Selected Fungal Natural Products with Antimicrobial Properties
Abstract
1. Introduction
2. Selected Examples of Antimicrobial Natural Products from Fungi
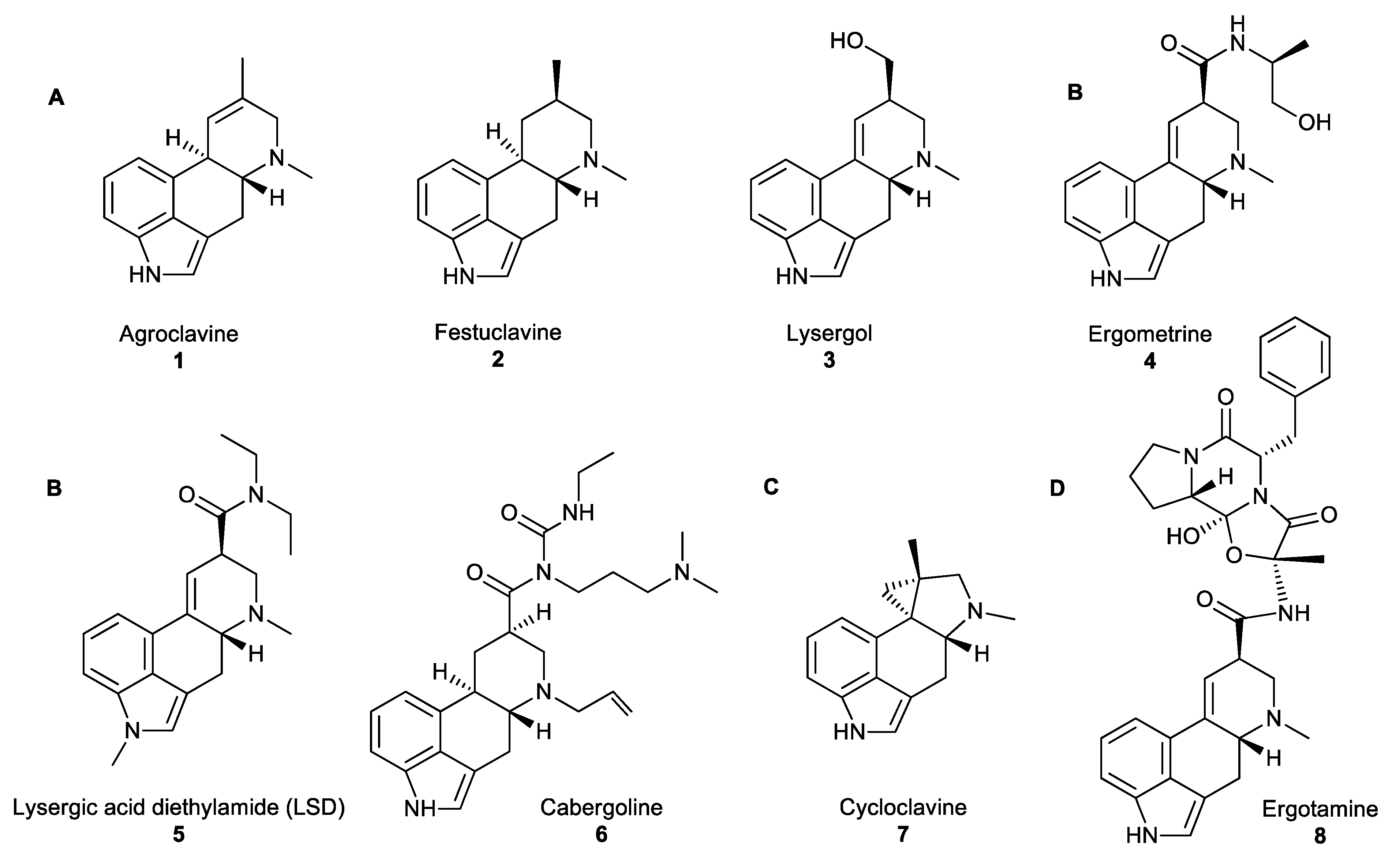
2.1. Ergot Alkaloids: Fungal Natural Products Derived from Amino Acids
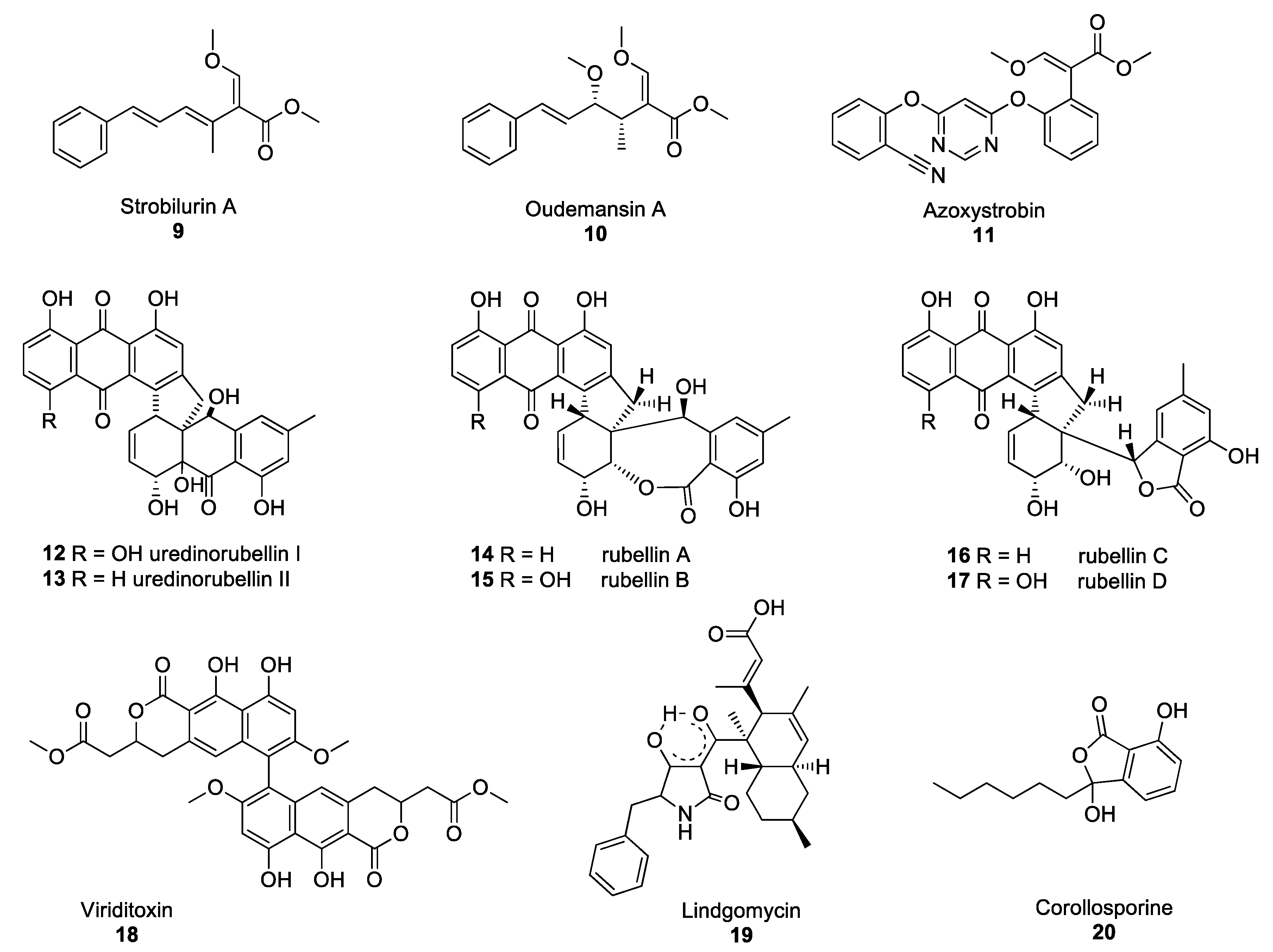
2.2. Fungal Polyketides
2.3. Peptidic Fungal Natural Products
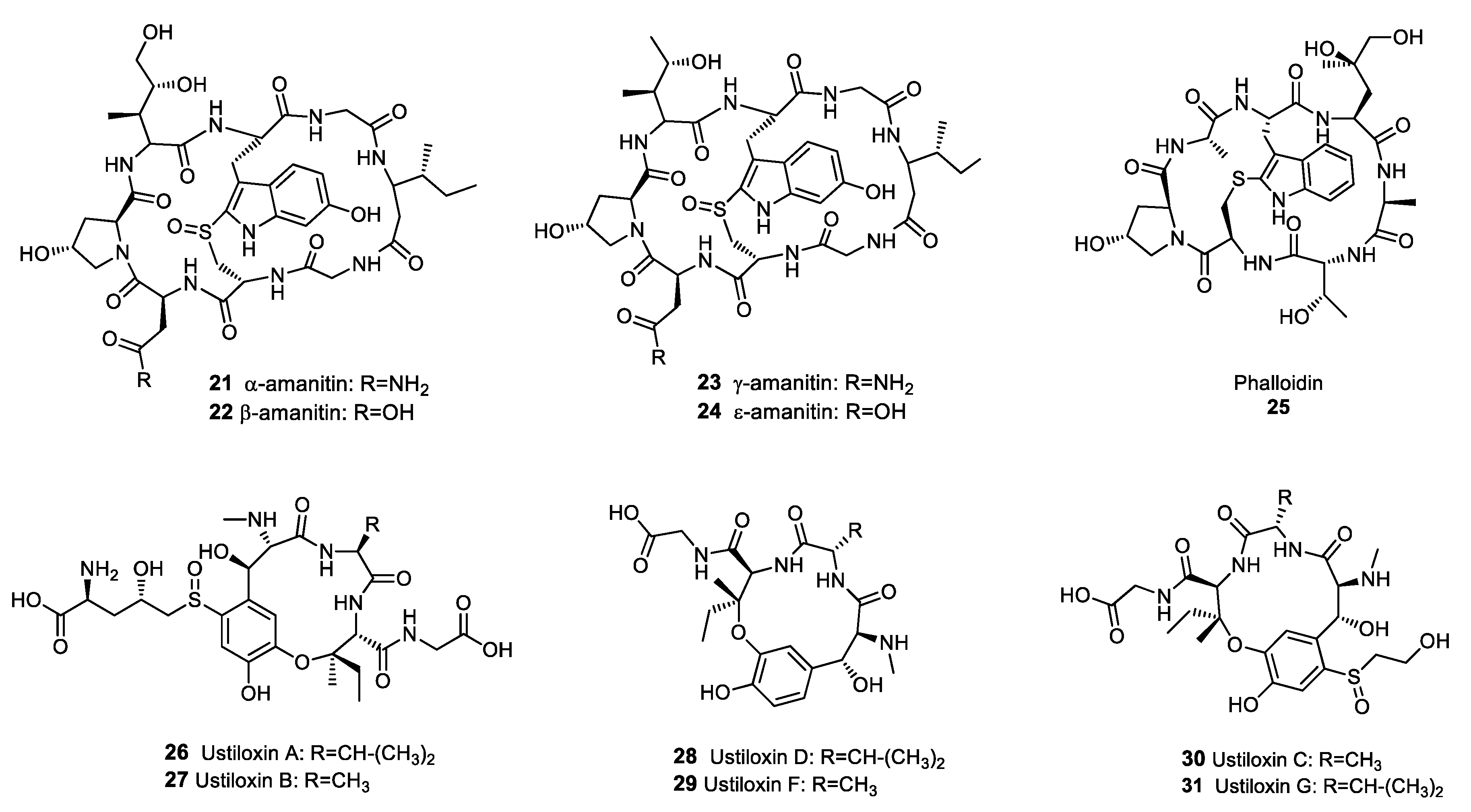
2.3.1. Ribosomally Synthesised and Post-Translationally Modified Peptides (RiPPs)
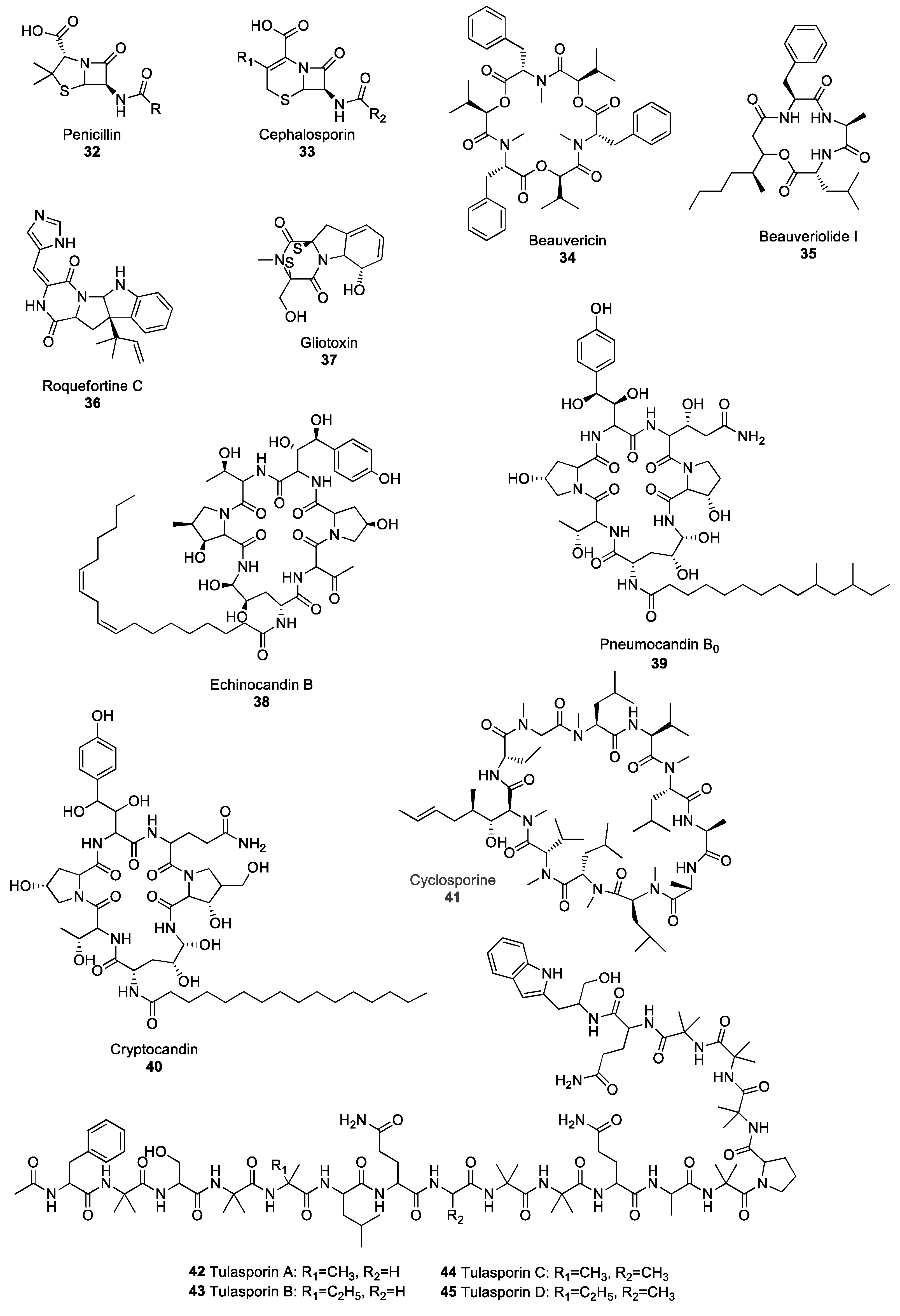
2.3.2. Nonribosomal Peptide Natural Products
β-lactam Antibiotics
Depsipeptides
Piperazines
