Inhibitory Effects of Eriodictyol-7-O-β-d-glucuronide and 5,7-Dihydroxy-4-chromene Isolated from Chrysanthemum zawadskii var. latilobum in FcεRI-Mediated Human Basophilic KU812F Cell Activation
Abstract
1. Introduction
2. Results
2.1. EDG and DC Isolation from CZL
2.2. EDG and DC Cytotoxicity
2.3. EDG and DC Effects on FcεRI Expression
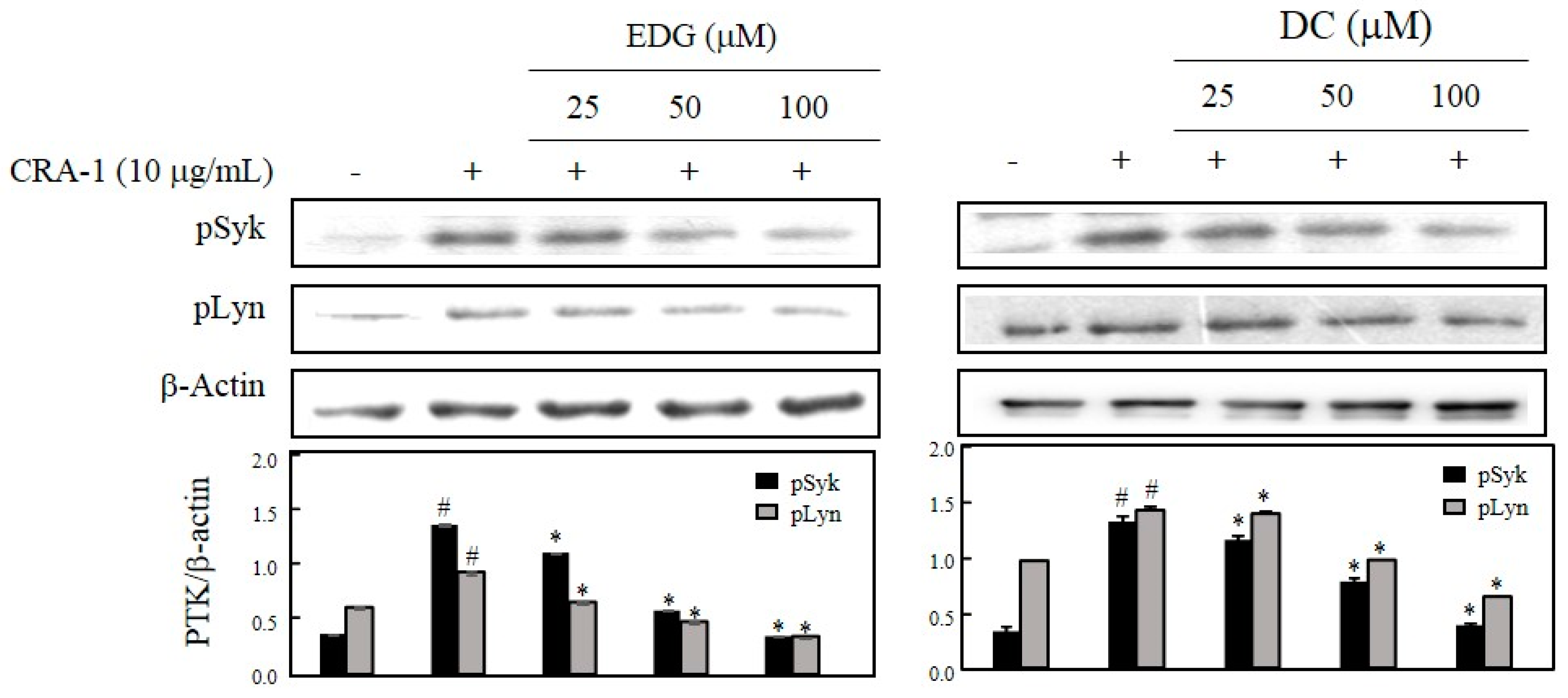
2.4. EDG and DC Effects on FcεRI-Mediated Activation of PTK, Syk, and Lyn
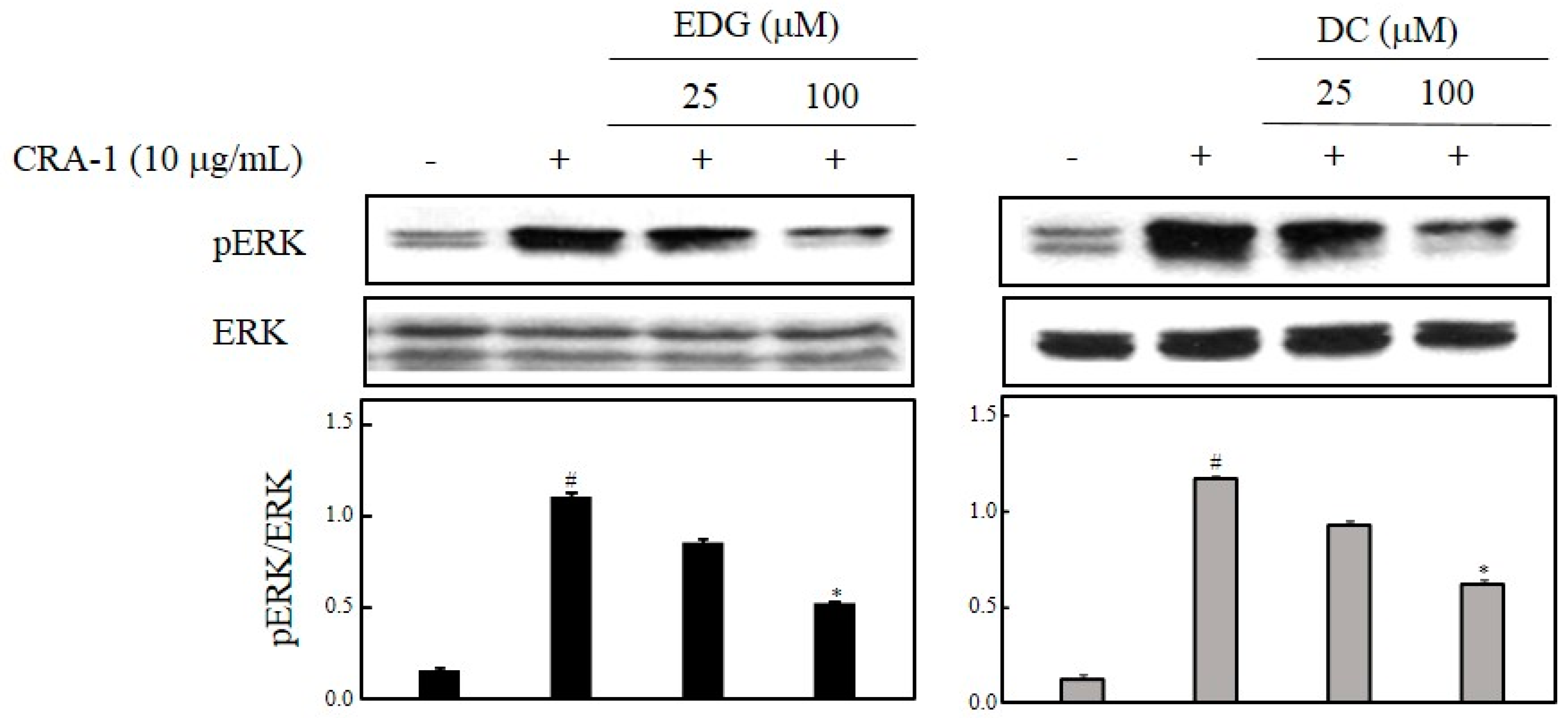
2.5. EDG and DC Effects on FcεRI-Mediated ERK ½ Activation
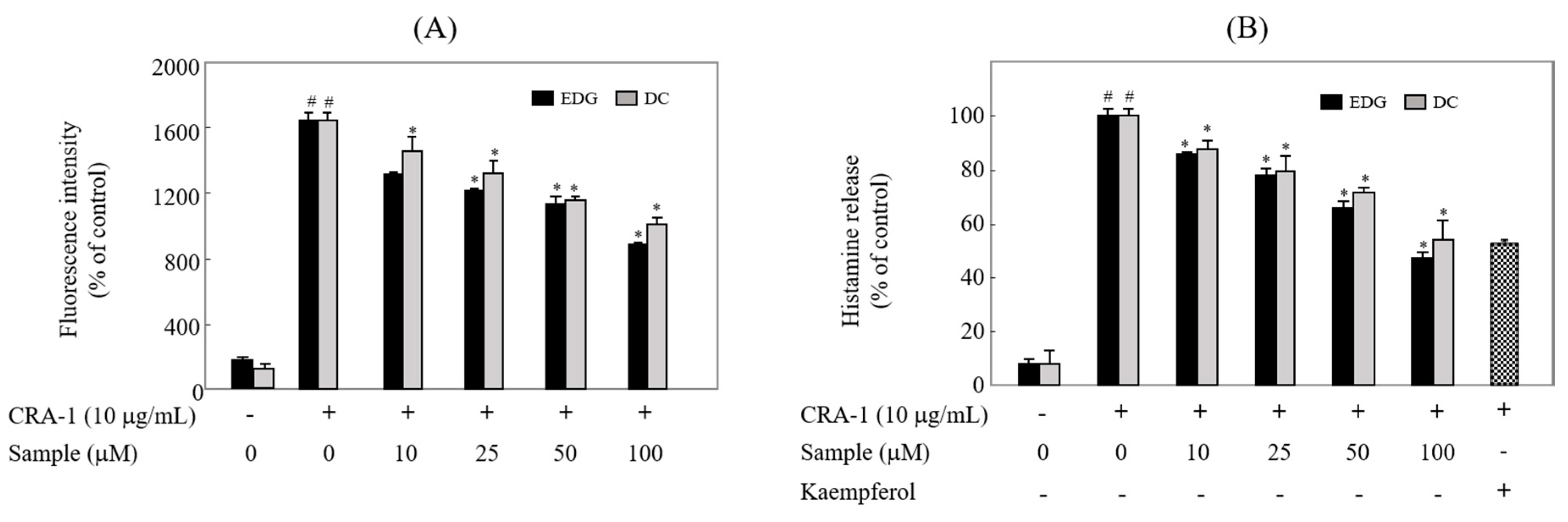
2.6. EDG and DC Inhibited FcεRI-Mediated Calcium Influx and Degranulation
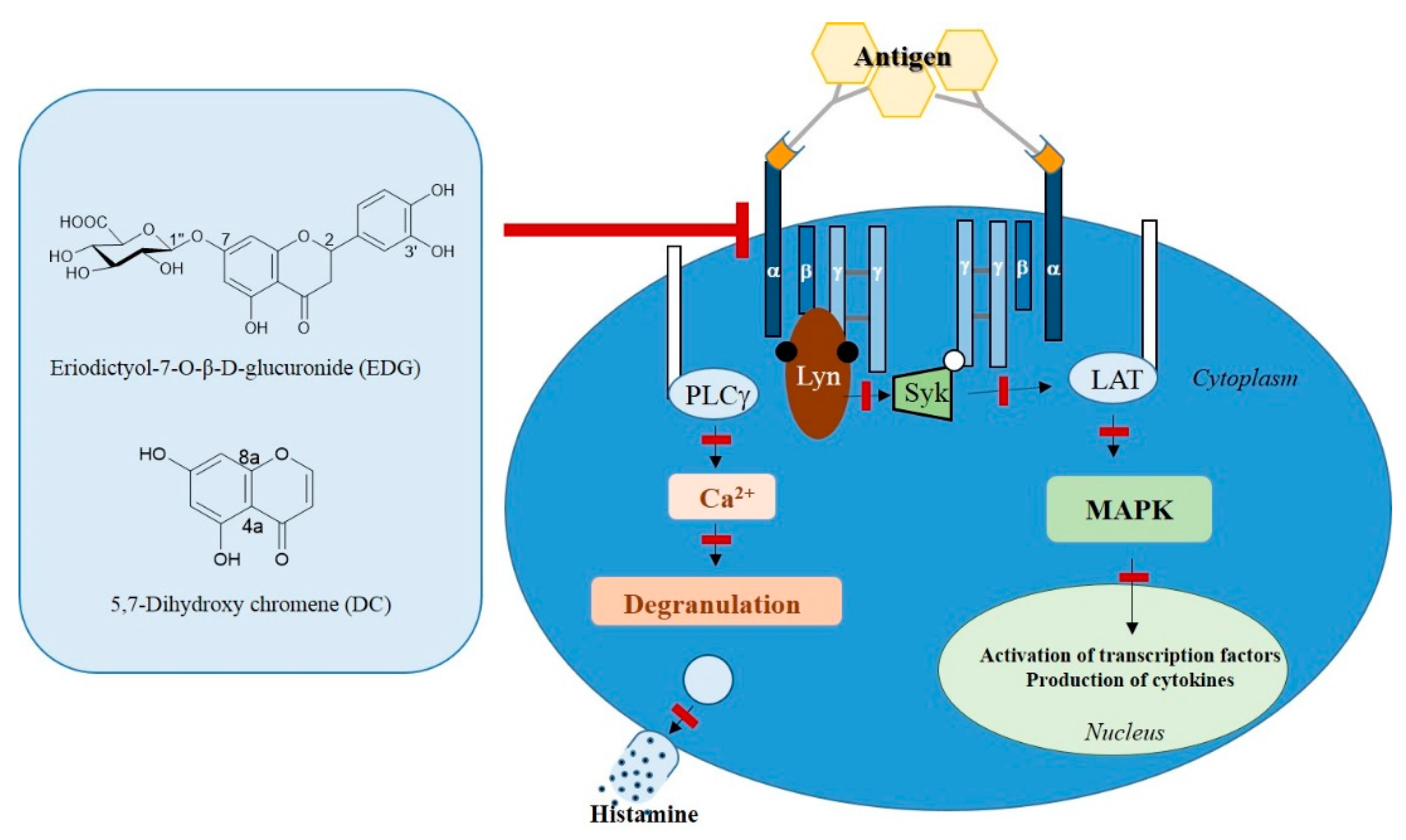
2.7. EDG and DC Effects on FcεRI-Mediated Signaling Pathway in KU812F Cells
3. Discussion
4. Materials and Methods
4.1. Plant Materials, Extraction, and Isolation
4.2. Cell Culture, Treatment, and Stimulation
4.3. Cell-Viability Assay
4.4. Flow Cytometric Analysis
4.5. Western Blot Analysis
4.6. Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
4.7. [Ca2+]i-Level Assay
4.8. Histamine-Release Assay
4.9. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Kepley, C.L. New approaches to allergen immunotherapy. Curr. Allergy Asthma Rep. 2006, 6, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Oppenheimer, J.J.; Casale, T.B. Next generation antihistamines: Therapeutic rationale, accomplishments and advances. Expert Opin. Invest. Drugs 2002, 11, 807–817. [Google Scholar]
