Natural Psychoplastogens As Antidepressant Agents
Abstract
1. Introduction
1.1. Depression
1.2. Psychoplastogens
2. Pathomechanisms Relevant to the Action of Psychoplastogens
2.1. Monoamine Hypothesis
2.2. Neurotrophic Hypothesis
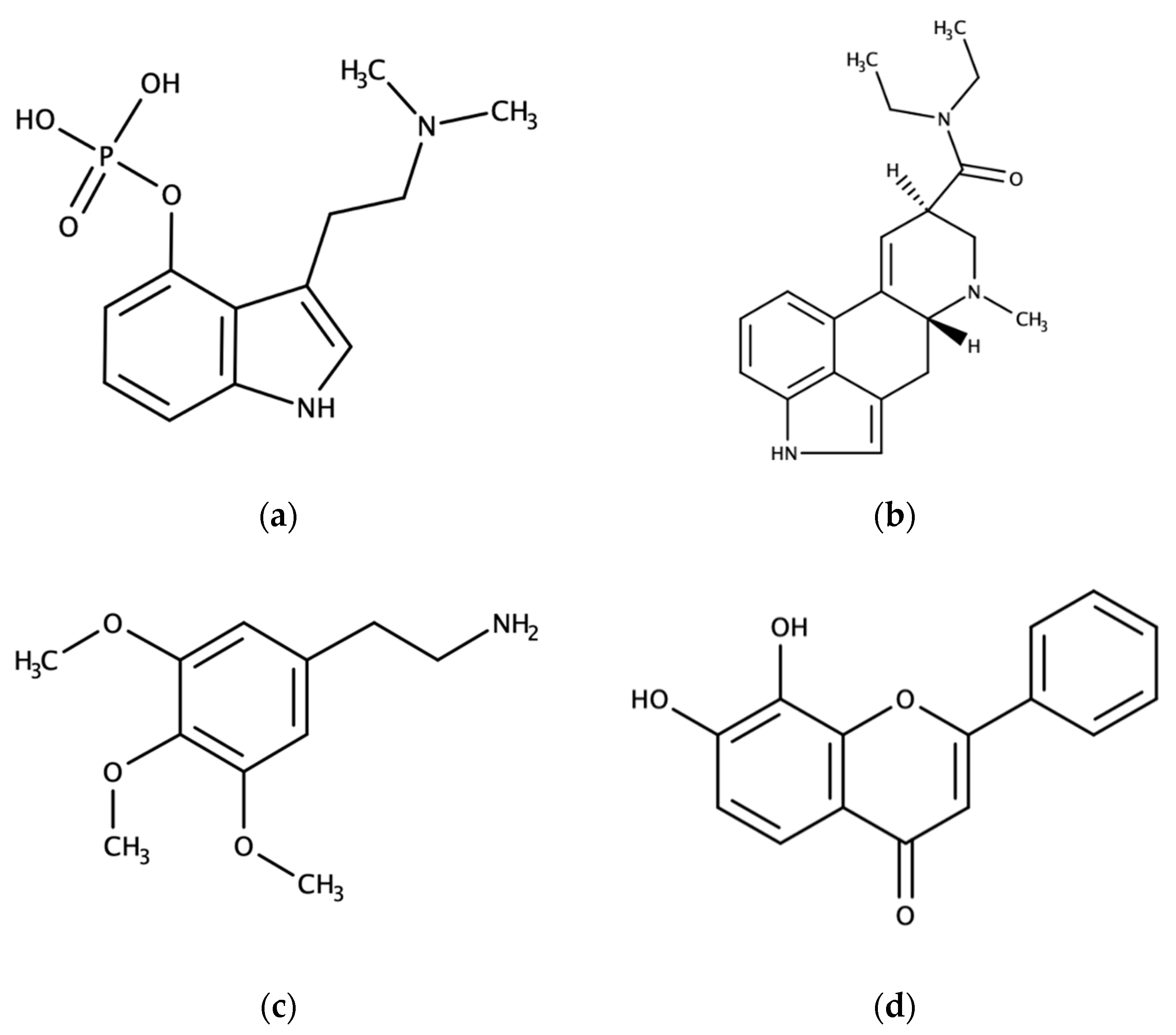
3. Natural Psychoplastogens with Potential in Clinical Practice
3.1. Serotonergic Psychedelics
3.1.1. Mechanism of Action
3.1.2. Animal Studies
3.1.3. Human Studies
3.2. 7,8-dihydroxyflavone and its Derivatives
3.2.1. Mechanism of Action
3.2.2. Animal Studies
3.2.3. Human Studies
4. Safety
5. Conclusions
Funding
Conflicts of Interest
References
- Lopez, A.D.; Murray, C.C.J.L. The Global Burden of Disease, 1990–2020. Nat. Med. 1998, 4, 1241. [Google Scholar] [CrossRef] [PubMed]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- Mann, J.J. The Medical Management of Depression. N. Engl. J. Med. 2005, 353, 1819–1834. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.L.; Nutt, D.J. Serotonin and Brain Function: A Tale of Two Receptors. J. Psychopharmacol. 2017, 31, 1091–1120. [Google Scholar] [CrossRef] [PubMed]
- Kupferberg, A.; Bicks, L.; Hasler, G. Social Functioning in Major Depressive Disorder. Neurosci. Biobehav. Rev. 2016, 69, 313–332. [Google Scholar] [CrossRef] [PubMed]
- Byers, A.L.; Yaffe, K. Depression and Risk of Developing Dementia. Nat. Rev. Neurol. 2011, 7, 323–331. [Google Scholar] [CrossRef]
- Huffman, J.C.; Celano, C.M.; Beach, S.R.; Motiwala, S.R.; Januzzi, J.L. Depression and Cardiac Disease: Epidemiology, Mechanisms, and Diagnosis. Cardiovasc. Psychiatry Neurol. 2013, 2013, 695925. [Google Scholar] [CrossRef]
- Ly, C.; Greb, A.C.; Cameron, L.P.; Wong, J.M.; Barragan, E.V.; Wilson, P.C.; Burbach, K.F.; Soltanzadeh Zarandi, S.; Sood, A.; Paddy, M.R.; et al. Psychedelics Promote Structural and Functional Neural Plasticity. Cell Rep. 2018, 23, 3170–3182. [Google Scholar] [CrossRef]
- Olson, D.E. Psychoplastogens: A Promising Class of Plasticity-Promoting Neurotherapeutics. J. Exp. Neurosci. 2018, 12, 1179069518800508. [Google Scholar] [CrossRef]
- FDA Approves New Nasal Spray Medication for Treatment-Resistant Depression; Available Only At a Certified Doctor’s Office or Clinic. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-new-nasal-spray-medication-treatment-resistant-depression-available-only-certified (accessed on 18 November 2019).