Lipopetides
Other Peptides
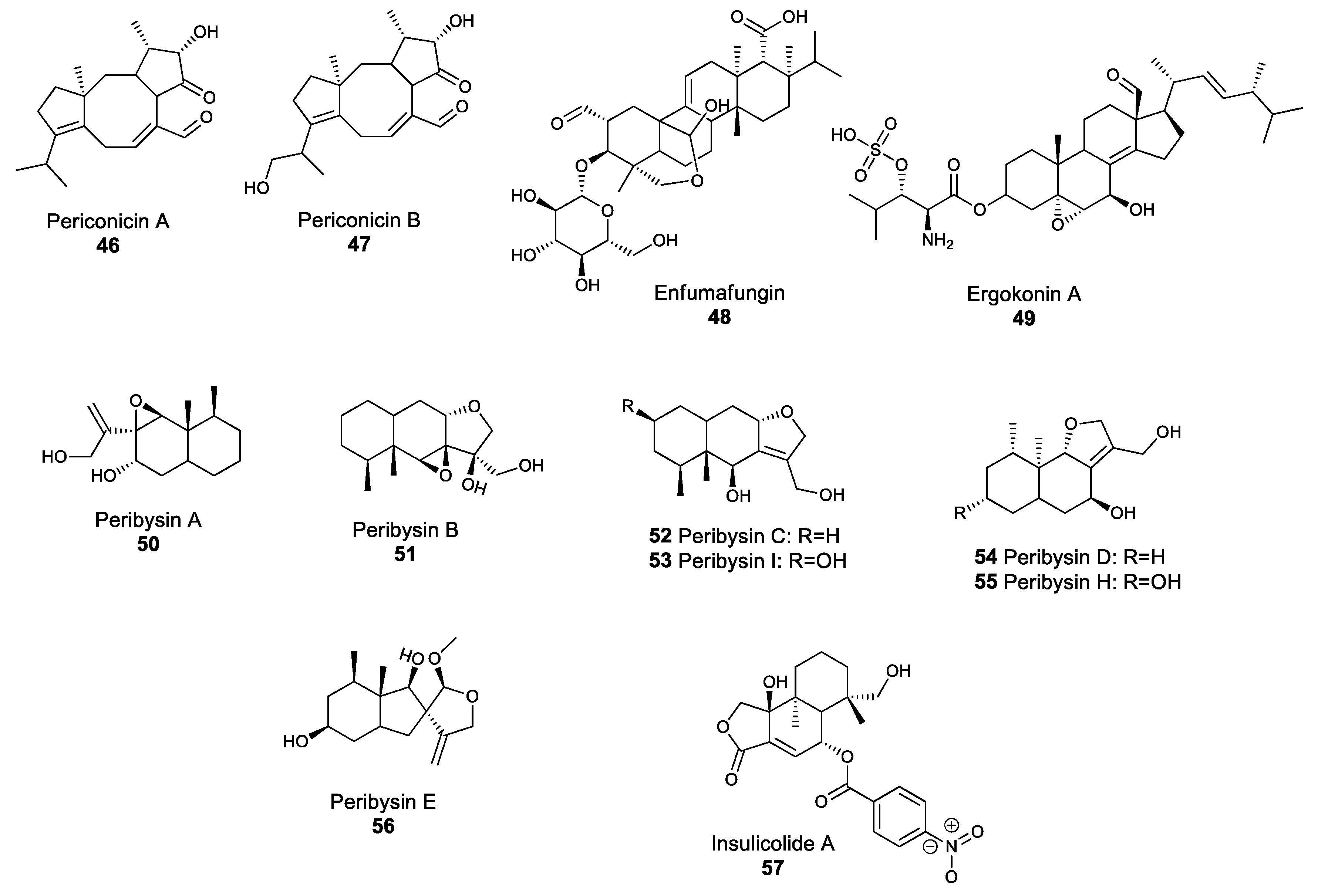
2.4. Terpenoid Compounds
3. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Schiff, P.L. Ergot and its alkaloids. Am. J. Pharm. Educ. 2006, 70, 98. [Google Scholar] [CrossRef] [PubMed]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach; John Wiley & Sons: West Sussex, UK, 2002; ISBN 0471496413. [Google Scholar]
- Mukherjee, J.; Menge, M. Progress and prospects of ergot alkaloid research. In New Products and New Areas of Bioprocess Engineering; Springer: Berlin/Heidelberg, Germany, 2000; pp. 1–20. [Google Scholar]
- Ligon, B.L. Penicillin: Its discovery and early development. Semin. Pediatr. Infect. Dis. WB Saunders 2004, 15. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Swierstra, J.; Wu, C.; Girard, G.; Choi, Y.H.; van Wamel, W.; Sandiford, S.K.; van Wezel, G.P. Eliciting antibiotics active against the ESKAPE pathogens in a collection of actinomycetes isolated from mountain soils. Microbiology 2014, 160, 1714–1725. [Google Scholar] [CrossRef] [PubMed]
- Versluis, D. Antibiotic Resistance Reservoirs. 2016. Available online: https://research.wur.nl/en/publications/antibiotic-resistance-reservoirs-the-cases-of-sponge-and-human-gu (accessed on 20 January 2020).
