The Effect of Thermomechanical Pretreatment on the Structure and Properties of Lignin-Rich Plant Biomass
Abstract
1. Introduction
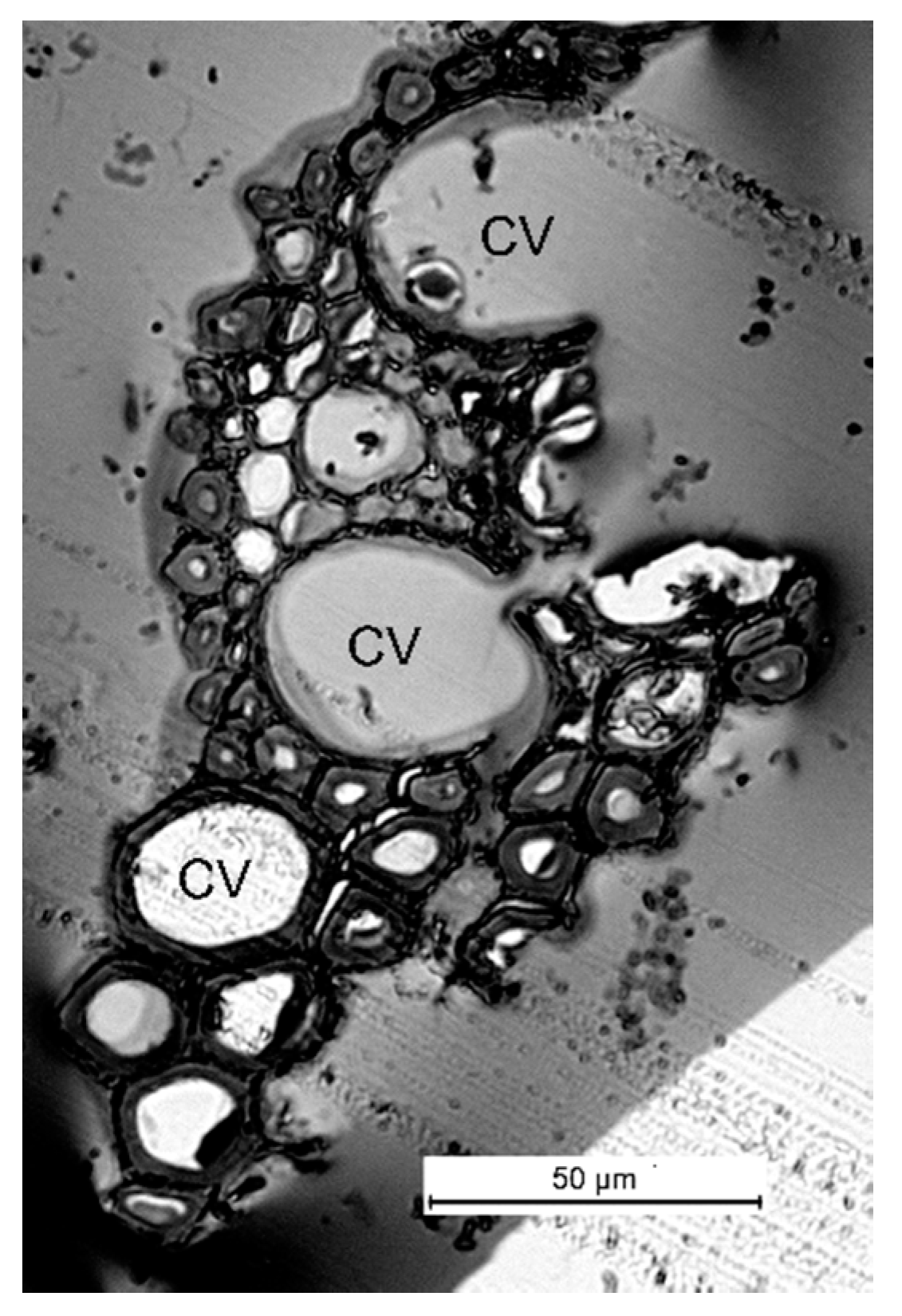
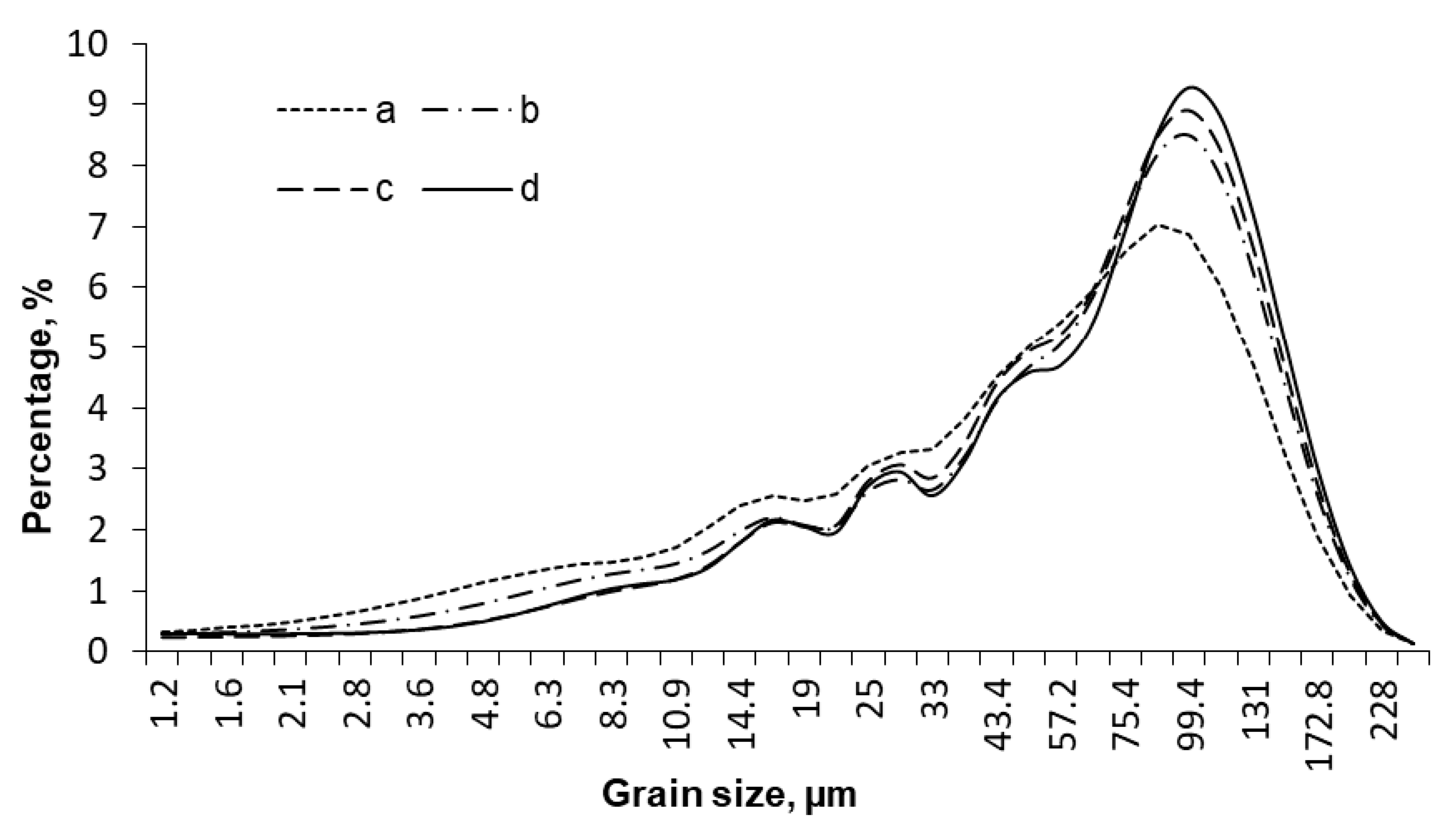
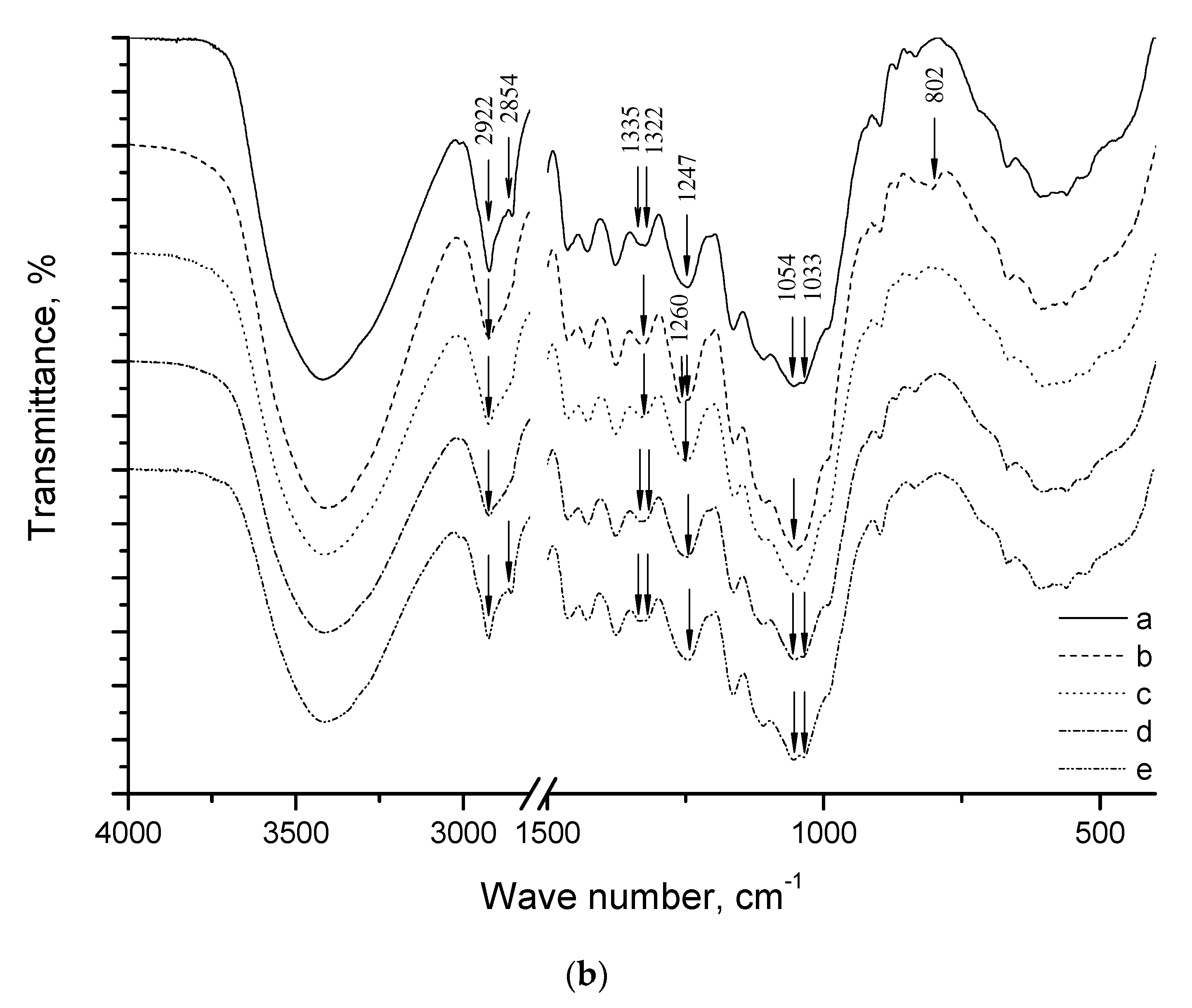
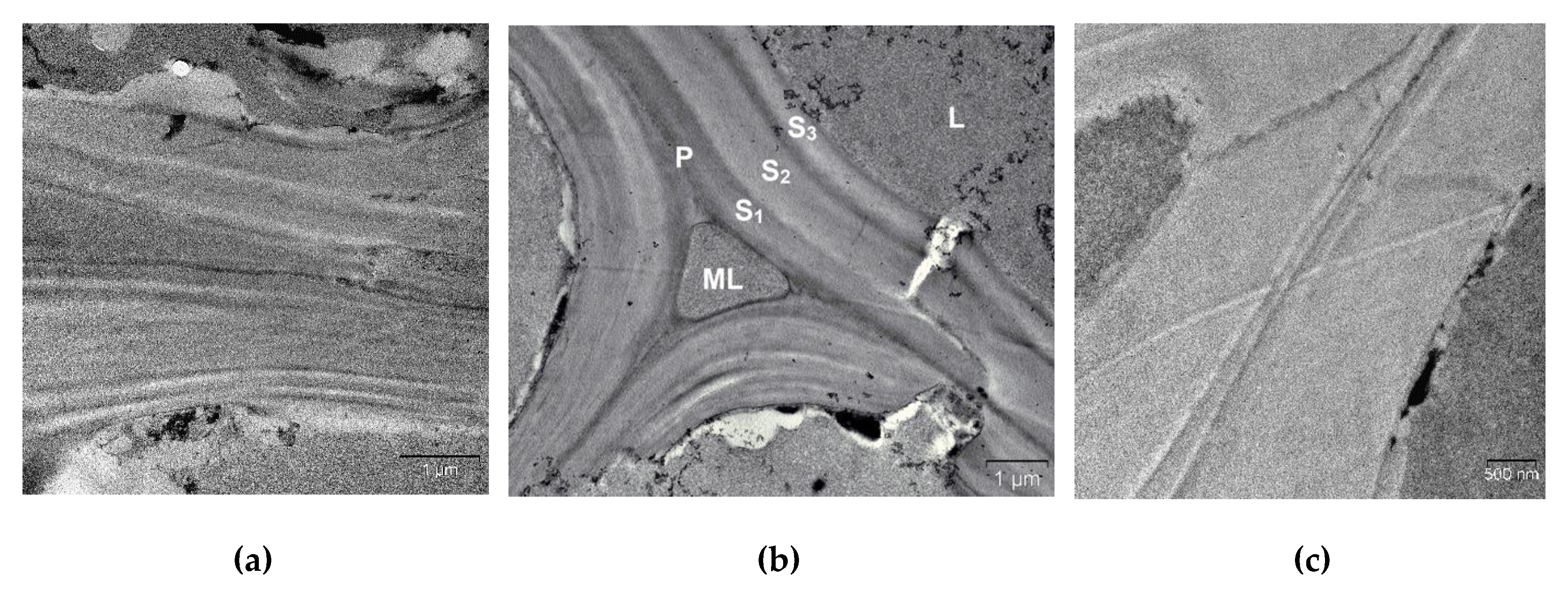
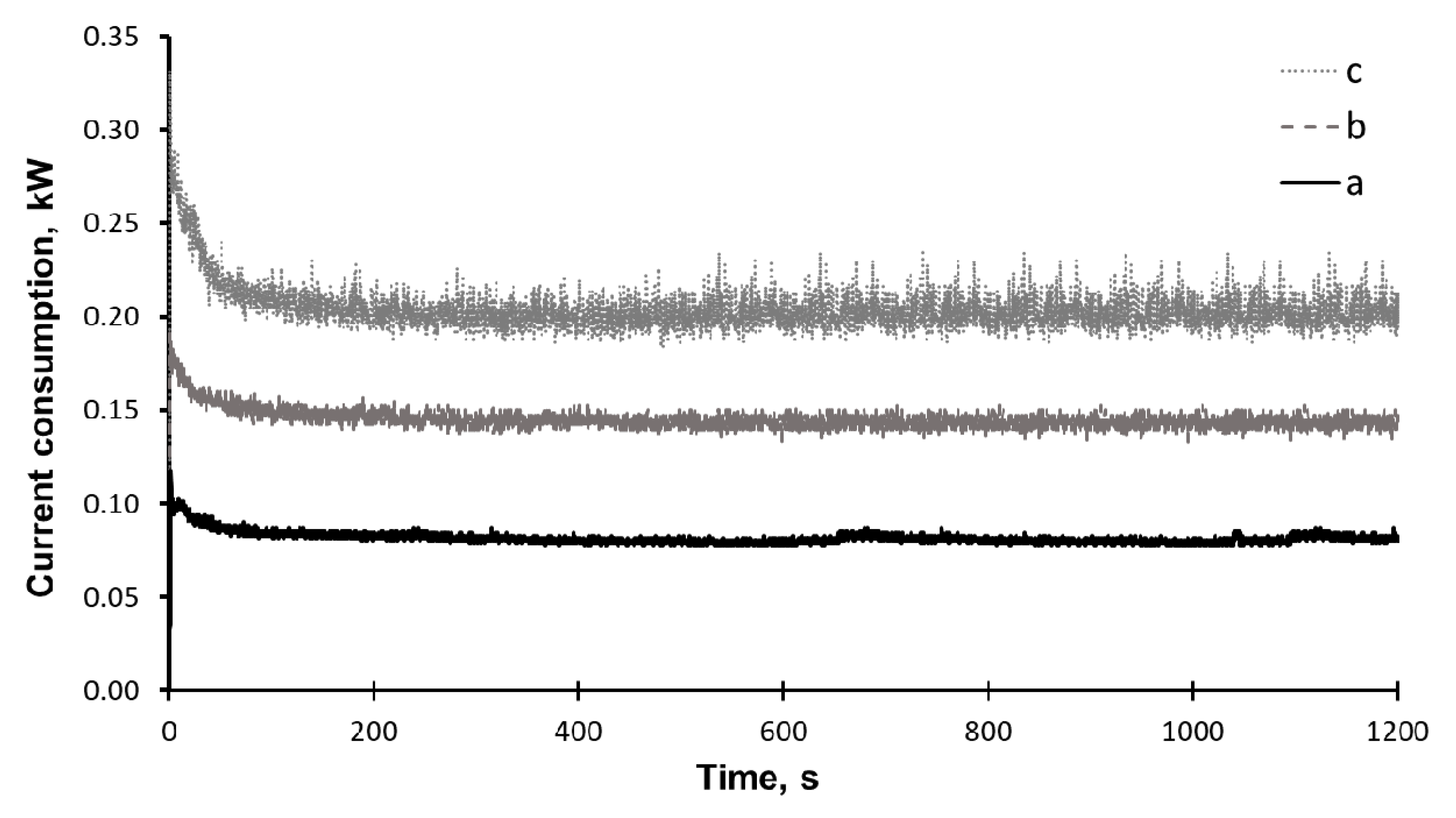
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gallezot, P. Conversion of biomass to selected chemical products. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef]
- Luterbacher, J.S.; Martin Alonso, D.; Dumesic, J.A. Targeted chemical upgrading of lignocellulosic biomass to platform molecules. Green Chem. 2014, 16, 4816–4838. [Google Scholar] [CrossRef]
- Zemnukhova, L.A.; Skiba, E.A.; Budaeva, V.V.; Panasenko, A.E.; Polyakova, N.V. Composition of inorganic components of oat husks and products of their chemical and enzymatic transformation. Russ. J. Appl. Chem. 2018, 91, 230–234. [Google Scholar] [CrossRef]
- Godoy, C.M.D.; Machado, D.L.; Costa, A.C.D. Batch and fed-batch enzymatic hydrolysis of pretreated sugarcane bagasse—Assays and modeling. Fuel 2019, 253, 392–399. [Google Scholar] [CrossRef]