- Stein, L.; Ray, O.S. Accelerated Recovery from Reserpine Depression by Monoamine Oxidase Inhibitors. Nature 1960, 188, 1199–1200. [Google Scholar] [CrossRef]
- Freis, E.D. Mental Depression in Hypertensive Patients Treated for Long Periods with Large Doses of Reserpine. N. Engl. J. Med. 1954, 251, 1006–1008. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.D.; Brossard, G.; Hautbois, C.; Roux, S. Rodent Models of Depression: Forced Swimming and Tail Suspension Behavioral Despair Tests in Rats and Mice. Curr. Protoc. Neurosci. 2001, 14, 8.10A.1–8.10A.10. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, D.; Forrest, L.R.; Schuldiner, S. The Ins and Outs of Vesicular Monoamine Transporters. J. Gen. Physiol. 2018, 150, 671–682. [Google Scholar] [CrossRef]
- Ruhé, H.G.; Khoenkhoen, S.J.; Ottenhof, K.W.; Koeter, M.W.; Mocking, R.J.; Schene, A.H. Longitudinal Effects of the SSRI Paroxetine on Salivary Cortisol in Major Depressive Disorder. Psychoneuroendocrinology 2015, 52, 261–271. [Google Scholar] [CrossRef]
- Santarelli, L.; Saxe, M.; Gross, C.; Surget, A.; Battaglia, F.; Dulawa, S.; Weisstaub, N.; Lee, J.; Duman, R.; Arancio, O.; et al. Requirement of Hippocampal Neurogenesis for the Behavioral Effects of Antidepressants. Science 2003, 301, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Sheline, Y.I.; Bardgett, M.E.; Csernansky, J.G. Correlated Reductions in Cerebrospinal Fluid 5-HIAA and MHPG Concentrations After Treatment with Selective Serotonin Reuptake Inhibitors. J. Clin. Psychopharmacol. 1997, 17, 11–14. [Google Scholar] [CrossRef]
- Bell, C.; Abrams, J.; Nutt, D. Tryptophan Depletion and Its Implications for Psychiatry. Br. J. Psychiatry 2001, 178, 399–405. [Google Scholar] [CrossRef]
- Mann, J.J.; Arango, V.; Marzuk, P.M.; Theccanat, S.; Reis, D.J. Evidence for the 5-HT Hypothesis of Suicide a Review of Post-Mortem Studies. Br. J. Psychiatry 1989, 155, 7–14. [Google Scholar] [CrossRef]
- Harro, J.; Tõnissaar, M.; Eller, M.; Kask, A.; Oreland, L. Chronic Variable Stress and Partial 5-HT Denervation by Parachloroamphetamine Treatment in the Rat: Effects on Behavior and Monoamine Neurochemistry. Brain Res. 2001, 899, 227–239. [Google Scholar] [CrossRef]
- Tõnissaar, M.; Mällo, T.; Eller, M.; Häidkind, R.; Kõiv, K.; Harro, J. Rat Behavior After Chronic Variable Stress and Partial Lesioning of 5-HT-ergic Neurotransmission: Effects of Citalopram. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 164–177. [Google Scholar] [CrossRef]
- Caspi, A.; Sugden, K.; Moffitt, T.E.; Taylor, A.; Craig, I.W.; Harrington, H.; Mcclay, J.; Mill, J.; Martin, J.; Braithwaite, A.; et al. Influence of Life Stress on Depression: Moderation by a Polymorphism in the 5-HTT Gene. Science 2003, 301, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Lüscher, C.; Malenka, R.C. NMDA Receptor-Dependent Long-Term Potentiation and Long-Term Depression (LTP/LTD). Cold Spring Harbor Perspect. Biol. 2012, 4. [Google Scholar] [CrossRef] [PubMed]
- Panja, D.; Bramham, C.R. BDNF Mechanisms in Late LTP Formation: A Synthesis and Breakdown. Neuropharmacology 2014, 76, 664–676. [Google Scholar] [CrossRef] [PubMed]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell. Mol. Neurobiol. 2018, 38, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Li, N.; Liu, R.J.; Duric, V.; Aghajanian, G. Signaling Pathways Underlying the Rapid Antidepressant Actions of Ketamine. Neuropharmacology 2012, 62, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Johansen, J.; Cain, C.; Ostroff, L.; Ledoux, J. Molecular Mechanisms of Fear Learning and Memory. Cell 2011, 147, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Mcewen, B.S. Glucocorticoids, Depression, and Mood Disorders: Structural Remodeling in the Brain. Metabolism 2005, 54, 20–23. [Google Scholar] [CrossRef]
- Arnsten, A.F. Stress Signalling Pathways That Impair Prefrontal Cortex Structure and Function. Nat. Rev. Neurosci. 2009, 10, 410–422. [Google Scholar] [CrossRef]
- Gourley, S.L.; Kedves, A.T.; Olausson, P.; Taylor, J.R. A History of Corticosterone Exposure Regulates Fear Extinction and Cortical NR2B, GluR2/3, and BDNF. Neuropsychopharmacology 2009, 34, 707–716. [Google Scholar] [CrossRef]
- Murray, F.; Smith, D.W.; Hutson, P.H. Chronic Low Dose Corticosterone Exposure Decreased Hippocampal Cell Proliferation, Volume and Induced Anxiety and Depression Like Behaviours in Mice. Eur. J. Pharmacol. 2008, 583, 115–127. [Google Scholar] [CrossRef]
