New Ionic Carbosilane Dendrons Possessing Fluorinated Tails at Different Locations on the Skeleton
Abstract
1. Introduction
2. Results and Discussion
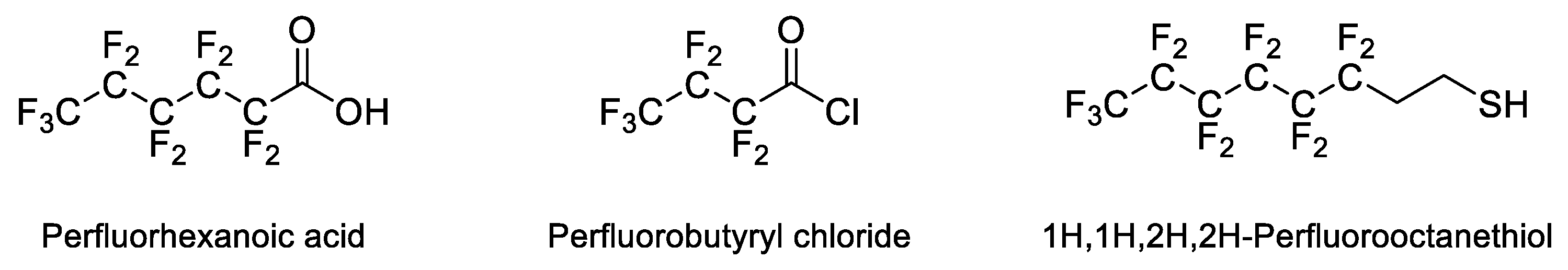
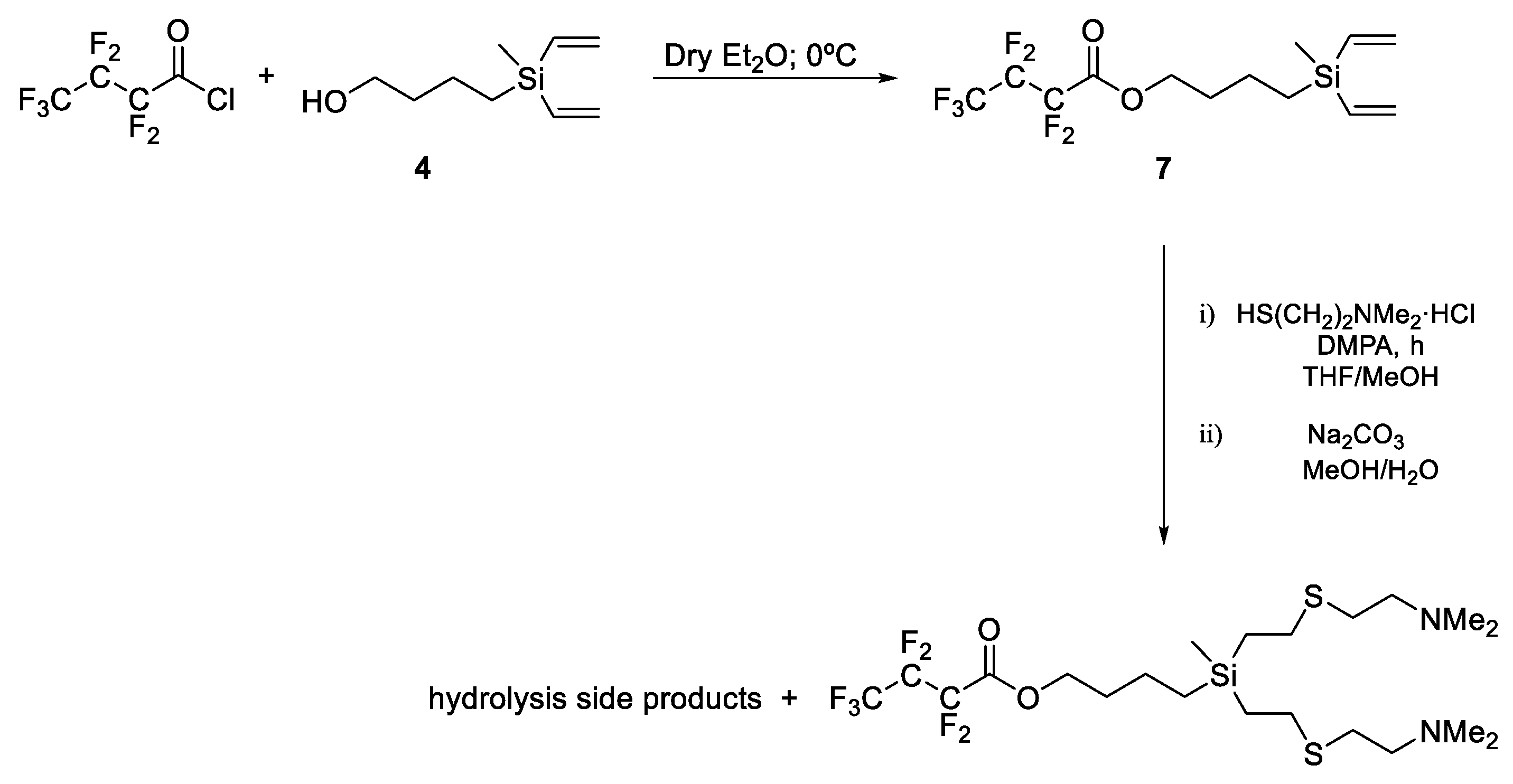
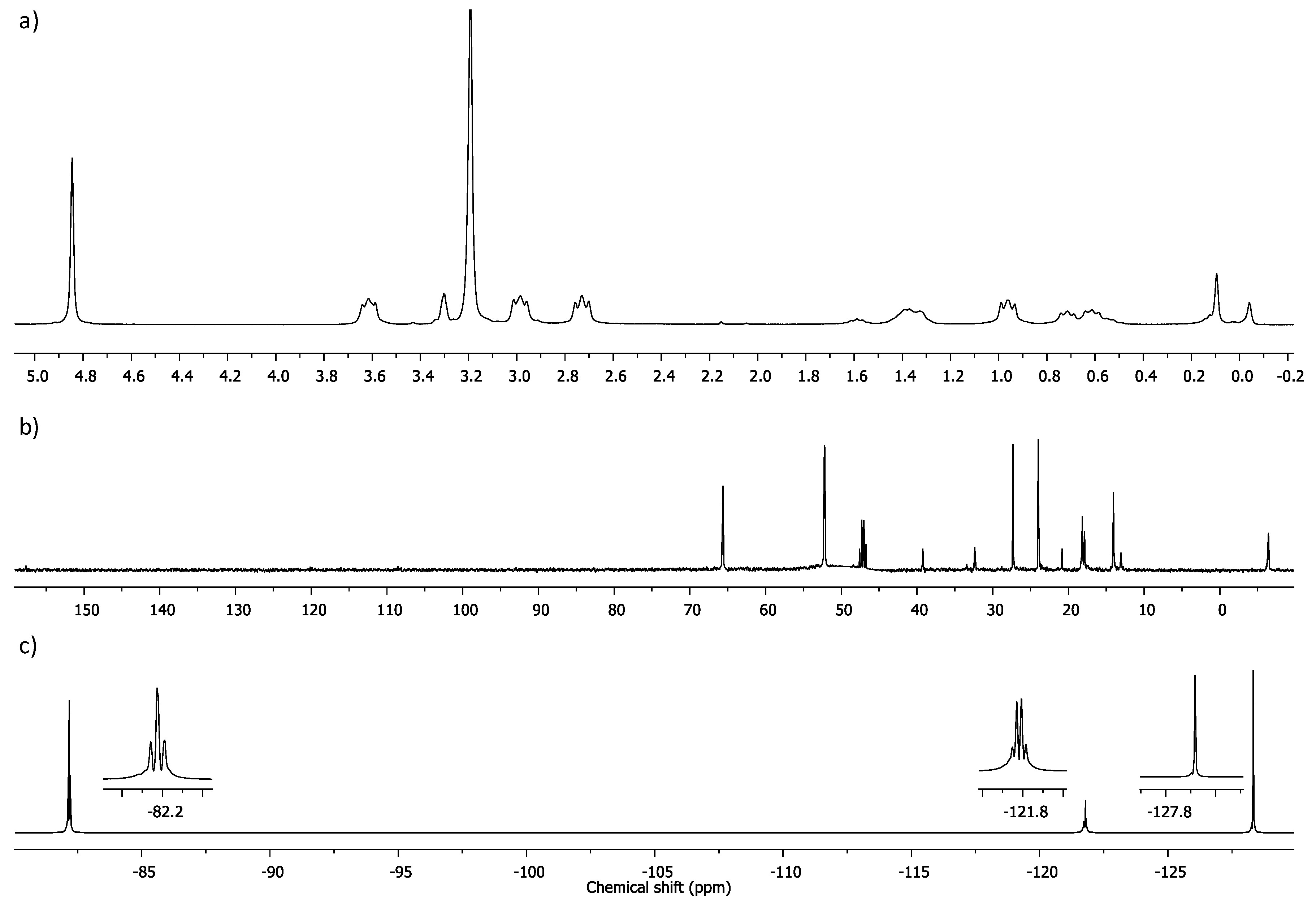
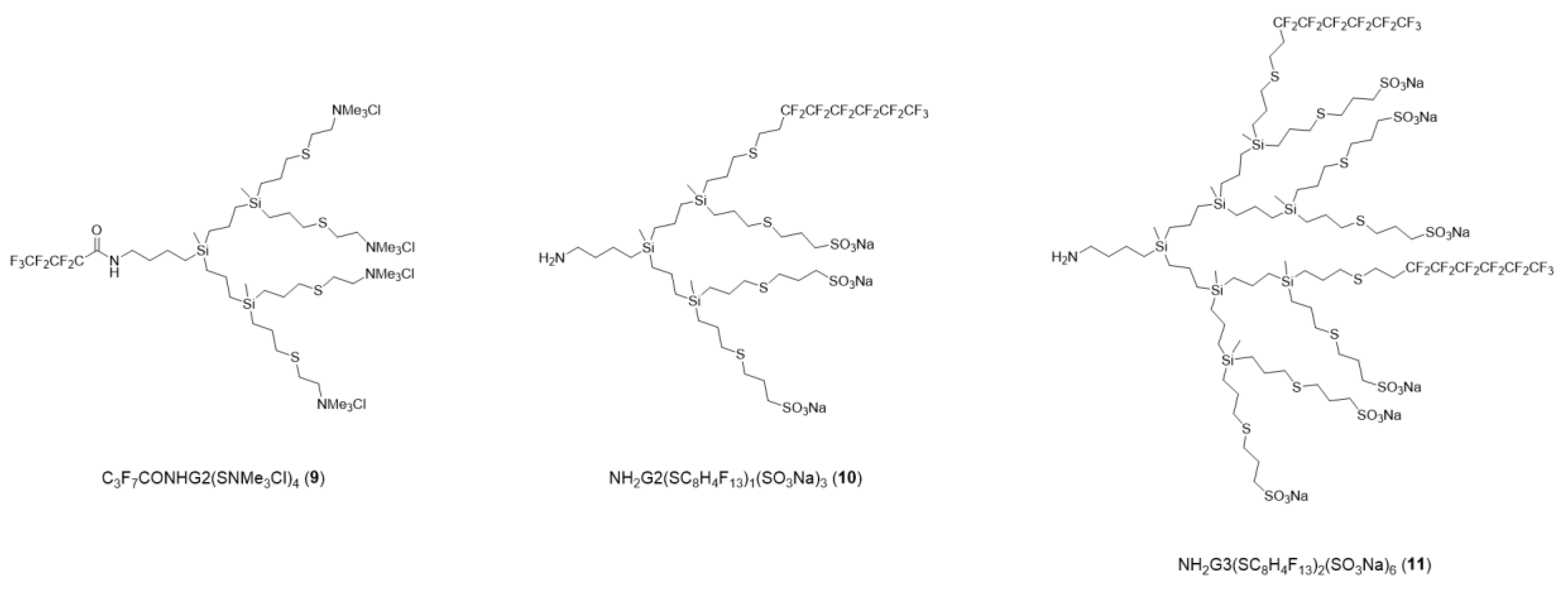
2.1. Dendrons with Perfluorinated Fatty Acids at the Focal Point
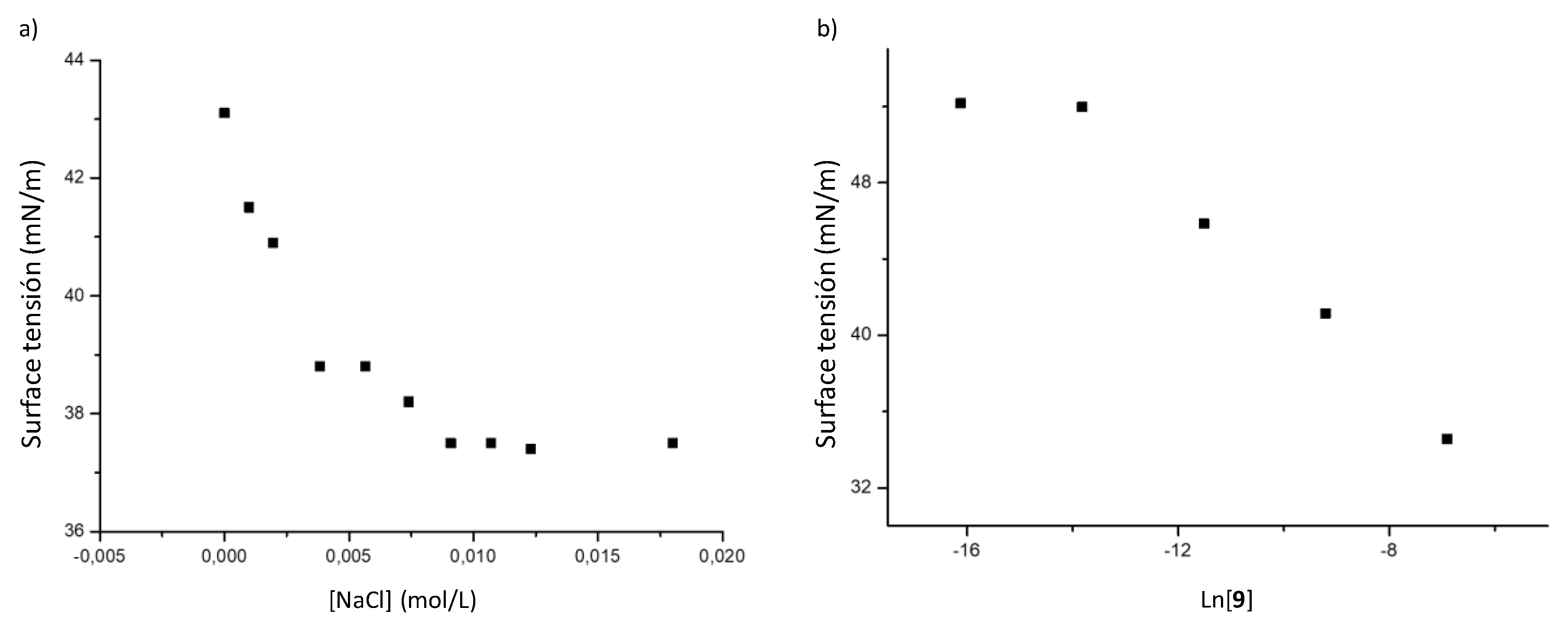
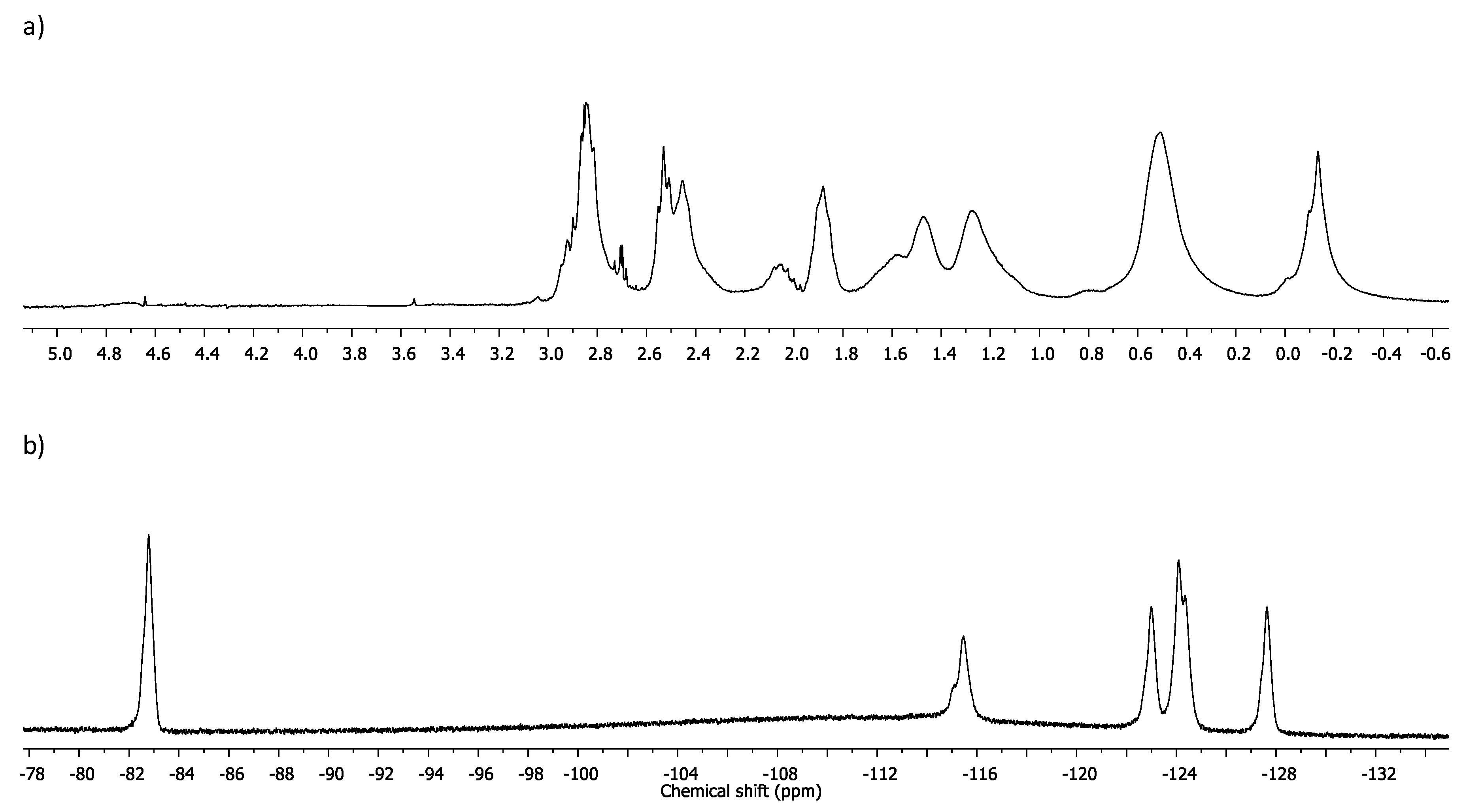
Self-Assembly Assay of Dendron C3F7CONHG2(SNMe3Cl)4 (9)
2.2. Dendrons with Perfluorinated Chains at the Periphery
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Surface Tension Measurements
4.3. Experimental Data
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Caminade, A.M. CHAPTER 7 The Role of Noncovalent Interactions in the Efficiency of Dendrimers in Catalysis. In Noncovalent Interactions in Catalysis; The Royal Society of Chemistry: London, UK, 2019; pp. 153–167. [Google Scholar]
- Yin, R.; Niu, Y.; Zhang, B.; Chen, H.; Yang, Z.; Yang, L.; Cu, Y. Removal of Cr(III) from aqueous solution by silica-gel/PAMAM dendrimer hybrid materials. Environ. Sci. Pollut. Res. 2019, 26, 18098–18112. [Google Scholar] [CrossRef]