- Kinet, J.P.; Blank, U.; Brini, A.; Jouvin, M.H.; Kuster, H.; Mejan, O.; Ra, C. The high affinity receptor for immunoglobulin E: A target for therapy of allergic diseases. Int. Arch. Allergy Immunol. 1991, 94, 51–55. [Google Scholar] [CrossRef]
- Kinet, J.P. The high-affinity IgE receptor (FcεRI): From physiology to pathology. Annu. Rev. Immunol. 1999, 17, 931–972. [Google Scholar] [CrossRef] [PubMed]
- Beaven, M.A.; Metzger, H. Signal transduction Fc receptors: The Fc&RI case. Immunol. Today 1993, 14, 222–226. [Google Scholar]
- Metzer, H. The high affinity receptor for IgE on mast cells. Clin. Exp. Immunol. 1991, 21, 269–279. [Google Scholar]
- He, S.; Zhang, H.; Zeng, X.; Chen, D.; Yang, P. Mast cells and basophils are essential for allergies: Mechanisms of allergic inflammation and a proposed procedure for diagnosis. Acta Pharmacol. Sin. 2013, 34, 1270–1283. [Google Scholar] [CrossRef]
- Hakimi, J.C.; Seals, J.A.; Kondas, L.; Pettine, W.; Danko, W.; Kochan, J. The α subunit of the human IgE receptor (FcεRI) is sufficient for high affinity IgE binding. J. Biol. Chem. 1990, 265, 22079–22081. [Google Scholar]
- Siraganian, R.P. Mast cell signal transduction from the high-affinity IgE receptor. Curr. Opin. Immunol. 2003, 15, 639–646. [Google Scholar] [CrossRef]
- Siraganian, R.P.; de Castro, R.O.; Barbu, E.A.; Zhang, J. Mast cell signaling: The role of protein tyrosine kinase Syk, its activation and screening methods for new pathway participants. FEBS Lett. 2010, 584, 4933–4940. [Google Scholar] [CrossRef]
- Rivera, J. Molecular adapters in FcεRI signaling and the allergic response. Curr. Opin. Immunol. 2002, 14, 688–693. [Google Scholar] [CrossRef]