- Berglin, N.; Tomani, P.; Salman, H.; Svard, S.H. Pilot-scale combustion studies with kraft lignin in a powder burner and a CFB boiler. Tappi J. 2010, 9, 24–30. [Google Scholar] [CrossRef]
- Mesfun, S.; Lundgren, J.; Grip, C.E.; Toffolo, A.; Nilsson, R.L.; Rova, U. Black liquor fractionation for biofuels production—A technoeconomic assessment. Bioresour. Technol. 2014, 166, 508–517. [Google Scholar] [CrossRef]
- Fang, Z.; Smith, R.L., Jr. Production of Biofuels and Chemicals from Lignin; Springer: Singapore, 2016; 435p. [Google Scholar] [CrossRef]
- Urazova, T.S.; Bychkov, A.L.; Lomovskii, O.I. Sorption capacity of lignocellulosic materials toward humic acids. Russ. Chem. Bull. 2015, 64, 1183–1188. [Google Scholar] [CrossRef]
- Kucharska, K.; Rybarczyk, P.; Holowacz, I.; Lukajtis, R.; Glinka, M.; Kaminski, M. Pretreatment of lignocellulosic materials as substrates for fermentation processes. Molecules 2018, 23, 2937. [Google Scholar] [CrossRef]
- Barakat, A.; Mayer-Laigle, C.; Solhy, A.; Arancon, R.A.D.; Vries, H.; Luque, R. Mechanical pretreatments of lignocellulosic biomass: Towards facile and environmentally sound technologies for biofuels production. Rsc Adv. 2014, 4, 48109–48127. [Google Scholar] [CrossRef]
- Bychkov, A.L.; Podgorbunskikh, E.M.; Bychkova, E.S.; Lomovsky, O.I. Current achievements in the mechanically pretreated conversion of plant biomass. Biotechnol. Bioeng. 2019, 116, 1234–1244. [Google Scholar] [CrossRef]
- Ashraf, M.T.; Thomsen, M.H.; Schmidt, J.E. Hydrothermal pretreatment and enzymatic hydrolysis of mixed green and woody lignocellulosics from arid regions. Bioresour. Technol. 2017, 238, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.W.; Zhu, M.Q.; Li, M.F.; Wei, Q.; Sun, R.C. Effects of hydrothermal treatment on enhancing enzymatic hydrolysis of rapeseed straw. Renew. Energy 2019, 134, 446–452. [Google Scholar] [CrossRef]
- Ahmad, F.; Silva, E.L.; Varesche, M.B.A. Hydrothermal processing of biomass for anaerobic digestion—A review. Renew. Sustain. Energy Rev. 2018, 98, 108–124. [Google Scholar] [CrossRef]
