Cu(II) Ion Adsorption by Aniline Grafted Chitosan and Its Responsive Fluorescence Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of a Tweezer-Like Adsorbent (CS-Ac-An)
2.3. Instrumentation
2.4. Sorption Studies
2.4.1. Equilibrium Experiments
2.4.2. Thermodynamic Studies
2.4.3. Kinetic Experiments
2.4.4. Isotherms and Modelling
2.4.5. Adsorbent Regeneration
2.5. UV-Vis and Fluorescence Spectroscopy
3. Results and Discussion
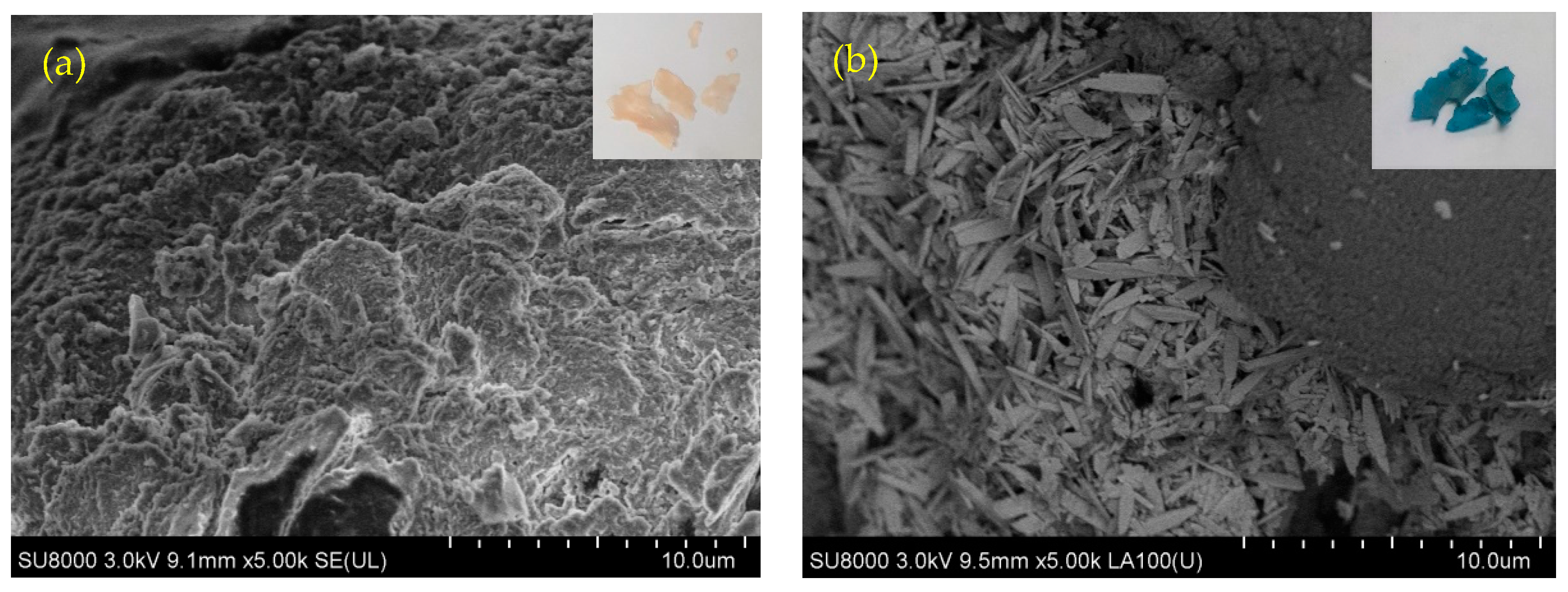
3.1. Characterization
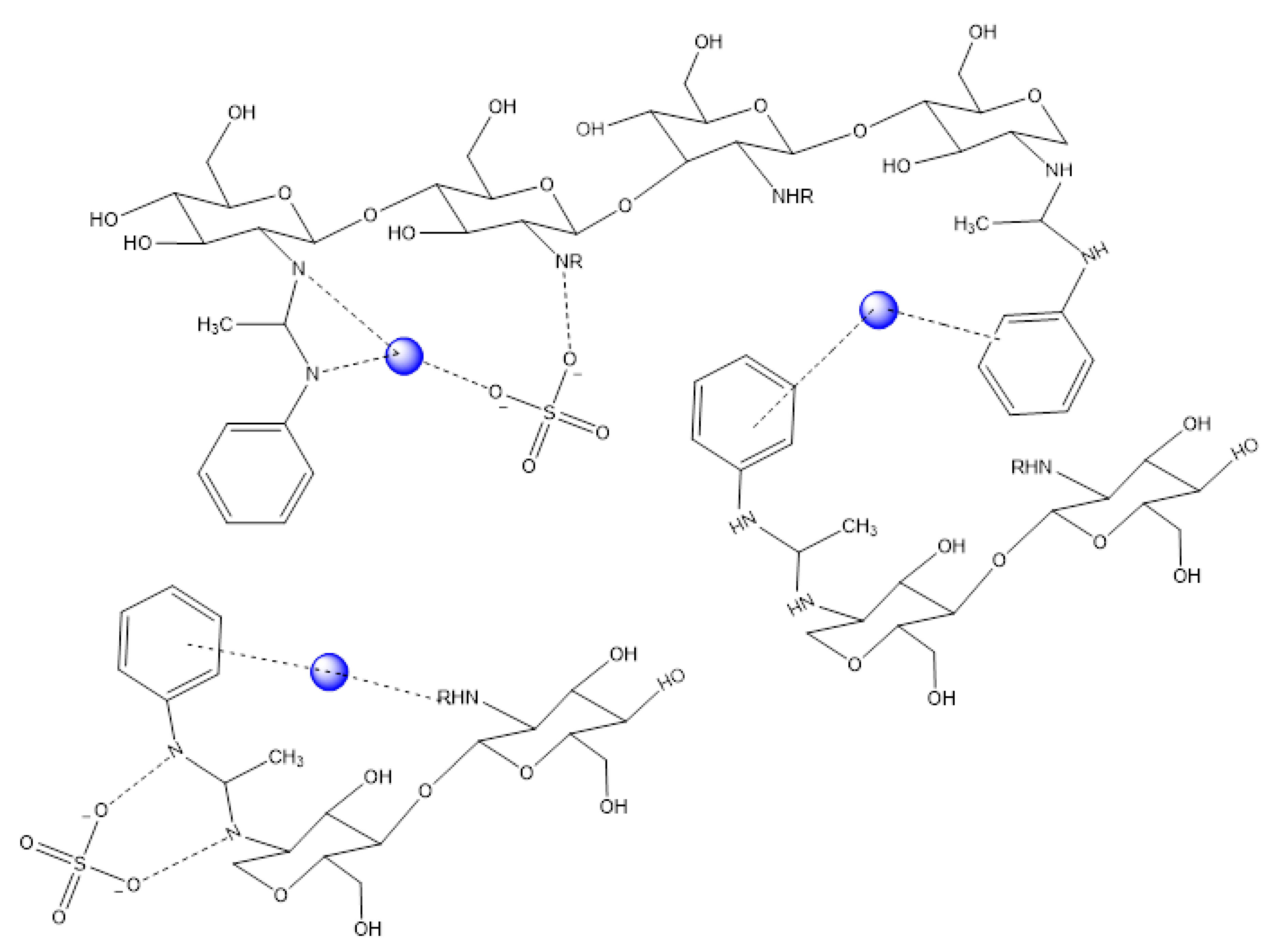
3.2. Adsorption Mechanism
3.2.1. FT-IR Spectroscopy
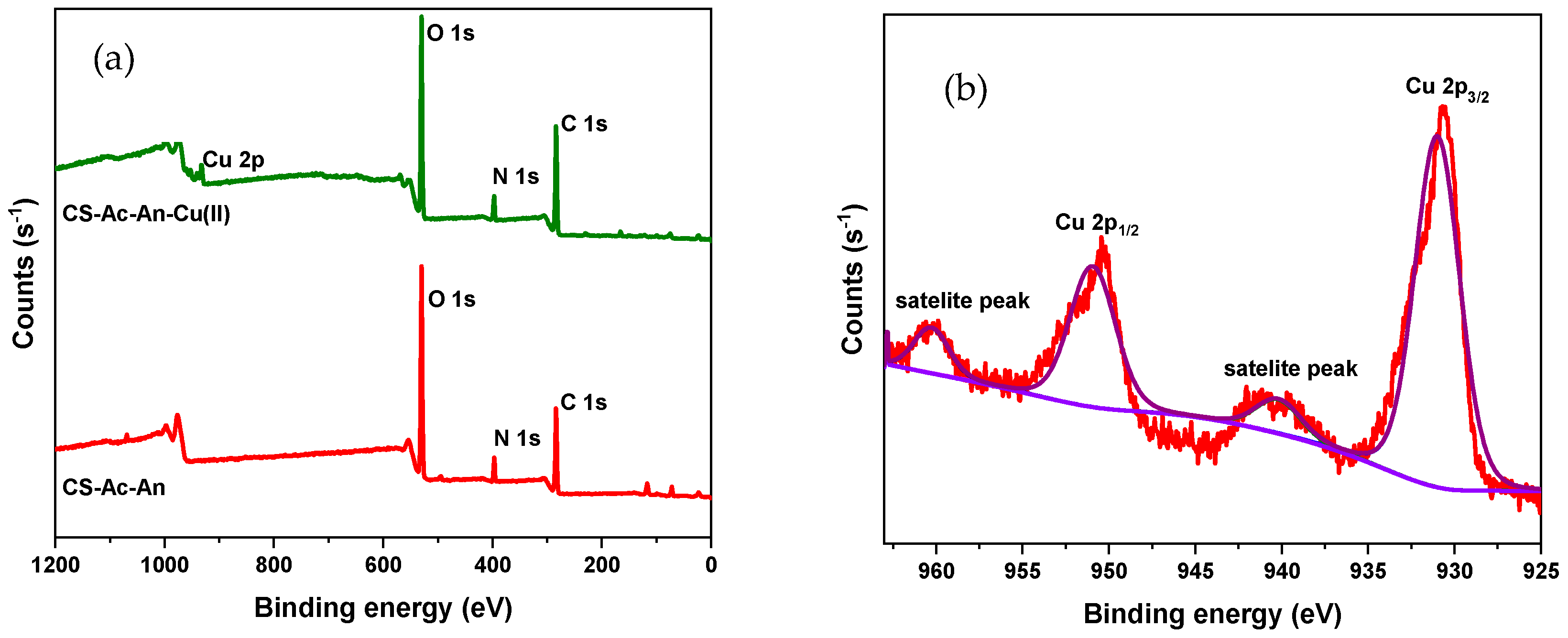