- Wellman, C.L. Dendritic Reorganization in Pyramidal Neurons in Medial Prefrontal Cortex After Chronic Corticosterone Administration. J. Neurobiol. 2001, 49, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Lakshminarasimhan, H.; Chattarji, S. Stress Leads to Contrasting Effects on the Levels of Brain Derived Neurotrophic Factor in the Hippocampus and Amygdala. PLoS ONE 2012, 7, e30481. [Google Scholar] [CrossRef] [PubMed]
- Vyas, A.; Mitra, R.; Rao, B.S.S.; Chattarji, S. Chronic Stress Induces Contrasting Patterns of Dendritic Remodeling in Hippocampal and Amygdaloid Neurons. J. Neurosci. 2002, 22, 6810–6818. [Google Scholar] [CrossRef] [PubMed]
- Bocchio-Chiavetto, L.; Bagnardi, V.; Zanardini, R.; Molteni, R.; Nielsen, M.G.; Placentino, A.; Giovannini, C.; Rillosi, L.; Ventriglia, M.; Riva, M.A.; et al. Serum and Plasma BDNF Levels in Major Depression: A Replication Study and Meta-Analyses. World. J. Biol. Psychiatry. 2010, 11, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Duman, R.; Sanacora, G. Serum Brain-Derived Neurotrophic Factor, Depression, and Antidepressant Medications: Meta-Analyses and Implications. Biol. Psychiatry 2008, 64, 527–532. [Google Scholar] [CrossRef]
- Montag, C.; Reuter, M.; Newport, B.; Elger, C.; Weber, B. The BDNF Val66Met Polymorphism Affects Amygdala Activity in Response to Emotional Stimuli: Evidence From a Genetic Imaging Study. NeuroImage 2008, 42, 1554–1559. [Google Scholar] [CrossRef]
- Gatt, J.M.; Nemeroff, C.B.; Dobson-Stone, C.; Paul, R.H.; Bryant, R.A.; Schofield, P.R.; Gordon, E.; Kemp, A.H.; Williams, L.M. Interactions Between BDNF Val66Met Polymorphism and Early Life Stress Predict Brain and Arousal Pathways to Syndromal Depression and Anxiety. Mol. Psychiatry 2009, 14, 681–695. [Google Scholar] [CrossRef]
- Dwivedi, Y.; Rizavi, H.S.; Conley, R.R.; Roberts, R.C.; Tamminga, C.A.; Pandey, G.N. Altered Gene Expression of Brain-Derived Neurotrophic Factor and Receptor Tyrosine Kinase B in Postmortem Brain of Suicide Subjects. Arch. Gen. Psychiatry 2003, 60, 804–815. [Google Scholar] [CrossRef]
- Jernigan, C.S.; Goswami, D.B.; Austin, M.C.; Iyo, A.H.; Chandran, A.; Stockmeier, C.A.; Karolewicz, B. The mTOR Signaling Pathway in the Prefrontal Cortex is Compromised in Major Depressive Disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 1774–1779. [Google Scholar] [CrossRef]
- Groves, J.O. Is it Time to Reassess the BDNF Hypothesis of Depression. Mol. Psychiatry 2007, 12, 1079–1088. [Google Scholar] [CrossRef]
- Nichols, D.E. Psychedelics. Pharmacol. Rev. 2016, 68, 264–355. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.W.; Liu, X.; Yepes, M.; Shepherd, K.R.; Miller, G.W.; Liu, Y.; Wilson, W.D.; Xiao, G.; Blanchi, B.; Sun, Y.E.; et al. A Selective TrkB Agonist with Potent Neurotrophic Activities by 7,8-Dihydroxyflavone. Proc. Natl. Acad. Sci. USA 2010, 107, 2687–2692. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Obianyo, O.; Chan, C.B.; Huang, J.; Xue, S.; Yang, J.J.; Zeng, F.; Goodman, M.; Ye, K. Biochemical and Biophysical Investigation of the Brain-Derived Neurotrophic Factor Mimetic 7,8-Dihydroxyflavone in the Binding and Activation of the TrkB Receptor. J. Biol. Chem. 2014, 289, 27571–27584. [Google Scholar] [CrossRef] [PubMed]
- Nutt, D. Psychedelic Drugs—A New Era in Psychiatry. Dialogues Clin. Neurosci. 2019, 21, 139. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qi, Q.; Xiao, G.; Li, J.; Luo, H.R.; Ye, K. O-Methylated Metabolite of 7,8-Dihydroxyflavone Activates TrkB Receptor and Displays Antidepressant Activity. Pharmacology 2013, 91, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Ray, T.S. Psychedelics and the Human Receptorome. PLoS ONE 2010, 5, e9019. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Erritzoe, D.; Williams, T.; Stone, J.M.; Reed, L.J.; Colasanti, A.; Tyacke, R.J.; Leech, R.; Malizia, A.L.; Murphy, K.; et al. Neural Correlates of the Psychedelic State as Determined by fMRI Studies with Psilocybin. Proc. Natl. Acad. Sci. USA 2012, 109, 2138–2143. [Google Scholar] [CrossRef]
- Aghajanian, G.K.; Marek, G.J. Serotonin Induces Excitatory Postsynaptic Potentials in Apical Dendrites of Neocortical Pyramidal Cells. Neuropharmacology 1997, 36, 589–599. [Google Scholar] [CrossRef]
- Kurrasch-Orbaugh, D.M.; Parrish, J.C.; Watts, V.J.; Nichols, D.E. A Complex Signaling Cascade Links the Serotonin2a Receptor to Phospholipase A2 Activation: The Involvement of Map Kinases. J. Neurochem. 2003, 86, 980–991. [Google Scholar] [CrossRef]
- González-Maeso, J.; Ang, R.L.; Yuen, T.; Chan, P.; Weisstaub, N.V.; López-Giménez, J.F.; Zhou, M.; Okawa, Y.; Callado, L.F.; Milligan, G.; et al. Identification of a Serotonin/glutamate Receptor Complex Implicated in Psychosis. Nature 2008, 452, 93–97. [Google Scholar] [CrossRef]
