Quercetin Protects against Lipopolysaccharide-Induced Intestinal Oxidative Stress in Broiler Chickens through Activation of Nrf2 Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals Treatment
2.2. Sample Collection
2.3. Intestinal Morphology
2.4. Measurement of Antioxidant Parameters
2.5. Real-Time Quantitative PCR
2.6. Determination of mtDNA Copy Number and Transmission Electron Microscopy (TEM) Analysis
2.7. Western Blot Analysis
2.8. Statistical Analysis
3. Results
3.1. Quercetin Impacts on Intestinal Morphology of Broiler Chickens.
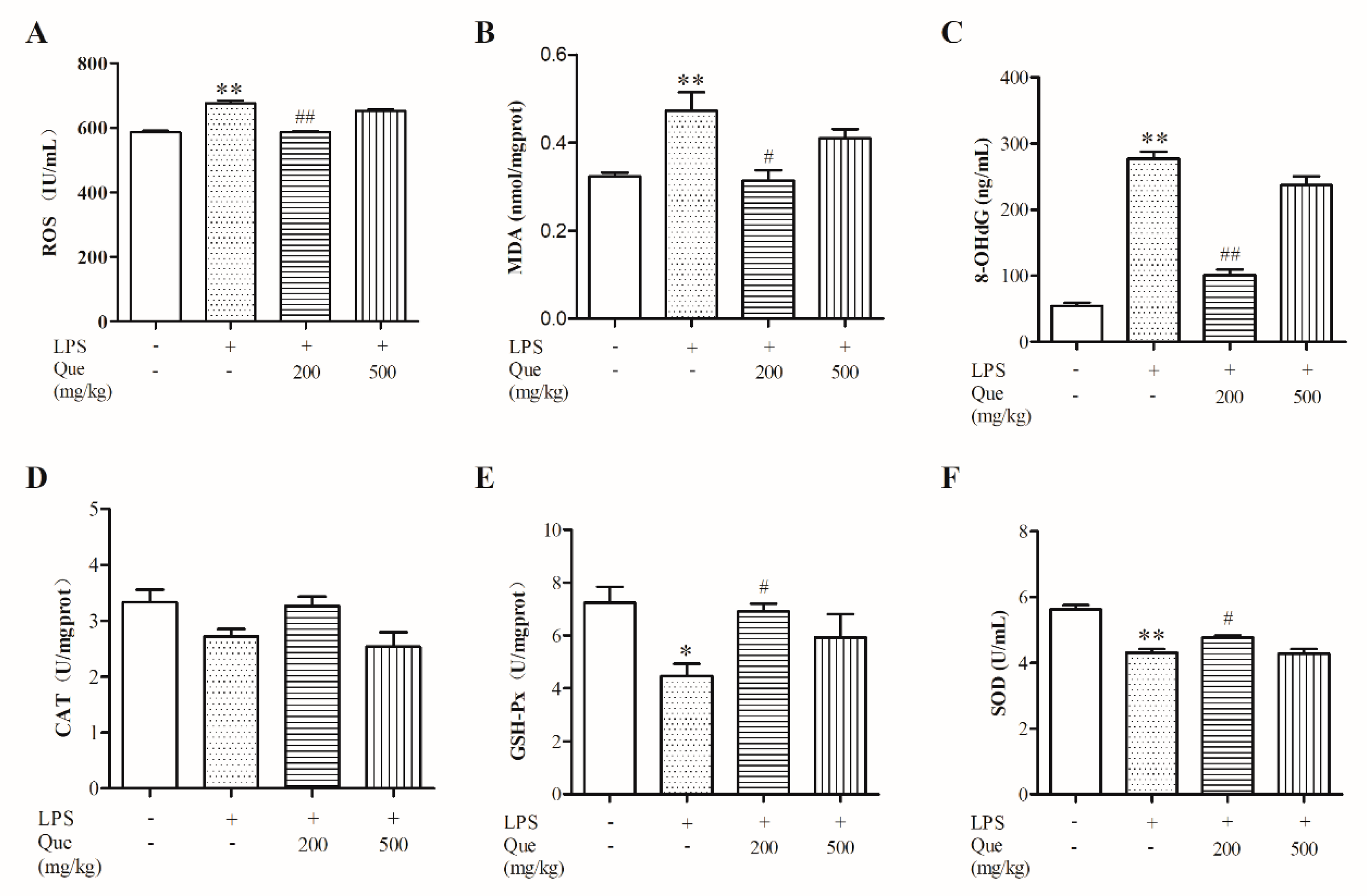
3.2. Effect of Quercetin on Oxidative Stress Induced by LPS
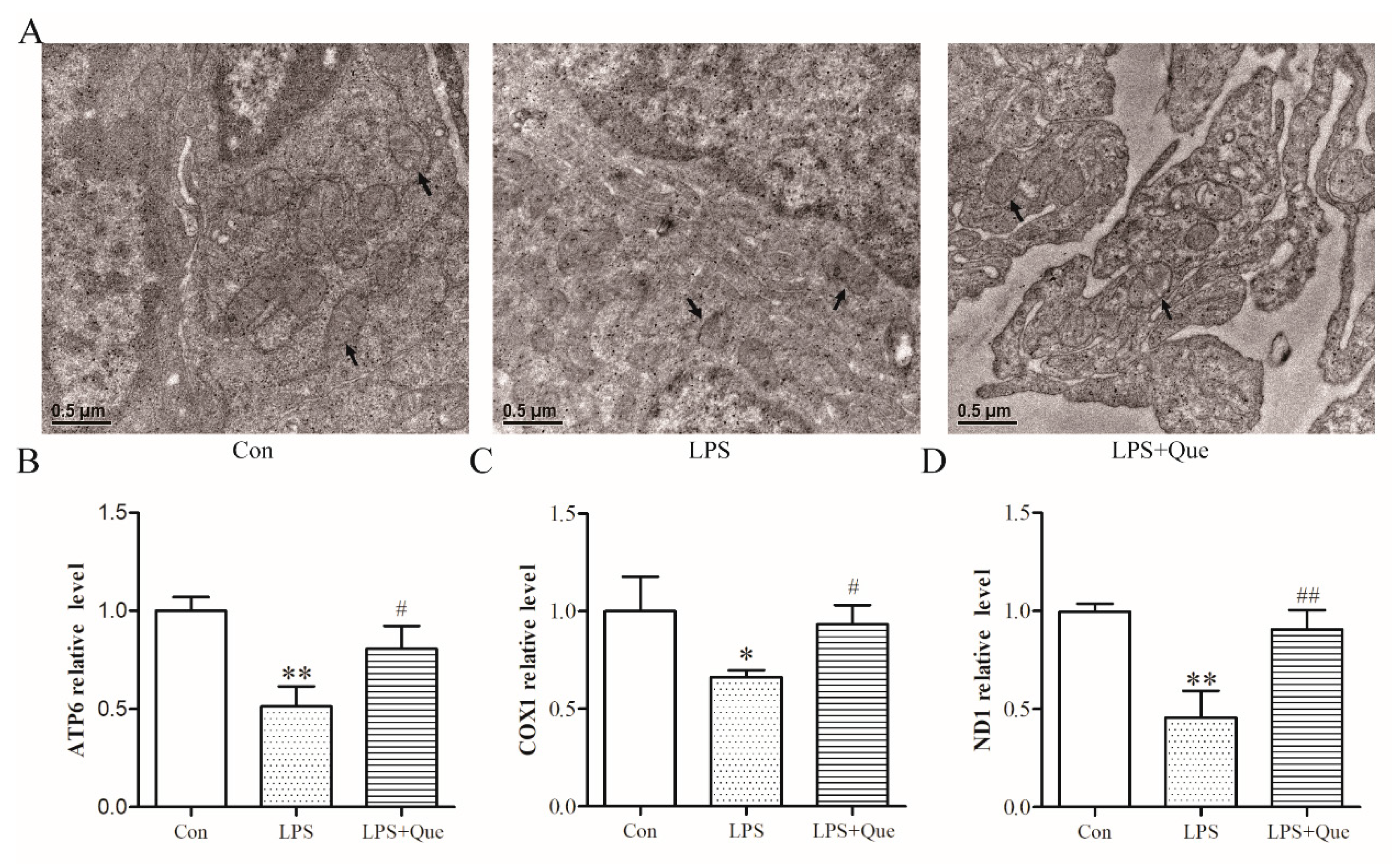
3.3. Effects of Quercetin on Jejunal Mitochondria of Broiler Chickens
3.4. Effects of Quercetin on Nrf2 Activation and Downstream Genes
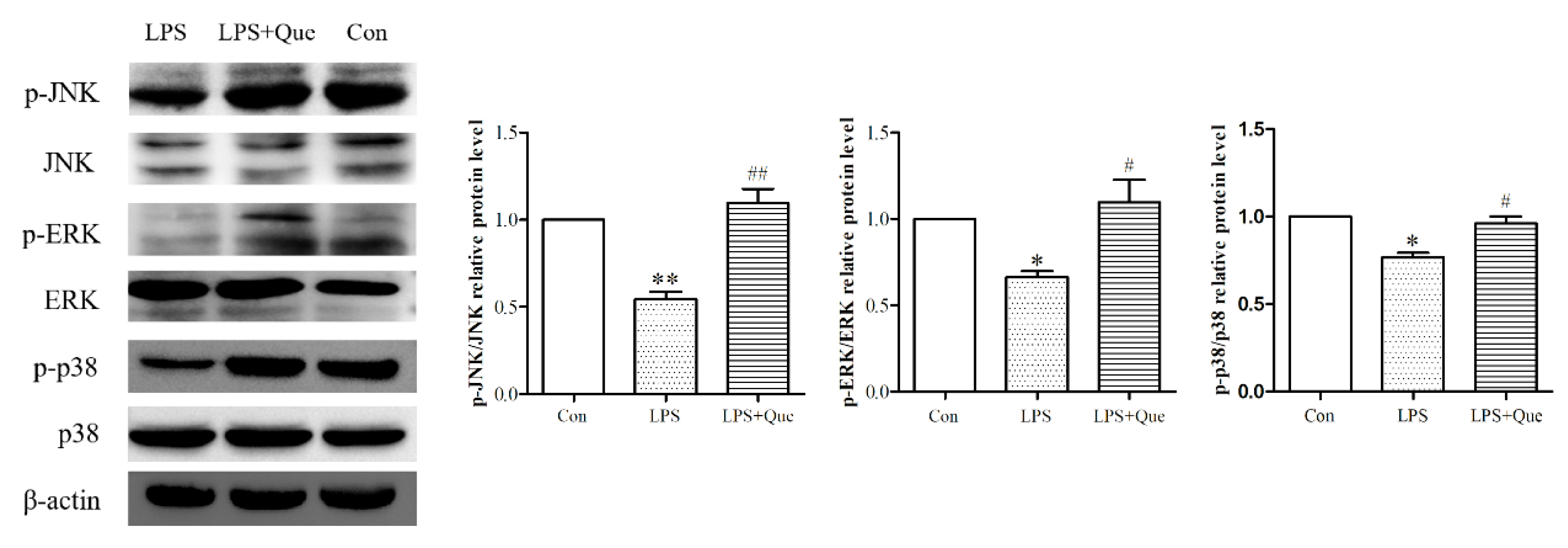
3.5. Quercetin Impacts on ERK, JNK, and p38 Phosphorylation Reduced by LPS
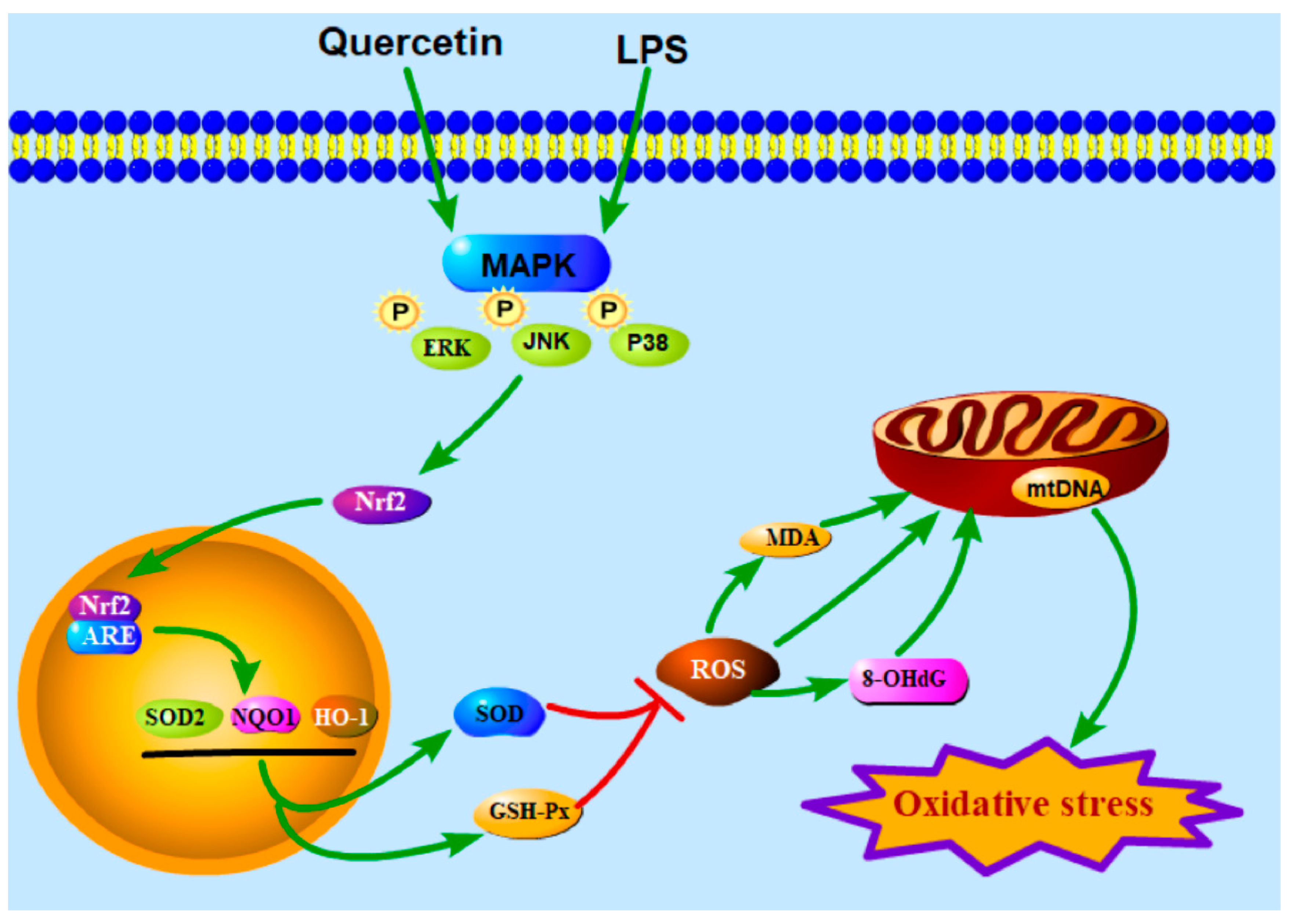
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Akaike, T. Host defense and oxidative stress signaling in bacterial infection. Nihon Saikingaku Zasshi 2015, 70, 339–349. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ateya, A.I.; Arafat, N.; Saleh, R.M.; Ghanem, H.M.; Naguib, D.; Radwan, H.A.; Elseady, Y.Y. Intestinal gene expressions in broiler chickens infected with Escherichia coli and dietary supplemented with probiotic, acidifier and synbiotic. Vet. Res. Commun. 2019, 43, 131–142. [Google Scholar] [CrossRef]
- Brandes, R.P.; Koddenberg, G.; Gwinner, W.; Kim, D.; Kruse, H.J.; Busse, R.; Mügge, A. Role of increased production of superoxide anions by NAD (P)H oxidase and xanthine oxidase in prolonged endotoxemia. Hypertension 1999, 33, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Apicella, M.A.; Griffiss, J.M.; Schneider, H. Isolation and characterization of lipopolysaccharides, lipooligosaccharides, and lipid A. Methods Enzym. 1994, 235, 242–252. [Google Scholar]
