Telodendrimer-Based Macromolecular Drug Design using 1,3-Dipolar Cycloaddition for Applications in Biology
Abstract
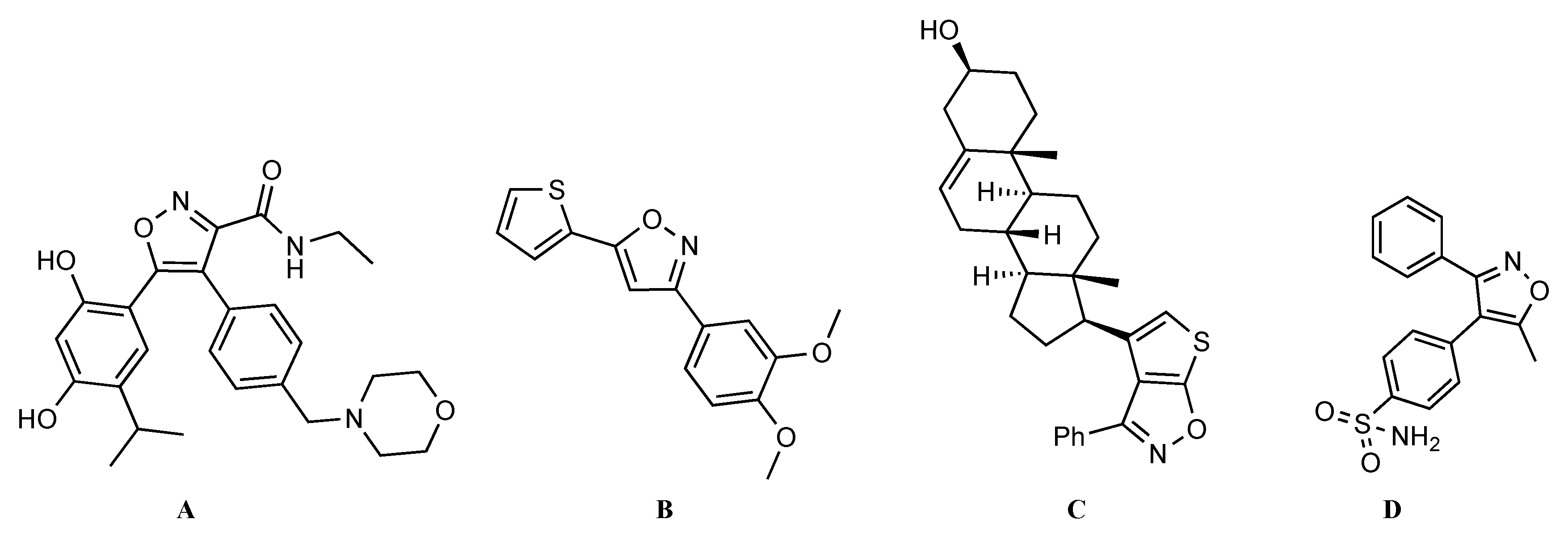
:1. Introduction
2. Results and Discussion
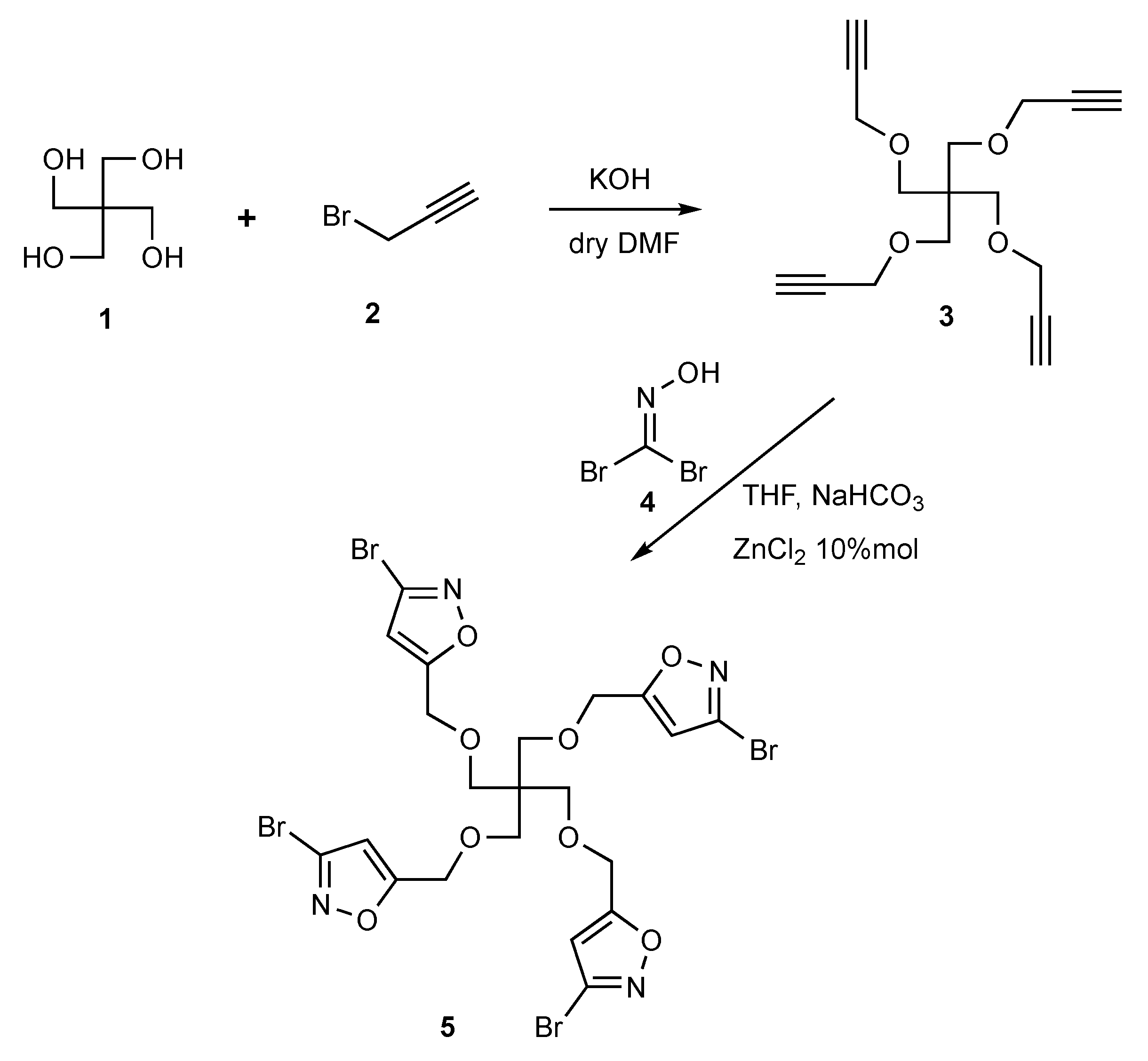
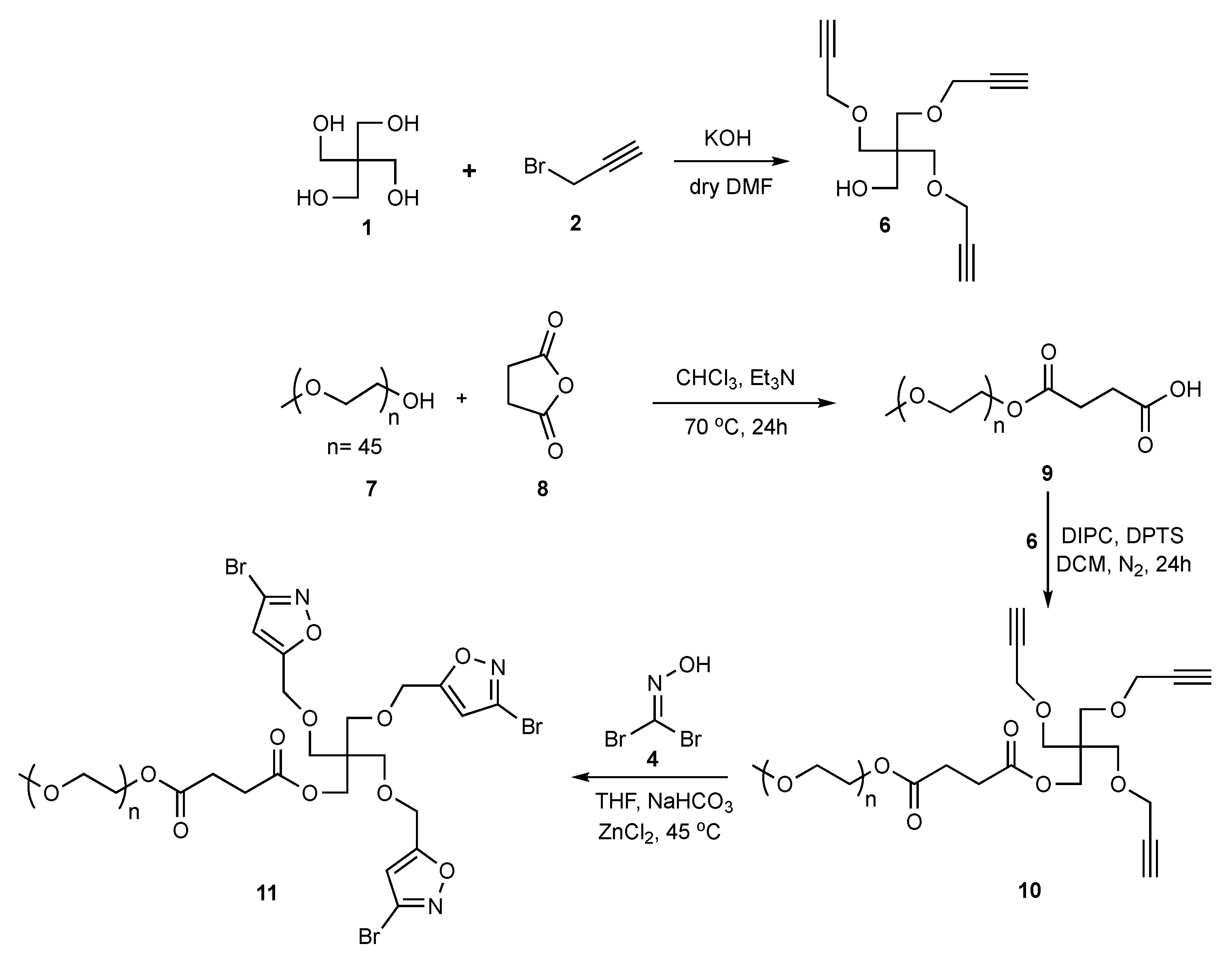
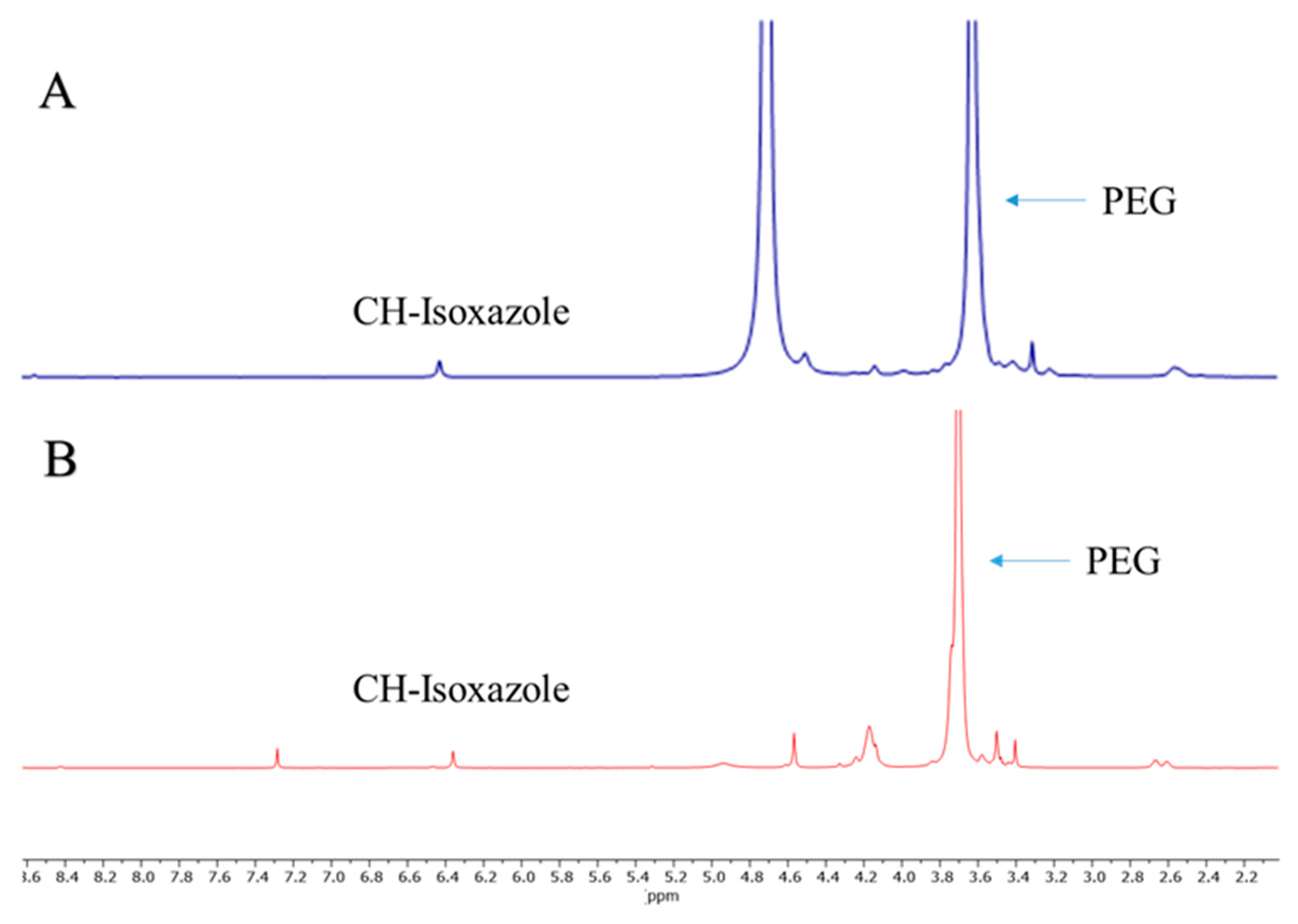
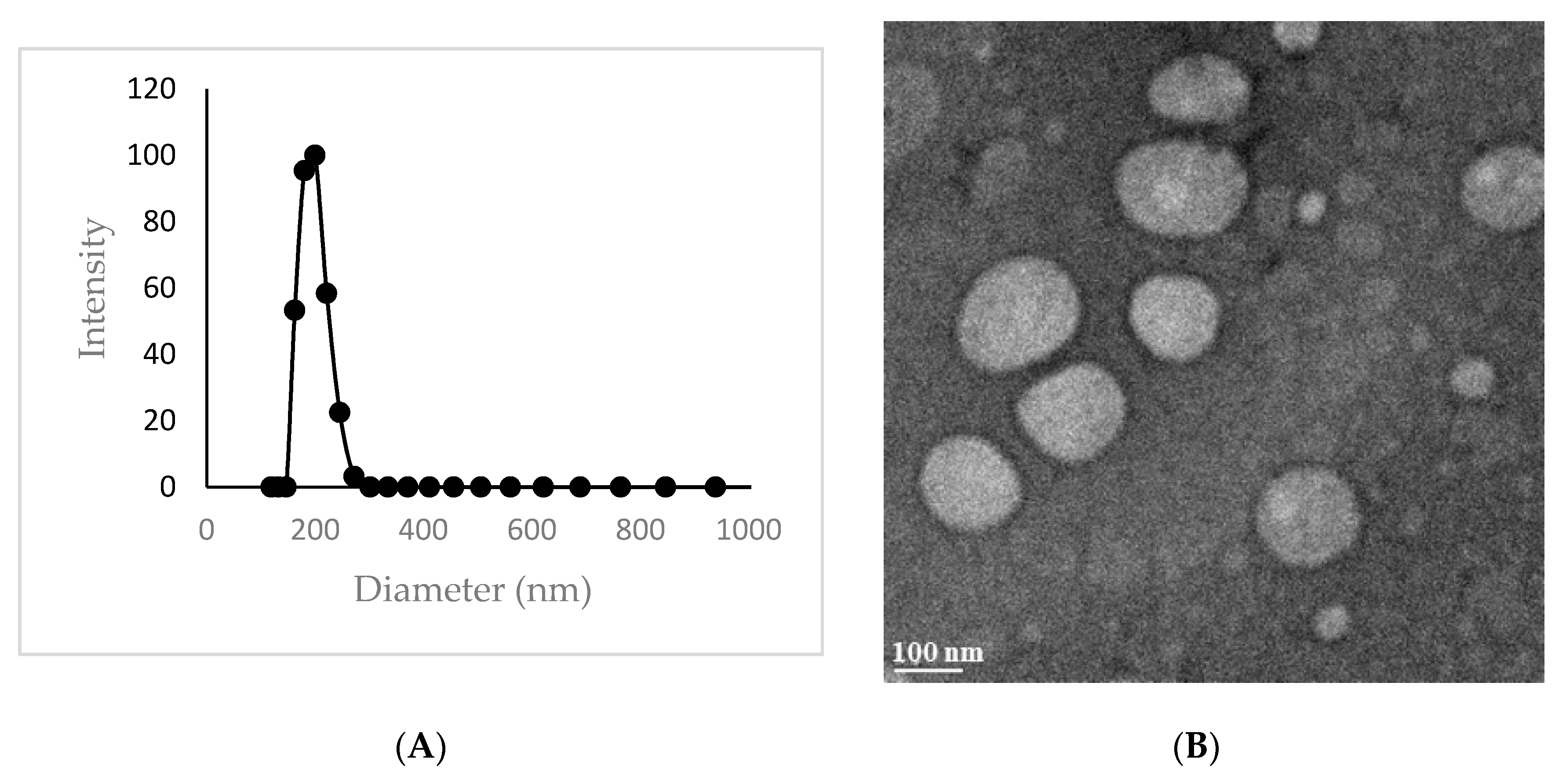
2.1. Synthesis
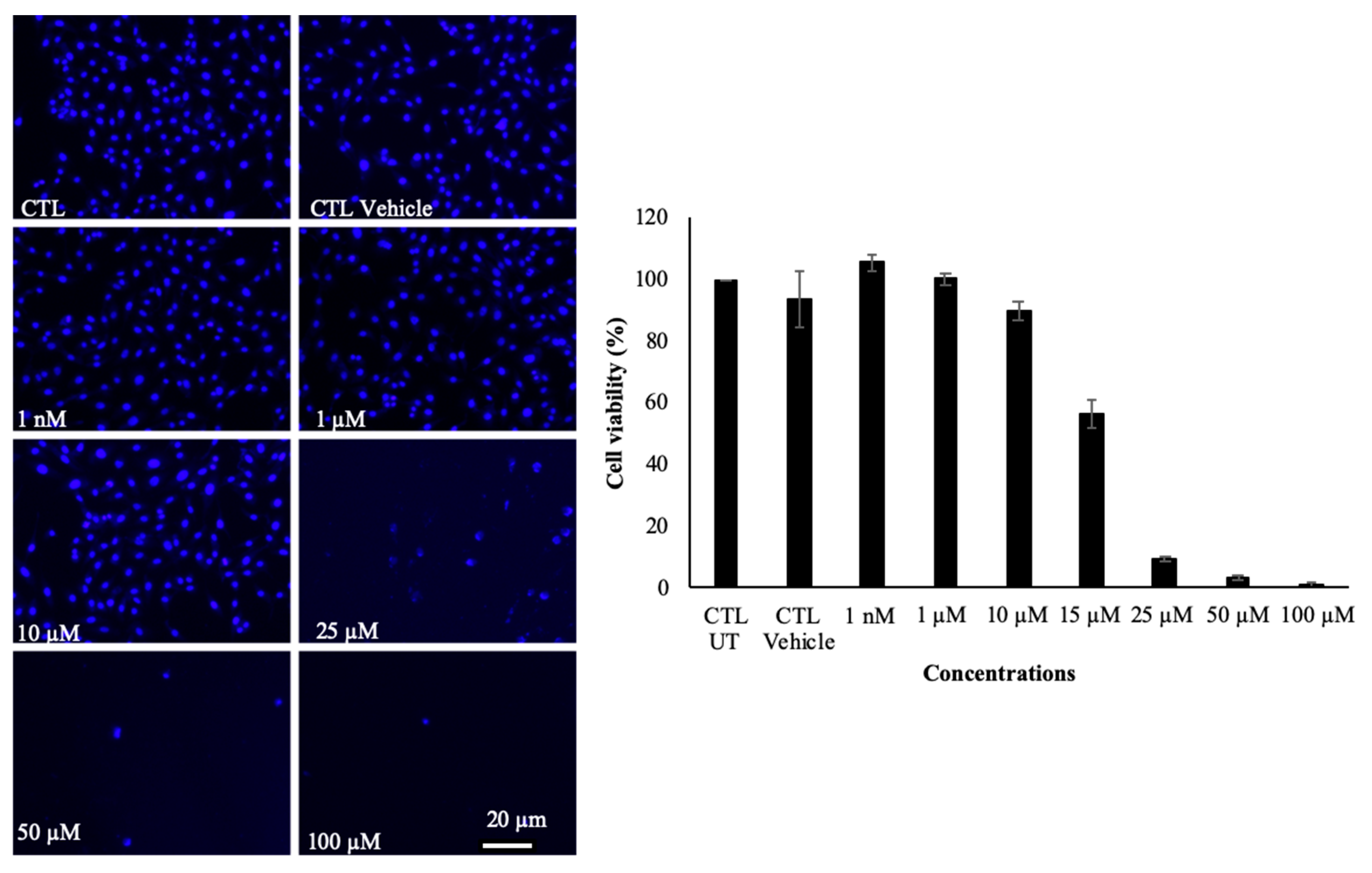
2.2. Isoxazole Telodendrimer Reduces Glioblastoma Cell Viability
3. Materials and Methods
3.1. General
3.2. Synthesis
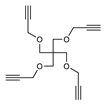

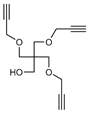

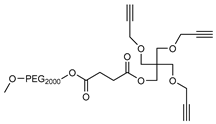
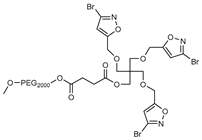
3.3. Methods
3.4. Cell Culture
3.5. Cell Viability Assay
3.6. Statistics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jensen, M.R.; Schoepfer, J.; Radimerski, T.; Massey, A.; Guy, C.T.; Brueggen, J.; Quadt, C.; Buckler, A.; Cozens, R.; Drysdale, M.J. NVP-AUY922: A small molecule HSP90 inhibitor with potent antitumor activity in preclinical breast cancer models. Breast Cancer Res. 2008, 10, R33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jancel, T.; Dudas, V. Management of uncomplicated urinary tract infections. West. J. Med. 2002, 176, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rakesh, K.S.; Jagadish, S.; Balaji, K.S.; Zameer, F.; Swaroop, T.R.; Mohan, C.D.; Jayarama, S.; Rangappa, K.S. 3, 5-disubstituted isoxazole derivatives: Potential inhibitors of inflammation and Cancer. Inflammation 2016, 39, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Kuz’min, V.E.; Artemenko, A.G.; Muratov, E.N.; Volineckaya, I.L.; Makarov, V.A.; Riabova, O.B.; Wutzler, P.; Schmidtke, M. Quantitative Structure−Activity Relationship Studies of [(Biphenyloxy) propyl] isoxazole Derivatives. Inhibitors of Human Rhinovirus 2 Replication. J. Med. Chem. 2007, 50, 4205–4213. [Google Scholar] [CrossRef]