- Pedziwiatr-Werbicka, E.; Milowska, K.; Dzmitruk, V.; Ionov, M.; Shcharbin, D.; Bryszewska, M. Dendrimers and hyperbranched structures for biomedical applications. Eur. Polym. J. 2019, 119, 61–73. [Google Scholar] [CrossRef]
- Wang, W.W.; Li, Z.M.; Su, L.; Wang, Q.R.; Wu, Y.L. Insight into the role of fluorinated dendrimers in ruthenium(II) catalyst for asymmetric transfer hydrogenation: The stabilizing effects from experimental and DFT approach. J. Mol. Catal. A Chem. 2014, 387, 92–102. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Suzuki, T.; Miura, A.; Fujikawa, H.; Tokito, S.; Taga, Y. Synthesis, Characterization, and Electron-Transport Property of Perfluorinated Phenylene Dendrimers. J. Am. Chem. Soc. 2000, 122, 1832–1833. [Google Scholar] [CrossRef]
- Bo, S.; Song, C.; Li, Y.; Yu, W.; Chen, S.; Zhou, X.; Yang, Z.; Zheng, X.; Jiang, Z.X. Design and Synthesis of Fluorinated Amphiphile as 19F MRI/Fluorescence Dual-Imaging Agent by Tuning the Self-Assembly. J. Org. Chem. 2015, 80, 6360–6366. [Google Scholar] [CrossRef]
- Wang, M.; Cheng, Y. The effect of fluorination on the transfection efficacy of surface-engineered dendrimers. Biomaterials 2014, 35, 6603–6613. [Google Scholar] [CrossRef]