- Sahoo, D.; Ummalyma, S.B.; Okram, A.K.; Pandey, A.; Sankar, M.; Sukumaran, R.K. Effect of dilute acid pretreatment of wild rice grass (Zizania latifolia) from Loktak Lake for enzymatic hydrolysis. Bioresour. Technol. 2018, 253, 252–255. [Google Scholar] [CrossRef]
- Bensah, E.C.; Kadar, Z.; Mensah, M.Y. Alkali and glycerol pretreatment of west african biomass for production of sugars and ethanol. Bioresour. Technol. Rep. 2019, 6, 123–130. [Google Scholar] [CrossRef]
- Inkrod, C.; Raita, M.; Champreda, V.; Laosiripojana, N. Characteristics of lignin extracted from different lignocellulosic materials via organosolv fractionation. Bioenergy Res. 2018, 11, 277–290. [Google Scholar] [CrossRef]
- Donohoe, B.S.; Decker, S.R.; Tucker, M.P.; Himmel, M.E.; Vinzant, T.B. Visualizing lignin coalescence and migration through maize cell walls following thermochemical pretreatment. Biotechnol. Bioeng. 2008, 101, 913–925. [Google Scholar] [CrossRef]
- Rahikainen, J.; Mikander, S.; Marjamaa, K.; Tamminen, T.; Lappas, A.; Viikari, L.; Kruus, K. Inhibition of enzymatic hydrolysis by residual lignins from softwood—Study of enzyme binding and inactivation on lignin-rich surface. Biotechnol. Bioeng. 2011, 108, 2823–2834. [Google Scholar] [CrossRef]