- Selvam, C.; Jachak, S.M.; Thilagavathi, R.; Chakraborti, A.K. Design, synthesis, biological evaluation and molecular docking of curcumin analogues as antioxidant, cyclooxygenase inhibitory and anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2005, 15, 1793–1797. [Google Scholar] [CrossRef]
- Chakraborti, S.; Dhar, G.; Dwivedi, V.; Das, A.; Poddar, A.; Chakraborti, G.; Basu, G.; Chakrabarti, P.; Surolia, A.; Bhattacharyya, B. Stable and potent analogues derived from the modification of the dicarbonyl moiety of curcumin. Biochemistry 2013, 52, 7449–7460. [Google Scholar] [CrossRef]
- Almahariq, M.; Tsalkova, T.; Mei, F.C.; Chen, H.; Zhou, J.; Sastry, S.K.; Schwede, F.; Cheng, X. A novel EPAC-specific inhibitor suppresses pancreatic cancer cell migration and invasion. Mol. Pharmacol. 2013, 83, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Mo, J.; Lin, H.Z.; Chen, Y.; Sun, H.-P. The recent progress of isoxazole in medicinal chemistry. Bioorg. Med. Chem. 2018, 26, 3065–3075. [Google Scholar] [CrossRef]
- Drysdale, M.J.; Dymock, B.W.; Finch, H.; Webb, P.; McDonald, E.; James, K.E.; Cheung, K.M.; Matthews, T.P. Isoxazole Compounds as Inhibitors of Heat Shock Proteins. U.S. Patent 2017326152 A1, 16 November 2017. [Google Scholar]
- FU, J.; Jin, X.; Karur, S.; Lapointe, G.; Madera, A.M.; Sweeney, Z.K. Isoxazole Hydroxamic Acid Compounds as LpxC Inhibitors. U.S. Patent 20180030004 A1, 1 February 2018. [Google Scholar]