- Wootten, D.; Christopoulos, A.; Marti-Solano, M.; Babu, M.M.; Sexton, P.M. Mechanisms of Signalling and Biased Agonism in G Protein-Coupled Receptors. Nat. Rev. Mol. Cell. Biol. 2018, 19, 638–653. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Villacreses, N.; Murphy, D.L.; Rapoport, S.I. 5-HT2A/2C Receptor Signaling Via Phospholipase A2 and Arachidonic Acid is Attenuated in Mice Lacking the Serotonin Reuptake Transporter. Psychopharmacology 2005, 180, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.A.; Maayani, S.; Goldfarb, J.; Scaramellini, C.; Leff, P.; Clarke, W.P. Effector Pathway-Dependent Relative Efficacy At Serotonin Type 2A and 2C Receptors: Evidence for Agonist-Directed Trafficking of Receptor Stimulus. Mol. Pharmacol. 1998, 54, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Puig, M.V.; Gulledge, A.T. Serotonin and Prefrontal Cortex Function: Neurons, Networks, and Circuits. Mol. Neurobiol. 2011, 44, 449–464. [Google Scholar] [CrossRef] [PubMed]
- Quirk, G.J.; Likhtik, E.; Pelletier, J.G.; Paré, D. Stimulation of Medial Prefrontal Cortex Decreases the Responsiveness of Central Amygdala Output Neurons. J. Neurosci. 2003, 23, 8800–8807. [Google Scholar] [CrossRef]
- Quirk, G.J.; Garcia, R.; González-Lima, F. Prefrontal Mechanisms in Extinction of Conditioned Fear. Biol. Psychiatry 2006, 60, 337–343. [Google Scholar] [CrossRef]
- Adhikari, A.; Lerner, T.N.; Finkelstein, J.; Pak, S.; Jennings, J.H.; Davidson, T.J.; Ferenczi, E.; Gunaydin, L.A.; Mirzabekov, J.J.; Ye, L.; et al. Basomedial Amygdala Mediates Top-Down Control of Anxiety and Fear. Nature 2015, 527, 179–185. [Google Scholar] [CrossRef]
- Dhonnchadha, B.Á.N.; Bourin, M.; Hascoët, M. Anxiolytic-Like Effects of 5-HT2 Ligands on Three Mouse Models of Anxiety. Behav. Brain Res. 2003, 140, 203–214. [Google Scholar] [CrossRef]
- Cameron, L.P.; Benson, C.J.; Dunlap, L.E.; Olson, D.E. Effects of N,N-Dimethyltryptamine on Rat Behaviors Relevant to Anxiety and Depression. ACS Chem. Neurosci. 2018, 9, 1582–1590. [Google Scholar] [CrossRef]
- Catlow, B.J.; Song, S.; Paredes, D.A.; Kirstein, C.L.; Sanchez-Ramos, J. Effects of Psilocybin on Hippocampal Neurogenesis and Extinction of Trace Fear Conditioning. Exp. Brain Res. 2013, 228, 481–491. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Roseman, L.; Haijen, E.; Erritzoe, D.; Watts, R.; Branchi, I.; Kaelen, M. Psychedelics and the Essential Importance of Context. J. Psychopharmacol. 2018, 32, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Petit, A.-C.; Quesseveur, G.; Gressier, F.; Colle, R.; David, D.J.; Gardier, A.M.; Ferreri, F.; Lépine, J.-P.; Falissard, B.; Verstuyft, C.; et al. Converging Translational Evidence for the Involvement of the Serotonin 2A Receptor Gene in Major Depressive Disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 54, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Jefsen, O.; Højgaard, K.; Christiansen, S.L.; Elfving, B.; Nutt, D.J.; Wegener, G.; Müller, H.K. Psilocybin Lacks Antidepressant-Like Effect in the Flinders Sensitive Line Rat. Acta Neuropsychiatr. 2019, 31, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Chen, M.C.; Qiu, M.H.; Lu, J. Ventromedial Prefrontal Cortex Regulates Depressive-Like Behavior and Rapid Eye Movement Sleep in the Rat. Neuropharmacology 2014, 86, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Koenigs, M.; Grafman, J. The Functional Neuroanatomy of Depression: Distinct Roles for Ventromedial and Dorsolateral Prefrontal Cortex. Behav. Brain Res. 2009, 201, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Siegle, G.J.; Steinhauer, S.R.; Thase, M.E.; Stenger, V.A.; Carter, C.S. Can’t Shake That Feeling: Event-Related fMRI Assessment of Sustained Amygdala Activity in Response to Emotional Information in Depressed Individuals. Biol. Psychiatry 2002, 51, 693–707. [Google Scholar] [CrossRef]
- Siegle, G.J.; Konecky, R.O.; Thase, M.E.; Carter, C.S. Relationships Between Amygdala Volume and Activity During Emotional Information Processing Tasks in Depressed and Never-depressed Individuals: An fMRI Investigation. Ann. N. Y. Acad. Sci. 2003, 985, 481–484. [Google Scholar] [CrossRef]
- Hamilton, J.P.; Farmer, M.; Fogelman, P.; Gotlib, I.H. Depressive Rumination, the Default-Mode Network, and the Dark Matter of Clinical Neuroscience. Biol. Psychiatry 2015, 78, 224–230. [Google Scholar] [CrossRef]
