Magnetic Solid-Phase Extraction of Organic Compounds Based on Graphene Oxide Nanocomposites
Abstract
1. Introduction
2. Preparation and Applications of GO for the MSPE of Organic Compounds
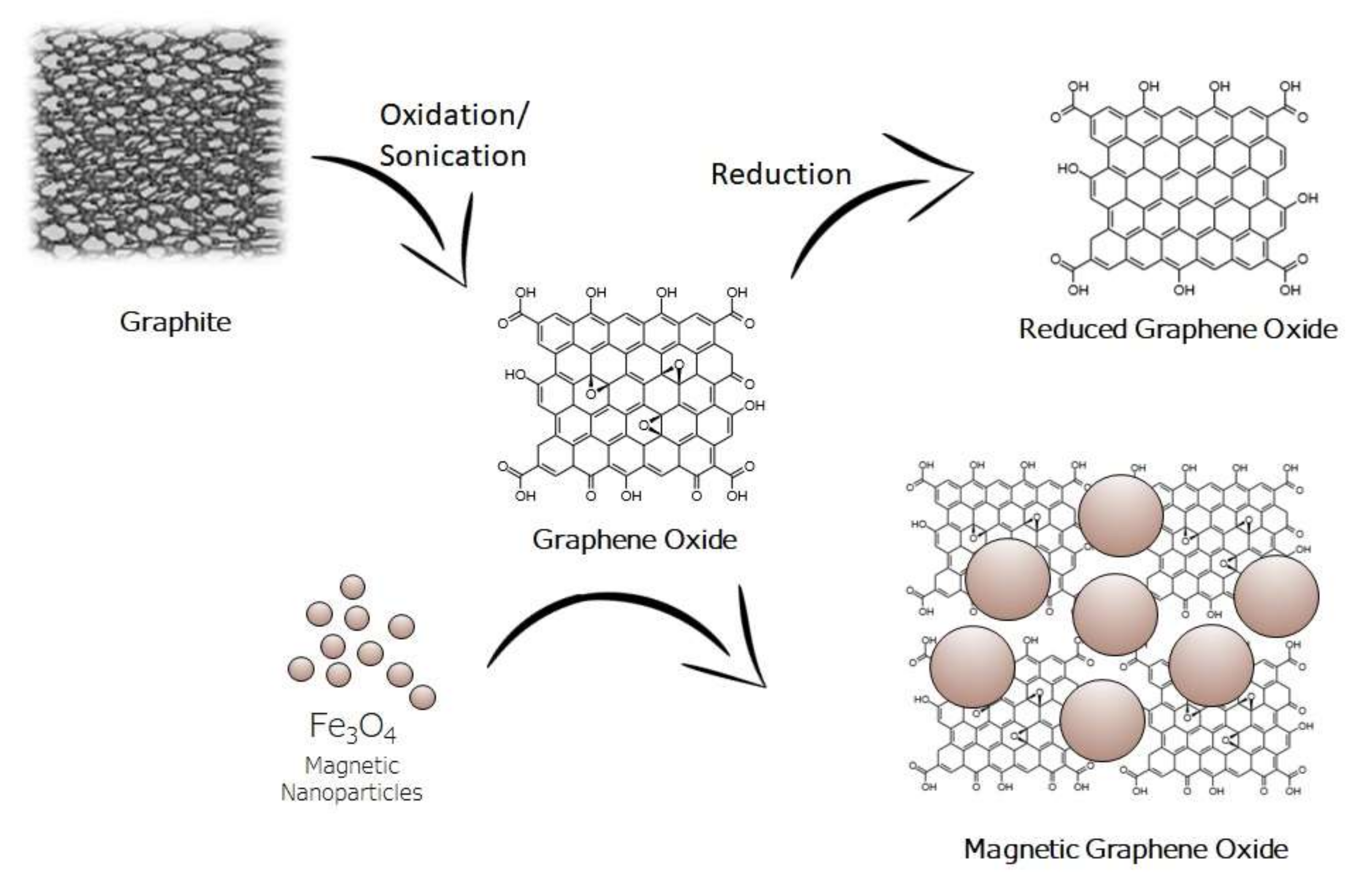
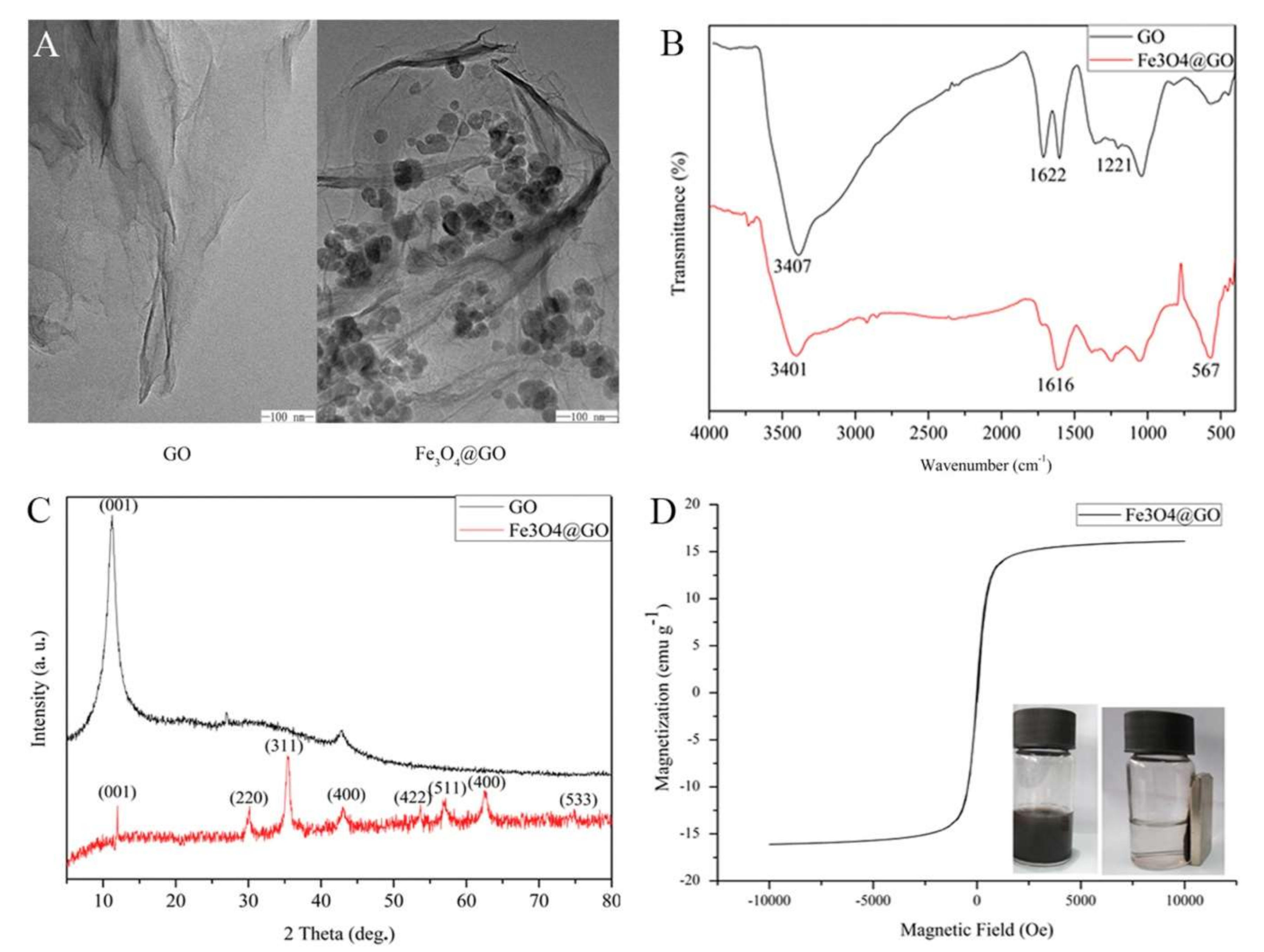
2.1. Nanocomposites of GO with Fe3O4 Nanoparticles
2.2. Nanocomposites of Reduced GO with Fe3O4 Nanoparticles
2.3. Functionalized Nanocomposites of GO with Fe3O4 Nanoparticles
2.4. Functionalized Nanocomposites of Magnetic GO with MIPs
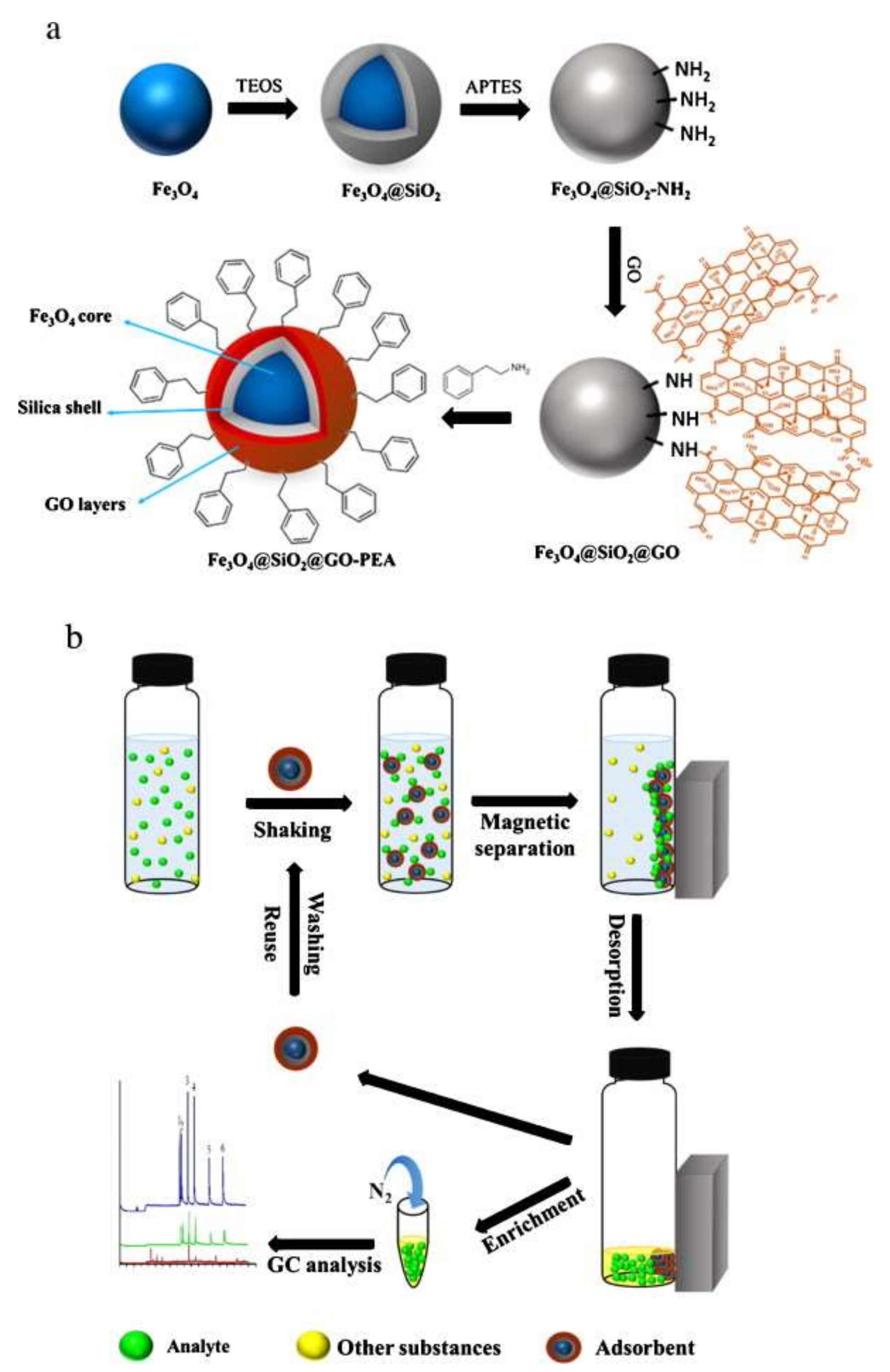
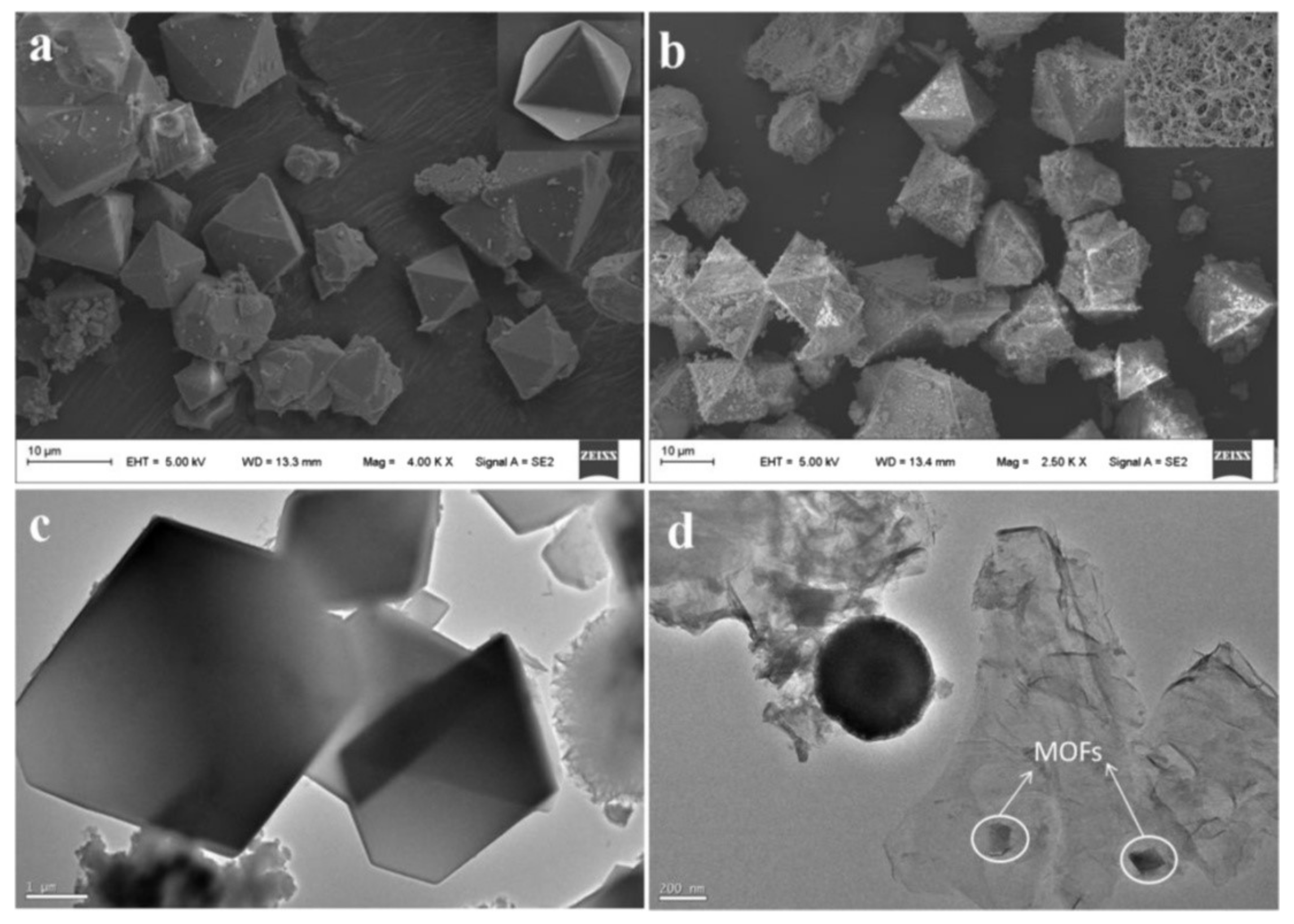
2.5. Functionalized Nanocomposites of Magnetic GO with MOFs
2.6. Functionalized Nanocomposites of Magnetic GO with MOPs
2.7. Applications of Magnetic GO Nanocomposites Modified with Ionic Liquids (ILs) and Deep Eutectic Solvents (DESs)
3. Conclusions
Funding
Conflicts of Interest
References
- Samanidou, V. Trends in Microextraction Techniques for Sample Preparation. Separations 2017, 5, 1. [Google Scholar] [CrossRef]
- Manousi, N.; Raber, G.; Papadoyannis, I. Recent Advances in Microextraction Techniques of antipsychotics in Biological Fluids Prior to Liquid Chromatography Analysis. Separations 2017, 4, 18. [Google Scholar] [CrossRef]
- Filippou, O.; Bitas, D.; Samanidou, V. Green approaches in sample preparation of bioanalytical samples prior to chromatographic analysis. J. Chromatogr. B 2017, 1043, 44–62. [Google Scholar] [CrossRef] [PubMed]
- Kissoudi, M.; Samanidou, V. Recent Advances in Applications of Ionic Liquids in Miniaturized Microextraction Techniques. Molecules 2018, 23, 1437. [Google Scholar] [CrossRef]
- Manousi, N.; Zachariadis, G. Determination of Volatile Compounds in Nut-Based Milk Alternative Beverages by HS-SPME Prior to GC-MS Analysis. Molecules 2019, 24, 3091. [Google Scholar] [CrossRef]
- Anthemidis, A.; Ioannou, K. Sequential injection ionic liquid dispersive liquid–liquid microextraction for thallium preconcentration and determination with flame atomic absorption spectrometry. Anal. Bioanal. Chem. 2012, 404, 685–691. [Google Scholar] [CrossRef]
- Karageorgou, E.; Manousi, N.; Samanidou, V.; Kabir, A.; Furton, K. Fabric phase sorptive extraction for the fast isolation of sulfonamides residues from raw milk followed by high performance liquid chromatography with ultraviolet detection. Food Chem. 2016, 196, 428–436. [Google Scholar] [CrossRef]
- Manousi, N.; Gomez-Gomez, B.; Madrid, Y.; Deliyanni, E.; Zachariadis, G. Determination of rare earth elements by inductively coupled plasma-mass spectrometry after dispersive solid phase extraction with novel oxidized graphene oxide and optimization with response surface methodology and central composite design. Microchem. J. 2019, 152, 104428. [Google Scholar] [CrossRef]
- Giakisikli, G.; Anthemidis, A. Magnetic materials as sorbents for metal/metalloid preconcentration and/or separation. A review. Analyt. Chim. Acta 2013, 789, 1–16. [Google Scholar] [CrossRef]
- Hemmati, M.; Rajabi, M.; Asghari, A. Magnetic nanoparticle based solid-phase extraction of heavy metal ions: A review on recent advances. Microchim. Acta 2018, 185. [Google Scholar] [CrossRef]
- Ahmadi, F.; Rajabi, M.; Faizi, F.; Rahimi-Nasrabadi, M.; Maddah, B. Magnetic solid-phase extraction of Zineb by C18-functionalised paramagnetic nanoparticles and determination by first-derivative spectrophotometry. Int. J. Environ. Anal. Chem. 2014, 94, 1123–1138. [Google Scholar] [CrossRef]
- Filippou, O.; Deliyanni, E.; Samanidou, V. Fabrication and evaluation of magnetic activated carbon as adsorbent for ultrasonic assisted magnetic solid phase dispersive extraction of bisphenol A from milk prior to high performance liquid chromatographic analysis with ultraviolet detection. J. Chromatogr. A 2017, 1479, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Shi, Y. Recent advances and applications of carbon nanotubes based composites in magnetic solid-phase extraction. Trac Trends Anal. Chem. 2019, 118, 652–665. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Samanidou, V.; Stalikas, C.D. Graphene-functionalized melamine sponges for microextraction of sulfonamides from food and environmental samples. J. Chromatogr. A 2017, 1522, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ziaei, E.; Mehdinia, A.; Jabbari, A. A novel hierarchical nanobiocomposite of graphene oxide–magnetic chitosan grafted with mercapto as a solid phase extraction sorbent for the determination of mercury ions in environmental water samples. Anal. Chim. Acta 2014, 850, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Gan, N.; Qiao, L.; Zhang, J.; Cao, Y.; Chen, Y. Magnetic metal-organic frameworks coated stir bar sorptive extraction coupled with GC-MS for determination of polychlorinated biphenyls in fish samples. Talanta 2015, 144, 1139–1145. [Google Scholar] [CrossRef]