- Yingfu, Z.; Songping, Z.; Shida, M.; Zhiguo, S.; Ping, W. Temperature sensitivity of cellulase adsorption on lignin and its impact on enzymatic hydrolysis of lignocellulosic biomass. J. Biotechnol. 2013, 166, 135–143. [Google Scholar] [CrossRef]
- Chow, S.Z.; Pickles, K.J. Thermal softening and degradation of wood and bark. Wood Fiber Sci. 1971, 3, 166–178. [Google Scholar]
- Kubo, S.; Uraki, Y.; Sano, Y. Thermomechanical analysis of isolated lignins. Holzforschung 1996, 50, 144–150. [Google Scholar] [CrossRef]
- Tejado, A.; Pena, C.; Labidi, J.; Echeverria, J.M.; Mondragon, I. Physico-chemical characterization of lignins from different sources for use in phenol–formaldehyde resin synthesis. Bioresour. Technol. 2007, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Ol’hov, Y.A.; Chernikov, S.S.; Mikhajlov, A.I. Solutionless analysis of molecular-mass distributions in vegetable polymers of lignin, cellulose and wood by the thermomechanical method. Chem. Plant Raw Mater. 2001, 2, 83–95. [Google Scholar]
- Chen, M.; Lu, J.; Cheng, Y.; Li, Q.; Wang, H. Novel process for the coproduction of xylo-oligosaccharide and glucose from reed scraps of reed pulp mill. Carbohydr. Polym. 2019, 215, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Cotana, F.; Cavalaglio, G.; Pisello, A.L.; Gelosia, M.; Ingles, D.; Pompili, E. Sustainable ethanol production from common reed (Phragmites australis) through simultaneuos saccharification and fermentation. Sustainability 2015, 7, 12149–12163. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Liu, M.; Cao, B.; Tan, H.; Wang, J.; Li, X. Responses of three different ecotypes of reed (Phragmites communis Trin.) to their natural habitats: Leaf surface micro-morphology, anatomy, chloroplast ultrastructure and physio-chemical characteristics. Plant Physiol. Biochem. 2012, 51, 159–167. [Google Scholar] [CrossRef]
- Debzi, E.M.; Chanzy, H.; Sugiyama, J.; Tekely, P.; Exoffier, G. The Iα→Iβ transformation of highly crystalline cellulose by annealing in various mediums. Macromolecules 1991, 24, 6816–6822. [Google Scholar] [CrossRef]
- Yamamoto, H.; Horii, F.; Odani, H. Structural changes of native cellulose crystals induced by annealing in aqueous alkaline and acidic solutions at high temperatures. Macromolecules 1989, 22, 4130–4132. [Google Scholar] [CrossRef]