- Ma, S.; Zhou, J.; Wali, A.R.M.; He, Y.; Xu, X.; Tang, J.Z.; Gu, Z. Self-assembly of pH-sensitive fluorinated peptide dendron functionalized dextran nanoparticles for on-demand intracellular drug delivery. J. Mater. Sci. Mater. Med. 2015, 26, 219. [Google Scholar] [CrossRef]
- Zieringer, M.; Wyszogrodzka, M.; Biskup, K.; Haag, R. Supramolecular behavior of fluorous polyglycerol dendrons and polyglycerol dendrimers with perfluorinated shells in water. New J. Chem. 2012, 36, 402–406. [Google Scholar] [CrossRef]
- Xiao, Q.; Rubien, J.D.; Wang, Z.; Reed, E.H.; Hammer, D.A.; Sahoo, D.; Heiney, P.A.; Yadavalli, S.S.; Goulian, M.; Wilner, S.E.; et al. Self-Sorting and Coassembly of Fluorinated, Hydrogenated, and Hybrid Janus Dendrimers into Dendrimersomes. J. Am. Chem. Soc. 2016, 138, 12655–12663. [Google Scholar] [CrossRef]
- Yang, Y.L.; Sheng, Y.J.; Tsao, H.K. Bilayered membranes of Janus dendrimers with hybrid hydrogenated and fluorinated dendrons: Microstructures and coassembly with lipids. Phys. Chem. Chem. Phys. 2019, 21, 15400–15407. [Google Scholar] [CrossRef]
- Oztuna, A.; Nazir, H. Pentafluoropropionic Anhydride Functionalized PAMAM Dendrimer as miRNA Delivery Reagent. J. Turk. Chem. Soc. Sect. A Chem. 2018, 5, 1295–1302. [Google Scholar] [CrossRef]