- Wang, M.; Gao, M.; Zhang, K.; Wang, L.; Wang, W.; Fu, Q.; Xia, Z.; Gao, D. Magnetic covalent organic frameworks with core-shell structure as sorbents for solid phase extraction of fluoroquinolones, and their quantitation by HPLC. Microchim. Acta 2019, 186. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Y.; Liu, H.; Liu, G.; Xu, X.; Li, L.; Lv, J.; Liu, Z.; Zhou, W.; Xu, D. Magnetic Solid-Phase Extraction of Dichlorodiphenyltrichloroethane and Its Metabolites from Environmental Water Samples Using Ionic Liquid Modified Magnetic Multiwalled Carbon Nanotube/Zeolitic Imidazolate Framework-8 as Sorbent. Molecules 2019, 24, 2758. [Google Scholar] [CrossRef]
- Kyzas, C.Z.; Deliyanni, E.A.; Bikiaris, D.N.; Mitropoulos, A.C. Graphene composites as dye adsorbents: Review. Chem. Eng. Res. Des. 2018, 129, 75–88. [Google Scholar] [CrossRef]
- Travlou, N.A.; Kyzas, C.Z.; Lazaridis, N.K.; Deliyanni, E.A. Functionalization of graphite oxide with magnetic chitosan for the preparation of a nanocomposite dye adsorbent. Langmuir. 2013, 29, 1657–1668. [Google Scholar] [CrossRef]
- Hummers, W.; Offeman, R. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Travlou, N.A.; Kalogirou, O.; Deliyanni, E. Magnetic graphene oxide: Effect of preparation route on Reactive Black 5 adsorption. Materials 2013, 6, 1360–1376. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Deliyanni, E.; Matis, K.A. Graphene oxide and its application as adsorbent to waste water treatment. J. Chem. Technol. Biotechnol 2014, 89, 196–205. [Google Scholar] [CrossRef]
- Rekos, K.; Kampouraki, Z.C.; Sarafidis, C.; Samanidou, V.; Deliyanni, E. Graphene Oxide Based Magnetic Nanocomposites with Polymers as Effective Bisphenol–A Nanoadsorbents. Materials 2019, 12, 1987. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano. Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Sun, J.; Liang, Q.; Han, Q.; Zhang, X.; Ding, M. One-step synthesis of magnetic graphene oxide nanocomposite and its application in magnetic solid phase extraction of heavy metal ions from biological samples. Talanta 2015, 132, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, E.; Dadfarnia, S.; Haji Shabani, A. Dispersive solid phase microextraction with magnetic graphene oxide as the sorbent for separation and preconcentration of ultra-trace amounts of gold ions. Talanta 2015, 141, 273–278. [Google Scholar] [CrossRef]
- AlKinani, A.; Eftekhari, M.; Gheibi, M. Ligandless dispersive solid phase extraction of cobalt ion using magnetic graphene oxide as an adsorbent followed by its determination with electrothermal atomic absorption spectrometry. Int. J. Environ. Anal. Chem. 2019, 1–18. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Babaei, A.; Zeeb, M.; Es-haghi, A. Magnetic dispersive solid-phase extraction based on graphene oxide/Fe3O4@polythionine nanocomposite followed by atomic absorption spectrometry for zinc monitoring in water, flour, celery and egg. J. Sci. Food Agric. 2018, 98, 3571–3579. [Google Scholar] [CrossRef]
- Wen, Y.; Niu, Z.; Ma, Y.; Ma, J.; Chen, L. Graphene oxide-based microspheres for the dispersive solid-phase extraction of non-steroidal estrogens from water samples. J. Chromatogr. A 2014, 1368, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Wang, Z.; Xia, J.; Chen, S.; Zhang, X.; Ding, M. Facile and tunable fabrication of Fe3O4/graphene oxide nanocomposites and their application in the magnetic solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. Talanta 2012, 101, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xiao, D.; Zhao, H.; He, H.; Peng, J.; Wang, C.; Zhang, C.; He, J. A nanocomposite consisting of graphene oxide and Fe3O4 magnetic nanoparticles for the extraction of flavonoids from tea, wine and urine samples. Microchim. Acta 2015, 182, 2299–2306. [Google Scholar] [CrossRef]
- Zeng, S.; Gan, N.; Weideman-Mera, R.; Cao, Y.; Li, T.; Sang, W. Enrichment of polychlorinated biphenyl 28 from aqueous solutions using Fe3O4 grafted graphene oxide. Chem. Eng. J. 2013, 218, 108–115. [Google Scholar] [CrossRef]
- Chen, X.; Hai, X.; Wang, J. Graphene/graphene oxide and their derivatives in the separation/isolation and preconcentration of protein species: A review. Anal. Chim. Acta 2016, 922, 1–10. [Google Scholar] [CrossRef]
- Sitko, R.; Zawisza, B.; Malicka, E. Graphene as a new sorbent in analytical chemistry. Trac Trends Anal. Chem. 2013, 51, 33–43. [Google Scholar] [CrossRef]
- Ibrahim, W.; Nodeh, H.; Sanagi, M. Graphene-Based Materials as Solid Phase Extraction Sorbent for Trace Metal Ions, Organic Compounds, and Biological Sample Preparation. Crit. Rev. Anal. Chem. 2015, 46, 267–283. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, J.; Jiang, G. Application of graphene in analytical sample preparation. Trac Trends Anal. Chem. 2012, 37, 1–11. [Google Scholar] [CrossRef]