- Raven, P.H.; Evert, R.F.; Eichhorn, S.E. Biology of Plants, 8th ed.; W.H. Freeman & Co Ltd.: New York, NY, USA, 2012; p. 900. [Google Scholar]
- Sannigrahi, P.; Kim, D.H.; Jung, S.; Ragauskas, A. Pseudo-lignin and pretreatment chemistry. Energy Env. Sci. 2011, 4, 1306–1310. [Google Scholar] [CrossRef]
- Meng, X.; Ragauskas, A.J. Pseudo-lignin formation during dilute acid pretreatment for cellulosic ethanol. Recent Adv. Petrochem. Sci. 2017, 1, 1–5. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

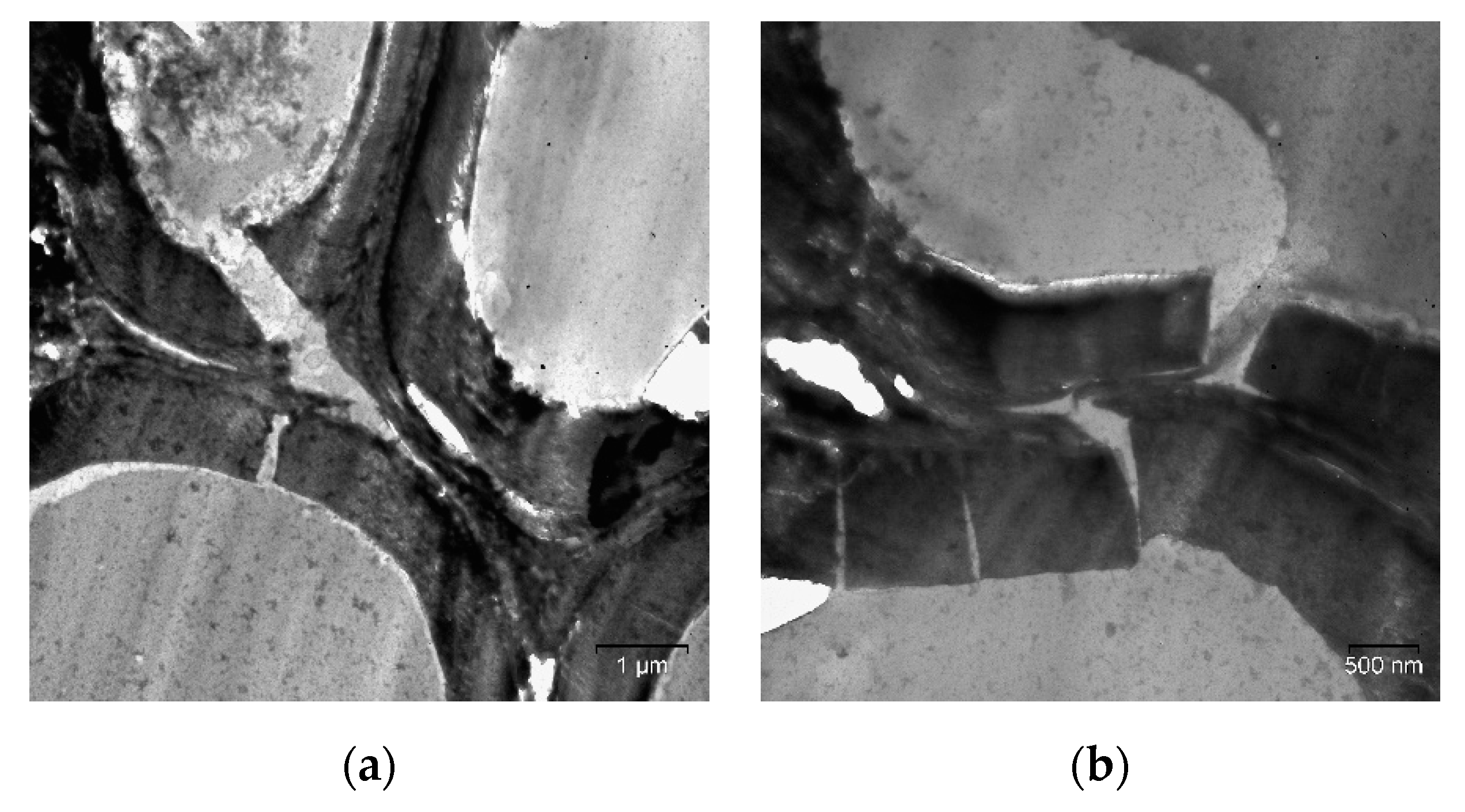
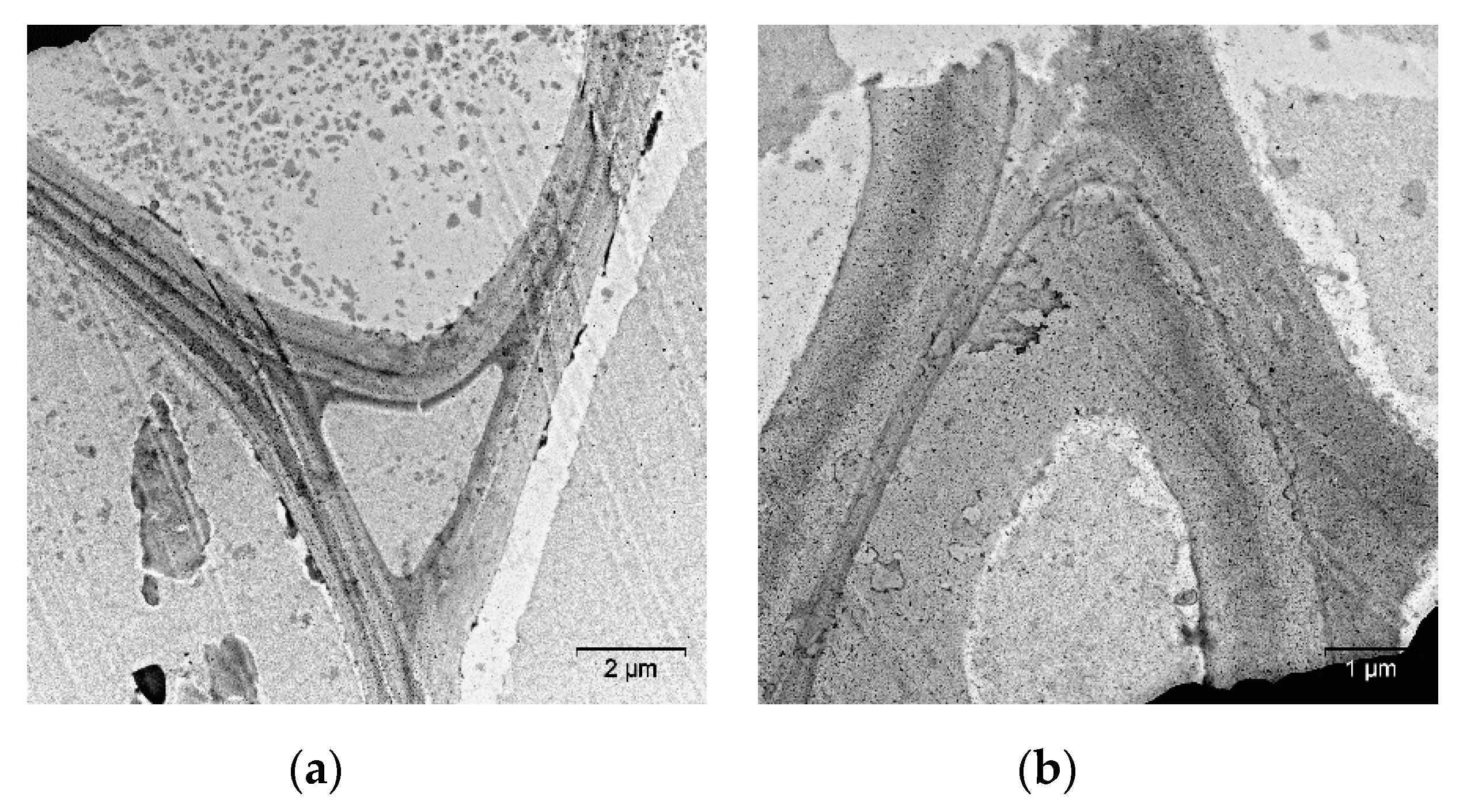

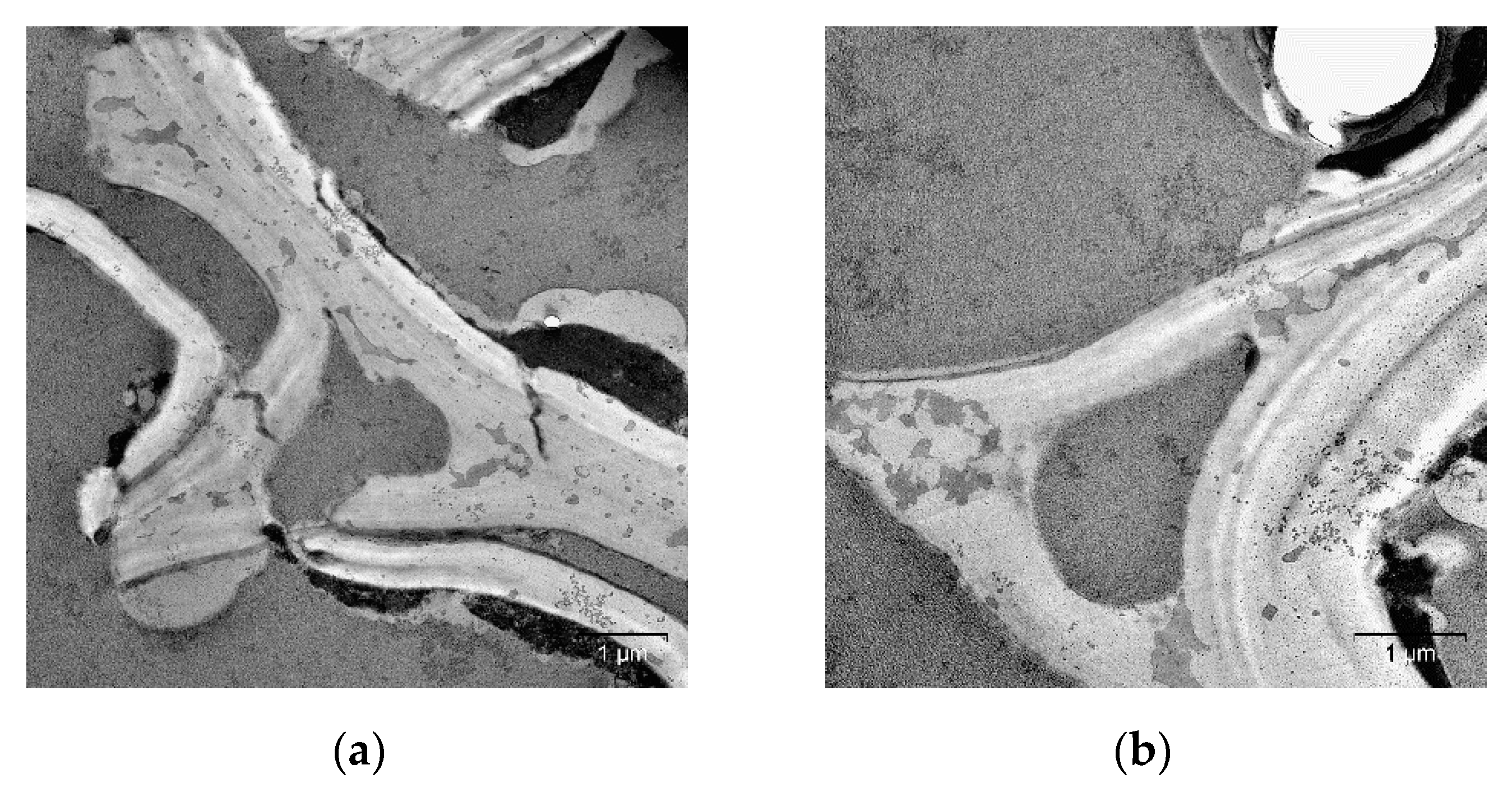

| Specimen | Average Grain Size, µm | Sspec, m2/g | Crystallinity Index, % |
|---|---|---|---|
| Initial reed biomass | 0.5–1 mm | 0.5 ± 0.1 | 72 ± 3 |
| Mechanical treatment at −196 °C | 45 ± 4 | 4.3 ± 0.3 | 60 ± 4 |
| Mechanical treatment at 10 °C | 54 ± 4 | 2.6 ± 0.2 | 61 ± 4 |
| Mechanical treatment at 100 °C | 65 ± 6 | 4.1 ± 0.3 | 66 ± 4 |