- Fu, J.P.; Jiang, S.Y.; Kordikowski, A.; Sweeney, Z.K. Crystalline Isoxazole Hydroxamic Acid Compounds. U.S. Patent 2017355684 A1, 14 December 2017. [Google Scholar]
- Foley Megan, A.C.; Kuntz, K.W.; Mills James, E.J.; Mitchell, L.H.; Munchhof, M.J.; Harvey, D.M. Isoxazole Carboxamide Compounds. U.S. Patent 2017190676 A1, 6 July 2017. [Google Scholar]
- Duggan, M.E. Deuterated Isoxazole Derivatives and Their Use as Metabotropic Glutamate Receptor Potentiators. WO2017089892 A1, 25 May 2017. [Google Scholar]
- Sun, O.Y.; Ma, J.; Wang, B.; He, Y.; Xing, X.; Shen, R.; Granger, B.; He, J.; Long, J.; Wang, G. Isoxazole Analogs As FXR Agonists And Methods Of Use Thereof. U.S. Patent 2017334893 A1, 23 November 2017. [Google Scholar]
- Sun, O.Y.; He, Y.; Shen, R.C.; Xing, X.; Granger, B.; Wang, B.; Ma, J.; He, J.; Long, J.; Wang, G. Isoxazole Derivatives As FXR Agonists And Methods Of Use Thereof. U.S. Patent 2017334894 A1, 23 November 2017. [Google Scholar]
- Das, A.M.; Hazarika, M.P.; Deka, B.P. Diosgenin Acetate-Isoxazole Derivatives, Process for Preparation Thereof and Their Antifungal Activity. WO2016135749 A1, 1 September 2016. [Google Scholar]
- Kotoku, M.; Maeba, T.; Seki, N.; Hirashima, S.; Fujioka, S.; Obika, S.; Yamanaka, H.; Yokota, M.; Sakai, T.; Hirata, K.; et al. Triazole-Isoxazole Compound And Medical USE Thereof. U.S. Patent 2016137639 A1, 19 May 2016. [Google Scholar]
- Cole, B.; Kolodziej, A. Isoxazole Compounds and Methods for the Treatment of Cystic Fibrosis. U.S. Patent 2016128984 A1, 12 May 2016. [Google Scholar]
- Yang, J.; Kopecek, J. Macromolecular therapeutics. J. Control. Rel. 2014, 288–303. [Google Scholar] [CrossRef] [Green Version]
- Fenton, O.S.; Olafson, K.N.; Pillai, P.S.; Mitchell, M.J.; Langer, R. Advances in biomaterials for drug delivery. Adv. Mater. 2018, 30, 1705328. [Google Scholar] [CrossRef]