- Ye, N.; Shi, P. Applications of Graphene-Based Materials in Solid-Phase Extraction and Solid-Phase Microextraction. Sep. Purif. Rev. 2014, 44, 183–198. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Li, W.; Yang, Y.; Qin, P.; Zhang, X.; Lu, M. Magnetic graphene oxide nanocomposites as the adsorbent for extraction and pre-concentration of azo dyes in different food samples followed by high-performance liquid chromatography analysis. Food Addit. Contam. A 2018, 35, 2099–2110. [Google Scholar] [CrossRef]
- Costa dos Reis, L.; Vidal, L.; Canals, A. Graphene oxide/Fe3O4 as sorbent for magnetic solid-phase extraction coupled with liquid chromatography to determine 2,4,6-trinitrotoluene in water samples. Anal. Bioanal. Chem. 2017, 409, 2665–2674. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Yu, L.; Ding, X.; Li, P.; Dai, X.; Chen, X.; Zhou, H.; Bai, Y.; Ding, J. Magnetic solid-phase extraction based on graphene oxide for the determination of lignans in sesame oil. Food Chem. 2017, 217, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Pashaei, Y.; Ghorbani-Bidkorbeh, F.; Shekarchi, M. Superparamagnetic graphene oxide-based dispersive-solid phase extraction for preconcentration and determination of tamsulosin hydrochloride in human plasma by high performance liquid chromatography-ultraviolet detection. J. Chromatogr. A 2017, 1499, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Taghvimi, A.; Hamishehkar, H.; Ebrahimi, M. Magnetic nano graphene oxide as solid phase extraction adsorbent coupled with liquid chromatography to determine pseudoephedrine in urine samples. J. Chromatogr. B 2016, 1009–1010, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Guo, H.; Zhang, Y.; Tang, X.; Lei, W.; Qi, R.; Chu, J.; Li, D.; Zhao, Q. Graphene oxide-Fe3O4 nanocomposite magnetic solid phase extraction followed by UHPLC-MS/MS for highly sensitive determination of eight psychoactive drugs in urine samples. Talanta 2019, 206, 120212. [Google Scholar] [CrossRef] [PubMed]
- Taghvimi, A.; Hamishehkar, H.; Ebrahimi, M. The application of magnetic nano graphene oxide in determination of methamphetamine by high performance liquid chromatography of urine samples. J. Iran. Chem. Soc. 2016, 13, 1471–1480. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, H. Magnetic graphene oxide as adsorbent for the determination of polycyclic aromatic hydrocarbon metabolites in human urine. J. Sep. Sci. 2014, 37, 2591–2598. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, X.; Li, X.; Li, C.; Wang, R.; Chen, M. Development of Ultra-sensitive Method for Determination of Trace Atrazine Herbicide in Environmental Water Using Magnetic Graphene Oxide-Based Solid-Phase Extraction Coupled with Dispersive Liquid-Liquid Microextraction Prior to Gas Chromatography-Mass Spectrometry. Water Air Soil Poll. 2018, 229. [Google Scholar]
- Ma, L.; Huang, J.; Zhou, M. Magnetic graphene oxide for efficient solid phase extraction of DEHP. IOP Conf. Ser. Mater. sci. eng. 2019, 544, 012063. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Tang, Y.; Li, X.; Zhang, X.; Li, C.; Xu, S. Magnetic solid-phase extraction based on Fe3O4/graphene oxide nanoparticles for the determination of malachite green and crystal violet in environmental water samples by HPLC. Int. J. Environ. Anal. Chem. 2018, 98, 215–228. [Google Scholar] [CrossRef]
- Arvand, M.; Masouleh, A. Magnetic solid-phase extraction of imatinib and doxorubicin as cytostatic drugs by Fe3O4/graphene oxide nanocomposite. J. Iran. Chem. Soc. 2017, 14, 1673–1682. [Google Scholar] [CrossRef]
- Shi, P.; Ye, N. Magnetite–graphene oxide composites as a magnetic solid-phase extraction adsorbent for the determination of trace sulfonamides in water samples. Anal. Methods 2014, 6, 9725–9730. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Xiao, C.; Chen, L.; Yang, P.; Liu, Q.; Wang, J.; Liu, X. Rapid Determination of Trace Sulfonamides in Milk by Graphene Oxide-Based Magnetic Solid Phase Extraction Coupled with HPLC–MS/MS. Food Anal. Methods 2016, 9, 2521–2530. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Qi, M.; Zhong, S.; Wang, W.; Wang, A.; Chen, J. Graphene-Fe3O4as a magnetic solid-phase extraction sorbent coupled to capillary electrophoresis for the determination of sulfonamides in milk. J. Sep. Sci. 2016, 39, 3818–3826. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, E.; Dadfarnia, S.; Haji Shabani, A.; Abbasi, A.; Rashidian Vaziri, M.; Behjat, A. Iron oxide functionalized graphene oxide as an efficient sorbent for dispersive micro-solid phase extraction of sulfadiazine followed by spectrophotometric and mode-mismatched thermal lens spectrometric determination. Talanta 2016, 147, 561–568. [Google Scholar] [CrossRef]