- Beckman, J.S.; Beckman, T.W.; Chen, J.; Marshall, P.A.; Freeman, B.A. Apparent hydroxyl radical production by peroxynitrite: Implications for endothelial injury from nitric oxide and superoxide. Proc Natl. Acad. Sci. USA 1990, 87, 1620–1624. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.J.; Wang, Y.Q.; Qi, Y.X. The protective effect of procyanidin against LPS-induced acute gut injury by the regulations of oxidative state. Springerplus 2016, 5, 1645. [Google Scholar] [CrossRef]
- Suzuki, K.; Yossapol, M.; Sugiyama, M.; Asai, T. Effects of Antimicrobial Administration on the Prevalence of Antimicrobial-Resistant Escherichia coli in Broiler Flocks. Jpn. J. Infect. Dis. 2019, 72, 179–184. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharm. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Shen, Y.; Ward, N.C.; Hodgson, J.M.; Puddey, I.B.; Wang, Y.; Zhang, D.; Maghzal, G.J.; Stocker, R.; Croft, K.D. Dietary quercetin attenuates oxidant-induced endothelial dysfunction and atherosclerosis in apolipoprotein E knockout mice fed a high-fat diet: A critical role for heme oxygenase-1. Free Radic Biol. Med. 2013, 65, 908–915. [Google Scholar] [CrossRef]
- Luo, M.; Tian, R.; Yang, Z.; Peng, Y.Y.; Lu, N. Quercetin suppressed NADPH oxidase-derived oxidative stress via heme oxygenase-1 induction in macrophages. Arch. Biochem. Biophys. 2019, 671, 69–76. [Google Scholar] [CrossRef]
