Adsorption and Desorption Properties of Total Flavonoids from Oil Palm (Elaeis guineensis Jacq.) Mature Leaf on Macroporous Adsorption Resins
Abstract
1. Introduction
2. Results and Discussion
2.1. Adsorption Kinetics of OPL Total Flavonoids on Selected MARs
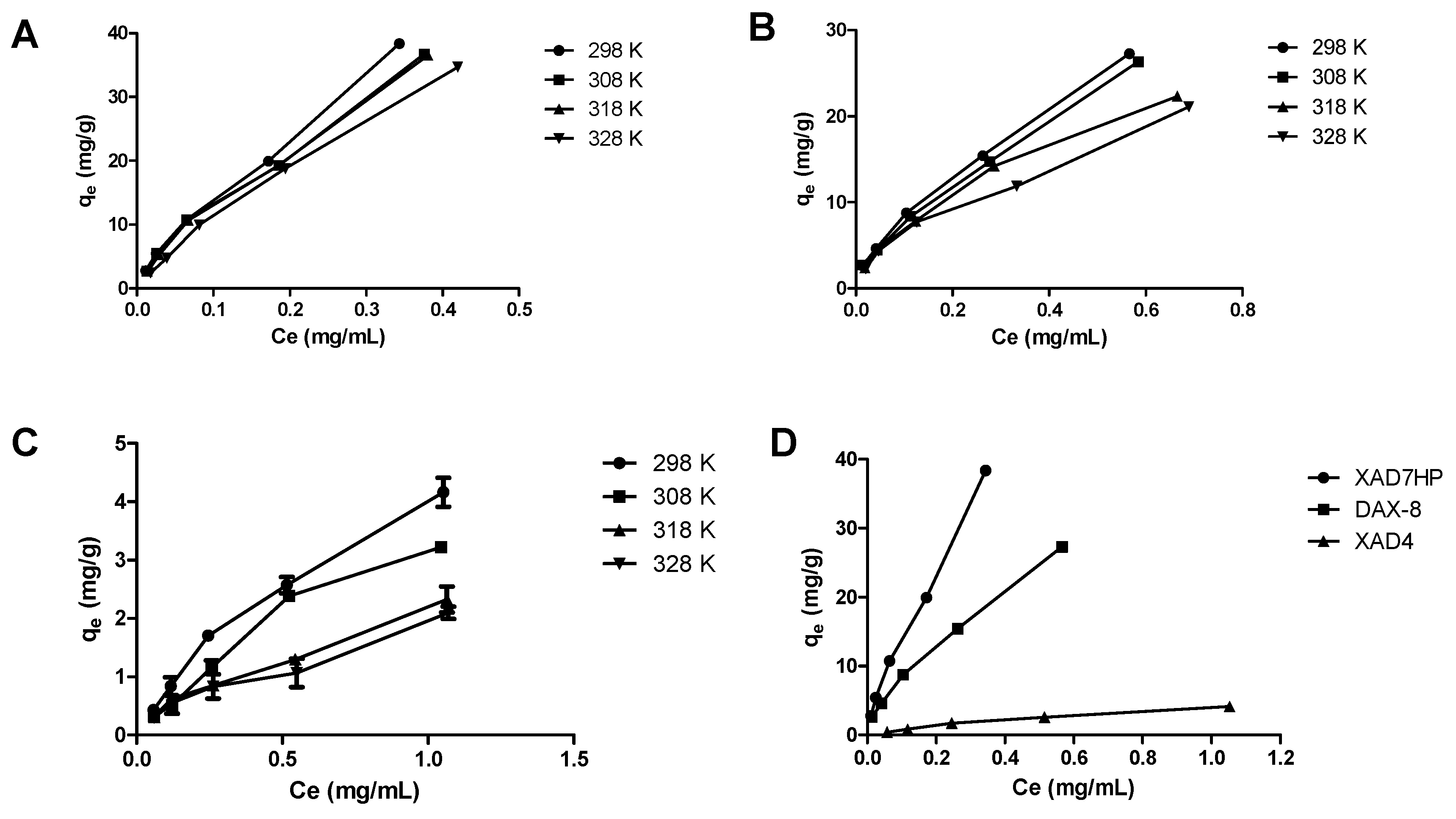
2.2. Adsorption Thermodynamics of OPL Total Flavonoids on the Selected MARs
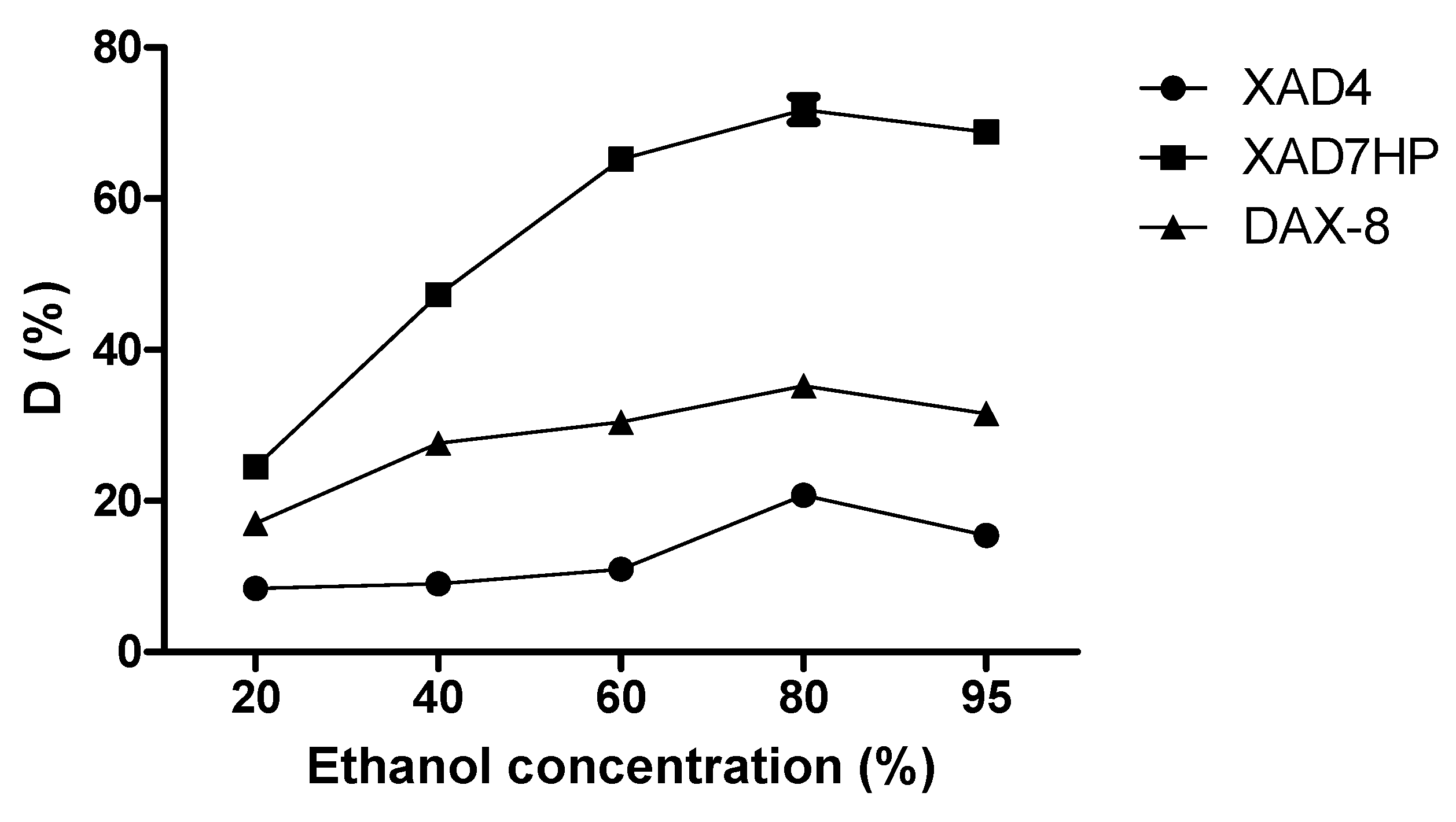
2.3. Static Desorption of OPL Total Flavonoids from the Selected MARs
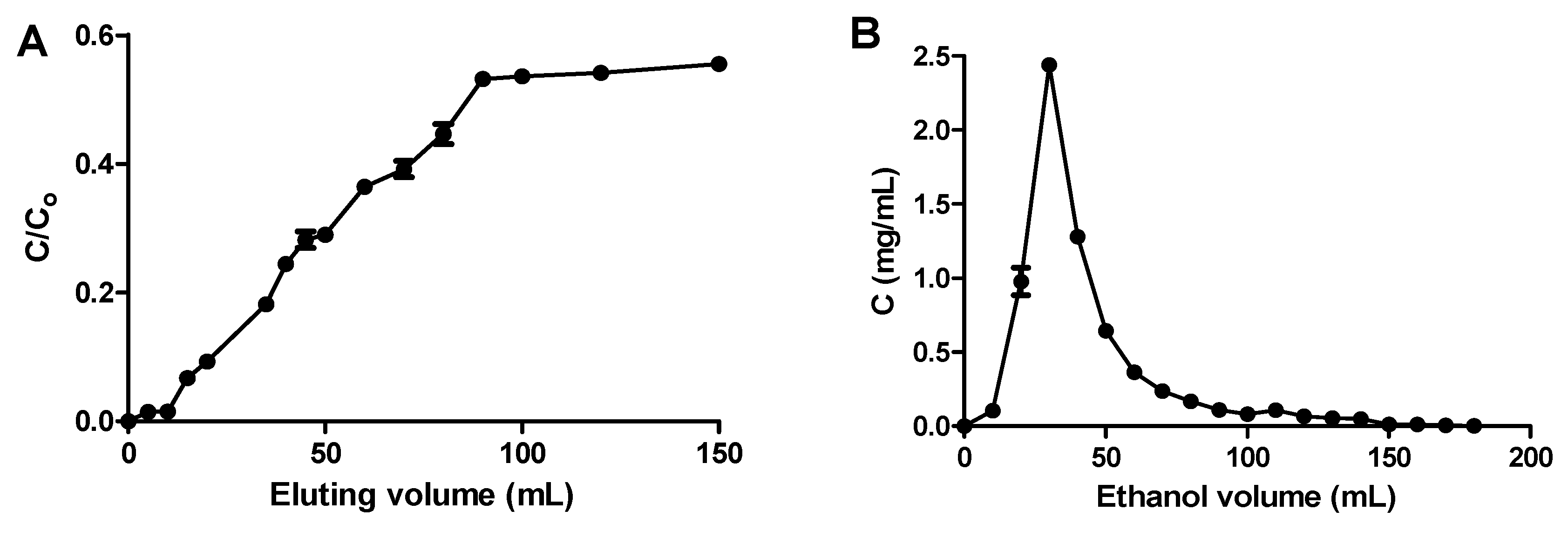
2.4. Dynamic Adsorption and Desorption of OPL Total Flavonoids from XAD7HP
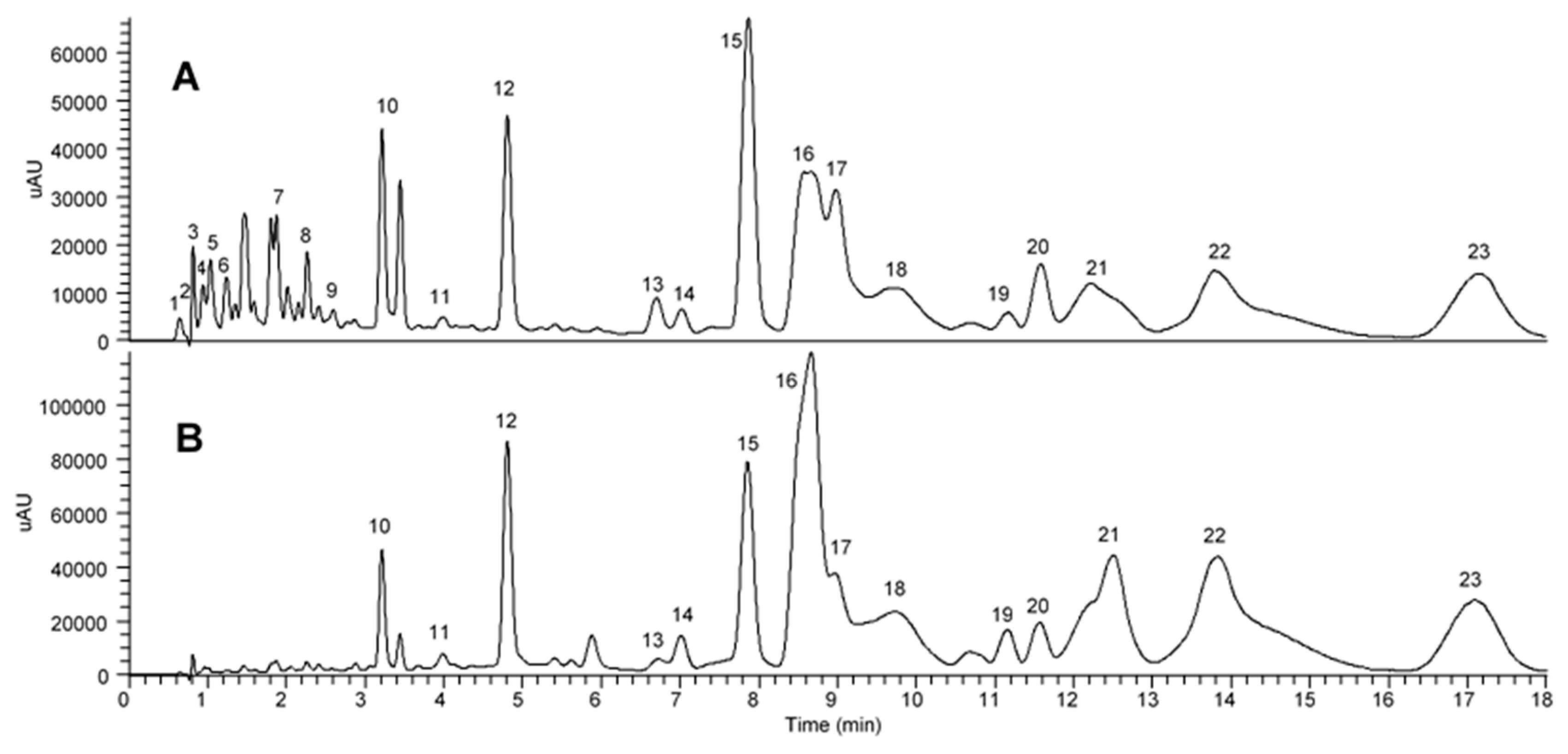
2.5. UHPLC–UV/PDA and UHPLC–MS/MS Analysis of Crude and Total Flavonoid-Enriched OPL Extracts
2.6. Free Radical Scavenging Activities of Crude and Enriched Fraction of OPL
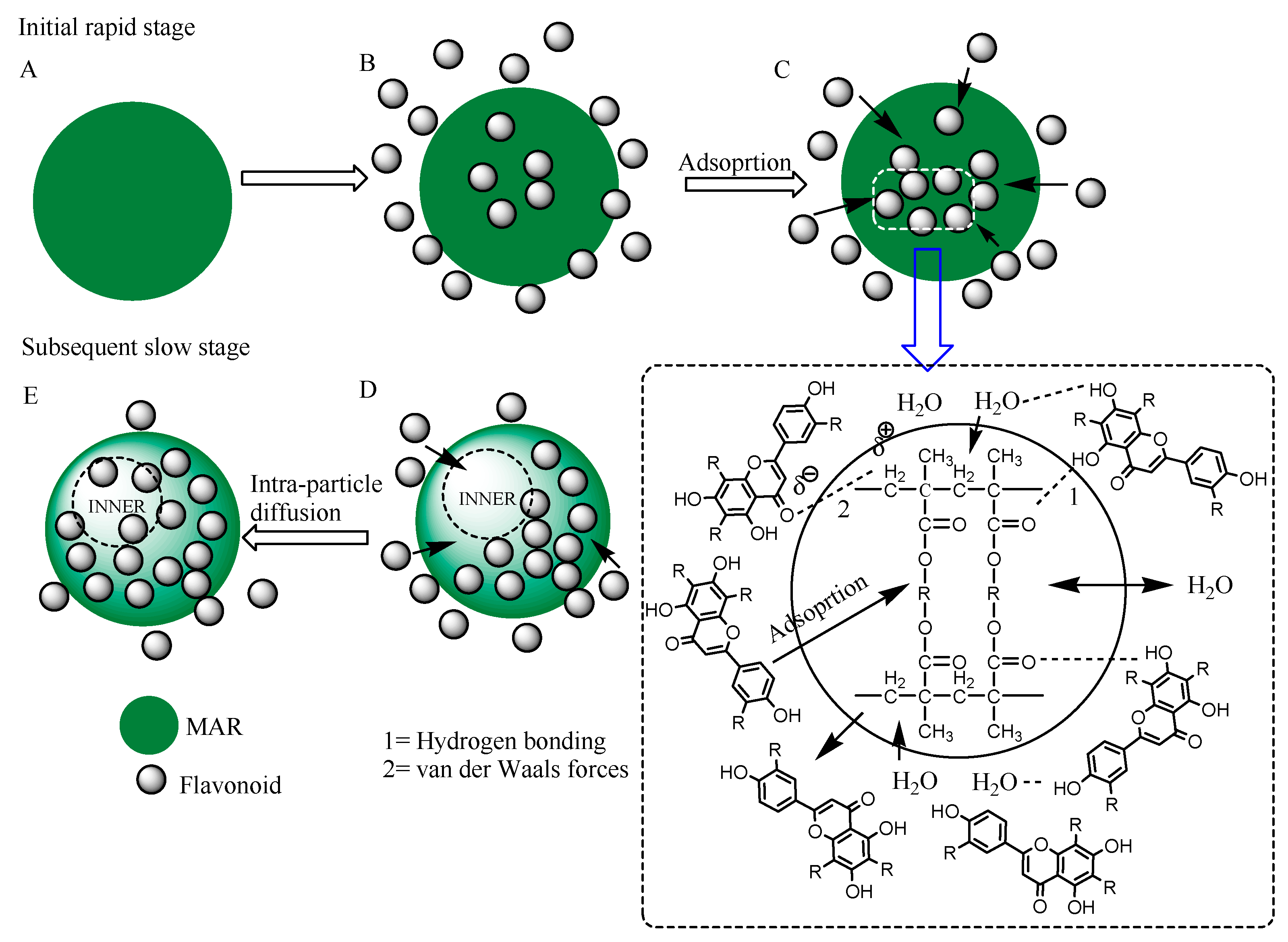
2.7. Adsorption Mechanism
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Adsorbents and Pretreatment
3.3. Preparation of OPL Extract
3.4. Determination of Total Flavonoid Content
3.5. Static Adsorption and Desorption Experiments
3.5.1. Adsorption Kinetics of OPL Total Flavonoids on the Selected MARs