- Zhou, H.X.; Chen, X.; Shen, Y.Q.; Li, L.; Chen, N.X.; Zhu, Z.C.; Castellanos, F.X.; Yan, C.G. Rumination and the Default Mode Network: Meta-Analysis of Brain Imaging Studies and Implications for Depression. NeuroImage 2019, 116287. [Google Scholar] [CrossRef]
- Andero, R.; Heldt, S.A.; Ye, K.; Liu, X.; Armario, A.; Ressler, K.J. Effect of 7,8-Dihydroxyflavone, a Small-Molecule TrkB Agonist, on Emotional Learning. Am. J. Psychiatry 2011, 168, 163–172. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Z.; Zhang, Z.; Liu, X.; Kang, S.S.; Zhang, Y.; Ye, K. The Prodrug of 7,8-Dihydroxyflavone Development and Therapeutic Efficacy for Treating Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2018, 115, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Studerus, E.; Kometer, M.; Hasler, F.; Vollenweider, F.X. Acute, Subacute and Long-Term Subjective Effects of Psilocybin in Healthy Humans: A Pooled Analysis of Experimental Studies. J. Psychopharmacol. 2011, 25, 1434–1452. [Google Scholar] [CrossRef] [PubMed]
- Vollenweider, F.X.; Vollenweider-Scherpenhuyzen, M.F.I.; Bäbler, A.; Vogel, H.; Hell, D. Psilocybin Induces Schizophrenia-Like Psychosis in Humans Via a Serotonin-2 Agonist Action. Neuroreport 1998, 9, 3897–3902. [Google Scholar] [CrossRef] [PubMed]
- Bogenschutz, M.P.; Johnson, M.W. Classic Hallucinogens in the Treatment of Addictions. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 64, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.L.; Bolstridge, M.; Rucker, J.; Day, C.M.; Erritzoe, D.; Kaelen, M.; Bloomfield, M.; Rickard, J.A.; Forbes, B.; Feilding, A.; et al. Psilocybin with Psychological Support for Treatment-Resistant Depression: An Open-Label Feasibility Study. Lancet Psychiatry 2016, 3, 619–627. [Google Scholar] [CrossRef]
- Ross, S.; Bossis, A.; Guss, J.; Agin-Liebes, G.; Malone, T.; Cohen, B.; Mennenga, S.E.; Belser, A.; Kalliontzi, K.; Babb, J.; et al. Rapid and Sustained Symptom Reduction Following Psilocybin Treatment for Anxiety and Depression in Patients with Life-Threatening Cancer: A Randomized Controlled Trial. J. Psychopharmacol. 2016, 30, 1165–1180. [Google Scholar] [CrossRef]
- LSD Therapy for Persons Suffering from Major Depression. Available online: https://clinicaltrials.gov/ct2/show/NCT03866252 (accessed on 18 November 2019).
- Carhart-Harris, R.L.; Bolstridge, M.; Day, C.M.J.; Rucker, J.; Watts, R.; Erritzoe, D.E.; Kaelen, M.; Giribaldi, B.; Bloomfield, M.; Pilling, S.; et al. Psilocybin with Psychological Support for Treatment-Resistant Depression: Six-Month Follow-Up. Psychopharmacology 2018, 235, 399–408. [Google Scholar] [CrossRef]
- Griffiths, R.R.; Johnson, M.W.; Carducci, M.A.; Umbricht, A.; Richards, W.A.; Richards, B.D.; Cosimano, M.P.; Klinedinst, M.A. Psilocybin Produces Substantial and Sustained Decreases in Depression and Anxiety in Patients with Life-Threatening Cancer: A Randomized Double-Blind Trial. J. Psychopharmacol. 2016, 30, 1181–1197. [Google Scholar] [CrossRef]
- Gasser, P.; Holstein, D.; Michel, Y.; Doblin, R.; Yazar-Klosinski, B.; Passie, T.; Brenneisen, R. Safety and Efficacy of Lysergic Acid Diethylamide-Assisted Psychotherapy for Anxiety Associated with Life-Threatening Diseases. J. Nerv. Ment. Dis. 2014, 202, 513–520. [Google Scholar] [CrossRef]
- Gasser, P.; Kirchner, K.; Passie, T. LSD-Assisted Psychotherapy for Anxiety Associated with a Life-Threatening Disease: A Qualitative Study of Acute and Sustained Subjective Effects. J. Psychopharmacol. 2015, 29, 57–68. [Google Scholar] [CrossRef]
- Barbosa, P.C.; Giglio, J.S.; Dalgalarrondo, P. Altered States of Consciousness and Short-Term Psychological After-Effects Induced by the First Time Ritual Use of Ayahuasca in an Urban Context in Brazil. J. Psychoactive Drugs 2005, 37, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.G.; Landeira-Fernandez, J.; Strassman, R.J.; Motta, V.; Cruz, A.P. Effects of Ayahuasca on Psychometric Measures of Anxiety, Panic-Like and Hopelessness in Santo Daime Members. J. Ethnopharmacol. 2007, 112, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Sanches, R.F.; De Lima Osório, F.; Dos Santos, R.G.; Macedo, L.R.H.; Maia-De-oliveira, J.P.; Wichert-Ana, L.; De Araujo, D.B.; Riba, J.; Crippa, J.A.S.; Hallak, J.E.C. Antidepressant Effects of a Single Dose of Ayahuasca in Patients with Recurrent Depression. J. Clin. Psychopharmacol. 2016, 36, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Palhano-Fontes, F.; Barreto, D.; Onias, H.; Andrade, K.C.; Novaes, M.M.; Pessoa, J.A.; Mota-Rolim, S.A.; Osório, F.L.; Sanches, R.; Dos Santos, R.G.; et al. Rapid Antidepressant Effects of the Psychedelic Ayahuasca in Treatment-Resistant Depression: A Randomized Placebo-Controlled Trial. Psychol. Med. 2019, 49, 655–663. [Google Scholar] [CrossRef]