- Wang, Y.; Wen, Y.; Ling, Y. Graphene Oxide-Based Magnetic Solid Phase Extraction Combined with High Performance Liquid Chromatography for Determination of Patulin in Apple Juice. Food Anal. Methods 2016, 10, 210–218. [Google Scholar] [CrossRef]
- Xiao, R.; Zhang, X.; Zhang, X.; Niu, J.; Lu, M.; Liu, X.; Cai, Z. Analysis of flavors and fragrances by HPLC with Fe3O4@GO magnetic nanocomposite as the adsorbent. Talanta 2017, 166, 262–267. [Google Scholar] [CrossRef]
- Bo, Z.; Shuai, X.; Mao, S.; Yang, H.; Qian, J.; Chen, J.; Yan, J.; Cen, K. Green preparation of reduced graphene oxide for sensing and energy storage applications. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef]
- Yuan, Y.; Sun, N.; Yan, H.; Han, D.; Row, K. Determination of indometacin and acemetacin in human urine via reduced graphene oxide-based pipette tip solid-phase extraction coupled to HPLC. Microchim. Acta 2015, 183, 799–804. [Google Scholar] [CrossRef]
- Chandra, V.; Park, J.; Chun, Y.; Lee, J.W.; Hwang, I.; Kim, K.S. Water-dispersible magnetite-reduced graphene oxide composites for arsenic removal. ACS Nano 2010, 4, 3979–3986. [Google Scholar] [CrossRef]
- Ai, L.; Zhang, C.; Chen, Z. Removal of methylene blue from aqueous solution by a solvothermal-synthesized graphene/magnetite composite. J. Hazard. Mater. 2011, 192, 1515–1524. [Google Scholar] [CrossRef]
- Gao, Y.; Ma, D.; Wang, C.; Guan, J.; Bao, X. Reduced graphene oxide as a catalyst for hydrogenation of nitrobenzene at room temperature. Chem. Commun. 2011, 47, 2432–2434. [Google Scholar] [CrossRef] [PubMed]
- Rowley-Neale, S.; Randviir, E.; Abo Dena, A.; Banks, C. An overview of recent applications of reduced graphene oxide as a basis of electroanalytical sensing platforms. Appl. Mater. Today 2018, 10, 218–226. [Google Scholar] [CrossRef]
- Qi, T.; Huang, C.; Yan, S.; Li, X.; Pan, S. Synthesis, characterization and adsorption properties of magnetite/reduced graphene oxide nanocomposites. Talanta 2015, 144, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Wang, C.; Luo, S.; Dong, R.; Tu, X.; Zeng, G. Adsorption of As (III) and As (V) from water using magnetite Fe3O4-reduced graphite oxide–MnO2 nanocomposites. Chem. Eng. J. 2012, 187, 45–52. [Google Scholar] [CrossRef]
- Yan, S.; Qi, T.; Chen, D.; Li, Z.; Li, X.; Pan, S. Magnetic solid phase extraction based on magnetite/reduced graphene oxide nanoparticles for determination of trace isocarbophos residues in different matrices. J. Chromatogr. A 2014, 1347, 30–38. [Google Scholar] [CrossRef]
- Wang, Q.; Jiao, L.; Du, H.; Wang, Y.; Yuan, H. Fe3O4 nanoparticles grown on graphene as advanced electrode materials for supercapacitors. J. Power Sources 2014, 245, 101–106. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, M.; Hao, Y.; Shi, X.; Wang, X. Effective extraction and simultaneous determination of Sudan dyes from tomato sauce and chili-containing foods using magnetite/reduced graphene oxide nanoparticles coupled with high-performance liquid chromatography. J. Sep. Sci. 2016, 39, 1749–1756. [Google Scholar] [CrossRef]
- Li, D.; Ma, X.; Wang, R.; Yu, Y. Determination of trace bisphenol A in environmental water by high-performance liquid chromatography using magnetic reduced graphene oxide based solid-phase extraction coupled with dispersive liquid–liquid microextraction. Anal. Bioanal.l Chem. 2016, 409, 1165–1172. [Google Scholar] [CrossRef]
- Mehdinia, A.; Rouhani, S.; Mozaffari, S. Microwave-assisted synthesis of reduced graphene oxide decorated with magnetite and gold nanoparticles, and its application to solid-phase extraction of organochlorine pesticides. Microchim. Acta 2016, 183, 1177–1185. [Google Scholar] [CrossRef]
- Li, N.; Chen, J.; Shi, Y. Magnetic polyethyleneimine functionalized reduced graphene oxide as a novel magnetic sorbent for the separation of polar non-steroidal anti-inflammatory drugs in waters. Talanta 2019, 191, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Li, N.A.; Chen, Y.; Shi, Y. Magnetic polyethyleneimine functionalized reduced graphene oxide as a novel magnetic solid-phase extraction adsorbent for the determination of polar acidic herbicides in rice. Anal. Chim. Acta 2017, 949, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Hu, X.; Zhao, F.; Zeng, B. Fe3O4/reduced graphene oxide-carbon nanotubes composite for the magnetic solid-phase extraction and HPLC determination of sulfonamides in milk. J. Sep. Sci. 2019, 42, 1058–1066. [Google Scholar] [PubMed]