3.5.2. Adsorption Thermodynamics of OPL Total Flavonoids on the Selected MARs
3.5.3. Static Desorption of OPL Total Flavonoids from the Selected MARs
3.6. Dynamic Sorption Experiments
3.7. Equations Used in This Study
3.8. UHPLC–UV/PDA and UHPLC–MS/MS Analysis of Crude and Total Flavonoid-Enriched Extracts
3.9. Determination of DPPH Free Radical Scavenging Activity
3.10. Determination of Nitric Oxide (NO) Free Radical Scavenging Activity
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hartley, C.W.S. The Oil Palm (Elaeis Guineensis Jacq.) Tropical Agriculture Series, 3rd ed.; Longman Scientific & Technical: London, UK, 1988; p. 761. [Google Scholar]
- Zeven, A.C. On the origin of the oil palm (elaeis guineensis jacq). Grana Palynol. 1964, 5, 121–123. [Google Scholar] [CrossRef]
- Schnell, R.J.; Priyadarshan, P.M. Genomics of Tree Crops; Springer: New York, NY, USA, 2012. [Google Scholar]
- Owoyele, B.V.; Owolabi, G.O. Traditional oil palm (Elaeis guineensis jacq.) and its medicinal uses: A review. Tang Humanit. Med. 2014, 416, 16. [Google Scholar] [CrossRef]