- Hamad, B. The antibiotics market. Nat. Rev. Drug Discov. 2010, 9, 675–676. [Google Scholar] [CrossRef]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef]
- Smith, E.; Lichten, C.A.; Taylor, J.; MacLure, C.; Lepetit, L.; Harte, E.; Martin, A.; Ghiga, I.; Pitchforth, E.; Sussex, J.; et al. Evaluation of the EC Action Plan Against the Rising Threats from Antimicrobial Resistance. RAND Corp. RB-9930-EC. 2018. Available online: https//www.rand.org/pubs/research_briefs/RB9930.html (accessed on 20 January 2020).
- Pimentel, D. Green revolution agriculture and chemical hazards. Sci. Total Environ. 1996, 188, S86–S98. [Google Scholar] [CrossRef]
- Schardl, C.L.; Panaccione, D.G.; Tudzynski, P. Ergot alkaloids–biology and molecular biology. Alkaloids Chem. Biol. 2006, 63, 45–86. [Google Scholar]
- Jakubczyk, D.; Caputi, L.; Hatsch, A.; Nielsen, C.A.F.; Diefenbacher, M.; Klein, J.; Molt, A.; Schröder, H.; Cheng, J.Z.; Naesby, M.; et al. Discovery and Reconstitution of the Cycloclavine Biosynthetic Pathway—Enzymatic Formation of a Cyclopropyl Group. Angew. Chem. Int. Ed. 2015, 54, 5117–5121. [Google Scholar] [CrossRef]
- Bräse, S. Privileged Scaffolds in Medicinal Chemistry; The Royal Society of Chemistry: Cambridge, UK, 2016; ISBN 978-1-78262-030-3. [Google Scholar]
- Keller, N.P.; Turner, G.; Bennett, J.W. Fungal secondary metabolism—From biochemistry to genomics. Nat. Rev. Microbiol. 2005, 3, 937–947. [Google Scholar] [CrossRef]
- Brookman, J.L.; Denning, D.W. Molecular genetics in Aspergillus fumigatus. Curr. Opin. Microbiol. 2000, 3, 468–474. [Google Scholar] [CrossRef]
- Perrone, G.; Susca, A.; Cozzi, G.; Ehrlich, K.; Varga, J.; Frisvad, J.C.; Meijer, M.; Noonim, P.; Mahakarnchanakul, W.; Samson, R.A. Biodiversity of Aspergillus species in some important agricultural products. Stud. Mycol. 2007, 59, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Florea, S.; Panaccione, D.G.; Schardl, C.L. Ergot Alkaloids of the Family Clavicipitaceae. Phytopathology 2017, 107, 504–518. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, D.; Caputi, L.; Stevenson, C.E.M.; Lawson, D.M.; O’Connor, S.E. Structural characterization of EasH (Aspergillus japonicus)—an oxidase involved in cycloclavine biosynthesis. Chem. Commun. 2016, 52, 14306–14309. [Google Scholar] [CrossRef]
- Yan, L.; Liu, Y. Insights into the Mechanism and Enantioselectivity in the Biosynthesis of Ergot Alkaloid Cycloclavine Catalyzed by Aj_EasH from Aspergillus japonicus. Inorg. Chem. 2019. [Google Scholar] [CrossRef]
- Anke, T.; Oberwinkler, F.; Steglich, W.; Schramm, G. The strobilurins—New antifungal antibiotics from the basidiomycete strobilurus tenacellus (Pers. ex Fr.) Sing. J. Antibiot. (Tokyo) 1977, 30, 806–810. [Google Scholar] [CrossRef]
- Anke, T.; Hecht, H.; Schramm, G.; Steglich, W. Antibiotics from basidiomycetes. IX Oudemansin, an antifungal antibiotic from Oudemansiella mucida (Schrader ex Fr.) Hoehnel (agaricales). J. Antibiot. (Tokyo) 1979, 32, 1112–1117. [Google Scholar] [CrossRef]
- Brandt, U.; Haase, U.; Schaegger, H.; von Jagow, G. Species specificity and mechanism of action of strobilurins. Dechema Monogr. 1993, 129, 27–38. [Google Scholar]
- Clough, J. The strobilurins, oudemansins, and myxothiazols, fungicidal derivatives of β-methoxyacrylic acid. Nat. Prod. Rep. 1993, 10, 565–574. [Google Scholar] [CrossRef]
- Godwin, J.; Anthony, V.; Clough, J.; Godfrey, C. ICIA5504: A novel, broad spectrum, systemic beta-methoxyacrylate fungicide. In Proceedings of the Brighton Crop Protection Conference—Pests and Diseases; British Crop Protection Council: Farnham, Surrey, UK, 23–26 November 1992; pp. 435–442. [Google Scholar]
- Torriani, S.F.F.; Brunner, P.C.; McDonald, B.A.; Sierotzki, H. QoI resistance emerged independently at least 4 times in European populations of Mycosphaerella graminicola. Pest Manag. Sci. 2009, 65, 155–162. [Google Scholar] [CrossRef]
- Leroux, P.; Gredt, M.; Leroch, M.; Walker, A.S. Exploring mechanisms of resistance to respiratory inhibitors in field strains of botrytis cinerea, the causal agent of gray mold. Appl. Environ. Microbiol. 2010, 76, 6615–6630. [Google Scholar] [CrossRef] [PubMed]
- Piszczek, J.; Pieczul, K.; Kiniec, A. First report of G143A strobilurin resistance in Cercospora beticola in sugar beet (Beta vulgaris) in Poland. J. Plant Dis. Prot. 2018, 125, 99–101. [Google Scholar] [CrossRef]
- Oliver, R.P. A reassessment of the risk of rust fungi developing resistance to fungicides. Pest Manag. Sci. 2014, 70, 1641–1645. [Google Scholar] [CrossRef] [PubMed]
- Isaka, M.; Palasarn, S.; Tobwor, P.; Boonruangprapa, T.; Tasanathai, K. Bioactive anthraquinone dimers from the leafhopper pathogenic fungus Torrubiella sp. BCC 28517. J. Antibiot. (Tokyo) 2012, 65, 571. [Google Scholar] [CrossRef]
- Miethbauer, S.; Heiser, I.; Liebermann, B. The phytopathogenic fungus Ramularia collo-cygni produces biologically active rubellins on infected barley leaves. J. Phytopathol. 2003, 151, 665–668. [Google Scholar] [CrossRef]
- Heiser, I.; Heß, M.; Schmidtke, K.-U.; Vogler, U.; Miethbauer, S.; Liebermann, B. Fatty acid peroxidation by rubellin B, C and D, phytotoxins produced by Ramularia collo-cygni (Sutton et Waller). Physiol. Mol. Plant Pathol. 2004, 64, 135–143. [Google Scholar] [CrossRef]