- Tagliazucchi, E.; Roseman, L.; Kaelen, M.; Orban, C.; Muthukumaraswamy, S.; Murphy, K.; Laufs, H.; Leech, R.; Mcgonigle, J.; Crossley, N.; et al. Increased Global Functional Connectivity Correlates with LSD-Induced Ego Dissolution. Curr. Biol. 2016, 26, 1043–1050. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Roseman, L.; Bolstridge, M.; Demetriou, L.; Pannekoek, J.N.; Wall, M.B.; Tanner, M.; Kaelen, M.; Mcgonigle, J.; Murphy, K.; et al. Psilocybin for Treatment-Resistant Depression: FMRI-Measured Brain Mechanisms. Sci. Rep. 2017, 7, 13187. [Google Scholar] [CrossRef]
- Garcia-Romeu, A.; Richards, W.A. Current Perspectives on Psychedelic Therapy: Use of Serotonergic Hallucinogens in Clinical Interventions. Int. Rev. Psychiatry 2018, 30, 291–316. [Google Scholar] [CrossRef]
- Kraehenmann, R.; Preller, K.H.; Scheidegger, M.; Pokorny, T.; Bosch, O.G.; Seifritz, E.; Vollenweider, F.X. Psilocybin-Induced Decrease in Amygdala Reactivity Correlates with Enhanced Positive Mood in Healthy Volunteers. Biol. Psychiatry 2015, 78, 572–581. [Google Scholar] [CrossRef]
- Grimm, O.; Kraehenmann, R.; Preller, K.H.; Seifritz, E.; Vollenweider, F.X. Psilocybin Modulates Functional Connectivity of the Amygdala During Emotional Face Discrimination. Eur. Neuropsychopharmacol. 2018, 28, 691–700. [Google Scholar] [CrossRef]
- Davis, A.K.; Barrett, F.S.; Griffiths, R.R. Psychological Flexibility Mediates the Relations Between Acute Psychedelic Effects and Subjective Decreases in Depression and Anxiety. J. Contextual. Behav. Sci. 2020, 15, 39–45. [Google Scholar] [CrossRef]
- Liu, X.; Chan, C.-B.; Jang, S.-W.; Pradoldej, S.; Huang, J.; He, K.; Phun, L.H.; France, S.; Xiao, G.; Jia, Y.; et al. A Synthetic 7,8-Dihydroxyflavone Derivative Promotes Neurogenesis and Exhibits Potent Antidepressant Effect. J. Med. Chem. 2010, 53, 8274–8286. [Google Scholar] [CrossRef] [PubMed]
- Boltaev, U.; Meyer, Y.; Tolibzoda, F.; Jacques, T.; Gassaway, M.; Xu, Q.; Wagner, F.; Zhang, Y.L.; Palmer, M.; Holson, E.; et al. Multiplex Quantitative Assays Indicate a Need for Reevaluating Reported Small-Molecule TrkB Agonists. Sci. Signal. 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.C.; Maguschak, K.A.; Ye, K.; Jang, S.W.; Myers, K.M.; Ressler, K.J. Prelimbic Cortical BDNF is Required for Memory of Learned Fear but Not Extinction or Innate Fear. Proc. Natl. Acad. Sci. USA 2010, 107, 2675–2680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.C.; Yao, W.; Dong, C.; Yang, C.; Ren, Q.; Ma, M.; Han, M.; Hashimoto, K. Comparison of Ketamine, 7,8-Dihydroxyflavone, and Ana-12 Antidepressant Effects in the Social Defeat Stress Model of Depression. Psychopharmacology 2015, 232, 4325–4335. [Google Scholar] [CrossRef] [PubMed]
- English, A.W.; Liu, K.; Nicolini, J.M.; Mulligan, A.M.; Ye, K. Small-Molecule TrkB Agonists Promote Axon Regeneration in Cut Peripheral Nerves. Proc. Natl. Acad. Sci. USA 2013, 110, 16217–16222. [Google Scholar] [CrossRef]
- Zeng, Y.; Lv, F.; Li, L.; Yu, H.; Dong, M.; Fu, Q. 7,8-Dihydroxyflavone Rescues Spatial Memory and Synaptic Plasticity in Cognitively Impaired Aged Rats. J. Neurochem. 2012, 122, 800–811. [Google Scholar] [CrossRef]
- Wetsel, W.C.; Rodriguiz, R.M.; Guillemot, J.; Rousselet, E.; Essalmani, R.; Kim, I.H.; Bryant, J.C.; Marcinkiewicz, J.; Desjardins, R.; Day, R.; et al. Disruption of the Expression of the Proprotein Convertase Pc7 Reduces BDNF Production and Affects Learning and Memory in Mice. Proc. Natl. Acad. Sci. USA 2013, 110, 17362–17367. [Google Scholar] [CrossRef]
- Gupta, V.; You, Y.; Gupta, V.; Klistorner, A.; Graham, S. TrkB Receptor Signalling: Implications in Neurodegenerative, Psychiatric and Proliferative Disorders. Int. J. Mol. Sci. 2013, 14, 10122–10142. [Google Scholar] [CrossRef]
- Tsai, T.; Klausmeyer, A.; Conrad, R.; Gottschling, C.; Leo, M.; Faissner, A.; Wiese, S. 7,8-Dihydroxyflavone Leads to Survival of Cultured Embryonic Motoneurons by Activating Intracellular Signaling Pathways. Mol. Cell. Neurosci. 2013, 56, 18–28. [Google Scholar] [CrossRef]
- Wang, B.; Wu, N.; Liang, F.; Zhang, S.; Ni, W.; Cao, Y.; Xia, D.; Xi, H. 7,8-Dihydroxyflavone, a Small-Molecule Tropomyosin-Related Kinase B (TrkB) Agonist, Attenuates Cerebral Ischemia and Reperfusion Injury in Rats. J. Mol. Histol. 2014, 45, 129–140. [Google Scholar] [CrossRef]