- Sabourian, P.; Tavakolian, M.; Yazdani, H.; Frounchi, M.; van de Ven, T.G.M.; Maysinger, D.; Kakkar, A. Stimuli-responsive chitosan as an advantageous platform for efficient delivery of bioactive agents. J. Control. Rel. 2020, 317, 216–231. [Google Scholar] [CrossRef] [PubMed]
- Bodratti, A.M.; Alexandridis, P. Amphiphilic block copolymers in drug delivery: Advances in formulation structure and performance. Expert Opin. Drug Deliv. 2018, 15, 1085–1104. [Google Scholar] [CrossRef] [PubMed]
- Agrahari, V.; Agrahari, V.; Mitra, A.K. Nanocarrier fabrication and macromolecule drug delivery: Challenges and opportunities. Ther. Deliv. 2016, 7, 257–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakkar, A.; Traverso, G.; Farokhzad, O.C.; Weissleder, R.; Langer, R. Evolution of macromolecular complexity in drug delivery systems. Nat. Rev. Chem 2017, 1, 63. [Google Scholar] [CrossRef]
- Moquin, A.; Sturn, J.; Zhang, I.; Ji, J.; von Celsing, R.; Vali, H.; Maysinger, D.; Kakkar, A. Unraveling aqueous self-assembly of telodendrimers to shed light on their efficacy in drug encapsulation. ACS Appl. Bio Mater. 2019, 2, 4515–4526. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, X.; Guo, D.; Luo, J.; Nangia, S. Drug-specific design of telodendrimer architecture for effective doxorubicin encapsulation. J. Phys. Chem. B 2016, 120, 9766–9777. [Google Scholar] [CrossRef]
- Xiao, K.; Suby, N.; Li, Y.; Lam, K.S. Telodendrimer-based nanocarriers for the treatment of ovarian cancer. Ther. Deliv. 2013, 4, 1279–1292. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Moquin, A.; Bomal, E.; Na, L.; Maysinger, D.; Kakkar, A. Telodendrimers for physical encapsulation and covalent linking of individual or combined therapeutics. Mol. Pharm. 