- Karuppagounder, V.; Arumugam, S.; Thandavarayan, R.A.; Sreedhar, R.; Giridharan, V.V.; Watanabe, K. Molecular targets of quercetin with anti-inflammatory properties in atopic dermatitis. Drug Discov. Today 2016, 21, 632–639. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Tao, L.; Zhai, J.; Gao, H.; Song, Y.; Qu, X. Quercetin protected against isoniazide-induced HepG2 cell apoptosis by activating the SIRT1/ERK pathway. J. Biochem. Mol. Toxicol. 2019, e22369. [Google Scholar] [CrossRef] [PubMed]
- Bournival, J.; Plouffe, M.; Renaud, J.; Provencher, C.; Martinoli, M.G. Quercetin and sesamin protect dopaminergic cells from MPP+-induced neuroinflammation in a microglial (N9)-neuronal (PC12) coculture system. Oxid. Med. Cell Longev. 2012, 2012, 921941. [Google Scholar] [CrossRef] [PubMed]
- Bachmetov, L.; Gal-Tanamy, M.; Shapira, A.; Vorobeychik, M.; Giterman-Galam, T.; Sathiyamoorthy, P.; Golan-Goldhirsh, A.; Benhar, I.; Tur-Kaspa, R.; Zemel, R. Suppression of hepatitis C virus by the flavonoid quercetin is mediated by inhibition of NS3 protease activity. J. Viral Hepat 2012, 19, e81–e88. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhang, S.; Chan, J.Y.; Zhang, D.D. Keap1 controls postinduction repression of the Nrf2-mediated antioxidant response by escorting nuclear export of Nrf2. Mol. Cell Biol. 2007, 27, 6334–6349. [Google Scholar] [CrossRef]
- Hafez, H.M.; Ibrahim, M.A.; Zedan, M.Z.; Hassan, M.; Hassanein, H. Nephroprotective effect of cilostazol and verapamil against thioacetamide-induced toxicity in rats may involve Nrf2/HO-1/NQO-1 signaling pathway. Toxicol. Mech. Methods 2019, 29, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ding, M.; Gao, X.R.; Ding, F. Pyrroloquinoline quinone rescues hippocampal neurons from glutamate-induced cell death through activation of Nrf2 and up-regulation of antioxidant genes. Genet. Mol. Res. 2012, 11, 2652–2664. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Ji, J.; Qu, H.; Wang, J.; Shu, D.M.; Wang, Y.; Liu, T.F.; Li, Y.; Luo, C.L. Effects of sodium butyrate on intestinal health and gut microbiota composition during intestinal inflammation progression in broilers. Poultry Sci. 2019, 98, 4449–4456. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Wei, H.K.; Xiang, Q.H.; Wang, J.; Zhou, Y.F.; Peng, J. Protective effect of quercetin on pig intestinal integrity after transport stress is associated with regulation oxidative status and inflammation. J. Vet. Med. Sci. 2016, 78, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Sukhotnik, I.; Moati, D.; Shaoul, R.; Loberman, B.; Pollak, Y.; Schwartz, B. Quercetin prevents small intestinal damage and enhances intestinal recovery during methotrexate-induced intestinal mucositis of rats. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Jia, S.; Ma, Q.; Zhong, M.; Gao, P.; Yu, Z.; Zhang, Y. Suppressive effects of subchronic aluminum overload on the splenic immune function may be related to oxidative stress in mice. Biol. Trace Elem. Res. 2014, 157, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Caddeo, C.; Gabriele, M.; Fernàndez-Busquets, X.; Valenti, D.; Fadda, A.M.; Pucci, L.; Manconi, M. Antioxidant activity of quercetin in Eudragit-coated liposomes for intestinal delivery. Int. J. Pharm. 2019, 565, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Cebecioglu, R.; Yildirim, M.; Akagunduz, D.; Korkmaz, I.; Tekin, H.O.; Atasever-Arslan, B.; Catal, T. Synergistic effects of quercetin and selenium on oxidative stress in endometrial adenocarcinoma cells. Bratisl Lek Listy 2019, 120, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Mahdavinia, M.; Alizadeh, S.; Raesi