- Xian, Z.; Jin, G.; Li, H.; Jiang, J.; Wang, C.; Zhu, L.; Jin, Z.; Li, L.; Piao, H.; Zheng, M.; et al. Imperatorin suppresses anaphylactic reaction and IgE-mediated allergic responses by inhibiting multiple steps of FceRI signaling in mast cells: IMP alleviates allergic responses in PCA. Biomed. Res. Int. 2019, 2019, 7823761. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.S.; Ha, T.J.; Hwang, S.W.; Jin, Y.M.; Nam, S.H.; Park, K.H.; Yang, M.S. Cytotoxic flavonoids from the whole plants of Chrysanthemum zawadskii Herbich var. latilobum Kitamura. J. Life Sci. 2006, 16, 746–749. [Google Scholar]
- Seo, J.Y.; Lim, S.S.; Park, J.A.; Lim, J.S.; Kim, H.J.; Kang, H.J.; Yoon Park, J.H.; Kim, J.S. Protection by Chrysanthemum zawadskii extract from liver damage of mice caused by carbon tetrachloride is maybe mediated by modulation of QR activity. Nutr. Res. Pract. 2010, 4, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Agrawal, P.; Yim, D.S.; Agarwal, C.; Agarwal, R. Acacetin inhibits cell growth and cell cycle progression, and induces apoptosis in human prostate cancer cells: Structure-activity relationship with linarin and linarin acetate. Carcinogenesis 2005, 26, 845–854. [Google Scholar] [CrossRef]
- Kim, B.; Lee, J.H.; Seo, M.J.; Eom, S.H.; Kim, W. Linarin down-regulates phagocytosis, pro-inflammatory cytokine production, and activation marker expression in RAW264.7 macrophages. Food Sci. Biotechnol. 2016, 25, 1437–1442. [Google Scholar] [CrossRef]