- Miethbauer, S.; Haase, S.; Schmidtke, K.-U.; Günther, W.; Heiser, I.; Liebermann, B. Biosynthesis of photodynamically active rubellins and structure elucidation of new anthraquinone derivatives produced by Ramularia collo-cygni. Phytochemistry 2006, 67, 1206–1213. [Google Scholar] [CrossRef]
- McGrann, G.R.D.; Andongabo, A.; Sjokvist, E.; Trivedi, U.; Dussart, F.; Kaczmarek, M.; Mackenzie, A.; Fountaine, J.M.; Taylor, J.M.G.; Paterson, L.J.; et al. The genome of the emerging barley pathogen Ramularia collo-cygni. BMC Genom. 2016, 17, 584. [Google Scholar] [CrossRef]
- Dussart, F.; Douglas, R.; Sjökvist, E.; Hoebe, P.N.; Spoel, S.H.; McGrann, G.R.D. Genome-Based Discovery of Polyketide-Derived Secondary Metabolism Pathways in the Barley Pathogen Ramularia collo-cygni. Mol. Plant-Microbe Interact. 2018, 31, 962–975. [Google Scholar] [CrossRef]
- Miethbauer, S.; Gaube, F.; Mollmann, U.; Dahse, H.-M.; Schmidtke, M.; Gareis, M.; Pickhardt, M.; Liebermann, B. Antimicrobial, antiproliferative, cytotoxic, and tau inhibitory activity of rubellins and caeruleoramularin produced by the phytopathogenic fungus Ramularia collo-cygni. Planta Med. 2009, 75, 1523–1525. [Google Scholar] [CrossRef]
- Schueffler, A.; Anke, T. Fungal natural products in research and development. Nat. Prod. Rep. 2014, 31, 1425–1448. [Google Scholar] [CrossRef] [PubMed]
- Royles, B.J.L. Naturally Occurring Tetramic Acids: Structure, Isolation, and Synthesis. Chem. Rev. 1995, 95, 1981–2001. [Google Scholar] [CrossRef]
- Marfori, E.C.; Bamba, T.; Kajiyama, S.; Fukusaki, E.; Kobayashi, A. Biosynthetic studies of the tetramic acid antibiotic trichosetin. Tetrahedron 2002, 58, 6655–6658. [Google Scholar] [CrossRef]
- Imhoff, J.F. Natural products from marine fungi—Still an underrepresented resource. Mar. Drugs 2016, 14, 19. [Google Scholar] [CrossRef]
- Liberra, K.; Jansen, R.; Lindequist, U. Corollosporine, a new phthalide derivative from the marine fungus Corollospora maritima Werderm. 1069. Pharmazie 1998, 53, 578–581. [Google Scholar] [CrossRef]
- Arnison, P.G.; Bibb, M.J.; Bierbaum, G.; Bowers, A.A.; Bugni, T.S.; Bulaj, G.; Camarero, J.A.; Campopiano, D.J.; Challis, G.L.; Clardy, J.; et al. Ribosomally synthesized and post-translationally modified peptide natural products: Overview and recommendations for a universal nomenclature. Nat. Prod. Rep. 2013, 30, 108–160. [Google Scholar] [CrossRef]
- Hudson, G.A.; Mitchell, D.A. RiPP antibiotics: Biosynthesis and engineering potential. Curr. Opin. Microbiol. 2018, 45, 61–69. [Google Scholar] [CrossRef]
- Hallen, H.E.; Luo, H.; Scott-craig, J.S.; Walton, J.D. Gene family encoding the major toxins of lethal Amanita mushrooms. Proc. Natl. Acad. Sci. USA 2007, 104, 19097–19101. [Google Scholar] [CrossRef]
- Wong, J.H. Fungal Toxins. In Handbook of Biologically Active Peptides, 2nd ed.; Elsevier Inc.: San Diego, CA, USA, 2013; ISBN 9780123850959. [Google Scholar]
- Moldenhauer, G.; Salnikov, A.V.; Lüttgau, S.; Herr, I.; Anderl, J.; Faulstich, H. Therapeutic potential of amanitin-conjugated anti-epithelial cell adhesion molecule monoclonal antibody against pancreatic carcinoma. J. Natl. Cancer Inst. 2012, 104, 622–634. [Google Scholar] [CrossRef]
- Wulf, E.; Deboben, A.; Bautz, F.A.; Faulstich, H.; Wieland, T. Fluorescent phallotoxin, a tool for the visualization of cellular actin. Proc. Natl. Acad. Sci. USA 1979, 76, 4498–4502. [Google Scholar] [CrossRef]
- Anderl, J.; Echner, H.; Faulstich, H. Chemical modification allows phallotoxins and amatoxins to be used as tools in cell biology. Beilstein J. Org. Chem. 2012, 8, 2072–2084. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Minami, A.; Igarashi, Y.; Izumikawa, M.; Umemura, M.; Nagano, N.; Machida, M.; Kawahara, T.; Shin-ya, K.; Gomi, K.; et al. Unveiling the biosynthetic pathway of the ribosomally synthesized and post-translationally modified peptide ustiloxin B in filamentous fungi. Angew. Chem. Int. Ed. 2016, 55, 8072–8075. [Google Scholar] [CrossRef] [PubMed]
- Koiso, Y.; Li, Y.; Iwasaki, S.; Hanaka, K.; Kobayashi, T.; Sonoda, R.; Fujita, Y.; Yaegashi, H.; Sato, Z. Ustiloxins, antimitotic cyclic peptides from false smut balls on rice panicles caused by ustilaginoidea virens. J. Antibiot. (Tokyo) 1994, 47, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Gaynes, R. The discovery of penicillin—New insights after more than 75 years of clinical use. Emerg. Infect. Dis. 2017, 23, 849–853. [Google Scholar] [CrossRef]
- Bo, G. Giuseppe Brotzu and the discovery of cephalosporins. Clin. Microbiol. Infect. 2000, 6, 6–8. [Google Scholar] [CrossRef]
- Byford, M.F.; Baldwin, J.E.; Shiau, C.Y.; Schofield, C.J. The mechanism of ACV synthetase. Chem. Rev. 1997, 97, 2631–2649. [Google Scholar] [CrossRef]
- Miller, E.L. The penicillins: A review and update. J. Midwifery Women’s Heal. 2002, 47, 426–434. [Google Scholar] [CrossRef]
- Tipper, D.J. Mode of action of beta-lactam antibiotics. Pharmacol. Ther. 1985, 27, 1–35. [Google Scholar] [CrossRef]
- Sabath, L. Mechanisms of resistance to beta-lactam antibiotics in strains of Staphylococcus aureus. Ann. Intern. Med. 1982, 97, 339–344. [Google Scholar] [CrossRef]