- Mohamed, S. Oil Palm Leaf: A New Functional Food Ingredient for Health and Disease Prevention. J. Food Process. Technol. 2014, 5, 2. [Google Scholar] [CrossRef]
- Jaffri, J.M.; Mohamed, S.; Ahmad, I.N.; Mustapha, N.M.; Manap, Y.A.; Rohimi, N. Effects of catechin-rich oil palm leaf extract on normal and hypertensive rats’ kidney and liver. Food Chem. 2011, 128, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Tahir, N.I.; Shaari, K.; Abas, F.; Parveez, G.K.A.; Ishak, Z.; Ramli, U.S. Characterization of apigenin and luteolin derivatives from oil palm (Elaeis guineensis Jacq.) Leaf using LC-ESI-MS/MS. J. Agric. Food Chem. 2012, 60, 11201–11210. [Google Scholar] [CrossRef]
- Xiao, J.; Capanoglu, E.; Jassbi, A.R.; Miron, A. Advance on the Flavonoid C-glycosides and Health Benefits. Crit. Rev. Food Sci. Nutr. 2016, 56, S29–S45. [Google Scholar] [CrossRef]
- Grand View Research. Flavonoids Market Size To Reach $1.06 Billion By 2025; Grand View Research Inc.: San Francisco, CA, USA, 2016. [Google Scholar]
- Wu, S.; Wang, Y.; Gong, G.; Li, F.; Ren, H.; Liu, Y. Adsorption and desorption properties of macroporous resins for flavonoids from the extract of Chinese wolfberry (Lycium barbarum L.). Food Bioprod. Process. 2015, 93, 148–155. [Google Scholar] [CrossRef]