- Öztuna, A.; Nazır, H. In vitro transfection potential of fluorinated G5 PAMAM dendrimers for miRNA delivery to MRC-5 cells. Eur. Res. J. 2017, 4, 92–100. [Google Scholar] [CrossRef][Green Version]
- Shumilkina, N.; Myakushev, V.; Tatarinova, E.; Buzin, M.; Voronina, N.; Laptinskaya, T.; Gallyamov, M.; Khokhlov, A.; Muzafarov, A. Synthesis and properties of fluorinated derivatives of carbosilane dendrimers of high generations. Polym. Sci. Ser. A 2006, 48, 1240–1247. [Google Scholar] [CrossRef]
- Sheremetyeva, N.; Serenko, O.; Tatarinova, E.; Buzin, M.; Drozdov, F.; Elmanovich, I.; Gallyamov, M.; Muzafarov, A. Synthesis and properties of carbosilane dendrimers with perfluorohexyl groups in the outer layer of the molecular structure. Russ. Chem. Bull. 2018, 67, 1440–1444. [Google Scholar] [CrossRef]
- Sologubov, S.S.; Markin, A.V.; Smirnova, N.N.; Rybakova, Y.A.; Novozhilova, N.A.; Tatarinova, E.A.; Muzafarov, A.M. Calorimetric study of carbosilane dendrimers of the third and sixth generations with phenylethyl terminal groups. J. Therm. Anal. Calorim. 2016, 125, 595–606. [Google Scholar] [CrossRef]