- Preheim, L.C.; Penn, R.G.; Sanders, C.C.; Goering, R.V.; Giger, D.K. Emergence of resistance to β-lactam and aminoglycoside antibiotics during moxalactam therapy of Pseudomonas aeruginosa infections. Antimicrob. Agents Chemother. 1982, 22, 1037–1041. [Google Scholar] [CrossRef]
- Hiramatsu, K.; Cui, L.; Kuroda, M.; Ito, T. The emergence and evolution of methicillin-resistant Staphylococcus aureus. Trends Microbiol. 2001, 9, 486–493. [Google Scholar] [CrossRef]
- Frère, J. Beta-lactamases and bacterial resistance to antibiotics. Mol. Microbiol. 1995, 16, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Solensky, R. Hypersensitivity reactions to beta-lactam antibiotics. Clin. Rev. Allergy Immunol. 2003, 24, 201–219. [Google Scholar] [CrossRef]
- Jarvis, M. Preventing the emergence of multidrug-resistant microorganisms through antimicrobial use controls: The complexity of the problem. Infect. Control Hosp. Epidemiol. 1996, 17, 490–495. [Google Scholar] [CrossRef]
- Macy, E. Penicillin and Beta-Lactam Allergy: Epidemiology and Diagnosis. Curr. Allergy Asthma Rep. 2014, 14, 1–7. [Google Scholar] [CrossRef]
- Stawikowski, M.; Cudic, P. Depsipeptide synthesis. Methods Mol. Biol. 2007, 386, 321–339. [Google Scholar]
- Wang, X.; Gong, X.; Li, P.; Lai, D.; Zhou, L. Structural diversity and biological activities of cyclic depsipeptides from fungi. Molecules 2018, 23, 169. [Google Scholar] [CrossRef]
- Anke, H.; Laatsch, H. Cyclic Peptides and Depsipeptides from Fungi. In Physiology and Genetics. The Mycota (A Comprehensive Treatise on Fungi as Experimental Systems for Basic and Applied Research); Anke, T., Weber, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 15, pp. 331–335. [Google Scholar]
- Umeyama, A.; Takahashi, K.; Grudniewska, A.; Shimizu, M.; Hayashi, S.; Kato, M.; Okamoto, Y.; Suenaga, M.; Ban, S.; Kumada, T.; et al. In Vitro antitrypanosomal activity of the cyclodepsipeptides, cardinalisamides A-C, from the insect pathogenic fungus Cordyceps cardinalis NBRC 103832. J. Antibiot. (Tokyo) 2014, 67, 163–166. [Google Scholar] [CrossRef]
- Haritakun, R.; Sappan, M.; Suvannakad, R.; Tasanathai, K.; Isaka, M. An antimycobacterial cyclodepsipeptide from the entomopathogenic fungus Ophiocordyceps communis BCC 16475. J. Nat. Prod. 2010, 73, 75–78. [Google Scholar] [CrossRef]
- Nilanonta, C.; Isaka, M.; Kittakoop, P.; Trakulnaleamsai, S.; Tanticharoen, M.; Thebtaranonth, Y. Precursor-directed biosynthesis of beauvericin analogs by the insect pathogenic fungus Paecilomyces tenuipes BCC 1614. Tetrahedron 2002, 58, 3355–3360. [Google Scholar] [CrossRef]
- Shin, C.G.; An, D.G.; Song, H.H.; Lee, C. Beauvericin and enniatins H, i and MK1688 are new potent inhibitors of human immunodeficiency virus type-1 integrase. J. Antibiot. (Tokyo) 2009, 62, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhan, J.; Wijeratne, E.M.K.; Burns, A.M.; Gunatilaka, A.A.L.; Molnár, I. Cytotoxic and antihaptotactic beauvericin analogues from precursor-directed biosynthesis with the insect pathogen Beauveria bassiana ATCC 7159. J. Nat. Prod. 2007, 70, 1467–1471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yan, K.; Zhang, Y.; Huang, R.; Bian, J.; Zheng, C.; Sun, H.; Chen, Z.; Sun, N.; An, R.; et al. High-throughput synergy screening identifies microbial metabolites as combination agents for the treatment of fungal infections. Proc. Natl. Acad. Sci. USA 2007, 104, 4606–4611. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, D.M.; Jia, J.F.; Peng, Q.L.; Tian, H.Y.; Wang, L.; Ye, W.C. Cyclodepsipeptides from the ascocarps and insect-body portions of fungus Cordyceps cicadae. Fitoterapia 2014, 97, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Patocka, J.; Nepovimova, E.; Kuca, K. A review on the synthesis and bioactivity aspects of beauvericin, a Fusarium mycotoxin. Front. Pharmacol. 2018, 9, 1338. [Google Scholar] [CrossRef]
- Mallebrera, B.; Prosperini, A.; Font, G.; Ruiz, M.J. In Vitro mechanisms of Beauvericin toxicity: A review. Food Chem. Toxicol. 2018, 111, 537–545. [Google Scholar] [CrossRef]
- Fukuda, T.; Tomoda, H.; Omura, S. New beauvericins, potentiators of antifungal miconazole activity, produced by Beauveria sp. FKI-1366. II. Structure elucidation. J. Antibiot. (Tokyo) 2004, 57, 117–124. [Google Scholar] [CrossRef]
- Nakaya, S.; Mizuno, S.; Ishigami, H.; Yamakawa, Y.; Kawagishi, H.; Ushimaru, T. New rapid screening method for anti-aging compounds using budding yeast and identification of beauveriolide I as a potent active compound. Biosci. Biotechnol. Biochem. 2012, 76, 1226–1228. [Google Scholar] [CrossRef]
- Fabrizio, P.; Longo, V.D. The chronological life span of Saccharomyces cerevisiae. Aging Cell 2003, 2, 73–81. [Google Scholar] [CrossRef]
- Taevernier, L.; Wynendaele, E.; De Vreese, L.; Burvenich, C.; De Spiegeleer, B. The mycotoxin definition reconsidered towards fungal cyclic depsipeptides. J. Environ. Sci. Heal. Part C Environ. Carcinog. Ecotoxicol. Rev. 2016, 34, 114–135. [Google Scholar] [CrossRef]
- Tedjiotsop Feudjio, F.; Dornetshuber, R.; Lemmens, M.; Hoffmann, O.; Lemmens-Gruber, R.; Berger, W. Beauvericin and enniatin: Emerging toxins and/or remedies? World Mycotoxin J. 2010, 3, 415–430. [Google Scholar] [CrossRef]
