Protective Effect of Genistein against Compound 48/80 Induced Anaphylactoid Shock via Inhibiting MAS Related G Protein-Coupled Receptor X2 (MRGPRX2)
Abstract
1. Introduction
2. Results
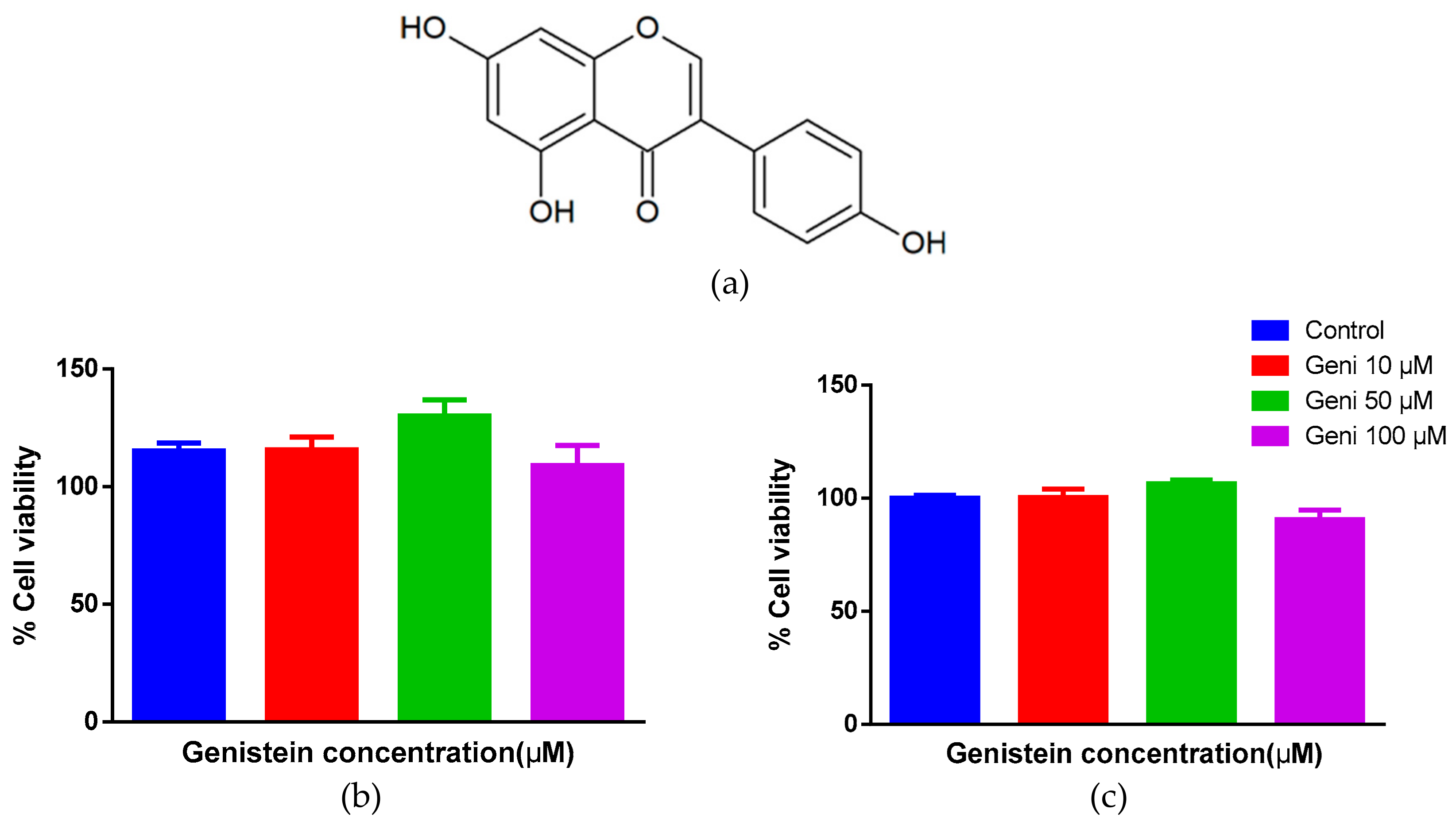
2.1. No Cytotoxicity of Genistein on Human Mast Cells and HTLA Cells
2.2. In-vitro Inhibitory Effect of Genistein on Mast Cell Degranulation, MRGPRX2 Activation and Calcium Flux
2.3. Genistein Binds at a Different Binding Site of MRGPRX2 as of Compound 48/80
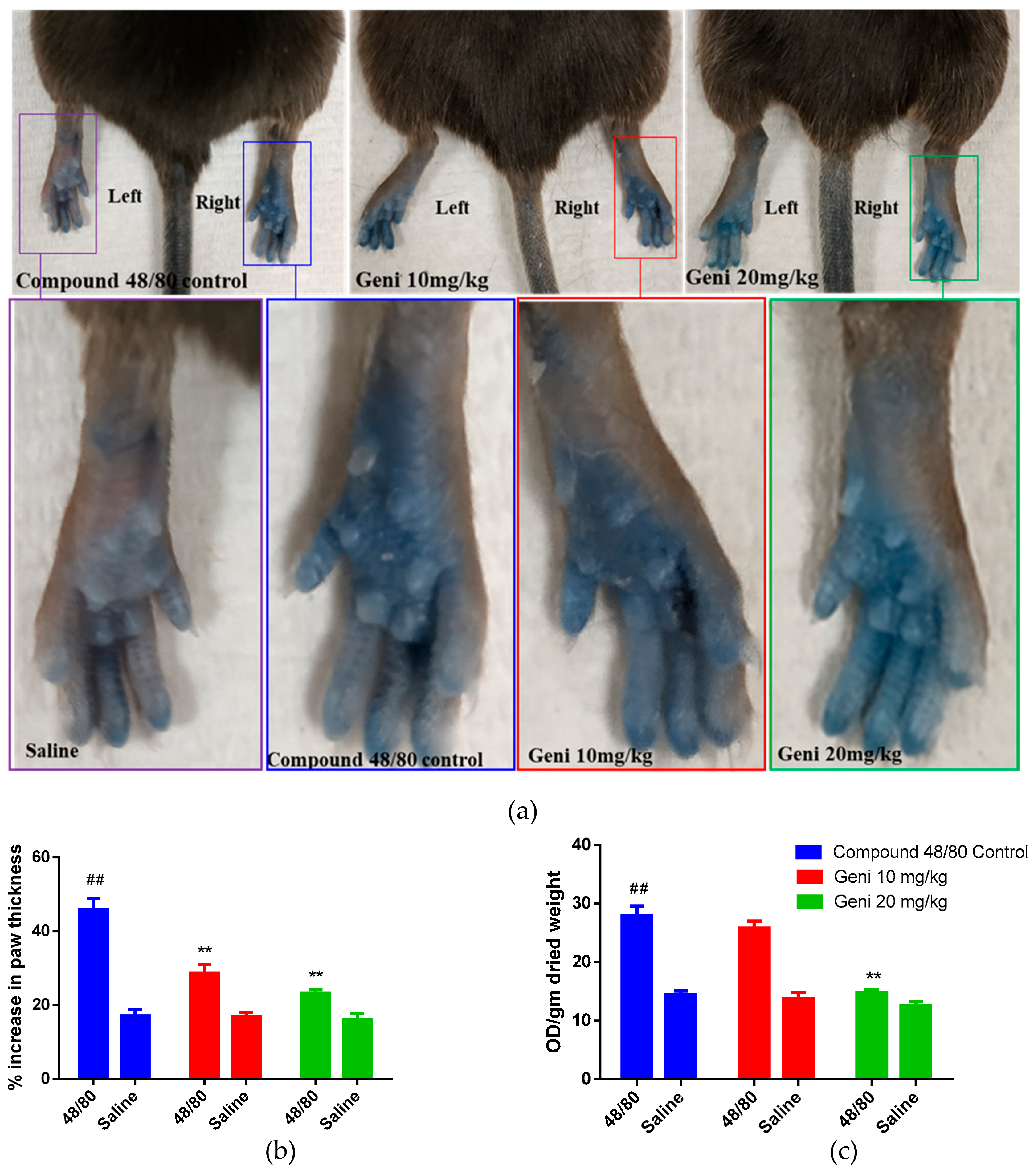
2.4. In-Vivo Inhibitory Effect of Genistein against Local Anaphylaxis Mice Model
3. Discussion
4. Materials and Methods
4.1. Drugs and Reagents
4.2. Drug Preparation
4.3. Cell Lines
4.4. Animals
4.5. Cell Toxicity Assay
4.6. Mast Cell Degranulation (β-Hexosaminidase) Assay
4.7. MRGPRX2 Activation Assay (PRESTO-Tango Assay)
4.8. Intracellular Ca2+ Flux Assay
4.9. Molecular Docking of Genistein
4.10. In-Vivo Anti-Anaphylactoid Activity
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Porebski, G.; Kwiecien, K.; Pawica, M.; Kwitniewski, M. Mas-related G protein-coupled receptor-X2 (MRGPRX2) in drug hypersensitivity reactions. Front. Immunol. 2018, 9, 3027. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Grant, J.A. Mediators of anaphylaxis. Immunol. Allergy Clin. N. Am. 2007, 27, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Lagopoulos, V.; Gigi, E. Anaphylactic and anaphylactoid reactions during the perioperative period. Hippokratia 2011, 15, 138. [Google Scholar] [PubMed]
- Fisher, M.; Baldo, B. Anaphylaxis during anaesthesia: Current aspects of diagnosis and prevention. Eur. J. Anaesthesiol. 1994, 11, 263. [Google Scholar] [PubMed]
- Levy, J.; Gottge, M.; Szlam, F.; Zaffer, R.; McCall, C. Weal and flare responses to intradermal rocuronium and cisatracurium in humans. Br. J. Anaesth. 2000, 85, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Mertes, P.M.; Laxenaire, M.-C.; Alla, F. Anaphylactic and anaphylactoid reactions occurring during anesthesia in France in 1999–2000. Anesthesiol. J. Am. Soc. Anesthesiol. 2003, 99, 536–545. [Google Scholar] [CrossRef]
- Decker, W.W.; Campbell, R.L.; Manivannan, V.; Luke, A.; Sauver, J.L.S.; Weaver, A.; Bellolio, M.F.; Bergstralh, E.J.; Stead, L.G.; Li, J.T. The etiology and incidence of anaphylaxis in Rochester, Minnesota: A report from the Rochester Epidemiology Project. J. Allergy Clin. Immunol. 2008, 122, 1161–1165. [Google Scholar] [CrossRef]