- Casado, M.A.; Roovers, J.; Stobart, S.R. Introduction of per (fluoroorganosilyl) peripheries into carbosilane dendrimers and related core-functionalized monodendrons gives rise to anomalous hydrodynamic and viscosimetric behavior. Chem. Commun. 2001, 313–314. [Google Scholar] [CrossRef]
- Peterson, K.L.; Srivastava, K.; Pierre, V.C. Fluorinated Paramagnetic Complexes: Sensitive and Responsive Probes for Magnetic Resonance Spectroscopy and Imaging. Front. Chem. 2018, 6. [Google Scholar] [CrossRef]
- Gomis, M.I.; Vestergren, R.; Borg, D.; Cousins, I.T. Comparing the toxic potency in vivo of long-chain perfluoroalkyl acids and fluorinated alternatives. Environ. Int. 2018, 113, 1–9. [Google Scholar] [CrossRef]
- Mahapatra, C.T.; Damayanti, N.P.; Guffey, S.C.; Serafin, J.S.; Irudayaraj, J.; Sepúlveda, M.S. Comparative in vitro toxicity assessment of perfluorinated carboxylic acids. J. Appl. Toxicol. 2017, 37, 699–708. [Google Scholar] [CrossRef]
- Wen, W.; Xia, X.; Zhou, D.; Wang, H.; Zhai, Y.; Lin, H.; Chen, J.; Hu, D. Bioconcentration and tissue distribution of shorter and longer chain perfluoroalkyl acids (PFAAs) in zebrafish (Danio rerio): Effects of perfluorinated carbon chain length and zebrafish protein content. Environ. Pollut. 2019, 249, 277–285. [Google Scholar] [CrossRef]