- Hsu, Y.L.; Kuo, P.L.; Lin, C.C. Acacetin inhibits the proliferation of HepG2 by blocking cell cycle progression and inducing apoptosis. Biochem. Pharmacol. 2004, 67, 823–829. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Lee, S.Y.; Yim, D.S. Biological activities of linarin from Chrysanthemum zawadskii var. latilobum. Yakhak Hoeji 2001, 45, 604–610. [Google Scholar]
- Han, S.; Sung, K.H.; Yim, D.; Lee, S.; Lee, C.K.; Ha, N.J.; Kim, K. The effect of linarin on LPS-induced cytokine production and nitric oxide inhibition in murine macrophage cell line RAW264.7. Arch. Pharm. Res. 2002, 25, 170–177. [Google Scholar] [CrossRef]
- Shim, S.Y.; Kang, H.S.; Sun, H.J.; Lee, Y.J.; Park, J.R.; Chun, S.S.; Song, Y.H.; Byun, D.S. Isolation and identification of flavonoids from Gujeolcho (Chrysanthemum zawadskii var. latilobum) as inhibitor of histamine release. Food Sci. Biotechnol. 2012, 21, 613–617. [Google Scholar] [CrossRef]
- Garg, N.; Luzzatto-Knaan, T.; Melnik, A.N.; Caraballo-Rodriguez, A.M.; Floros, D.J.; Petras, D.; Gregor, R.; Dorrestein, P.C.; Phelan, V.V. Natural products as mediators of disease. Nat. Prod. Rep. 2017, 34, 194–219. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Je, I.G.; Song, J.; Fei, X.; Lee, S.; Yang, H.; Kang, W.; Jang, Y.H.; Seo, S.Y.; Kim, S.H. SG-SP1 suppresses mast cell-mediated allergic inflammation via inhibition of FcεRI signaling. Front. Immunol. 2020, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- Rakhmanova, V.; Park, S.; Lee, S.; Kim, Y.H.; Shin, J. 3-Benzyl-5-((2-nitrophenoxy) methyl)-dihydrofuran-2(3H)-one suppresses FcεRI-mediated mast cell degranulation via the inhibition of mTORC2-Akt signaling. Biochem. Biophys. Res. Commun. 