2017, 14, 2607–2615. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Y.; Zhao, W.; Chen, Y.; Zhang, P.; Li, J.; Venkataramanan, R.; Li, S. PEG-farnesyl thiosalicylic acid telodendrimer micelles as an improved formulation for targeted delivery of paclitaxel. Mol. Pharm. 2014, 11, 2807–2814. [Google Scholar] [CrossRef]
- Padwa, A.; Pearson, W.H. Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products; Wiley: New York, NY, USA, 2003; Volume 59. [Google Scholar]
- Balalaie, S.; Shamakli, M.; Nikbakht, A.; Alavijeh, N.S.; Rominger, F.; Rostamizadeh, S.; Bijanzadeh, H.R. Direct access to isoxazolino and isoxazolo benzazepines from 2-((hydroxyimino) methyl) benzoic acid via a post-Ugi heteroannulation. Org. Biomol. Chem. 2017, 15, 5737–5742. [Google Scholar] [CrossRef]
- Ladd, E.; Sheikhi, A.; Li, N.; van de Ven, T.; Kakkar, A. Design and synthesis of dendrimers with facile surface group functionalization, and an evaluation of their bactericidal efficacy. Molecules 2017, 22, 868. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, H.; Bazgir, A. Lewis Acid Catalyzed Regio-and Diastereoselective Synthesis of Spiroisoxazolines via One-Pot Sequential Knoevenagel Condensation/1,3-Dipolar Cycloaddition Reaction. Synthesis 2019, 51, 1669–1679. [Google Scholar] [CrossRef]
- Sharma, A.; Mejía, D.; Regnaud, A.l.; Uhlig, N.; Li, C.-J.; Maysinger, D.; Kakkar, A. Combined A3 coupling and click chemistry approach for the synthesis of dendrimer-based biological tools. ACS Macro Lett. 2014, 3, 1079–1083. [Google Scholar] [CrossRef]