- Gutierrez-Ulloa, C.E.; Buyanova, M.Y.; Apartsin, E.K.; Venyaminova, A.G.; de la Mata, F.J.; Valiente, M.; Gómez, R. Amphiphilic carbosilane dendrons as a novel synthetic platform toward micelle formation. Org. Biomol. Chem. 2017, 15, 7352–7364. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Paniagua, E.; Peña-González, C.E.; Galán, M.; Gómez, R.; de la Mata, F.J.; Sánchez-Nieves, J. Thiol-Ene Synthesis of Cationic Carbosilane Dendrons: A New Family of Synthons. Organometallics 2013, 32, 1789–1796. [Google Scholar] [CrossRef]
- Martínez, Á.; Fuentes-Paniagua, E.; Baeza, A.; Sánchez-Nieves, J.; Cicuéndez, M.; Gómez, R.; de la Mata, F.J.; González, B.; Vallet-Regí, M. Mesoporous Silica Nanoparticles Decorated with Carbosilane Dendrons as New Non-viral Oligonucleotide Delivery Carriers. Chem. A Eur. J. 2015, 21, 15651–15666. [Google Scholar] [CrossRef] [PubMed]
- Rub, M.A.; Azum, N.; Asiri, A.M. Self-association behavior of an amphiphilic drug nortriptyline hydrochloride under the influence of inorganic salts. Russ. J. Phys. Chem. B 2016, 10, 1007–1013. [Google Scholar] [CrossRef]