- Wan, P.; Sheng, Z.; Han, Q.; Zhao, Y.; Cheng, G.; Li, Y. Enrichment and purification of total flavonoids from Flos Populi extracts with macroporous resins and evaluation of antioxidant activities in vitro. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 945–946, 68–74. [Google Scholar] [CrossRef]
- Li, C.; Zheng, Y.; Wang, X.; Feng, S.; Di, D. Simultaneous separation and purification of flavonoids and oleuropein from Olea europaea L. (olive) leaves using macroporous resin. J. Sci. Food Agric. 2011, 91, 2826–2834. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, M.; Sun-Waterhouse, D.; Zhuang, M.; Chen, H.; Feng, M.; Lin, L. Absorption and desorption behaviour of the flavonoids from Glycyrrhiza glabra L. leaf on macroporous adsorption resins. Food Chem. 2015, 168, 538–545. [Google Scholar] [CrossRef]
- Huang, S.; Lin, B.; Li, B.; Tan, B.; Hong, Y. Purification of total flavonoids from loquat leaves by macroporous resin and corresponding antioxidant capacity. BIO Web Conf. 2017, 8, 3010. [Google Scholar] [CrossRef]
- Lou, S.; Chen, Z.; Liu, Y.; Ye, H.; Di, D. Synthesis of functional adsorption resin and its adsorption properties in purification of flavonoids from Hippophae rhamnoides L. leaves. Ind. Eng. Chem. Res. 2012, 51, 2682–2696. [Google Scholar] [CrossRef]
- Scordino, M.; Di Mauro, A.; Passerini, A.; Maccarone, E. Adsorption of Flavonoids on Resins: Cyanidin 3-Glucoside. J. Agric. Food Chem. 2004, 52, 1965–1972. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Wang, H.; Yu, J.; Liang, C.; Ye, W.; Li, P. Enrichment and purification of total flavonoid C-glycosides from abrus mollis extracts with macroporous resins. Ind. Eng. Chem. Res. 2012, 51, 7349–7354. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, T.; Xiao, W.; Wang, Z.; Ding, G.; Zhao, L. Enrichment and Purification of Total Ginkgo Flavonoid O-Glycosides from Ginkgo Biloba Extract with Macroporous Resin and Evaluation of Anti-Inflammation Activities In Vitro. Molecules 2018, 23, 1167. [Google Scholar] [CrossRef]
- Ayranci, E.; Hoda, N. Adsorption kinetics and isotherms of pesticides onto activated carbon-cloth. Chemosphere 2005, 60, 1600–1607. [Google Scholar] [CrossRef]