- Mertes, P.; Laxenaire, M. Anaphylactic and anaphylactoid reactions occurring during anaesthesia in France. Seventh epidemiologic survey (January 2001-December 2002). Ann. Fr. D’anesthesie Et De Reanim. 2004, 23, 1133–1143. [Google Scholar] [CrossRef]
- Bohlke, K.; Davis, R.L.; DeStefano, F.; Marcy, S.M.; Braun, M.M.; Thompson, R.S.; Team, V.S.D. Epidemiology of anaphylaxis among children and adolescents enrolled in a health maintenance organization. J. Allergy Clin. Immunol. 2004, 113, 536–542. [Google Scholar] [CrossRef]
- Gupta, R.; Sheikh, A.; Strachan, D.P.; Anderson, H.R. Time trends in allergic disorders in the UK. Thorax 2007, 62, 91–96. [Google Scholar] [CrossRef]
- Moneret-Vautrin, D.; Morisset, M.; Flabbee, J.; Beaudouin, E.; Kanny, G. Epidemiology of life-threatening and lethal anaphylaxis: A review. Allergy 2005, 60, 443–451. [Google Scholar] [CrossRef]
- Subramanian, H.; Gupta, K.; Ali, H. Roles of Mas-related G protein–coupled receptor X2 on mast cell–mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseases. J. Allergy Clin. Immunol. 2016, 138, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, E.Z.M.; Jamur, M.C.; Oliver, C. Mast cell function: A new vision of an old cell. J. Histochem. Cytochem. 2014, 62, 698–738. [Google Scholar] [CrossRef]
- McNeil, B.D.; Pundir, P.; Meeker, S.; Han, L.; Undem, B.J.; Kulka, M.; Dong, X. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 2015, 519, 237. [Google Scholar] [CrossRef]
- Tatemoto, K.; Nozaki, Y.; Tsuda, R.; Konno, S.; Tomura, K.; Furuno, M.; Ogasawara, H.; Edamura, K.; Takagi, H.; Iwamura, H. Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochem. Biophys. Res. Commun. 2006, 349, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Solinski, H.J.; Gudermann, T.; Breit, A. Pharmacology and signaling of MAS-related G protein–coupled receptors. Pharmacol. Rev. 2014, 66, 570–597. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.P.; Battey, J.; Benson, H.E.; Benya, R.V.; Bonner, T.I.; Davenport, A.P.; Eguchi, S.; Harmar, A.; Holliday, N.; Jensen, R.T. Class A Orphans (version 2019.5) in the IUPHAR/BPS Guide to Pharmacology Database. Iuphar/Bps Guide Pharmacol. Cite 2019, 2019. [Google Scholar] [CrossRef]