- Palladino, P.; Ragone, R. Ionic strength effects on the critical micellar concentration of ionic and nonionic surfactants: The binding model. Langmuir 2011, 27, 14065–14070. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, K.; Hato, M.; Hayashi, T. Physicochemical properties of aqueous solutions of fluorinated surfactants. J. Phys. Chem. 1972, 76, 909–914. [Google Scholar] [CrossRef]
- Sánchez-Nieves, J.; Ortega, P.; Muñoz-Fernández, M.Á.; Gómez, R.; de la Mata, F.J. Synthesis of carbosilane dendrons and dendrimers derived from 1, 3, 5-trihydroxybenzene. Tetrahedron 2010, 66, 9203–9213. [Google Scholar] [CrossRef]
- Galán, M.; Fuentes-Paniagua, E.; de la Mata, F.J.; Gómez, R. Heterofunctionalized Carbosilane Dendritic Systems: Bifunctionalized Dendrons as Building Blocks versus Statistically Decorated Dendrimers. Organometallics 2014, 33, 3977–3989. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
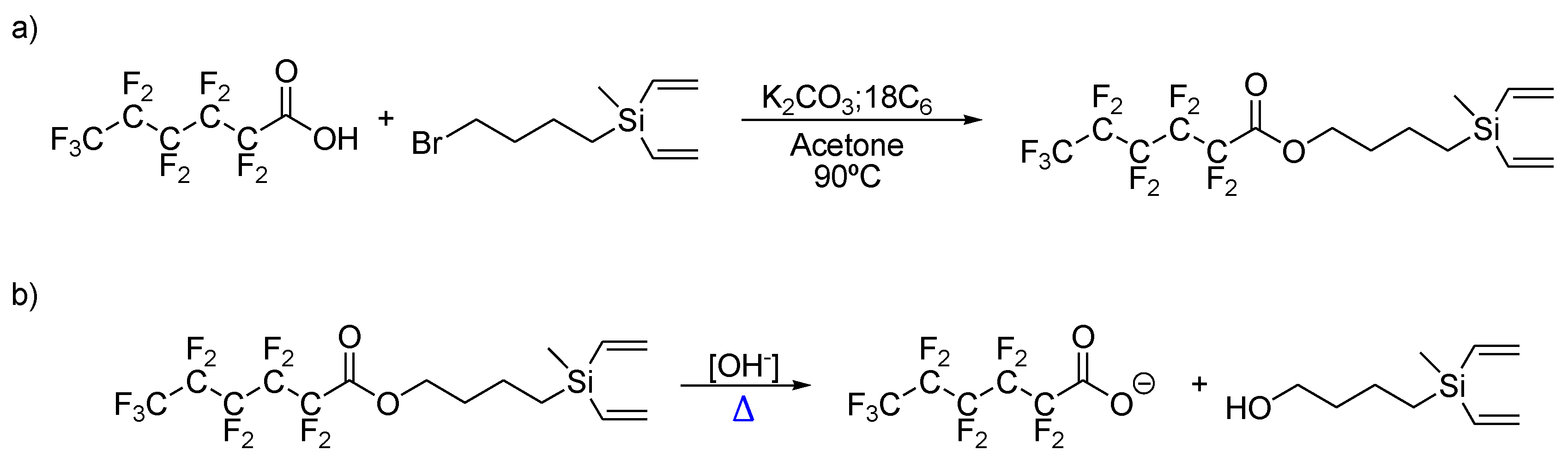



| Base | Equivalents | Temperature | Esterification (%) | Hydrolysis (%) |
|---|---|---|---|---|
| K2CO3 | 1 | 90 | 50 | 50 |
| K2CO3 | 1 | r.t. | 0 | 0 |
| K2CO3 | 0.5 | 90 | 65 | 35 |
| K2CO3 | 0.5 | r.t. | 0 | 0 |
| NaHCO3 | 1 | 90 | 70 | 30 |
| NaHCO3 | 1 | r.t. | 0 | 0 |