- Pinsrithong, S.; Bunkoed, O. Hierarchical porous nanostructured polypyrrole-coated hydrogel beads containing reduced graphene oxide and magnetite nanoparticles for extraction of phthalates in bottled drinks. J. Chromatogr. A 2018, 1570, 19–27. [Google Scholar] [CrossRef]
- Mahpishanian, S.; Sereshti, H. One-step green synthesis of β-cyclodextrin/iron oxide-reduced graphene oxide nanocomposite with high supramolecular recognition capability: Application for vortex-assisted magnetic solid phase extraction of organochlorine pesticides residue from honey samples. J. Chromatogr. A. 2017, 1485, 32–43. [Google Scholar]
- Akbarzade, S.; Chamsaz, M.; Rounaghi, G.; Ghorbani, M. Zero valent Fe-reduced graphene oxide quantum dots as a novel magnetic dispersive solid phase microextraction sorbent for extraction of organophosphorus pesticides in real water and fruit juice samples prior to analysis by gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 2017, 410, 429–439. [Google Scholar]
- Yilmaz, E.; Ulusoy, H.; Demir, Ö.; Soylak, M. A new magnetic nanodiamond/graphene oxide hybrid (Fe3O4@ND@GO) material for pre-concentration and sensitive determination of sildenafil in alleged herbal aphrodisiacs by HPLC-DAD system. J. Chromatogr. B 2018, 1084, 113–121. [Google Scholar] [CrossRef]
- Musevi, S.; Mohammad-Rezaei, R.; Razmi, H. Magnetic solid-phase extraction of malachite green using soluble eggshell membrane protein doped with magnetic graphene oxide nanocomposite. Int. J. Environ. Anal. Chem. 2018, 98, 1242–1252. [Google Scholar] [CrossRef]
- Lotfi, Z.; Mousavi, H.; Sajjadi, S. Amino-terminated hyper-branched polyamidoamine polymer grafted magnetic graphene oxide nanosheets as an efficient sorbent for the extraction of selective serotonin reuptake inhibitors from plasma samples. Anal. Methods 2017, 9, 4504–4513. [Google Scholar] [CrossRef]
- Shi, P.; Ye, N. Investigation of the adsorption mechanism and preconcentration of sulfonamides using a porphyrin-functionalized Fe3O4-graphene oxide nanocomposite. Talanta 2015, 143, 219–225. [Google Scholar] [CrossRef]
- Pan, S.; Zhou, L.; Zhao, Y.; Chen, X.; Shen, H.; Cai, M.; Jin, M. Amine-functional magnetic polymer modified graphene oxide as magnetic solid-phase extraction materials combined with liquid chromatography-tandem mass spectrometry for chlorophenols analysis in environmental water. J. Chromatogr. A 2014, 1362, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Amiri, A.; Baghayeri, M.; Sedighi, M. Magnetic solid-phase extraction of polycyclic aromatic hydrocarbons using a graphene oxide/Fe3O4@polystyrene nanocomposite. Microchim. Acta 2018, 185. [Google Scholar] [CrossRef] [PubMed]
- Amiri, A.; Baghayeri, M.; Hamidi, E. Poly(pyrrole-co-aniline)@graphene oxide/Fe3O4 sorbent for the extraction and preconcentration of polycyclic aromatic hydrocarbons from water samples. New, J. Chem. 2018, 42, 16744–16751. [Google Scholar] [CrossRef]
- Daryakenary, M.; Zeeb, M. Trace determination of chlorpheniramine in human plasma using magnetic dispersive solid-phase extraction based on a graphene oxide/Fe3O4@polythionine nanocomposite combined with high-performance liquid chromatography. RSC Adv. 2017, 7, 53210–53218. [Google Scholar] [CrossRef]
- Zeeb, M.; Farahani, H. Graphene oxide/Fe3O4@polythionine nanocomposite as an efficient sorbent for magnetic solid-phase extraction followed by high-performance liquid chromatography for the determination of duloxetine in human plasma. Chem. Pap. 2017, 72, 15–27. [Google Scholar] [CrossRef]
- Ji, W.; Zhang, M.; Duan, W.; Wang, X.; Zhao, H.; Guo, L. Phytic acid-stabilized super-amphiphilic Fe3O4-graphene oxide for extraction of polycyclic aromatic hydrocarbons from vegetable oils. Food Chem. 2017, 235, 104–110. [Google Scholar] [CrossRef]
- Abdolmohammad-Zadeh, H.; Talleb, Z. Magnetic solid phase extraction of gemfibrozil from human serum and pharmaceutical wastewater samples utilizing a β-cyclodextrin grafted graphene oxide-magnetite nano-hybrid. Talanta 2015, 134, 387–393. [Google Scholar] [CrossRef]
- Chen, X.; Pan, S.; Ye, M.; Li, X.; Zhao, Y.; Jin, M. Magnetic solid-phase extraction based on a triethylenetetramine-functionalized magnetic graphene oxide composite for the detection of ten trace phenolic environmental estrogens in environmental water. J. Sep. Sci. 2016, 39, 762–768. [Google Scholar] [CrossRef]
- Cheng, Z.; Du, F.; Qin, Q.; Sun, L.; Zeng, Q.; Ruan, G.; Li, J. Graphene oxide composites for magnetic solid-phase extraction of trace cytokinins in plant samples followed by liquid chromatography-tandem mass spectrometry. J. Sep. Sci. 2018, 41, 2386–2392. [Google Scholar] [CrossRef]
- Markus, A.; Gbadamosi, A.; Yusuff, A.; Agi, A.; Oseh, J. Magnetite-sporopollenin/graphene oxide as new preconcentration adsorbent for removal of polar organophosphorus pesticides in vegetables. Environ. Sci. Poll. Res. 2018, 25, 35130–35142. [Google Scholar] [CrossRef]