Vanani, A.; Dehghani, M.A.; Shirani, M.; Alipour, M.; Shahmohammadi, H.A.; Rafiei Asl, S. Effects of quercetin on bisphenol A-induced mitochondrial toxicity in rat liver. Iran. J. Basic Med. Sci. 2019, 22, 499–505. [Google Scholar]
- Carrasco-Pozo, C.; Mizgier, M.L.; Speisky, H.; Gotteland, M. Differential protective effects of quercetin, resveratrol, rutin and epigallocatechin gallate against mitochondrial dysfunction induced by indomethacin in Caco-2 cells. Chem. Biol. Interact. 2012, 195, 199–205. [Google Scholar] [CrossRef]
- Qiu, L.; Luo, Y.; Chen, X. Quercetin attenuates mitochondrial dysfunction and biogenesis via upregulated AMPK/SIRT1 signaling pathway in OA rats. Biomed. Pharm. 2018, 103, 1585–1591. [Google Scholar] [CrossRef]
- Chen, Z.; Lei, L.; Wen, D.; Yang, L. Melatonin attenuates palmitic acid-induced mouse granulosa cells apoptosis via endoplasmic reticulum stress. J. Ovarian Res. 2019, 12, 43. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Li, W.; Ao, H.; Zhang, Y.; Zhou, R.; Li, K. Effects of melatonin on the synthesis of estradiol and gene expression in pig granulosa cells. J. Pineal Res. 2019, 66, e12546. [Google Scholar] [CrossRef]
- Tanigawa, S.; Fujii, M.; Hou, D.X. Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free Radic. Biol. Med. 2007, 42, 1690–1703. [Google Scholar] [CrossRef]
- Bahar, E.; Kim, J.Y.; Yoon, H. Quercetin Attenuates Manganese-Induced Neuroinflammation by Alleviating Oxidative Stress through Regulation of Apoptosis, iNOS/NF-κB and HO-1/Nrf2 Pathways. Int. J. Mol. Sci. 2017, 18, 1989. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, H.; Gao, Y.; Li, L.; Tang, C.; Wen, G.; Zhou, Y.; Zhou, M.; Mao, L.; Fan, Y. Protective Effects of Quercetin on Mitochondrial Biogenesis in Experimental Traumatic Brain Injury via the Nrf2 Signaling Pathway. PLoS ONE 2016, 11, e0164237. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.; Nussler, A.; Liu, L.; Hao, L.; Song, F.; Schirmeier, A.; Nussler, N. Quercetin protects human hepatocytes from ethanol-derived oxidative stress by inducing heme oxygenase-1 via the MAPK/Nrf2 pathways. J. Hepatol. 2007, 47, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.; Forman, H.J. Redox signaling and the MAP kinase pathways. Biofactors 2003, 17, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.S.; Jun, M.; Kong, A.N. Nrf2: A potential molecular target for cancer chemoprevention by natural compounds. Antioxid Redox Signal. 2006, 8, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Ravagna, A.; Colombrita, C.; Scapagnini, G.; Guagliano, E.; Calvani, M.; Butterfield, D.A.; Giuffrida Stella, A.M. Acetylcarnitine induces heme oxygenase in rat astrocytes and protects against oxidative stress: Involvement of the transcription factor Nrf2. J. Neurosci Res. 2005, 79, 509–521. [Google Scholar] [CrossRef]
- Sun, G.Y.; Chen, Z.; Jasmer, K.J.; Chuang, D.Y.; Gu, Z.; Hannink, M.; Simonyi, A. Quercetin Attenuates Inflammatory Responses in BV-2 Microglial Cells: Role of MAPKs on the Nrf2 Pathway and Induction of Heme Oxygenase-1. PLoS ONE 2015, 10, e0141509. [Google Scholar] [CrossRef]
- Lin, H.C.; Cheng, T.H.; Chen, Y.C.; Juan, S.H. Mechanism of heme oxygenase-1 gene induction by quercetin in rat aortic smooth muscle cells. Pharmacology 2004, 71, 107–112. [Google Scholar] [CrossRef]
