. S 2012, 19, 597–608. [Google Scholar] [CrossRef][Green Version]
- Czarnota, J.; Masłoń, A.; Zdeb, M. Powdered keramsite as unconventional method of AGS technology support in GSBR reactor with minimum-optimum OLR. E3S Web Conf. 2018, 44, 00024. [Google Scholar] [CrossRef]
- Czarnota, J.; Masłoń, A. Biogranulation and Physical Properties of Aerobic Granules in Reactors at Low Organic Loading Rate and with Powdered Ceramsite Added. J. Ecol. Eng. 2019, 20, 202–210. [Google Scholar] [CrossRef]
- Szaja, A.; Łagód, G.; Jaromin-Gleń, K.; Montusiewicz, A. The Effect of bioaugmentation with Archaea on the oxygen uptake rate in a sequencing batch reactor. Water 2018, 10, 575. [Google Scholar] [CrossRef]
- Polus, M.; Anielak, A.M. The use of Archaea in the bioaugmentation of activated sludge as a method for the biological removal of nitrogen compounds. Tech. Trans. 2017, 5, 83–95. [Google Scholar] [CrossRef]
- Wang, Y.; Sabba, F.; Bott, C.; Nerenberg, R. Using kinetics and modeling to predict denitrification fluxes in elemental sulfur (SO) based biofilms. Biotechnol. Bioeng. 2019, 116, 2698–2709. [Google Scholar] [CrossRef]
- Roots, P.; Sabba, F.; Rosenthal, A.F.; Wang, Y.; Yuan, O.; Rieger, L.; Yang, F.; Kozak, J.A.; Zhang, H.; Wells, G.F. Integrated shortcut nitrogen and biological phosphorus removal from mainstream wastewater: Process operation and modeling. Environ. Sci. 2020. [Google Scholar] [CrossRef]
- Grübel, K.; Wacławek, S.; Kuglarz, M.; Wacławek, M.; Černík, M. Improvement of the thermophilic anaerobic digestion and hygienisation of waste activated sludge by synergistic pretreatment. J. Environ. Sci. Health 2019, 54, 694–700. [Google Scholar] [CrossRef]
- Wacławek, S.; Grübel, K.; Chłąd, Z.; Dudziak, M.; Černík, M. The impact of oxone on disintegrati, on and dewaterability of waste activated sludge. Water Environ. Res. 2016, 88, 152–157. [Google Scholar] [CrossRef]
- Lapo, B.; Demey, H.; Zapata, J.; Romero, C.; Sastre, A.M. Sorption of Hg(II) and Pb(II) Ions on Chitosan-Iron(III) from Aqueous Solutions: Single and Binary Systems. Polymers 2018, 10, 367. [Google Scholar] [CrossRef]
- Attar, K.; Demey, H.; Bouazza, D.; Sastre, A.M. Sorption and Desorption Studies of Pb(II) and Ni(II) from Aqueous Solutions by a New Composite Based on Alginate and Magadiite Materials. Polymers 2019, 11, 340. [Google Scholar] [CrossRef]
- Demey, H.; Barron-Zambrano, J.; Mhadhbi, T.; Miloudi, H.; Yang, Z.; Ruiz, M.; Sastre, A.M. Boron Removal from Aqueous Solutions by Using a Novel Alginate-Based Sorbent: Comparison with Al2O3 Particles. Polymers 2019, 11, 1509. [Google Scholar] [CrossRef]
- De Lucas, A.; Rodríguez, L.; Villaseñor, J.; Fernández, F.J. Fermentation of Agro-Food Wastewaters by Activated Sludge. Wat. Res. 2007, 41, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Piechna, P.; Żubrowska-Sudoł, M. Respirometric Activity of Activated Sludge and Biofilm in IFAS-MBBR System. J. Ecol. Eng. 2017, 18, 145–151. [Google Scholar] [CrossRef][Green Version]
- Fernández, F.J.; Castro, M.C.; Rodrigo, M.A.; Cañizares, P. Reduction of Aeration Costs by Tuning a Multi-Set Point On/Off Controller: A Case Study. Control Eng. Pract. 2011, 19, 1231–1237. [Google Scholar] [CrossRef]
- Goel, R.; Mino, T.; Satoh, H.; Matsuo, T. Modeling Hydrolysis Processes Considering Intracellular Storage. Water Sci. Technol. 1999, 39, 97–105. [Google Scholar] [CrossRef]