| NaHCO3 | 0.5 | 90 | 301 | 251 |
| NaHCO3 | 0.5 | r.t. | 0 | 0 |
| NEt3 | 1 | 90 | 15 | 85 |
| NEt3 | 1 | r.t. | 0 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mencia, G.; Lozano-Cruz, T.; Valiente, M.; de la Mata, J.; Cano, J.; Gómez, R. New Ionic Carbosilane Dendrons Possessing Fluorinated Tails at Different Locations on the Skeleton. Molecules 2020, 25, 807. https://doi.org/10.3390/molecules25040807
Mencia G, Lozano-Cruz T, Valiente M, de la Mata J, Cano J, Gómez R. New Ionic Carbosilane Dendrons Possessing Fluorinated Tails at Different Locations on the Skeleton. Molecules. 2020; 25(4):807. https://doi.org/10.3390/molecules25040807
Chicago/Turabian StyleMencia, Gabriel, Tania Lozano-Cruz, Mercedes Valiente, Javier de la Mata, Jesús Cano, and Rafael Gómez. 2020. "New Ionic Carbosilane Dendrons Possessing Fluorinated Tails at Different Locations on the Skeleton" Molecules 25, no. 4: 807. https://doi.org/10.3390/molecules25040807
APA StyleMencia, G., Lozano-Cruz, T., Valiente, M., de la Mata, J., Cano, J., & Gómez, R. (2020). New Ionic Carbosilane Dendrons Possessing Fluorinated Tails at Different Locations on the Skeleton. Molecules, 25(4), 807. https://doi.org/10.3390/molecules25040807