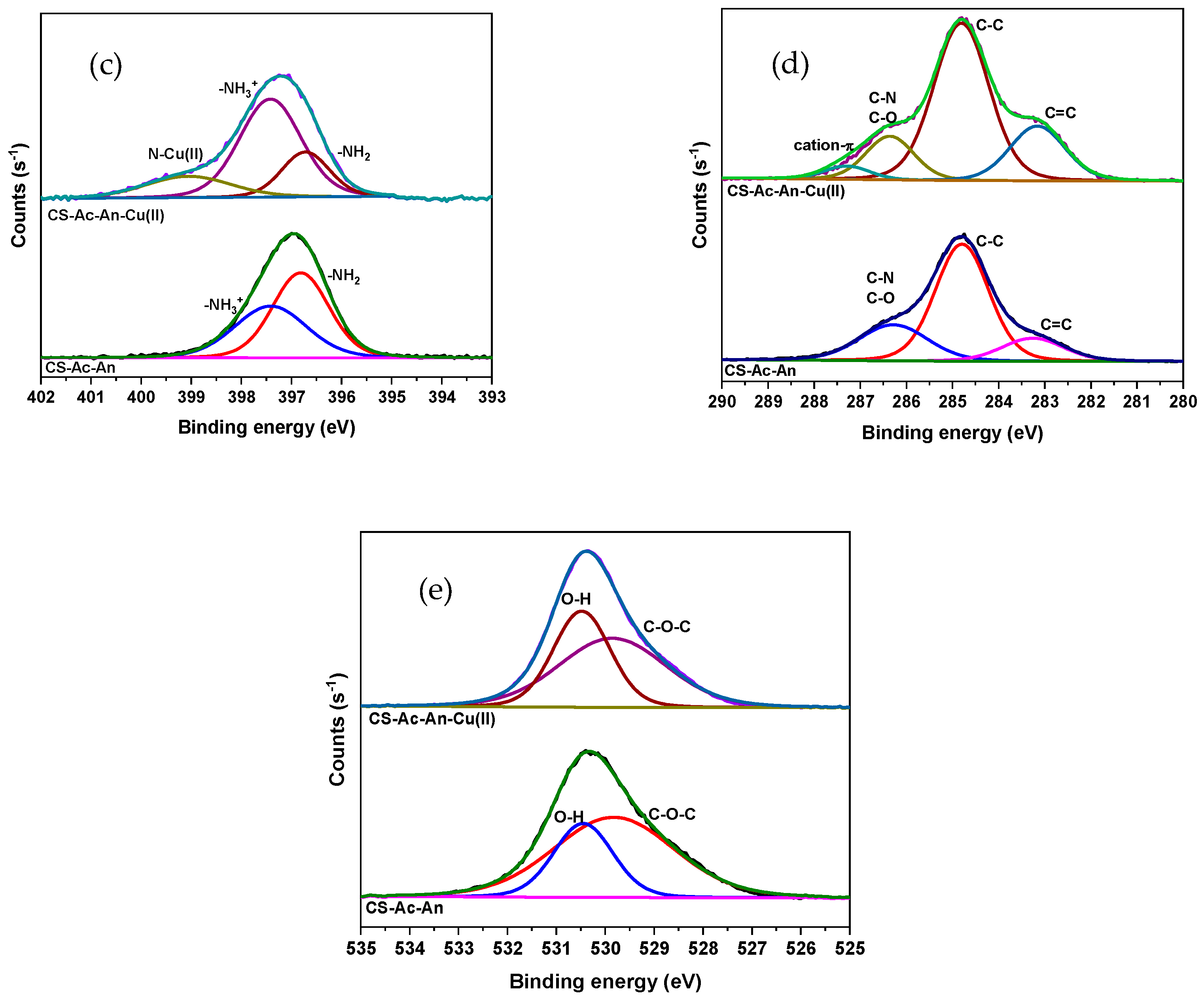
3.2.2. XPS Analysis
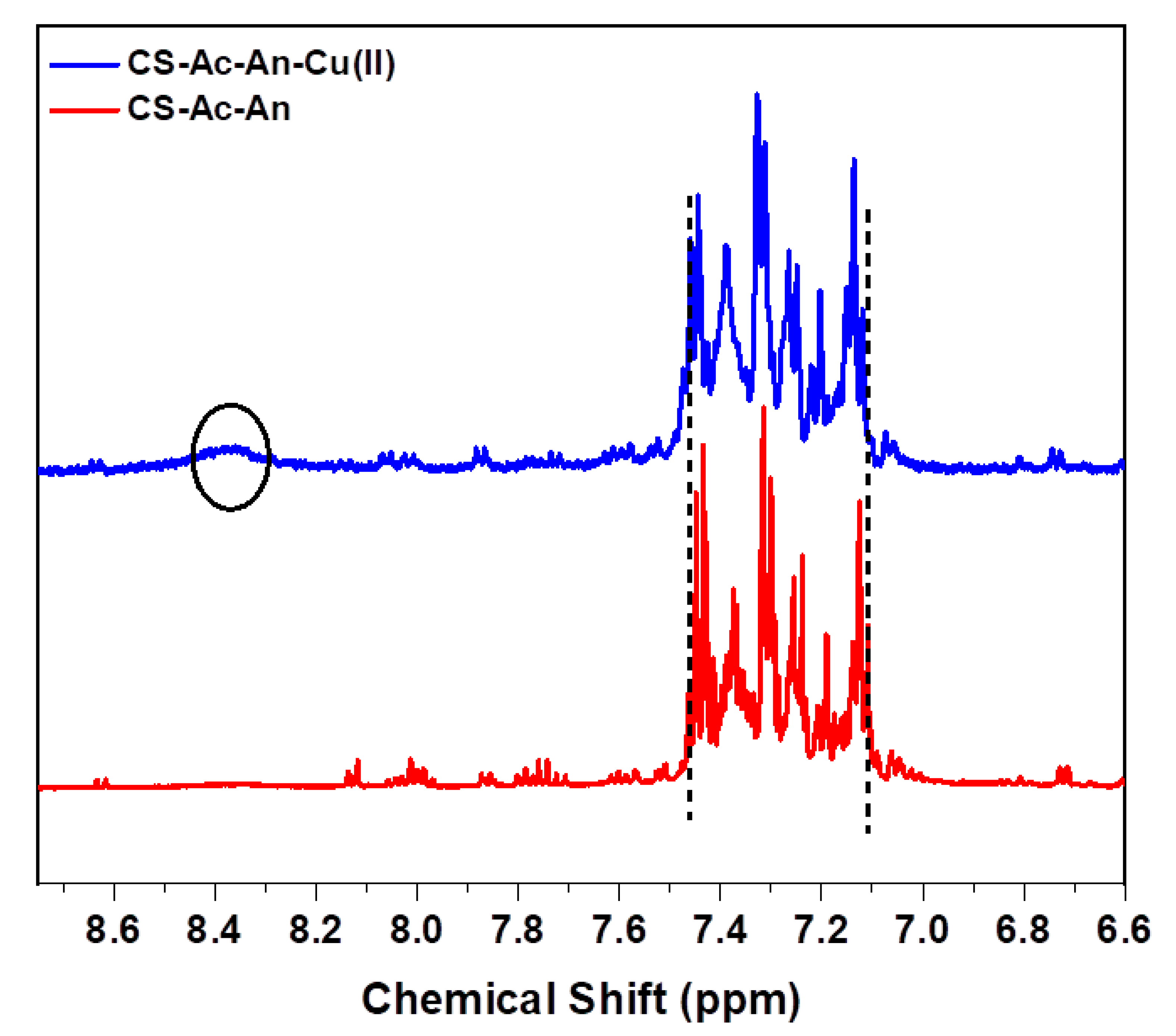
3.2.3. 1H-NMR Spectroscopy
3.3. Adsorption Behavior
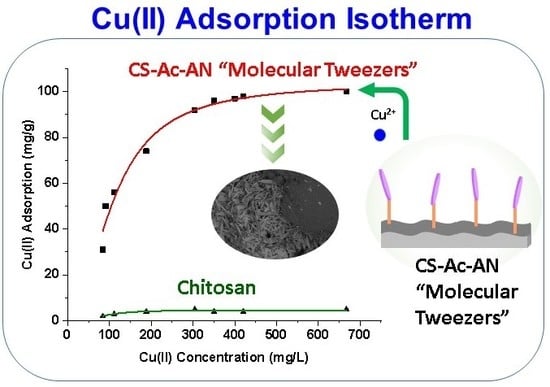
3.3.1. Equilibrium
3.3.2. Temperature Effects
3.3.3. Kinetic Results
3.3.4. Adsorbent Regeneration
3.4. Fluorescence Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ghazban, F.; Parizanganeh, A.; Zamani, A. Assessment of Heavy Metal Pollution in Water and Sediments from the Ghalechay River, Baychebagh Copper Mine Area, Iran. Soil Sediment. Contam. 2015, 24, 172–190. [Google Scholar] [CrossRef]