- Mahpishanian, S.; Sereshti, H.; Baghdadi, M. Superparamagnetic core–shells anchored onto graphene oxide grafted with phenylethyl amine as a nano-adsorbent for extraction and enrichment of organophosphorus pesticides from fruit, vegetable and water samples. J. Chromatogr. A 2015, 1406, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Kechagia, M.; Samanidou, V.; Kabir, A.; Furton, K.G. One-pot synthesis of a multi-template molecularly imprinted polymer for the extraction of six sulfonamide residues from milk before high-performance liquid chromatography with diode array detection. J. Sep. Sci. 2018, 41, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Cormack, P.A.G.; Elorza, A.Z. Molecularly imprinted polymers: Synthesis and characterisation. J. Chromatogr. B 2004, 804(1), 173–182. [Google Scholar] [CrossRef] [PubMed]
- Masqueè, N.; Marceè, R.M.; Borrull, F. Molecularly imprinted polymers: New tailor-made materials for selective solid-phase extraction. TrAC–Trends Anal. Chem. 2001, 20, 477–485. [Google Scholar] [CrossRef]
- Ning, F.; Peng, H.; Li, J.; Chen, L.; Xiong, H. Molecularly Imprinted Polymer on Magnetic Graphene Oxide for Fast and Selective Extraction of 17β-Estradiol. J. Agric. Food Chem. 2014, 62, 7436–7443. [Google Scholar] [CrossRef]
- Barati, A.; Kazemi, E.; Dadfarnia, S.; Haji Shabani, A. Synthesis/characterization of molecular imprinted polymer based on magnetic chitosan/graphene oxide for selective separation/preconcentration of fluoxetine from environmental and biological samples. J. Ind. Eng. Chem. 2017, 46, 212–221. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, H. Hydrothermal synthesis of a metal-organic framework containing large rectangular channels. J. Am. Chem. Soc. 1995, 117, 10401–10402. [Google Scholar] [CrossRef]
- Hashemi, B.; Zohrabi, P.; Raza, N.; Kim, K. Metal-organic frameworks as advanced sorbents for the extraction and determination of pollutants from environmental, biological, and food media. Trends Anal. Chem. 2017, 97, 65–82. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Bandosz, T.J. Building MOF Nanocomposites with Oxidized Graphitic Carbon Nitride Nanospheres: The Effect of Framework Geometry on the Structural Heterogeneity. Molecules 2019, 24, 4529. [Google Scholar] [CrossRef]
- Zhou, H.; Long, J.R.; Yaghi, O.M. Introduction to metal-organic frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yagh, O.M. The chemistry and applications of metal-organic frameworks. Science. 2013, 341. [Google Scholar] [CrossRef] [PubMed]
- Pourbahman, F.; Zeeb, M.; Monzavi, A.; Homami, S. Simultaneous trace monitoring of prokinetic drugs in human plasma using magnetic dispersive micro-solid phase extraction based on a new graphene oxide/metal–organic framework-74/Fe3O4/polytyramine nanoporous composite in combination with HPLC. Chem. Pap. 2019, 73, 3135–3150. [Google Scholar] [CrossRef]
- Cao, X.; Jiang, Z.; Wang, S.; Hong, S.; Li, H.; Shao, Y.; She, Y.; Wang, J.; Jin, F.; Jin, M. One-pot synthesis of magnetic zeolitic imidazolate framework/grapheme oxide composites for the extraction of neonicotinoid insecticides from environmental water samples. J. Sep. Sci. 2017, 40, 4747–4756. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yao, W.; Zhou, C.; Wang, J.; Zhao, H. Fabrication of magnetic zeolitic imidazolate framework-7 supported graphene oxide for the extraction of fungicides from environmental water and soil samples. Int. J. Environ. Anal. Chem. 2019, 1–10. [Google Scholar] [CrossRef]
- Liu, G.; Li, L.; Huang, X.; Zheng, S.; Xu, D.; Xu, X.; Zhang, Y.; Lin, H. Determination of triazole pesticides in aqueous solution based on magnetic graphene oxide functionalized MOF-199 as solid phase extraction sorbents. Micropor. Mesopor. Mater. 2018, 270, 258–264. [Google Scholar] [CrossRef]
- Hua, X.; Gao, G.; Pan, S. High-affinity graphene oxide-encapsulated magnetic Zr-MOF for pretreatment and rapid determination of the photosensitizers hematoporphyrin and hematoporphyrin monomethyl ether in human urine prior to UPLC-HRMS. Anal. Bioanal. Chem. 2018, 410, 7749–7764. [Google Scholar] [CrossRef]
- Wang, X.; Ma, X.; Huang, P.; Wang, J.; Du, P.; Du, X.; Lu, X. Magnetic Cu-MOFs embedded within graphene oxide nanocomposites for enhanced preconcentration of benzenoid-containing insecticides. Talanta 2018, 181, 112–117. [Google Scholar] [CrossRef]
- Shahrebabak, S.M.; Saber-Tehrani, M.; Faraji, M.; Shabanian, M.; Aberoomand-Azar, P. Simultaneous magnetic solid phase extraction of acidic and basic pesticides using triazine-based polymeric network modified magnetic nanoparticles/graphene oxide nanocomposite in water and food samples. Microchem. J. 2019, 146, 630–639. [Google Scholar] [CrossRef]