- Orhon, D.; Cokgor, E.U.; Sozen, S. Dual Hydrolysis Model of the Slowly Biodegradable Substrate in Activated Sludge Systems. Biotechnol. Tech. 1998, 12, 737–741. [Google Scholar] [CrossRef]
- Drewnowski, J.; Makinia, J. Modeling Hydrolysis of Slowly Biodegradable Organic Compounds in Biological Nutrient Removal Activated Sludge Systems. Water Sci. Technol. 2013, 67, 2067–2074. [Google Scholar] [CrossRef]
- Makinia, J. Performance Prediction of Full-Scale Biological Nutrient Removal Systems Using Complex Activated Sludge Models; ISAH: Hannover, Germany, 2006. [Google Scholar]
- Roeleveld, P.J.; van Loosdrecht, M.C.M. Experience with Guidelines for Wastewater Characterisation in the Netherlands. Water Sci. Technol. 2002, 45, 77–87. [Google Scholar] [CrossRef]
- Ekama, G.A.; Dold, P.L.; Marais, G.v.R. Procedures for Determining Influent COD Fractions and the Maximum Specific Growth Rate of Heterotrophs in Activated Sludge. Water Sci. Technol. 1986, 18, 91–114. [Google Scholar] [CrossRef]
- Henze, M.; Grady, C.P.L., Jr.; Gujer, W.; Marais, G.v.R.; Matsuo, T. Activated Sludge Model No. 1, IAWPRC Scientific and Technical Reports, No. 1; IAWQ: London, UK, 1987. [Google Scholar]
- Kappeler, J.; Gujer, W. Estimation of Kinetic Parameters of Heterotrophic Biomass Under Aerobic Conditions & Characterization of Wastewater for Activated Sludge Model. Water Sci. Technol. 1992, 25, 125–139. [Google Scholar] [CrossRef]
- Lesouef, A.; Payraudeau, M.; Rogalla, F.; Kleiber, B. Optimizing Nitrogen Removal Reactor Configurations by On-Site Calibration of the IAWPRC ASM. Water Sci. Technol. 1992, 25, 105–123. [Google Scholar] [CrossRef]
- Petersen, B.; Gernaey, K.; Henze, M.; Vanrolleghem, P.A. Evaluation of an ASM1 Calibration Procedure on a Municipal-Industrial Wastewater Treatment Plant. J. Hydroinformatics 2002, 4, 15–38. [Google Scholar] [CrossRef]
- Koch, G.; Kuhni, M.; Gujer, W.; Siegrist, H. Calibration and Validation of Activated Sludge Model No. 3 for Swiss Municipal Wastewater. Wat. Res. 2000, 34, 3580–3590. [Google Scholar] [CrossRef]
- Meijer, S.C.F.; van Loosdrecht, M.C.M.; Heijnen, J.J. Metabolic Modeling of Full-Scale Biological Nitrogen and Phosphorus Removing WWTP’s. Wat. Res. 2001, 35, 2711–2723. [Google Scholar] [CrossRef]
- Henze, M.; Gujer, W.; Mino, T.; Matsuo, T.; Wentzel, M.C.; Marais, G.v.R.; van Loosdrecht, M. Activated Sludge Model No.2D, ASM2D. Water Sci. Technol. 1999, 39, 165–182. [Google Scholar] [CrossRef]
- Anderson, J.S.; Hyunook, K.; McAvoy, T.J.; Hao, O.J. Control of an Alternating Aerobic–Anoxic Activated Sludge System—Part 1: Development of a Linearization-Based Modeling Approach. Control Eng. Pract. 2000, 8, 271–278. [Google Scholar] [CrossRef]
- Melcer, H.; Dold, P.L.; Jones, R.M.; Bye, C.M.; Takacs, I.; Stensel, H.D.; Wilson, A.W.; Sun, P.; Bury, S. Methods for Wastewater Characterisation in Activated Sludge Modeling; Water Environment Research Foundation, IWA Publishing and Water Environment Federation: Alexandria, VA, USA; London, UK, 2003. [Google Scholar]
- Makinia, J.; Czerwionka, K. Transformations and Removal Potential of Dissolved Organic Nitrogen in Biological Nutrient Removal (BNR) Activated Sludge Systems; Raport WERF/45/2007 as part of the research program Water Environment Research Foundation (WERF) Research Program (USA) “Efficient, Cost-Effective Nutrient Removal from Wastewater—Limit of Treatment N Removal Issues”; Politechnika Gdańska: Gdańsk, Poland, 2009. [Google Scholar]