- Islam, S.; Ahmed, K.; Raknuzzaman, M.; Mamnun, H.; Islam, M.K. Heavy metal pollution in surface water and sediment: A preliminary assessment of an urban river in a developing country. Ecol. Indic. 2015, 48, 282–291. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Y.; Zheng, L.; Liu, B.; Liu, J.; Wang, X. Assessment of Heavy Metal Pollution in the Sediment of the Main Tributaries of Dongting Lake, China. Water 2018, 10, 1060. [Google Scholar] [CrossRef]
- Bhargava, P.; Gupta, N.; Vats, S.; Goel, R. Health Issues and Heavy Metals. Austin J. Environ.Tox. 2017, 3, 1–8. [Google Scholar]
- Jolly, J.L. Technical Report, The U.S. Copper-base Scrap Industry and Its By-products; Copper Development Association Inc.: New York, NY, USA, 2013. [Google Scholar]
- Ashish, B.; Neeti, K.; Himanshu, K. Copper Toxicity: A Comprehensive Study. Res. J. Recent Sci. 2013, 2, 58–67. [Google Scholar]
- European Copper Institute, Copper Alliance, About the Copper Market, Demand and Economic Value. Available online: https://copperalliance.eu/about-us/market/ (accessed on 20 January 2020).
- What are EPA’s Drinking Water Regulations for Copper? Available online: https://safewater.zendesk.com/hc/en-us/articles/211402978-4-What-are-EPA-s-drinking-water-regulations-for-copper-or-copper- (accessed on 1 March 2018).