- Jansen, J.C.; Esposito, E.; Fuoco, A.; Carta, M. Microporous Organic Polymers: Synthesis, Characterization, and Applications. Polymers 2019, 11, 844. [Google Scholar] [CrossRef]
- Li, Q.; Razzaque, S.; Jin, S.; Tan, B. Morphology design of microporous organic polymers and their potential applications: An overview. Sci. China Chem. 2017, 60, 1056–1066. [Google Scholar] [CrossRef]
- Cousins, K.; Zhang, R. Highly Porous Organic Polymers for Hydrogen Fuel Storage. Polymers 2019, 11, 690. [Google Scholar] [CrossRef] [PubMed]
- Faraji, M.; Shabanian, M.; Aryanasab, F. Efficient removal of anionic dyes from aqueous media using newly in situ synthesized triazine-based nitrogen-rich network modified magnetic nanoparticles. J. Iran. Chem. Soc. 2018, 15, 733–741. [Google Scholar] [CrossRef]
- Kaur, P.; Hupp, J.T.; Nguyen, S.T. Porous Organic Polymers in Catalysis: Opportunities and Challenges. ACS Catal. 2011, 1, 819–835. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, S. Recent Advancements in the Synthesis of Covalent Triazine Frameworks for Energy and Environmental Applications. Polymers 2018, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Hu, Y.; Li, G. Conjugated microporous polymers with built-in magnetic NPs for excellent enrichment of trace hydroxylated polycyclic aromatic hydrocarbons in human urine. Anal. Chem. 2016, 88, 6930–6938. [Google Scholar]
- Yang, W.; Wu, X.; Liu, T.; Wang, T.; Hou, X. Triazine-based conjugated microporous polymer composite for magnetic solid phase extraction of 5-nitroimidazoles coupled with UPLC-MS/MS for quantification. Analyst 2018, 143, 5744–5753. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Stalikas, C. Carbon-Based Nanomaterials Functionalized with Ionic Liquids for Microextraction in Sample Preparation. Separations 2017, 4, 14. [Google Scholar] [CrossRef]
- Han, D.; Row, K.H. Recent applications of ionic liquids in separation technology. Molecules 2010, 15, 2405–2426. [Google Scholar] [CrossRef]
- Serrano, M.; Chatzimitakos, T.; Gallego, M.; Stalikas, C.D. 1-butyl-3-aminopropyl imidazolium functionalized graphene oxide as a nanoadsorbent for the simultaneous extraction of steroids and β-blockers via dispersive solid-phase microextraction. J. Chromatogr. A 2016, 1436, 9–18. [Google Scholar] [CrossRef]
- Smith, E.; Abbott, A.; Ryder, K. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Xu, K.; Wang, Y.; Ding, X.; Huang, Y.; Li, N.; Wen, Q. Magnetic solid-phase extraction of protein with deep eutectic solvent immobilized magnetic graphene oxide nanoparticles. Talanta 2016, 148, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Wasserscheid, P.; Welton, T. Ionic Liquids in Synthesis, 2nd ed.; Wiley-VCH: Berlin, Germany, 2003. [Google Scholar]
- Cai, M.-Q.; Su, J.; Hu, J.-Q.; Wang, Q.; Dong, C.-Y.; Pan, S.-D.; Jin, M.-C. Planar graphene oxide-based magnetic ionic liquid nanomaterial for extraction of chlorophenols from environmental water samples coupled with liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2016, 1459, 38–46. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, H.; Xiao, D.; Chuong, P.; He, J.; He, H. Mixed hemimicelles solid-phase extraction of cephalosporins in biological samples with ionic liquid-coated magnetic graphene oxide nanoparticles coupled with high-performance liquid chromatographic analysis. J. Chromatogr. A 2016, 1454, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, H.; Zhang, Z.; Wu, X.; Chen, W.; Zhu, Y.; Fang, C.; Zhao, Y. Three-dimensional ionic liquid functionalized magnetic graphene oxide nanocomposite for the magnetic dispersive solid phase extraction of 16 polycyclic aromatic hydrocarbons in vegetable oils. J. Chromatogr. A 2017, 1489, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Lamei, N.; Ezoddin, M.; Ardestani, M.S.; Abdi, K. Dispersion of magnetic graphene oxide nanoparticles coated with a deep eutectic solvent using ultrasound assistance for preconcentration of methadone in biological and water samples followed by GC-FID and GC-MS. Anal. Bioanal. Chem. 2017, 409, 6113–6121. [Google Scholar] [CrossRef]

| Analyte | Sample Matrix | Synthetic Route | Analytical Technique | LODs (ng mL−1) | Recovery (%) | Reusability | Reference |
|---|---|---|---|---|---|---|---|
| Biological Samples | |||||||
| Tamsulosin hydrochloride | Plasma | Mix/Stirring | HPLC-UV | 0.17 | 98.1–101.4 | At least 4 times | [43] |
| Pseudoephedrine | Urine | Chemical coprecipitation | HPLC-UV | 25 | 96.42 | N.A. | [44] |
| Psychoactive drugs | Urine | Chemical coprecipitation | UHPLC-MS/MS | 0.02–0.2 | 80.4–105.5 | At least 10 times | [45] |
| Methamphetamine | Urine | Chemical coprecipitation | HPLC-UV | 30 | 93.5 | At least 1 time | [46] |