- Insel, G.; Orhon, D.; Vanrolleghem, P.A. Identification and Modelling of Aerobic Hydrolysis – Application of Optimal Experimental Design. J. Chem. Tech. Biotech. 2003, 78, 437–445. [Google Scholar] [CrossRef]
- Sollfrank, U.; Gujer, W. Characterisation of Domestic Wastewater for Mathematical Modelling of the Activated Sludge Process. Water Sci. Technol. 1991, 23, 1057–1066. [Google Scholar] [CrossRef]
- Insel, G.; Karahan-Gul, O.; Orhon, D.; Vanrolleghem, P.A.; Henze, M. Important Limitations in the Modeling of Activated Sludge: Based Calibration of the Hydrolysis Process. Water Sci. Technol. 2002, 45, 23–36. [Google Scholar] [CrossRef]
- Li, Q.; Li, P.; Zhu, P.; Wu, J.; Liang, S. Effects of Exogenous Organic Carbon Substrates on Nitrous Oxide Emissions During the Denitrification Process of Sequence Batch Reactors. Environ. Eng. Sci. 2008, 25, 1221–1228. [Google Scholar] [CrossRef]
- Levine, A.D.; Tchobanoglous, G.; Asano, T. Size distribution of particulate contaminants in wastewater and their impact on treatability. Wat. Res. 1991, 25, 911–922. [Google Scholar] [CrossRef]
- Hvitved-Jacobsen, T.; Vollertsen, J.; Tanaka, N. Wastewater quality changes during transport in sewers—An integrated anaerobic and aerobic model concept for carbon and sulfur microbial transformations. Water Sci. Technol. 1999, 39, 233–249. [Google Scholar] [CrossRef]
- Morgenroth, E.; Kommedal, R.; Harremoes, P. Processes and modeling of hydrolysis of particulate organic matter in aerobic wastewater treatment—A review. Water Sci. Technol. 2002, 45, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Maruéjouls, T.; Lessard, P.; Vanrolleghem, P.A. Impact of particle property distribution on hydrolysis rates in integrated wastewater modelling. In Proceedings of the 13th International Conference on Urban Drainage (13ICUD), Sarawak, Malaysia, 7–12 September 2014. [Google Scholar]
- Vavilin, V.A.; Fernandez, B.; Palatsi, J.; Flotats, X. Hydrolysis kinetics in anaerobic degradation of particulate organic material: An overview. Waste Manag. 2008, 28, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Spérandio, M.; Paul, E. Estimation of wastewater biodegradable COD fractions by combining respirometric experiments in various So/Xo ratios. Wat. Res. 2000, 34, 1233–1246. [Google Scholar] [CrossRef]
- Dimock, R.; Morgenroth, E. The influence of particle size on microbial hydrolysis of protein particles in activated sludge. Wat. Res. 2006, 40, 2064–2074. [Google Scholar] [CrossRef] [PubMed]
- Makinia, J.; Drewnowski, J.; Swinarski, M.; Czerwionka, K.; Kaszubowska, M.; Majtacz, J. The Impact of Precipitation and External Carbon Source Addition on Biological Nutrient Removal in Activated Sludge Systems—Experimental Investigation and Mathematical Modeling. Water Prac. Tech. 2012, 7. [Google Scholar] [CrossRef]
- Swinarski, M.; Makinia, J.; Stensel, H.D.; Czerwionka, K.; Drewnowski, J. Modeling External Carbon Addition in Biological Nutrient Removal Processes with an Extension of the International Water Association Activated Sludge Model. Water Environ. Res. 2012, 84, 646–655. [Google Scholar] [CrossRef]
- Drewnowski, J.; Makinia, J.; Kopec, Ł.; Fernandez-Morales, F.J. Modelization of Nutrient Removal Processes at a Large WWTP Using a Modified ASM2d Model. Int. J. Environ. Res. Public Health 2018, 15, 2817. [Google Scholar] [CrossRef]