- Yli-Koski, M.; Palokangas, M.; Haarahiltunen, A.; Vainola, H.; Storgards, J.; Holmberg, H.; Sinkkonen, J. Detection of low-level copper contamination in p-type silicon by means of microwave photoconductive decay measurements. J. Phys. 2002, 14, 13119–13125. [Google Scholar] [CrossRef]
- Wen, T.; Qu, F.; Li, N.B.; Luo, H.Q. A facile, sensitive, and rapid spectrophotometric method for copper(II) ion detection in aqueous media using polyethyleneimine. Arab. J. Chem. 2017, 10, 1680–1685. [Google Scholar] [CrossRef]
- Shamuyarira, K.K.; Gumbo, J.R. Assessment of Heavy Metals in Municipal Sewage Sludge: A Case Study of Limpopo Province, South Africa. Int. J. Environ. Res. Public Health 2014, 11, 2569–2579. [Google Scholar] [CrossRef]
- Oliviera, P.R.; Lamy-Mendes, A.C.; Pissinati Rezende, E.I.; Sálvio Mangrich, A.; Marcolino Junior, L.H.; Bergamini, M.F. Electrochemical determination of copper ions in spirit drinks using carbon paste electrode modified with biochar. Food Chem. 2015, 171, 426–431. [Google Scholar] [CrossRef]
- Al-Saydeh, S.A.; El-naas, M.H.; Zaidi, S.J. Copper removal from industrial wastewater: A comprehensive review. J. Ind. Eng. Chem. 2017, 56, 35–44. [Google Scholar] [CrossRef]
- Radaideh, J.A.; Abdulgader, H.; Barjenbruch, M. Evaluation of Absorption Process for Heavy Metals Removal found in Pharmaceutical Wastewater. J. Med. Toxicol. Clin. Forensic Med. 2017, 3, 1–12. [Google Scholar] [CrossRef][Green Version]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of Copper (II) onto Different Adsorbents. J. Dispers. Sci. Technol. 2010, 31, 918–930. [Google Scholar] [CrossRef]
- Turan, N.G.; Ozgonenel, O. Study of Montmorillonite Clay for the Removal of Copper (II) by Adsorption: Full Factorial Design Approach and Cascade Forward Neural Network. Sci. World J. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Li, H.; He, H.; Lin, R.; Zuo, P. Adsorption performance of carboxylated multi-wall carbon nanotube-Fe3O4 magnetic hybrids for Cu (II) in water. New Carbon Mater. 2014, 29, 15–25. [Google Scholar] [CrossRef]
- White, R.L.; White, C.M.; Turgut, H.; Massoud, A.; Tian, Z.R. Comparative studies on copper adsorption by graphene oxide and functionalized graphene oxide nanoparticles. J. Taiwan Inst. Chem. Eng. 2018, 85, 18–28. [Google Scholar] [CrossRef]
- Demiral, H.; Gungor, C. Adsorption of copper (II) from aqueous solutions on activated carbon prepared from grape bagasse. J. Clean. Prod. 2016, 124, 103–113. [Google Scholar] [CrossRef]
- Chu, K.H. Removal of copper from aqueous solution by chitosan in prawn shell: Adsorption equilibrium and kinetics. J. Hazard. Mater. 2002, 90, 77–95. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmed, S.; Swami, B.L.; Ikram, S. Preparation and characterization of antibacterial thiosemicarbazide chitosan as efficient Cu (II) adsorbent. Carbohydr. Polym. 2015, 132, 164–172. [Google Scholar] [CrossRef]
- Zalloum, H.M.; Al-Qodah, Z.; Mubarak, M.S. Copper Adsorption on Chitosan-Derived Schiff Bases. J. Macromol. Sci. Part A 2009, 46, 45–57. [Google Scholar] [CrossRef]
- Agarwal, A.; Vaishali, A. Chitosan Based Adsorbent: A Remedy to Handle Industrial Waste Water. Int. J. Eng. Sci. 2017, 6, 34–49. [Google Scholar]
- Chitosan-Based Adsorbents for Wastewater Treatment; Nasar, A., Ed.; Materials Research Forum LLC: Millersville, PA, USA, 2018; ISBN 9781945291746. [Google Scholar]
- Jayakumar, R.; Prabaharan, M.; Reis, R.L.; Mano, J.F. Graft copolymerized chitosan- present status and applications. Carbohydr. Polym. 2005, 62, 142–158. [Google Scholar] [CrossRef]
- Crini, G.; Morin-Crini, N.; Fatin-Rouge, N.; Deon, S.; Fievet, P. Metal removal from aqueous media by polymer-assisted ultrafiltration with chitosan. Arab. J. Chem. 2017, 10, 3826–3839. [Google Scholar] [CrossRef]