- Sharma, A.; Kakkar, A. Designing dendrimer and miktoarm polymer based multi-tasking nanocarriers for efficient medical therapy. Molecules 2015, 20, 16987–17015. [Google Scholar] [CrossRef]
- Moquin, A.; Sharma, A.; Cui, Y.; Lau, A.; Maysinger, D.; Kakkar, A. Asymmetric AB3 Miktoarm Star Polymers: Synthesis, Self-Assembly, and Study of Micelle Stability Using AF4 for Efficient Drug Delivery. Macromol. Biosci. 2015, 15, 1744–1754. [Google Scholar] [CrossRef]
- Lam, T.; Avti, P.K.; Pouliot, P.; Tardif, J.-C.; Rhéaume, É.; Lesage, F.; Kakkar, A. Surface engineering of SPIONs: Role of phosphonate ligand multivalency in tailoring their efficacy. Nanotechnology 2016, 27, 415602. [Google Scholar] [CrossRef]
- Nakahara, Y.; Kida, T.; Nakatsuji, Y.; Akashi, M. New fluorescence method for the determination of the critical micelle concentration by photosensitive monoazacryptand derivative. Langmuir 2005, 21, 6688–6695. [Google Scholar] [CrossRef]
- Eccles, S.A.; Massey, A.; Raynaud, F.I.; Sharp, S.Y.; Box, G.; Valenti, M.; Patterson, L.; de Haven Brandon, A.; Gowan, S.; Boxall, F. NVP-AUY922: A novel heat shock protein 90 inhibitor active against xenograft tumor growth, angiogenesis, and metastasis. Cancer Res. 2008, 68, 2850–2860. [Google Scholar] [CrossRef] [Green Version]
- Felip, E.; Barlesi, F.; Besse, B.; Chu, Q.; Gandhi, L.; Kim, S.-W.; Carcereny, E.; Sequist, L.V.; Brunsvig, P.; Chouaid, C. Phase 2 Study of the HSP-90 Inhibitor AUY922 in Previously Treated and Molecularly Defined Patients with Advanced Non–Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, 576–584. [Google Scholar] [CrossRef] [Green Version]