- Chow, J.M.; Shen, S.C.; Huan, S.K.; Lin, H.Y.; Chen, Y.C. Quercetin, but not rutin and quercitrin, prevention of H2O2-induced apoptosis via anti-oxidant activity and heme oxygenase 1 gene expression in macrophages. Biochem. Pharm. 2005, 69, 1839–1851. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Ingredients (g/kg) | Values | Calculation of nutrients (g/kg)y | Values |
|---|---|---|---|
| Corn | 586.267 | Crude protein | 21 |
| Soya bean meal (46%) | 320.722 | Metabolism energy (MJ/kg) | 3050 |
| Soybean oil | 35.259 | Moisture | 12.3807 |
| Corn gluten meal | 20 | Dry matter | 87.6193 |
| Limestone | 11.712 | Crude fat | 6.1026 |
| DL-methionine (98%) | 2.301 | Crude fiber | 3.1719 |
| Lysine (98%) | 3.829 | Crude ash | 5.5485 |
| L-threonine | 0.525 | Calcium | 0.9 |
| Choline chloride (60%) | 1 | Total phosphorus | 0.6361 |
| calcium hydrogen phosphate | 10.315 | Available phosphorus | 0.41 |
| Salt | 3.32 | Sodium | 0.1492 |
| Vitamin-Trace mineral premix z | 3.7 | Chlorine | 0.2266 |
| Phytase (10000 units) | 0.1 | Lysine | 1.35 |
| L-tryptophan (20%) | 0.95 | Methionine | 0.55 |
| Sulfur-containing amino acid | 0.878 | ||
| Threonine | 0.83 | ||
| Total | 1000 | Tryptophan | 0.25 |
| Genes | Primer Sequence (5′– 3′) | Size (bp) | Tm (°C) |
|---|---|---|---|
| HO-1 | F: AAGAGCCAGGAGAACGGTCA | 121 | 57 |
| R: AAGAGCCAGGAGAACGGTCA | |||
| SOD2 | F: CTTGGTCGCAAGGCAGAAG | 120 | 57 |
| R: ACGTAGGTGGCGTGGTGTT | |||
| NQO1 | F: TCGCCGAGCAGAAGAAGATTGAAG | 192 | 57 |
| R: CGGTGGTGAGTGACAGCATGG | |||
| β-actin | F: GTGCTATGTTGCTCTAGACTTCG | 174 | 57 |
| R: ATGCCACAGGATTCCATACC | |||
| ND1 | F: GCGCCCCATTTGACCTAACA | 85 | 58 |
| R: AATATGGCGAATGGTCCGGC | |||
| COX1 | F: TCCTTACCCGTCCTAGCAGC | 134 | 58 |
| R: TCGGGGTGACCGAAGAATCA | |||
| ATP6 | F: GATCAACAACCGCCTCTCCA | 111 | 58 |
| R: GAGGTGAGTAGGAGGGCTCA | |||
| AGRT | F: TGGCCATAGTGCATCCAGTG | 199 | 58 |