2020, 521, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Cemerski, S.; Chu, S.Y.; Moore, G.L.; Muchhal, U.S.; Desjarlais, J.R.; Szymkowski, D.E. Suppression of mast cell degranulation through a dual-targeting tandem IgE-IgG Fc domain biologic engineered to bind with high affinity to FcεRIIb. Immunol. Lett. 2012, 143, 34–43. [Google Scholar] [CrossRef]
- Kadam, P.D.; Chuan, H.H. Rectocutaneous fistula with transmigration of the suture: A rare delayed complication of vault fixation with the sacrospinous ligament. Intl. Urogynecol. J. 2016, 27, 155–157. [Google Scholar] [CrossRef]
- Medzhitov, R.; Horng, T. Transcriptional control of the inflammatory response. Nat. Rev. Immunol. 2009, 9, 692–703. [Google Scholar] [CrossRef]
- Johnson, G.L.; Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef]
- Schottelius, A.J.G.; Baldwin, A.S., Jr. A role for transcription factor NF-kappa B in intestinal inflammation. Int. J. Colorectal Dis. 1999, 14, 18–28. [Google Scholar] [CrossRef]
- Blank, U.; Ra, C.; Miller, L.; White, K.; Metzer, H.; Kinet, J.P. Complete structure and expression in transfected cells of high affinity IgE receptor. Nature 1989, 337, 187–189. [Google Scholar] [CrossRef]
- Miller, L.; Blank, U.; Metzer, H.; Kinet, J.P. Expression of high affinity binding of human immunoglobulin E by transfected cells. Science 1989, 244, 334–337. [Google Scholar] [CrossRef]
- Ra, C.; Jouvin, M.H.; Kinet, J.P. Complete structure of the mouse mast cell receptor for IgE (FcεRI) and surface expression of chimeric receptors (rat-mouse-human) on transfected cells. J. Biol. Chem. 1989, 264, 15323–15327. [Google Scholar] [PubMed]