- Kamal, A.; Bharathi, E.V.; Reddy, J.S.; Ramaiah, M.J.; Dastagiri, D.; Reddy, M.K.; Viswanath, A.; Reddy, T.L.; Shaik, T.B.; Pushpavalli, S. Synthesis and biological evaluation of 3,5-diaryl isoxazoline/isoxazole linked 2, 3-dihydroquinazolinone hybrids as anticancer agents. Eur. J. Med. Chem. 2011, 46, 691–703. [Google Scholar] [CrossRef]
- Kepp, O.; Galluzzi, L.; Zitvogel, L.; Kroemer, G. Pyroptosis–A cell death modality of its kind? Eur. J. Immunol. 2010, 40, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Aglietti, R.A.; Dueber, E.C. Recent Insights into the Molecular Mechanisms Underlying Pyroptosis and Gasdermin Family Functions. Trends Immunol. 2017, 38, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, S.B.; Miao, E.A. Gasdermins: Effectors of Pyroptosis. Trends Cell Biol. 2017, 27, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Stockwell, B.R. The hallmarks of ferroptosis. Ann. Rev. Can. Biol. 2019, 3, 35–54. [Google Scholar] [CrossRef]
- Dixon, S.J. Ferroptosis: Bug or feature? Immunol. Rev. 2017, 277, 150–157. [Google Scholar] [CrossRef]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016, 26, 165–176. [Google Scholar] [CrossRef] [Green Version]
- Angeli, J.P.F.; Shah, R.; Pratt, D.A.; Conrad, M. Ferroptosis Inhibition: Mechanisms and Opportunities. Trends Pharmacol. Sci. 2017, 38, 489–498. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [Green Version]
- Shen, Z.; Song, J.; Yung, B.C.; Zhou, Z.; Wu, A.; Chen, X. Emerging Strategies of Cancer Therapy Based on Ferroptosis. Adv. Mater. 2018, 30, 1704007. [Google Scholar] [CrossRef]
- Wolfrum, C.; Shi, S.; Jayaprakash, K.N.; Jayaraman, M.; Wang, G.; Pandey, R.K.; Rajeev, K.G.; Nakayama, T.; Charrise, K.; Ndungo, E.M. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat. Biotechnol. 2007, 25, 1149. [Google Scholar] [CrossRef]