- Fletcher, A.J.; Uygur, Y.; Thomas, K.M. Role of Surface Functional Groups in the Adsorption Kinetics of Water Vapor on Microporous Activated Carbons. J. Phys. Chem. C 2007, 111, 8349–8359. [Google Scholar] [CrossRef]
- Thien, D.; An, N.; Hoa, N. Preparation of Fully Deacetylated Chitosan for Adsorption of Hg(II) Ion from Aqueous Solution. Chem. Sci. J. Thien 2015, 6, 1–8. [Google Scholar]
- Chauhan, S. Modification of chitosan for sorption of metal ions. J. Chem. Pharm. Res. 2015, 7, 49–55. [Google Scholar]
- Vafakish, B.; Wilson, L.D. Surface Modified Chitosan: An Adsorption Study of a Biopolymer “Tweezer-Like” System with Fluorescein. Surfaces 2019, 35. [Google Scholar] [CrossRef]
- Langmuir, I. The Constitution and Fundumentals Properties of Solids and Liquids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Sips, R. On the Structure of a Catalyst Surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Ho, Y.S.; Mckay, G. Sorption of dye from aqueous solution by peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Sreelatha, G.; Ageetha, V.; Parmar, J.; Padmaja, P. Equilibrium and Kinetic Studies on Reactive Dye Adsorption Using Palm Shell Powder (An Agrowaste) and Chitosan. J. Chem. Eng. Data 2011, 56, 35–42. [Google Scholar] [CrossRef]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. Comparative adsorption of metal and dye on flake- and bead-types of chitosans prepared from fishery wastes. J. Hazard. Mater. 2000, 73, 63–75. [Google Scholar] [CrossRef]
- Cheng, Z.; Liu, R.; Jiang, X. Spectroscopic studies on the interaction between tetrandrine and two serum albumins by chemometrics methods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 115, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Mende, M.; Schwarz, D.; Steinbach, C.; Boldt, R.; Schwarz, S. Simultaneous adsorption of heavy metal ions and anions from aqueous solutions on chitosan—Investigated by spectrophotometry and SEM-EDX analysis. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 510, 275–282. [Google Scholar] [CrossRef]
- García-Sánchez, M.E.; Perez-Fonseca, A.A.; Gomez, C.; Gonzalez-Reynoso, O.; Vazquez-Lepe, M.O.; Gonzalez-Nunez, R.; Manriquez-Gonzalez, R.; Robledo-Oritz, J.R. Improvement of Pb (II) Adsorption Capacity by Controlled Alkali Treatment to Chitosan Supported onto Agave Fiber-HDPE Composites. Macromol. Symp. 2017, 374, 1–7. [Google Scholar]
- Zhang, H.; Dang, Q.; Liu, C.; Cha, D.; Yu, Z.; Zhu, W.; Fan, B. Uptake of Pb (II) and Cd (II) on Chitosan Microsphere Surface Successively Grafted by Methyl Acrylate and Diethylenetriamine. ACS Appl. Mater. Interface 2017, 9, 11144–11155. [Google Scholar] [CrossRef]
- Vieira, R.S.; Oliveira, M.L.M.; Guibal, E.; Rodríguez-Castellón, E.; Beppu, M.M. Copper, mercury and chromium adsorption on natural and crosslinked chitosan films: An XPS investigation of mechanism. Colloids Surfaces A Physicochem. Eng. Asp. 2011, 374, 108–114. [Google Scholar] [CrossRef]
- Huang, Z.; Zheng, X.; Lv, W.; Wang, M.; Yang, Q.; Kang, F. Adsorption of Lead (II) Ions from Aqueous Solution on Low-Temperature Exfoliated Graphene Nanosheets. Langmuir 2011, 27, 7558–7562. [Google Scholar] [CrossRef]
- Shao, D.; Hu, J.; Wang, X. Plasma Induced Grafting Multiwalled Carbon Nanotube with Chitosan and Its Application for Removal of UO2, Cu and Pb from Aqueous Solutions. Plasma Process. Polym 2010, 7, 977–985. [Google Scholar] [CrossRef]
- Rosillo-lopez, M.; Salzmann, C.G. Studies on the adsorption behaviors of Pb (II) onto an acyl-thiourea resin. RSC Adv. 2018, 8, 1316–1323. [Google Scholar]