- Navinés-Ferrer, A.; Serrano-Candelas, E.; Lafuente, A.; Muñoz-Cano, R.; Martín, M.; Gastaminza, G. MRGPRX2-mediated mast cell response to drugs used in perioperative procedures and anaesthesia. Sci. Rep. 2018, 8, 11628. [Google Scholar] [CrossRef]
- Olivera, A.; Beaven, M.A.; Metcalfe, D.D. Mast cells signal their importance in health and disease. J. Allergy Clin. Immunol. 2018, 142, 381–393. [Google Scholar] [CrossRef]
- Ring, J.; Beyer, K.; Biedermann, T.; Bircher, A.; Duda, D.; Fischer, J.; Friedrichs, F.; Fuchs, T.; Gieler, U.; Jakob, T. Guideline for acute therapy and management of anaphylaxis. Allergo J. Int. 2014, 23, 96–112. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 125124. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-C.; Wang, C.-C.; Lu, J.-W.; Lee, C.-H.; Chen, S.-C.; Ho, Y.-J.; Peng, Y.-J. Chondroprotective Effects of Genistein against Osteoarthritis Induced Joint Inflammation. Nutrients 2019, 11, 1180. [Google Scholar] [CrossRef] [PubMed]
- Abron, J.D.; Singh, N.P.; Price, R.L.; Nagarkatti, M.; Nagarkatti, P.S.; Singh, U.P. Genistein induces macrophage polarization and systemic cytokine to ameliorate experimental colitis. PLoS ONE 2018, 13, e0199631. [Google Scholar] [CrossRef] [PubMed]
- Weng, L.; Zhang, F.; Wang, R.; Ma, W.; Song, Y. A review on protective role of genistein against oxidative stress in diabetes and related complications. Chem. Biol. Interact. 2019, 310, 108665. [Google Scholar] [CrossRef] [PubMed]
- Braxas, H.; Rafraf, M.; Hasanabad, S.K.; Jafarabadi, M.A. Effectiveness of genistein supplementation on metabolic factors and antioxidant status in postmenopausal women with type-2 diabetes mellitus. Can. J. Diabetes 2019, 43, 490–497. [Google Scholar] [CrossRef]
- Sahin, K.; Yenice, E.; Bilir, B.; Orhan, C.; Tuzcu, M.; Sahin, N.; Ozercan, I.H.; Kabil, N.; Ozpolat, B.; Kucuk, O. Genistein Prevents Development of Spontaneous Ovarian Cancer and Inhibits Tumor Growth in Hen Model. Cancer Prev. Res. 2019, 12, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, C.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Sureda, A.; Nabavi, S.F.; Devi, K.P.; Loizzo, M.R.; Tundis, R. Genistein and cancer: Current status, challenges, and future directions. Adv. Nutr. 2015, 6, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.-M.; Lin, Y.-J.; Hsu, T.-H.; Ruan, T. Genistein Suppressing the ROS-Induced Hypersensitivity of Rat Vagal Lung C-Fiber Afferents through an ERα-Mediated Mechanism. Chin. J. Physiol. 2018, 61, 14–24. [Google Scholar] [CrossRef]