| R: ACGATGAATGATGACGGGCA |
| Item | Cony | LPSx | 200 mg/kg Que + LPSw | 500 mg/kg Que + LPSv |
|---|---|---|---|---|
| Duodenum | ||||
| Villi height (μm) | 718.1±29.1a | 582.8±23.3b | 749.6±26.9a | 566.4±8.7b |
| Crypt depth (μm) | 103.1±10.6b | 155.5±9.4a | 104.6±11.2b | 139.7±13.5a |
| Jejunum | ||||
| Villi height (μm) | 414.4±8.5b | 362.9±6.5c | 463.9±4.5a | 338.9±13.5c |
| Crypt depth (μm) | 60.4±7.8b | 85.3±5.6a | 70.5±9.1b | 86.7±10.5a |
| Ileum | ||||
| Villi height (μm) | 413.7±16.7a | 325.0±10.6b | 356.0±13.6a | 304.8±11.9b |
| Crypt depth (μm) | 50.2±6.1c | 86.4±5.2a | 54.2±7.5c | 68.1±9.5b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Xu, G.; Dong, Y.; Li, M.; Yang, L.; Lu, W. Quercetin Protects against Lipopolysaccharide-Induced Intestinal Oxidative Stress in Broiler Chickens through Activation of Nrf2 Pathway. Molecules 2020, 25, 1053. https://doi.org/10.3390/molecules25051053
Sun L, Xu G, Dong Y, Li M, Yang L, Lu W. Quercetin Protects against Lipopolysaccharide-Induced Intestinal Oxidative Stress in Broiler Chickens through Activation of Nrf2 Pathway. Molecules. 2020; 25(5):1053. https://doi.org/10.3390/molecules25051053
Chicago/Turabian StyleSun, Lei, Gaoqing Xu, Yangyunyi Dong, Meng Li, Lianyu Yang, and Wenfa Lu. 2020. "Quercetin Protects against Lipopolysaccharide-Induced Intestinal Oxidative Stress in Broiler Chickens through Activation of Nrf2 Pathway" Molecules 25, no. 5: 1053. https://doi.org/10.3390/molecules25051053
APA StyleSun, L., Xu, G., Dong, Y., Li, M., Yang, L., & Lu, W. (2020). Quercetin Protects against Lipopolysaccharide-Induced Intestinal Oxidative Stress in Broiler Chickens through Activation of Nrf2 Pathway. Molecules, 25(5), 1053. https://doi.org/10.3390/molecules25051053