- Singha, N.R.; Mahapatra, M.; Karmakar, M.; Mondal, H.; Dutta, A.; Deb, M.; Mitra, M.; Roy, C.; Chattopadhyay, P.K.; Maiti, D. In situ allocation of a monomer in pectin-g-terpolymer hydrogels and effect of comonomer compositions on superadsorption of metal ions/dyes. ACS Omega 2018, 3, 4163–4180. [Google Scholar] [CrossRef]
- Wang, N.; Xu, X.; Li, H.; Yuan, L.; Yu, H. Enhanced Selective Adsorption of Pb (II) from Aqueous Solutions by One-Pot Synthesis of Xanthate-Modified Chitosan Sponge: Behaviors and Mechanisms. Ind. Eng. Chem. Res. 2016, 55, 12222–12231. [Google Scholar] [CrossRef]
- Cheng, J.; Zhu, W.; Tang, Y.; Xu, Y.; Li, Z.; Chen, K.; Jiang, H. Effect of cation-pi interaction on NMR: A theoretical investigation on complexes of Li, Na, Be, and Mg with aromatics. Chem. Phys. Lett. 2006, 422, 455–460. [Google Scholar] [CrossRef]
- Mcewen, I. Broadening of H-NMR signals in the spectra of heparin and OSCS by paramagnetic transition metal ions. The use of EDTA to sharpen the signals. J. Pharm. Biomed. Anal. 2010, 51, 733–735. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Gupta, R.K.; Shahid, M.; Maiti, B.; Misra, A.; Pandey, D.S. Synthesis and Characterization of Electroactive Ferrocene Derivatives: Ferrocenylimidazoquinazoline as a Multichannel Chemosensor Selectively for Hg and Pb Ions in an Aqueous Environment. Inorg. Chem. 2012, 51, 298–311. [Google Scholar] [CrossRef]
- Rouzi, K.; Abulikemu, A.; Zhao, J.; Wu, B. A study on the synthesis and anion recognition of a chitosan-urea receptor. RSC Adv. 2017, 7, 50920–50927. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, Y.; Wu, J.; Zheng, J.; Zhao, X.; Lu, B.; Chen, F. Chitosan-coated mesoporous microspheres of calcium silicate hydrate: Environmentally friendly synthesis and application as a highly efficient adsorbent for heavy metal ions. J. Colloid Interface Sci. 2014, 418, 208–215. [Google Scholar] [CrossRef]
- Wan, M.; Kan, C.; Rogel, B.D.; Dalida, M.L.P. Adsorption of copper (II) and lead (II) ions from aqueous solution on chitosan-coated sand. Carbohydr. Polym. 2010, 80, 891–899. [Google Scholar] [CrossRef]
- Ngah, W.S.W.A.N.; Isa, I.M. Comparison Study of Copper Ion Adsorption on Chitosan, Dowex A-1, and Zerolit 225. J. Appl. Polym. Sci. 1998, 67, 1067–1070. [Google Scholar] [CrossRef]
- Monier, M. Adsorption of Hg, Cu and Zn ions from aqueous solution using formaldehyde cross-linked modified chitosan-thioglyceraldehyde Schiff’s base. Int. J. Biol. Macromol. 2012, 50, 773–781. [Google Scholar] [CrossRef]
- Jiang, W.; Chen, X.; Pan, B.; Zhang, Q.; Teng, L.; Chen, Y.; Liu, L. Spherical polystyrene-supported chitosan thin film of fast kinetics and high capacity for copper removal. J. Hazard. Mater. 2014, 276, 295–301. [Google Scholar] [CrossRef]
- Charpentier, T.V.J.; Neville, A.; Lanigan, J.L.; Barker, R.; Smith, M.J.; Richardson, T. Preparation of Magnetic Carboxymethylchitosan Nanoparticles for Adsorption of Heavy Metal Ions. ACS Omega 2016, 1, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Zia, Q.; Tabassum, M.; Lu, Z.; Khawar, M.T.; Song, J.; Gong, H.; Meng, J.; Li, Z.; Li, J. Porous poly(L–lactic acid)/chitosan nanofibres for copper ion adsorption. Carbohydr. Polym. 2020, 227, 115343. [Google Scholar] [CrossRef] [PubMed]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Milosavljević, N.B.; Ristić, M.D.; Perić-Grujić, A.A.; Filipović, J.M.; Štrbac, S.B.; Rakočević, Z.L.; Kalagasidis Krušić, M.T. Hydrogel based on chitosan, itaconic acid and methacrylic acid as adsorbent of Cd2+ ions from aqueous solution. Chem. Eng. J. 2010, 165, 554–562. [Google Scholar] [CrossRef]