- Jiang, M.; Peng, Q.; Liu, X.; Jin, J.; Hou, Z.; Zhang, J.; Mori, S.; Ross, C.A.; Ye, K.; Duan, W. Small-Molecule TrkB Receptor Agonists Improve Motor Function and Extend Survival in a Mouse Model of Huntington’s Disease. J. Mol. Histol. 2013, 22, 2462–2470. [Google Scholar] [CrossRef] [PubMed]
- Castello, N.A.; Nguyen, M.H.; Tran, J.D.; Cheng, D.; Green, K.N.; Laferla, F.M. 7,8-Dihydroxyflavone, a Small Molecule TrkB Agonist, Improves Spatial Memory and Increases Thin Spine Density in a Mouse Model of Alzheimer Disease-Like Neuronal Loss. PLoS ONE 2014, 9, e91453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, X.; Schroeder, J.P.; Chan, C.-B.; Song, M.; Yu, S.P.; Weinshenker, D.; Ye, K. 7,8-Dihydroxyflavone Prevents Synaptic Loss and Memory Deficits in a Mouse Model of Alzheimer’s Disease. Neuropsychopharmacology 2014, 39, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Rucker, J.J.H.; Iliff, J.; Nutt, D.J. Psychiatry & the Psychedelic Drugs. Past, Present & Future. Neuropharmacology 2018, 142, 200–218. [Google Scholar] [CrossRef]
- Johansen, P.-Ø.; Krebs, T.S. Psychedelics Not Linked to Mental Health Problems or Suicidal Behavior: A Population Study. J. Psychopharmacol. 2015, 29, 270–279. [Google Scholar] [CrossRef]
- Santos, R.G.; Osório, F.L.; Crippa, J.A.; Riba, J.; Zuardi, A.W.; Hallak, J.E. Antidepressive, Anxiolytic, and Antiaddictive Effects of Ayahuasca, Psilocybin and Lysergic Acid Diethylamide (LSD): A Systematic Review of Clinical Trials Published in the Last 25 Years. Ther. Adv. Psychopharmacol. 2016, 6, 193–213. [Google Scholar] [CrossRef]
- Wegener, G.; Rujescu, D. The Current Development of CNS Drug Research. Int. J. Neuropsychopharmacol. 2013, 16, 1687–1693. [Google Scholar] [CrossRef]
- Mithoefer, M.C.; Mithoefer, A.T.; Feduccia, A.A.; Jerome, L.; Wagner, M.; Wymer, J.; Holland, J.; Hamilton, S.; Yazar-Klosinski, B.; Emerson, A.; et al. 3,4-Methylenedioxymethamphetamine (MDMA)-Assisted Psychotherapy for Post-Traumatic Stress Disorder in Military Veterans, Firefighters, and Police Officers: A Randomised, Double-Blind, Dose-Response, Phase 2 Clinical Trial. The Lancet Psychiatry 2018, 5, 486–497. [Google Scholar] [CrossRef]
- Nutt, D.J.; King, L.A.; Phillips, L.D. Drug Harms in the UK: A Multicriteria Decision Analysis. Lancet 2010, 376, 1558–1565. [Google Scholar] [CrossRef]
- Ren, Q.; Zhang, J.-C.; Fujita, Y.; Ma, M.; Wu, J.; Hashimoto, K. Effects of TrkB Agonist 7,8-Dihydroxyflavone on Sensory Gating Deficits in Mice After Administration of Methamphetamine. Pharmacol. Biochem. Behav. 2013, 106, 124–127. [Google Scholar] [CrossRef]
- Coull, J.A.; Beggs, S.; Boudreau, D.; Boivin, D.; Tsuda, M.; Inoue, K.; Gravel, C.; Salter, M.W.; De Koninck, Y. BDNF From Microglia Causes the Shift in Neuronal Anion Gradient Underlying Neuropathic Pain. Nature 2005, 438, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, L.; Zhan, Y.; Li, D.; Zhang, Y.; Wang, G.; Zhang, M. Contribution of BDNF/TrkB Signalling in the rACC to the Development of Pain-Related Aversion Via Activation of ERK in Rats with Spared Nerve Injury. Brain Res. 2017, 1671, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, L.E.; Azinfar, A.; Ly, C.; Cameron, L.P.; Viswanathan, J.; Tombari, R.J.; Myers-Turnbull, D.; Taylor, J.C.; Grodzki, A.C.; Lein, P.J.; et al. Identification of Psychoplastogenic N,N-Dimethylaminoisotryptamine (isoDMT) Analogues Through Structure-Activity Relationship Studies. J. Med. Chem. 2020, 63, 1142–1155. [Google Scholar] [CrossRef] [PubMed]
| Study | Substance | Dosage | Timing | Strain | Main Findings |
|---|---|---|---|---|---|
| Andero et al., 2011 [71] | 7,8-DHF | 5 mg/kg i.p. | 60 min before procedures | C57BL/6J mice | Activation of amygdalar TrkB receptors by 7,8-DHF. 7,8-DHF enhanced fear acquisition and extinction. |
| Cameron et al., 2018 [60] | DMT | 10 mg/kg i.p. | 60 min before procedures; in FST, 30 min after pre-test and 6 h and 1 h prior to test | SD rats | Decreased exploratory behavior of rats in OF and EPM. The initial increase in freezing in fear conditioning was affected, but contextual and cued fear memory was not affected in the following days. Decreased freezing in cued fear extinction but not in context fear extinction. Reduced immobility in FST was equal to the effects of ketamine. |
| Catlow et al., 2013 [61] | psilocybin | 0.1–1.5 mg/kg i.p. | 1 d before procedures | C57BL/6J mice | Acquisition of conditioned fear response was intact in C57BL/6J mice. In extinction learning, low doses (0.1 mg/kg and 0.5 mg/kg) showed initial increase in freezing, but in later trials, they facilitated fear extinction. Low dose (0.1 mg/kg) caused trend towards increase, but high dose (1 mg/kg) caused decrease in hippocampal neurogenesis. |