- Wu, L.C. Immunoglobulin E receptor signaling and asthma. J. Biol. Chem. 2011, 286, 32891–32897. [Google Scholar] [CrossRef] [PubMed]
- Shore, P.A.; Burkhalter, A.; Cohn, V.H. A method for the fluorometric assay of histamine in tissues. J. Pharmacol. Exp. Ther. 1959, 127, 183–186. [Google Scholar]
Sample Availability: Samples of the compounds used in this research are not available from the authors. |



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.; Shim, S.-Y. Inhibitory Effects of Eriodictyol-7-O-β-d-glucuronide and 5,7-Dihydroxy-4-chromene Isolated from Chrysanthemum zawadskii var. latilobum in FcεRI-Mediated Human Basophilic KU812F Cell Activation. Molecules 2020, 25, 994. https://doi.org/10.3390/molecules25040994
Lee M, Shim S-Y. Inhibitory Effects of Eriodictyol-7-O-β-d-glucuronide and 5,7-Dihydroxy-4-chromene Isolated from Chrysanthemum zawadskii var. latilobum in FcεRI-Mediated Human Basophilic KU812F Cell Activation. Molecules. 2020; 25(4):994. https://doi.org/10.3390/molecules25040994
Chicago/Turabian StyleLee, Mina, and Sun-Yup Shim. 2020. "Inhibitory Effects of Eriodictyol-7-O-β-d-glucuronide and 5,7-Dihydroxy-4-chromene Isolated from Chrysanthemum zawadskii var. latilobum in FcεRI-Mediated Human Basophilic KU812F Cell Activation" Molecules 25, no. 4: 994. https://doi.org/10.3390/molecules25040994
APA StyleLee, M., & Shim, S.-Y. (2020). Inhibitory Effects of Eriodictyol-7-O-β-d-glucuronide and 5,7-Dihydroxy-4-chromene Isolated from Chrysanthemum zawadskii var. latilobum in FcεRI-Mediated Human Basophilic KU812F Cell Activation. Molecules, 25(4), 994. https://doi.org/10.3390/molecules25040994