- Drewnowski, J.; Remiszewska-Skwarek, A.; Fudala-Ksiazek, S.; Luczkiewicz, A.; Kumari, S.; Bux, F. The evaluation of COD fractionation and modeling as a key factor for appropriate optimization and monitoring of modern cost-effective activated sludge systems. J. Environ. Sci. Health 2019, 54, 736–744. [Google Scholar] [CrossRef]
- Drewnowski, J.; Makinia, J.; Szaja, A.; Łagód, G.; Kopeć, Ł.; Aguilar, J.A. Comparative study of balancing SRT by using modified ASM2d in control and operation strategy at full-scale WWTP. Water 2019, 11. [Google Scholar] [CrossRef]
- Maruéjouls, T.; Lessard, P.; Wipliez, B.; Pelletier, G.; Vanrolleghem, P.A. Characterization of the potential impact of retention tank emptying on wastewater primary treatment: A new element for CSO management. Water Sci. Technol. 2011, 64, 1898–1905. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, J.; Remiszewska-Skwarek, A.; Duda, S.; Łagód, G. Aeration Process in Bioreactors as the Main Energy Consumer in a Wastewater Treatment Plant. Review of Solutions and Methods of Process Optimization. Processes 2019, 7, 311. [Google Scholar] [CrossRef]
- Maruéjouls, T.; Lessard, P.; Vanrolleghem, P.A. Integrated urban wastewater systems: Prediction of particle settling velocity distributions along the sewer- retention tank-primary clarifier system. In Proceedings of the 7th International Conference on Sewer Processes and Networks (SPN7), Sheffield, UK, 28–30 August 2013. [Google Scholar]
- Drewnowski, J.; Makinia, J. The Role of Colloidal and Particulate Organic Compounds in Denitrification and EBPR Occurring in a Full-Scale Activated Sludge System. Water Sci. Technol. 2011, 63, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Mamais, D.; Jenkins, D.; Pitt, P.A. Rapid Physical Chemical Method for the Determination of Readily Biodegradable Soluble COD in Municipal Wastewater. Wat. Res. 1993, 27, 195–197. [Google Scholar] [CrossRef]
- Hydromantis, Inc. GPS-X 5.0. User’s Guide and Technical Reference; Hydromantis Inc.: Hamilton, ON Canada, 2007. [Google Scholar]
- Nelder, J.A.; Mead, R. A Simplex Method for Function Minimization. Comput. J. 1965, 7, 308–313. [Google Scholar] [CrossRef]
- American Public Health Association. Standard Methods for Examination of Water and Wastewater, 18th ed.; American Public Health Association: Washington, DC, USA, 1992. [Google Scholar]
| Wschod WWTP | COD Fraction | Calculated Values During the Study Period | |||||
|---|---|---|---|---|---|---|---|
| Winter | Spring | Summer | |||||
| Concentration COD | Concentration COD | Concentration COD | |||||
| [g COD/m3] | [%] | [g COD/m3] | [%] | [g COD/m3] | [%] | ||
| Settled wastewater fractionation | SI | 38.9 | 6.2 | 37.1 | 5.4 | 36.1 | 4.5 |
| SF | 64.1 | 10.3 | 66.1 | 9.6 | 64.9 | 8.2 | |
| SA | 85.0 | 13.6 | 87.7 | 12.7 | 86.0 | 10.8 | |
| XI | 133.5 | 21.4 | 181.3 | 26.2 | 257.4 | 32.4 | |
| XS | 302.5 | 48.5 | 318.8 | 46.1 | 349.6 | 44.1 | |
| Total COD | 624.0 | 100.0 | 691.0 | 100.0 | 794.0 | 100.0 | |
| Series | Wschod WWTP | ||||
|---|---|---|---|---|---|
| Settled Wastewater | Colloidal Fraction | Estimation of XSH (S/C) | |||
| CODinf | CODf(1.2),inf | CODf(0.1),inf | CODcol,inf | % of SBCOD | |
| [g COD/m3] | [g COD/m3] | ||||
| 1 | 650 | 237 | 213 | 24 | 7.5 |
| 2 | 630 | 188 | 152 | 36 | 11.8 |
| 3 | 590 | 182 | 143 | 39 | 13.6 |
| 4 | 548 | 164 | 131 | 33 | 12.3 |