- Takasugi, M.; Muta, E.; Yamada, K.; Arai, H. A new method to evaluate anti-allergic effect of food component by measuring leukotriene B 4 from a mouse mast cell line. Cytotechnology 2018, 70, 177–184. [Google Scholar] [CrossRef]
- Kim, D.H.; Jung, W.S.; Kim, M.E.; Lee, H.W.; Youn, H.Y.; Seon, J.K.; Lee, H.N.; Lee, J.S. Genistein inhibits pro-inflammatory cytokines in human mast cell activation through the inhibition of the ERK pathway. Int. J. Mol. Med. 2014, 34, 1669–1674. [Google Scholar] [CrossRef]
- Robertson, N.; Rappas, M.; Doré, A.S.; Brown, J.; Bottegoni, G.; Koglin, M.; Cansfield, J.; Jazayeri, A.; Cooke, R.M.; Marshall, F.H. Structure of the complement C5a receptor bound to the extra-helical antagonist NDT9513727. Nature 2018, 553, 111. [Google Scholar] [CrossRef]
- Colovos, C.; Yeates, T.O. Verification of protein structures: Patterns of nonbonded atomic interactions. Protein Sci. 1993, 2, 1511–1519. [Google Scholar] [CrossRef]
- Gordon, J.R.; Burd, P.R.; Galli, S.J. Mast cells as a source of multifunctional cytokines. Immunol. Today 1990, 11, 458–464. [Google Scholar] [CrossRef]
- Mukai, K.; Tsai, M.; Saito, H.; Galli, S.J. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol. Rev. 2018, 282, 121–150. [Google Scholar] [CrossRef] [PubMed]
- Grimes, J.; Desai, S.; Charter, N.W.; Lodge, J.; Santos, R.M.; Isidro-Llobet, A.; Mason, A.M.; Wu, Z.; Wolfe, L.A.; Anantharaman, L. MrgX2 is a promiscuous receptor for basic peptides causing mast cell pseudo-allergic and anaphylactoid reactions. Pharmacol. Res. Perspect. 2019, 7, e00547. [Google Scholar] [CrossRef]
- Wang, N.; Che, D.; Zhang, T.; Liu, R.; Cao, J.; Wang, J.; Zhao, T.; Ma, P.; Dong, X.; He, L. Saikosaponin A inhibits compound 48/80-induced pseudo-allergy via the Mrgprx2 pathway in vitro and in vivo. Biochem. Pharmacol. 2018, 148, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, H.; Furuno, M.; Edamura, K.; Noguchi, M. Novel MRGPRX2 antagonists inhibit IgE-independent activation of human umbilical cord blood-derived mast cells. J. Leukoc. Biol. 2019, 106, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Che, D.; Li, C.; Cao, J.; Wang, J.; Ma, P.; Zhao, T.; An, H.; Zhang, T. Quercetin inhibits Mrgprx2-induced pseudo-allergic reaction via PLCγ-IP3R related Ca2+ fluctuations. Int. Immunopharmacol. 