- Scott, P.M.; Merrien, M.A.; Polonsky, J. Roquefortine and isofumigaclavine A, metabolites from Penicillium roqueforti. Experientia 1976, 32, 140–142. [Google Scholar] [CrossRef]
- Arnold, D.; Scott, P.; McGuire, P.; Harwig, J.; Nera, E. Acute toxicity studies on roquefortine and PR toxin, metabolites of the Penicillium roqueforti in the mouse. Food Cosmet. Toxicol. 1978, 16, 369–371. [Google Scholar] [CrossRef]
- Lowes, N.R.; Smith, R.A.; Beck, B.E. Roquefortine in the stomach contents of dogs suspected of strychnine poisoning in Alberta. Can. Vet. J. 1992, 33, 535–538. [Google Scholar] [PubMed]
- Eriksen, G.S.; Jäderlund, K.H.; Moldes-Anaya, A.; Schönheit, J.; Bernhoft, A.; Jæger, G.; Rundberget, T.; Skaar, I. Poisoning of dogs with tremorgenic Penicillium toxins. Med. Mycol. 2010, 48, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, H.; Oldenburg, E.; Weissbach, F. Incidence of penicillium roqueforti and roquefortine C in silages. J. Sci. Food Agric. 1998, 76, 565–572. [Google Scholar] [CrossRef]
- Driehuis, F.; Spanjer, M.C.; Scholten, J.M.; Te Giffel, M.C. Occurrence of mycotoxins in feedstuffs of dairy cows and estimation of total dietary intakes. J. Dairy Sci. 2008, 91, 4261–4271. [Google Scholar] [CrossRef]
- Bünger, J.; Westphal, G.; Mönnich, A.; Hinnendahl, B.; Hallier, E.; Müller, M. Cytotoxicity of occupationally and environmentally relevant mycotoxins. Toxicology 2004, 202, 199–211. [Google Scholar] [CrossRef]
- Finoli, C.; Vecchio, A.; Galli, A.; Dragoni, I. Roquefortine C occurrence in blue cheese. J. Food Prot. 2001, 64, 246–251. [Google Scholar] [CrossRef]
- Seto, Y.; Kogami, Y.; Shimanuki, T.; Takahashi, K.; Matsuura, H.; Yoshihara, T. Production of phleichrome by Cladosporium phlei as stimulated by diketopiperadines of Epichloe typhina. Biosci. Biotechnol. Biochem. 2005, 69, 1515–1519. [Google Scholar] [CrossRef]
- Sutton, P.; Waring, P.; Mullbacher, A. Exacerbation of invasive aspergillosis by the immunosuppressive fungal metabolite, gliotoxin. Immunol Cell Biol 1996, 74, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Pahl, B.H.L.; Kraub, B.; Schulze-osthoff, K.; Decker, T.; Traenckner, E.B.; Myersfl, C.; Parksfl, T.; Warring, P.; Miihlbacher, I.I.A.; Czernilofiky, A.; et al. The immunosuppressive fungal metabolite gliotoxin specifically inhibits transcription factor NF-kB. J. Exp. Med. 1996, 183, 1829–1840. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.J.; Ghosh, S.; Okoli, I.; Mylonakis, E. Antifungal activity of microbial secondary metabolites. PLoS ONE 2011, 6, e25321. [Google Scholar] [CrossRef] [PubMed]
- Nyfeler, R.; Keller-Schierlein, W. Metabolites of microorganisms. 143. Echinocandin B, a novel polypeptide-antibiotic from Aspergillus nidulans var. echinulatus: Isolation and structural components. Helv. Chim. Acta 1974, 57, 2459–2477. [Google Scholar] [PubMed]
- De La Cruz, M.; Martín, J.; González-Menéndez, V.; Pérez-Victoria, I.; Moreno, C.; Tormo, J.R.; El Aouad, N.; Guarro, J.; Vicente, F.; Reyes, F.; et al. Chemical and physical modulation of antibiotic activity in Emericella species. Chem. Biodivers. 2012, 9, 1095–1113. [Google Scholar] [CrossRef] [PubMed]
- Denning, D. Echinocandins: A new class of antifungal. J. Antimicrob. Chemother. 2002, 49, 889–891. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.E.; Sesin, D.F.; Joshua, H.; Wilson, K.E.; Kempf, A.J.; Goklen, K.A.; Kuehner, D.; Gailliot, P.; Gleason, C.; White, R.; et al. Pneumocandins from Zalerion arboricola I. Discovery and isolation. J. Antibiot. (Tokyo) 1992, 45, 1853–1866. [Google Scholar] [CrossRef][Green Version]
- Schmatz, D.M.; Abruzzo, G.; Powles, M.A.; Balkovec, J.M.; Black, R.M.; Nollstadt, K.; Mcfadden, D.C.; Bartizal, K. Pneumocandins from Zalerion arboricola IV. Biological evaluation of natural and semisynthetic pneumocandins for activity against Pneumocystis carinii and Candida species. J. Antibiot. (Tokyo) 1992, 45, 1886–1891. [Google Scholar] [CrossRef]
- Balkovec, J.; Hughes, D.; Masurekar, P.; Sable, C.; Schwartz, R.; Singh, S. Discovery and development of first in class antifungal caspofungin (CANCIDAS®)—A case study. Nat. Prod. Rep. 2014, 31, 15–34. [Google Scholar] [CrossRef]
- Strobel, G.A.; Miller, R.V.; Martinez-Miller, C.; Condron, M.M.; Teplow, D.B.; Hess, W.M. Cryptocandin, a potent antimycotic from the endophytic fungus Cryptosporiopsis cf. quercina. Microbiology 1999, 145, 1919–1926. [Google Scholar] [CrossRef]
- Strobel, G.; Daisy, B. Bioprospecting for microbial endophytes and their natural products. Microbiol. Mol. Biol. Rev. 2003, 67, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Weber, G.; Schörgendorfer, K.; Schneider-Scherzer, E.; Leitner, E. The peptide synthetase catalyzing cyclosporine production in Tolypocladium niveum is encoded by a giant 45.8-kilobase open reading frame. Curr. Genet. 1994, 26, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Feng, P.; Yin, Y.; Bushley, K.; Spatafora, J.W.; Wang, C. Cyclosporine biosynthesis in Tolypocladium inflatum benefits fungal adaptation to the environment. MBio 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Koyasu, S. Mechanisms of action of cyclosporine. Immunopharmacology 2000, 47, 119–125. [Google Scholar] [CrossRef]