| 5 | 451 | 138 | 106 | 32 | 14.5 |
| 6 | 821 | 191 | 154 | 37 | 9.2 |
| 7 | 620 | 176 | 132 | 44 | 14.5 |
| 8 | 1390 | 247 | 174 | 73 | 10.7 |
| 9 | 1001 | 236 | 172 | 64 | 13.0 |
| 10 | 797 | 165 | 112 | 53 | 13.6 |
| Ave. value | 750 | 192 | 149 | 44 | 12 |
| Model | Study Period | Type of Sample | Contribution of the COD Particulate Component [%] | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Xinog | XPP | XPHA | XSTO | XPAO | XA | XH | XI | XSH | XS | % | |||
| Modified ASM2d | Winter | SW | 12.0 | 3.4 | 0.7 | 0.0 | 12.9 | 1.3 | 19.6 | 6.1 | 0.8 | 43.2 | 100.0 |
| SW c–f | 12.8 | 4.3 | 0.8 | 0.0 | 12.6 | 1.2 | 18.9 | 1.7 | 0.5 | 47.2 | 100.0 | ||
| Spring | SW | 8.3 | 2.4 | 0.6 | 0.0 | 13.7 | 0.8 | 23.4 | 9.2 | 1.3 | 40.3 | 100.0 | |
| SW c–f | 10.2 | 3.2 | 0.7 | 0.0 | 14.3 | 0.8 | 21.4 | 2.5 | 0.7 | 46.2 | 100.0 | ||
| Summer | SW | 12.3 | 3.1 | 0.6 | 0.0 | 9.8 | 1.0 | 18.8 | 7.3 | 1.0 | 46.1 | 100.0 | |
| SW c–f | 13.1 | 3.5 | 0.6 | 0.0 | 10.4 | 1.1 | 17.5 | 2.9 | 0.5 | 50.3 | 100.0 | ||
| Experiment | Study Period | Process Rate | MARD [%] | |||
|---|---|---|---|---|---|---|
| Wschod WWTP | ||||||
| Settled Wastewater | Settled Wastewater (C–F) | |||||
| ASM2d | Modified ASM2d | ASM2d | Modified ASM2d | |||
| OUR batch test | Winter | Oxygen uptake | 29.5 | 14.6 | 45.8 | 30.3 |
| COD utilization | 4.3 | 3.8 | 5.0 | 3.1 | ||
| Summer | Oxygen uptake | 11.3 | 9.7 | 18.9 | 11.8 | |
| COD utilization | 4.8 | 5.5 | 5.2 | 5.4 | ||
| Spring | Oxygen uptake | 16.2 | 15.8 | 23.3 | 19.2 | |
| COD utilization | 4.3 | 2.7 | 4.4 | 4.5 | ||
| Parameter | PE | Q [m3/d] | SRT [d] | MLSS [kg/m3] | COD [g COD/m3] | SCOD [g COD/m3] | TP [g P/m3)] | TN [g N/m3] | N-NH4 [g N/m3] | N-NO3 [g N/m3] |
|---|---|---|---|---|---|---|---|---|---|---|
| Influent | 574,000 | 81,600 | x | x | 626 ± 82 | 194 ± 38 | 14.9 ± 2.6 | 81.2 ± 5.0 | 58.9 ± 3.4 | 7.4 ± 0.64 |
| Effluent | 48 ± 4.2 | 0.60 ± 0.1 | 11.1 ± 1.1 | 1.20 ± 0.75 | ||||||
| Reactor | T = 11.8–20.5 °C | 21 ± 2.9 | 5.45 ± 0.56 | x | x | x | x | x | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drewnowski, J.; Szeląg, B.; Xie, L.; Lu, X.; Ganesapillai, M.; Deb, C.K.; Szulżyk-Cieplak, J.; Łagód, G. The Influence of COD Fraction Forms and Molecules Size on Hydrolysis Process Developed by Comparative OUR Studies in Activated Sludge Modelling. Molecules 2020, 25, 929. https://doi.org/10.3390/molecules25040929
Drewnowski J, Szeląg B, Xie L, Lu X, Ganesapillai M, Deb CK, Szulżyk-Cieplak J, Łagód G. The Influence of COD Fraction Forms and Molecules Size on Hydrolysis Process Developed by Comparative OUR Studies in Activated Sludge Modelling. Molecules. 2020; 25(4):929. https://doi.org/10.3390/molecules25040929
Chicago/Turabian StyleDrewnowski, Jakub, Bartosz Szeląg, Li Xie, Xi Lu, Mahesh Ganesapillai, Chinmoy Kanti Deb, Joanna Szulżyk-Cieplak, and Grzegorz Łagód. 2020. "The Influence of COD Fraction Forms and Molecules Size on Hydrolysis Process Developed by Comparative OUR Studies in Activated Sludge Modelling" Molecules 25, no. 4: 929. https://doi.org/10.3390/molecules25040929
APA StyleDrewnowski, J., Szeląg, B., Xie, L., Lu, X., Ganesapillai, M., Deb, C. K., Szulżyk-Cieplak, J., & Łagód, G. (2020). The Influence of COD Fraction Forms and Molecules Size on Hydrolysis Process Developed by Comparative OUR Studies in Activated Sludge Modelling. Molecules, 25(4), 929. https://doi.org/10.3390/molecules25040929