2019, 66, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Li, C.; Ding, Y.; Hu, S.; An, H. Inhibitory function of Shikonin on MRGPRX2-mediated pseudo-allergic reactions induced by the secretagogue. Phytomedicine 2019, 68, 153149. [Google Scholar] [CrossRef]
- Alley, M.C.; Scudiero, D.A.; Monks, A.; Hursey, M.L.; Czerwinski, M.J.; Fine, D.L.; Abbott, B.J.; Mayo, J.G.; Shoemaker, R.H.; Boyd, M.R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988, 48, 589–601. [Google Scholar]
- Kuehn, H.S.; Radinger, M.; Gilfillan, A.M. Measuring mast cell mediator release. Curr. Protoc. Immunol. 2010, 91, 7–38. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Ranieri, G. Tryptase, a novel angiogenic factor stored in mast cell granules. Exp. Cell Res. 2015, 332, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Koibuchi, Y.; Ichikawa, A.; Nakagawa, M.; Tomita, K. Histamine release induced from mast cells by active components of compound 4880. Eur. J. Pharmacol. 1985, 115, 163–170. [Google Scholar] [CrossRef]
- Abramo, F.; Pirone, A.; Lenzi, C.; Della Valle, M.F.; Vidali, S.; Vannozzi, I.; Miragliotta, V. Development of a short-term canine full-thickness skin organ culture method under serum-free conditions. Am. J. Anim. Vet. Sci. 2016, 11, 61–69. [Google Scholar] [CrossRef][Green Version]
- Schemann, M.; Kugler, E.M.; Buhner, S.; Eastwood, C.; Donovan, J.; Jiang, W.; Grundy, D. The mast cell degranulator compound 48/80 directly activates neurons. PLoS ONE 2012, 7, e52104. [Google Scholar] [CrossRef]
- Hughes, J.P.; Rees, S.; Kalindjian, S.B.; Philpott, K.L. Principles of early drug discovery. Br. J. Pharmacol. 2011, 162, 1239–1249. [Google Scholar] [CrossRef]
- Li, J.; Fu, A.; Zhang, L. An overview of scoring functions used for protein–ligand interactions in molecular docking. Interdiscip. Sci. Comput. Life Sci. 2019, 1–9. [Google Scholar] [CrossRef]
- Lengauer, T.; Rarey, M. Computational methods for biomolecular docking. Curr. Opin. Struct. Biol. 1996, 6, 402–406. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y. I-TASSER server: New development for protein structure and function predictions. Nucleic Acids Res. 2015, 43, W174–W181. [Google Scholar] [CrossRef]