- Marik, T.; Tyagi, C.; Balázs, D.; Urbán, P.; Szepesi, Á.; Bakacsy, L.; Endre, G.; Rakk, D.; Szekeres, A.; Andersson, M.A.; et al. Structural diversity and bioactivities of peptaibol compounds from the longibrachiatum clade of the filamentous fungal genus trichoderma. Front. Microbiol. 2019, 10, 1434. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Chen, L.; Wang, X.W.; Zhang, T.; Zhao, P.B.; Song, X.Y.; Sun, C.Y.; Chen, X.L.; Zhou, B.C.; Zhang, Y.Z. Antimicrobial peptaibols from Trichoderma pseudokoningii induce programmed cell death in plant fungal pathogens. Microbiology 2012, 158, 166–175. [Google Scholar] [CrossRef]
- Otto, A.; Laub, A.; Haid, M.; Porzel, A.; Schmidt, J.; Wessjohann, L.; Arnold, N. Tulasporins A-D, 19-residue peptaibols from the mycoparasitic fungus sepedonium tulasneanum. Nat. Prod. Commun. 2016, 11, 1821–1824. [Google Scholar] [CrossRef]
- Viterbo, A.; Wiest, A.; Brotman, Y.; Chet, I.; Kenerley, C. The 18mer peptaibols from Trichoderma virens elicit plant defence responses. Mol. Plant Pathol. 2007, 8, 737–746. [Google Scholar] [CrossRef]
- Kim, S.; Shin, D.S.; Lee, T.; Oh, K.B. Periconicins, two new fusicoccane diterpenes produced by an endophytic fungus Periconia sp. with antibacterial activity. J. Nat. Prod. 2004, 67, 448–450. [Google Scholar] [CrossRef]
- Pelaez, F.; Cabello, A.; Platas, G.; Díez, M.T.; Del Val, A.G.; Basilio, A.; Martán, I.; Vicente, F.; Bills, G.F.; Giacobbe, R.A.; et al. The discovery of enfumafungin, a novel antifungal compound produced by an endophytic Hormonema species biological activity and taxonomy of the producing organisms. Syst. Appl. Microbiol. 2000, 23, 333–343. [Google Scholar] [CrossRef]
- Vicente, M.F.; Cabello, A.; Platas, G.; Basilio, A.; Díez, M.T.; Dreikorn, S.; Giacobbe, R.A.; Onishi, J.C.; Meinz, M.; Kurtz, M.B.; et al. Antimicrobial activity of ergokonin A from Trichoderma longibrachiatum. J. Appl. Microbiol. 2001, 91, 806–813. [Google Scholar] [CrossRef]
- Onishi, J.; Meinz, M.; Thompson, J.; Curotto, J.; Dreikorn, S.; Rosenbach, M.; Douglas, C.; Abruzzo, G.; Flattery, A.; Kong, L.; et al. Discovery of novel antifungal (1,3)-β-D-glucan synthase inhibitors. Antimicrob. Agents Chemother. 2000, 44, 368–377. [Google Scholar] [CrossRef]
- Elissawy, A.M.; El-Shazly, M.; Ebada, S.S.; Singab, A.N.B.; Proksch, P. Bioactive terpenes from marine-derived fungi. Mar. Drugs 2015, 13, 1966–1992. [Google Scholar] [CrossRef]
- Yamada, T.; Doi, M.; Miura, A.; Harada, W.; Hiramura, M.; Minoura, K.; Tanaka, R.; Numata, A. Absolute stereostructures of cell-adhesion inhibitors, peribysins A, E, F and G, produced by a sea hare-derived Periconia sp. J. Antibiot. (Tokyo) 2005, 58, 185–191. [Google Scholar] [CrossRef]
- Fang, W.; Lin, X.; Zhou, X.; Wan, J.; Lu, X.; Yang, B.; Ai, W.; Lin, J.; Zhang, T.; Tu, Z.; et al. Cytotoxic and antiviral nitrobenzoyl sesquiterpenoids from the marine-derived fungus Aspergillus ochraceus Jcma1F17. Med. Chem. Commun. 2014, 5, 701–705. [Google Scholar] [CrossRef]
- Wainwright, M. Moulds in ancient and more recent medicine. Mycologist 1989, 3, 21–23. [Google Scholar] [CrossRef]
- Torjesen, I. Drug development: The journey of a medicine from lab to shelf. Pharm. J. 2015. [Google Scholar]
- Masurekar, P.S.; Fountoulakis, J.M.; Hallada, T.C.; Sosa, M.S.; Kaplan, L. Pneumocandins from zalerion arboricola II. Modification of product spectrum by mutation and medium manipulation. J. Antibiot. (Tokyo) 1992, 45, 1867–1874. [Google Scholar] [CrossRef][Green Version]
- Kavanagh, F.; Hervey, A.; Robbins, W.J. Antibiotic Substances from Basidiomycetes: VIII. Pleurotus multilus (Fr.) Sacc. and Pleurotus passeckerianus Pilat. Proc. Natl. Acad. Sci. USA 1951, 37, 570–574. [Google Scholar]
- Bailey, A.M.; Alberti, F.; Kilaru, S.; Collins, C.M.; De Mattos-Shipley, K.; Hartley, A.J.; Hayes, P.; Griffin, A.; Lazarus, C.M.; Cox, R.J.; et al. Identification and manipulation of the pleuromutilin gene cluster from Clitopilus passeckerianus for increased rapid antibiotic production. Sci. Rep. 2016, 6, 25202. [Google Scholar] [CrossRef]
- Stierle, A.; Strobel, G.; Stierle, D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science 1993, 260, 214–216. [Google Scholar] [CrossRef]
- Manzoni, M.; Rollini, M. Biosynthesis and biotechnological production of statins by filamentous fungi and application of these cholesterol-lowering drugs. Appl. Microbiol. Biotechnol. 2002, 58, 555–564. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakubczyk, D.; Dussart, F. Selected Fungal Natural Products with Antimicrobial Properties. Molecules 2020, 25, 911. https://doi.org/10.3390/molecules25040911
Jakubczyk D, Dussart F. Selected Fungal Natural Products with Antimicrobial Properties. Molecules. 2020; 25(4):911. https://doi.org/10.3390/molecules25040911
Chicago/Turabian StyleJakubczyk, Dorota, and Francois Dussart. 2020. "Selected Fungal Natural Products with Antimicrobial Properties" Molecules 25, no. 4: 911. https://doi.org/10.3390/molecules25040911
APA StyleJakubczyk, D., & Dussart, F. (2020). Selected Fungal Natural Products with Antimicrobial Properties. Molecules, 25(4), 911. https://doi.org/10.3390/molecules25040911