| PAH metabolites | Urine | Mix/Agitation | UHPLC-MS | 0.01–0.15 | 98.3–125.2 | N.A. | [47] |
| Environmental Samples | |||||||
| PCB 28 | Water | Chemical coprecipitation | GC-MS | 0.027–0.059 | 77.2–99.7 | N.A. | [34] |
| Atrazine | Water | Chemical coprecipitation | GC-MS | 0.6 × 10−3 | 96–102 | N.A. | [48] |
| DEHP | Water | Solvothermal approach | HPLC-DAD | 0.35 | 91.6–106.5 | N.A. | [49] |
| PAHs | Water | Chemical coprecipitation | HPLC-UV | 0.09–0.19 | 76.8–101.2 | N.A. | [32] |
| TNT | Water | Mix/Stirring | HPLC-UV | 0.3 | 87–120 | Up to 6 times | [41] |
| Malachite Green, Crystal Violet | Water | Chemical coprecipitation | HPLC-UV | 0.091–0.12 | 91.5–116 | N.A. | [50] |
| Imatinib, doxorubicin | Water | Mix/Stirring | HPLC-UV | 1.8–1.9 | 88.4–96.7 | N.A. | [51] |
| Sulfonamides | Water | Chemical coprecipitation | HPLC-DAD | 50–100 | 67.4–119.9 | N.A. | [52] |
| Food Samples | |||||||
| Sulfonamides | Milk | Chemical coprecipitation | HPLC-MS/MS | 0.02–0.13 | 73.4–97.4 | At least 6 times | [53] |
| Sulfonamides | Milk | Solvothermal approach | CE-DAD | 0.89–2.31 | 62.7–104.8 | At least 3 times | [54] |
| Sulfadiazine | Milk, honey, water | Chemical coprecipitation | Spectrophotometry | 340 | 94.3–100.7 | At least 10 times | [55] |
| Patulin | Apple juice | Chemical coprecipitation | HPLC-UV | 2.3 ng g−1 | 68.7–83.6 | At least 10 times | [56] |
| Flavors, fragrances | Orange juice, chocolate, fruit sugar | Chemical coprecipitation | HPLC-DAD | 20–40 | 71.5–112.4 | At least 5 times | [57] |
| Flavonoids | Tea, wine, urine | Solvothermal approach | HPLC-DAD | 0.2–6.0 | 82.0–101.4 | N.A. | [33] |
| Azo dyes | Jelly, candy, plum candy | Solvothermal approach | HPLC-UV | 0.36–2.23 ng g−1 | 73.2–107.7 | Up to 6 times | [40] |
| Lignans | Sesame oil | Hydroxylation, sonication | HPLC-UV | 20–50 ng g−1 | 84.6–86.8 | N.A. | [42] |
| Analyte | Sample Matrix | Functional Group | Analytical Technique | LODs (ng mL−1) | Recovery (%) | Reusability | Reference |
|---|---|---|---|---|---|---|---|
| Biological Samples | |||||||
| Chlorpheniramine | Plasma | Polythionine | HPLC-UV | 0.4 | 87.9–96.4 | At least 6 times | [84] |
| Duloxetine | Plasma | Polythionine | HPLC-UV | 0.5 | 87 | At least 9 times | [85] |
| Gemfibrozil | Serum | β-CD | Spectrofluorometry | 3 × 10−3 | 96.0–104.0 | At least 50 times | [87] |
| SSRIs | Plasma | PAMAM | HPLC-UV | 0.3–0.9 | 89.1–97.9 | Up to 20 times | [78] |
| Environmental Samples | |||||||
| Sulphonamide | Water | Porphyrin | HPLC-DAD | 200 | 83.7–116.7 | At least 8 times | [80] |
| Chlorophenols | Water | Poly (DVB-co-GMA) | HPLC-MS/MS | 0.6–9.2 | 86.4–99.8 | N.A. | [81] |
| PAHs | Water | Polystyrene | GC-FID | 3 × 10−3–10 × 10−3 | 69.5–88.7 | N.A. | [82] |
| PAHs | Water | Poly(pyrrole-co-aniline) | GC-FID | 0.003–0.01 | 50.4–78.3 | At least 20 times | [83] |
| Estrogens | Water | Triethylenetetramine | LC-MS/MS | 0.15–1.5 × 10−3 | 88.5–105.6 | N.A. | [88] |
| Malachite green | Water | SEP | UV Vis | 0.2 | 96.3 | At least 50 times | [79] |
| Food Samples | |||||||
| Organophosphorus pesticides | Vegetables | Sporopollenin | GC-ECD | 0.02–0.05 | 81.0–120.0 | N.A. | [90] |
| Organophosphorus pesticides | Fruit, vegetables, water | Phenylethyl amine | GC-NPD | 0.02–0.1 | >80.4 | At least 30 times | [91] |
| PAHs | Vegetable oils | Phytic acid | HPLC-DAD | 0.06–0.15 ng g−1 | 85.6–102.3 | At least 20 time | [86] |
| sildenafil citrate | Herbal supplementary products | Nanodiamond | HPLC-DAD | 1.49 | 94.0–104.1 | At least 10 times | [77] |
| Agricultural Samples | |||||||
| Cytokinins | Plants | Silica | HPLC-MS/MS | 93–120 × 103 | 78.9–97.3 | At least 30 times | [89] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manousi, N.; Rosenberg, E.; Deliyanni, E.; Zachariadis, G.A.; Samanidou, V. Magnetic Solid-Phase Extraction of Organic Compounds Based on Graphene Oxide Nanocomposites. Molecules 2020, 25, 1148. https://doi.org/10.3390/molecules25051148
Manousi N, Rosenberg E, Deliyanni E, Zachariadis GA, Samanidou V. Magnetic Solid-Phase Extraction of Organic Compounds Based on Graphene Oxide Nanocomposites. Molecules. 2020; 25(5):1148. https://doi.org/10.3390/molecules25051148
Chicago/Turabian StyleManousi, Natalia, Erwin Rosenberg, Eleni Deliyanni, George A. Zachariadis, and Victoria Samanidou. 2020. "Magnetic Solid-Phase Extraction of Organic Compounds Based on Graphene Oxide Nanocomposites" Molecules 25, no. 5: 1148. https://doi.org/10.3390/molecules25051148
APA StyleManousi, N., Rosenberg, E., Deliyanni, E., Zachariadis, G. A., & Samanidou, V. (2020). Magnetic Solid-Phase Extraction of Organic Compounds Based on Graphene Oxide Nanocomposites. Molecules, 25(5), 1148. https://doi.org/10.3390/molecules25051148