- Sheik, S.; Sundararajan, P.; Hussain, A.; Sekar, K. Ramachandran plot on the web. Bioinformatics 2002, 18, 1548–1549. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, W.; Gao, S.; Xu, H.; Wu, B.; Kulkarni, K.; Singh, R.; Tang, L.; Hu, M. Simultaneous determination of genistein and its four phase II metabolites in blood by a sensitive and robust UPLC–MS/MS method: Application to an oral bioavailability study of genistein in mice. J. Pharm. Biomed. Anal. 2010, 53, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Suzuki, Y.; Ikeda, Y.; Umemura, K. Genistein, an isoflavone included in soy, inhibits thrombotic vessel occlusion in the mouse femoral artery and in vitro platelet aggregation. Eur. J. Pharmacol. 2002, 455, 53–57. [Google Scholar] [CrossRef]
- Radu, M.; Chernoff, J. An in vivo assay to test blood vessel permeability. Jove (J. Vis. Exp.) 2013, 73, e50062. [Google Scholar] [CrossRef]
- Rådinger, M.; Jensen, B.M.; Kuehn, H.S.; Kirshenbaum, A.; Gilfillan, A.M. Generation, isolation, and maintenance of human mast cells and mast cell lines derived from peripheral blood or cord blood. Curr. Protoc. Immunol. 2010, 90, 7–37. [Google Scholar]
- Slater, T.; Sawyer, B.; Sträuli, U. Studies on succinate-tetrazolium reductase systems: III. Points of coupling of four different tetrazolium salts III. Points of coupling of four different tetrazolium salts. Biochim. Et Biophys. Acta 1963, 77, 383–393. [Google Scholar] [CrossRef]
- Kroeze, W.K.; Sassano, M.F.; Huang, X.-P.; Lansu, K.; McCorvy, J.D.; Giguère, P.M.; Sciaky, N.; Roth, B.L. PRESTO-Tango as an open-source resource for interrogation of the druggable human GPCRome. Nat. Struct. Mol. Biol. 2015, 22, 362. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.; Weissig, H.; Shindyalov, I.; Bourne, P. Th e protein data bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Yang, J.; Yan, R.; Roy, A.; Xu, D.; Poisson, J.; Zhang, Y. The I-TASSER Suite: Protein structure and function prediction. Nat. Methods 2015, 12, 7. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminformatics 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Schrodinger, L. The PyMOL Molecular Graphics System, version 1.8. 2015.