| Mechanical treatment at 180 °C | 63 ± 5 | 5.0 ± 0.3 | 67 ± 5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podgorbunskikh, E.M.; Bychkov, A.L.; Ryabchikova, E.I.; Lomovsky, O.I. The Effect of Thermomechanical Pretreatment on the Structure and Properties of Lignin-Rich Plant Biomass. Molecules 2020, 25, 995. https://doi.org/10.3390/molecules25040995
Podgorbunskikh EM, Bychkov AL, Ryabchikova EI, Lomovsky OI. The Effect of Thermomechanical Pretreatment on the Structure and Properties of Lignin-Rich Plant Biomass. Molecules. 2020; 25(4):995. https://doi.org/10.3390/molecules25040995
Chicago/Turabian StylePodgorbunskikh, Ekaterina M., Aleksey L. Bychkov, Elena I. Ryabchikova, and Oleg I. Lomovsky. 2020. "The Effect of Thermomechanical Pretreatment on the Structure and Properties of Lignin-Rich Plant Biomass" Molecules 25, no. 4: 995. https://doi.org/10.3390/molecules25040995
APA StylePodgorbunskikh, E. M., Bychkov, A. L., Ryabchikova, E. I., & Lomovsky, O. I. (2020). The Effect of Thermomechanical Pretreatment on the Structure and Properties of Lignin-Rich Plant Biomass. Molecules, 25(4), 995. https://doi.org/10.3390/molecules25040995