- Igberase, E.; Osifo, P.; Ofomaja, A. The adsorption of copper (II) ions by polyaniline graft chitosan beads from aqueous solution: Equilibrium, kinetic and desorption studies. J. Environ. Chem. Eng. 2014, 2, 362–369. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Bhatnagar, A.; Bikiaris, D.N.; Kyzas, G.Z. Chitin adsorbents for toxic metals: A review. Int. J. Mol. Sci. 2017, 18, 114. [Google Scholar] [CrossRef]
- Ma, Z.; Di, N.; Zhang, F.; Gu, P.; Liu, S.; Liu, P. Kinetic and Thermodynamic Studies on the Adsorption of Zn onto Chitosan-aluminium Oxide Composite Material. Int. J. Chem. 2011, 3, 18–23. [Google Scholar]
- Dragan, E.S.; Apopei Loghin, D.F.; Cocarta, A.I. Efficient sorption of Cu by composite chelating sorbents based on potato starch-graft-Polyamidoxime embedded in chitosan beads. ACS Appl. Mater. Interfaces 2014, 6, 16577–16592. [Google Scholar] [CrossRef]
- Rabelo, R.B.; Vieira, R.S.; Luna, F.M.T.; Guibal, E.; Beppu, M.M. Adsorption of Copper(II) and Mercury(II) Ions onto Chemically-Modified Chitosan Membranes: Equilibrium and Kinetic Properties. Adsorpt. Sci. Technol. 2012, 30, 1–21. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Z.; Li, R.; Wang, J.J.; Ali, A. Removal of Pb (II) and Cd (II) ions from aqueous solution by thiosemicarbazide modified chitosan. Int. J. Biol. Macromol. 2016, 86, 876–884. [Google Scholar] [CrossRef]
- Ashokkumar, P.; Ramakrishnan, V.T.; Ramamurthy, P. Photoinduced Electron Transfer (PET) Based Zn Fluorescent Probe: Transformation of Turn-On Sensors into Ratiometric Ones with Dual Emission in Acetonitrile. J. Phys. Chem. A 2011, 115, 14292–14299. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Kim, M.H.; Yoon, Y.I.; Park, W.H. Fluorescent Property of Chitosan Oligomer and Its Application as a Metal Ion Sensor. Mar. Drugs 2017, 15, 105. [Google Scholar] [CrossRef] [PubMed]
- Nolan, E.M.; Lippard, S.J. A “Turn-On” Fluorescent Sensor for the Selective Detection of Mercuric Ion in Aqueous Media. J. Am. Chem. Soc. 2003, 125, 14270–14271. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Yin, J.; Tao, Z.; Liu, Y.; Lin, X.; Deng, J.; Wang, S. An Ultrasensitive Fluorescence Sensor with Simple Operation for Cu Specific Detection in Drinking Water. ACS Omega 2018, 3, 3045–3050. [Google Scholar] [CrossRef]
- Meng, Q.; Su, W.; He, C.; Duan, C. Novel chitosan-based fluorescent materials for the selective detection and adsorption of Fe in water and consequent bio-imaging applications. Talanta 2012, 97, 456–461. [Google Scholar] [CrossRef]
- Geng, Z.; Zhang, H.; Xiong, Q.; Zhang, Y.; Zhao, H.; Wang, G. A fluorescent chitosan hydrogel detection platform for the sensitive and selective determination of trace mercury(II) in water. J. Mater. Chem. A 2015, 3, 19455–19460. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

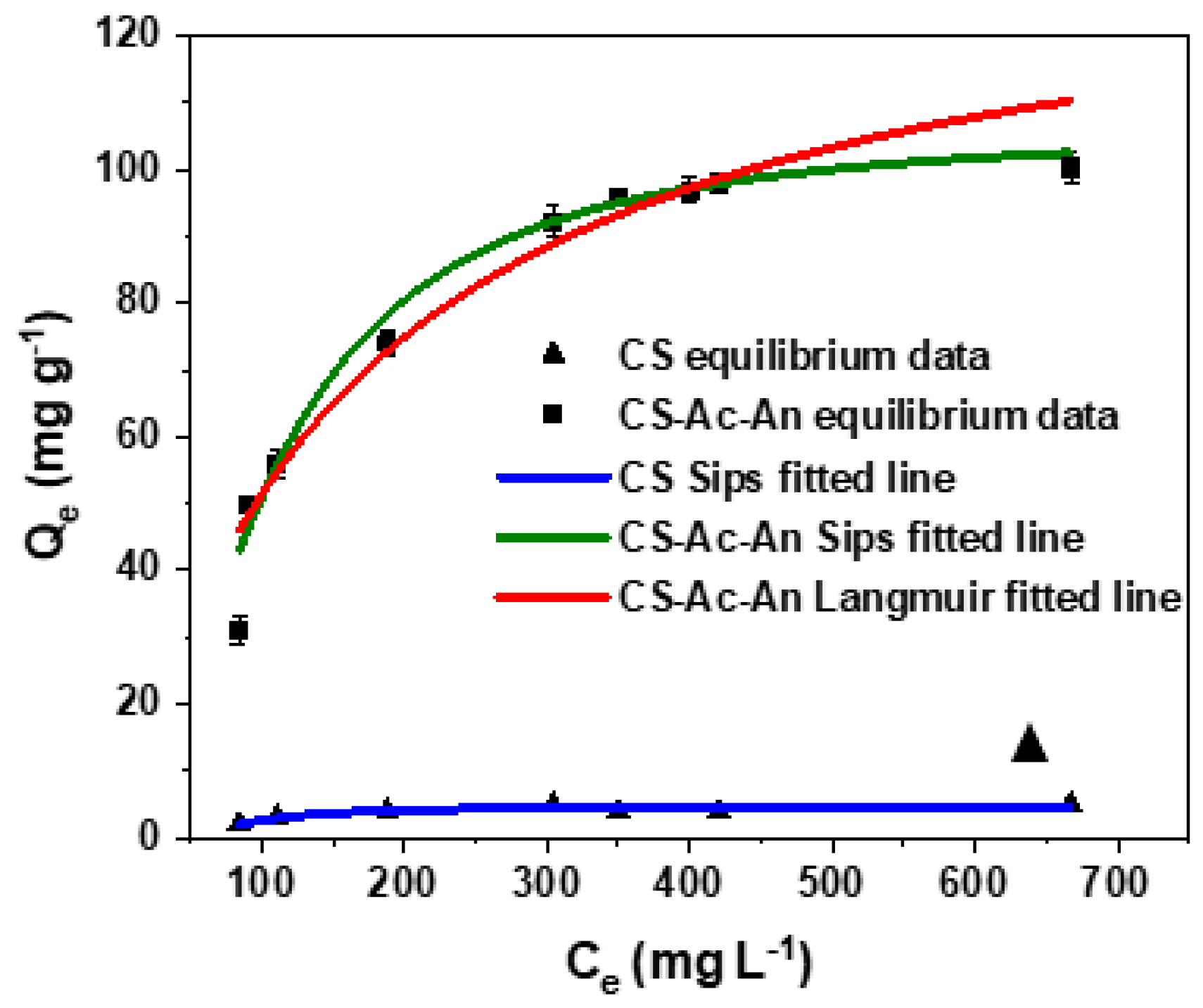


| CS | CS-Ac-An | ||||||
|---|---|---|---|---|---|---|---|
| Qm (mg g−1) | Ks (L g−1) | ns | Adj-R2 | Qm (mg g−1) | Ks (L g−1) | ns | Adj-R2 |
| 4.56 | 11.2 | 2.88 | 0.851 | 106.6 | 9.52 | 1.85 | 0.978 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vafakish, B.; Wilson, L.D. Cu(II) Ion Adsorption by Aniline Grafted Chitosan and Its Responsive Fluorescence Properties. Molecules 2020, 25, 1052. https://doi.org/10.3390/molecules25051052
Vafakish B, Wilson LD. Cu(II) Ion Adsorption by Aniline Grafted Chitosan and Its Responsive Fluorescence Properties. Molecules. 2020; 25(5):1052. https://doi.org/10.3390/molecules25051052
Chicago/Turabian StyleVafakish, Bahareh, and Lee D. Wilson. 2020. "Cu(II) Ion Adsorption by Aniline Grafted Chitosan and Its Responsive Fluorescence Properties" Molecules 25, no. 5: 1052. https://doi.org/10.3390/molecules25051052
APA StyleVafakish, B., & Wilson, L. D. (2020). Cu(II) Ion Adsorption by Aniline Grafted Chitosan and Its Responsive Fluorescence Properties. Molecules, 25(5), 1052. https://doi.org/10.3390/molecules25051052