- Research, D.E.S. Desmond Molecular Dynamics System, D. E. Shaw Research, version 3.4; Schrödinger: New York, NY, USA, 2017. [Google Scholar]
- Dassault Systèmes BIOVIA. Discovery Studio Modeling Environment; Dassault Systèmes: San Diego, CA, USA, 2017. [Google Scholar]
Sample Availability: Sample of the compound (Genistein) is available from the authors. |


| In-vitro assays | EC50 | E Max | IC50 | ||||
|---|---|---|---|---|---|---|---|
| Graded Concentration of Compound 48/80 | Genistein +10 µM Compound 48/80 | ||||||
| Buffer | Genistein (50 µM) | Genistein (100 µM) | Buffer | Genistein (50 µM) | Genistein (100 µM) | ||
| β-Hex | 2.38 × 10−6 M | 4.48 × 10−6 M | 5.76 × 10−6 M | 63.81 | 46.99 | 35.43 | 2.76 × 10−5 M |
| PRESTO-tango | 1.98 × 10−6 M | 2.02 × 10−6 M | 2.18 × 10−6 M | 21,483 | 15,848 | 11,997 | 3.83 × 10−5 M |
| Ca2+ flux | 4.94 × 10−6 M | 4.07 × 10−6 M | 3.61 × 10−6 M | 107,462 | 74,457 | 60,570 | 3.16 × 10−5 M |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, M.; Singh, K.; Duraisamy, K.; Allam, A.A.; Ajarem, J.; Kwok Chong CHOW, B. Protective Effect of Genistein against Compound 48/80 Induced Anaphylactoid Shock via Inhibiting MAS Related G Protein-Coupled Receptor X2 (MRGPRX2). Molecules 2020, 25, 1028. https://doi.org/10.3390/molecules25051028
Kumar M, Singh K, Duraisamy K, Allam AA, Ajarem J, Kwok Chong CHOW B. Protective Effect of Genistein against Compound 48/80 Induced Anaphylactoid Shock via Inhibiting MAS Related G Protein-Coupled Receptor X2 (MRGPRX2). Molecules. 2020; 25(5):1028. https://doi.org/10.3390/molecules25051028
Chicago/Turabian StyleKumar, Mukesh, Kailash Singh, Karthi Duraisamy, Ahmed A. Allam, Jamaan Ajarem, and Billy Kwok Chong CHOW. 2020. "Protective Effect of Genistein against Compound 48/80 Induced Anaphylactoid Shock via Inhibiting MAS Related G Protein-Coupled Receptor X2 (MRGPRX2)" Molecules 25, no. 5: 1028. https://doi.org/10.3390/molecules25051028
APA StyleKumar, M., Singh, K., Duraisamy, K., Allam, A. A., Ajarem, J., & Kwok Chong CHOW, B. (2020). Protective Effect of Genistein against Compound 48/80 Induced Anaphylactoid Shock via Inhibiting MAS Related G Protein-Coupled Receptor X2 (MRGPRX2). Molecules, 25(5), 1028. https://doi.org/10.3390/molecules25051028