| Chen et al., 2018 [72] | R13 - 7,8-DHF prodrug | 7.25–24.6 mg/kg p.o. | daily, from 2 to 5 mo of age | 5XFAD mice | Increased bioavailability, a reversal of hippocampal synaptic loss, an increase in hippocampal LTP magnitude, a decrease in Aβ deposition in the hippocampus and frontal cortex, and improvement in spatial learning and memory in 5XFAD mice. |
| Dhonnchadha et al., 2002 [59] | DOI | 0.25–4 mg/kg i.p. | 30 min before procedures | Swiss mice | Anxiolytic effect in four-plate test (increase in punished passages) and EPM (increased number of open arm entries). No effects in light/dark box test. |
| Jefsen et al., 2019 [64] | psilocin, psilocybin | 0.5–2 mg/kg for psilocin; 2–10 mg/kg for psilocybin | single dose 4–24 h before procedures; 3 doses in 3 d, last injection 8 d before procedures | FSL rats | Psilocin and psilocybin failed to promote any effects on behavior in FST. |
| Study | Substance | Dosage | Timing | Design | N | Main Findings |
|---|---|---|---|---|---|---|
| Carhart-Harris et al., 2016, 2017, 2018 [76,79,88] | psilocybin | 10 and 25 mg p.o. | low and high dose 7 days apart at the beginning of the sessions | open-label; no control group; low dose for safety assessment | 12–20 | The decrease in depressive symptoms and anxiety at 1 week, 3 months, and 6 months post-treatment. Decreased CBF in structures of the temporal lobe, including the amygdala, 1-day post-treatment. The decrease in amygdala CBF correlated with a reduction in depressive symptoms. |
| Davis et al., 2020 [92] | psilocybin mushrooms, psilocybin, LSD, ayahuasca, mescaline, DMT, etc. | single recreational dose | questionnaires 3 mo before and 3 mo after the psychedelic experience | an internet-based cross-sectional survey of recreational users | 985 | Acute effects were associated with decreases in depression and/or anxiety. The decrease was fully mediated by psychological flexibility. |
| Gasser et al., 2014, 2015 [81,82] | LSD | 20 µg (active placebo) or 200 µg | single dose at the beginning of the session | open-label; cross-over; with initial blinding | 12 | Decrease in state anxiety at 2 months and sustained at 12 months follow-up. No changes in trait anxiety. Subjective reports of a higher quality of life. |
| Griffiths et al., 2016 [80] | psilocybin | 1 or 3 mg/70 kg (active placebo) and 22 or 30 mg/70 kg p.o. | low or high dose at the beginning of 2 sessions; 38 d between the sessions | randomized, double-blind, cross-over trial | 51 | High dose produced a decrease in depressive symptoms and anxiety in cancer patients with life-threatening diagnoses. It also increased the quality of life, life meaning, and optimism. Changes were sustained at a 6-month follow-up. The effects were mediated by mystical-type experiences on session day. |
| Palhano-Fontes et al., 2019 [86] | DMT, harmine, harmaline, tetrahydroharmine (ayahuasca) | 0.36, 1.86, 0.24, 1.2 mg/kg p.o. | single dose at the beginning of the session | randomized, double-blind, cross-over trial | 29 | Substantial decrease in depression symptoms on day 1, day 2, and day 7 post-treatment. Response rate was 64% vs. 27% in favor of ayahuasca. Remission rate showed a trend towards significance at day 7 (36% vs. 7%). |
| Ross et al., 2016 [77] | psilocybin | 0.3 mg/kg p.o. | single dose at the beginning of the session | randomized, double-blind, cross-over trial | 29 | Immediate, substantial, and sustained decrease in depression symptoms and anxiety in cancer patients. The effects were significant after session 1 until cross-over 7 weeks later. After cross-over, both groups showed a substantial reduction in depression symptoms and anxiety compared to baseline. These effects were sustained at a 6.5-month follow-up and were mediated by mystical-like experiences on session day. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benko, J.; Vranková, S. Natural Psychoplastogens As Antidepressant Agents. Molecules 2020, 25, 1172. https://doi.org/10.3390/molecules25051172
Benko J, Vranková S. Natural Psychoplastogens As Antidepressant Agents. Molecules. 2020; 25(5):1172. https://doi.org/10.3390/molecules25051172
Chicago/Turabian StyleBenko, Jakub, and Stanislava Vranková. 2020. "Natural Psychoplastogens As Antidepressant Agents" Molecules 25, no. 5: 1172. https://doi.org/10.3390/molecules25051172
APA StyleBenko, J., & Vranková, S. (2020). Natural Psychoplastogens As Antidepressant Agents. Molecules, 25(5), 1172. https://doi.org/10.3390/molecules25051172