- Li, Z.; Tan, S.; Li, S.; Shen, Q.; Wang, K. Cancer drug delivery in the nano era: An overview and perspectives. Oncol. Rep. 2017, 38, 611–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franco, Y.L.; Vaidya, T.R.; Ait-Oudhia, S. Anticancer and cardio-protective effects of liposomal doxorubicin in the treatment of breast cancer. Breast Cancer 2018, 10, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, L.; You, X.; Huang, J.; Chen, Y.; Chen, L.; Zhu, Y.; Zhang, Y.; Liu, X.; Wu, J.; Hai, Q. Human albumin fragments nanoparticles as PTX carrier for improved anti-cancer efficacy. Front. Pharmacol. 2018, 9, 582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Not available. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yazdani, H.; Kaul, E.; Bazgir, A.; Maysinger, D.; Kakkar, A. Telodendrimer-Based Macromolecular Drug Design using 1,3-Dipolar Cycloaddition for Applications in Biology. Molecules 2020, 25, 857. https://doi.org/10.3390/molecules25040857
Yazdani H, Kaul E, Bazgir A, Maysinger D, Kakkar A. Telodendrimer-Based Macromolecular Drug Design using 1,3-Dipolar Cycloaddition for Applications in Biology. Molecules. 2020; 25(4):857. https://doi.org/10.3390/molecules25040857
Chicago/Turabian StyleYazdani, Hossein, Esha Kaul, Ayoob Bazgir, Dusica Maysinger, and Ashok Kakkar. 2020. "Telodendrimer-Based Macromolecular Drug Design using 1,3-Dipolar Cycloaddition for Applications in Biology" Molecules 25, no. 4: 857. https://doi.org/10.3390/molecules25040857
APA StyleYazdani, H., Kaul, E., Bazgir, A., Maysinger, D., & Kakkar, A. (2020). Telodendrimer-Based Macromolecular Drug Design using 1,3-Dipolar Cycloaddition for Applications in Biology. Molecules, 25(4), 857. https://doi.org/10.3390/molecules25040857