- Lorenc-Grabowska, E.; Gryglewicz, G. Adsorption of lignite-derived humic acids on coal-based mesoporous activated carbons. J. Colloid Interface Sci. 2005, 284, 416–423. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Hall, K.R.; Eagleton, L.C.; Acrivos, A.; Vermeulen, T. Pore and Solid Diffusion Kinetics in Fixed-Bed Adsorption Under Constant Pattern Conditions. I&EC Fundam. 1966, 5, 212–223. [Google Scholar]
- Wang, R.; Peng, X.; Wang, L.; Tan, B.; Liu, J.; Feng, Y.; Yang, S. Preparative purification of peoniflorin and albiflorin from peony rhizome using macroporous resin and medium-pressure liquid chromatography. J. Sep. Sci. 2012, 35, 1985–1992. [Google Scholar] [CrossRef]
- Gökmen, V.; Serpen, A. Equilibrium and kinetic studies on the adsorption of dark colored compounds from apple juice using adsorbent resin. J. Food Eng. 2002, 53, 221–227. [Google Scholar] [CrossRef]
- Lin, L.; Zhao, H.; Dong, Y.; Yang, B.; Zhao, M. Macroporous resin purification behavior of phenolics and rosmarinic acid from Rabdosia serra (MAXIM.) HARA leaf. Food Chem. 2012, 130, 417–424. [Google Scholar] [CrossRef]
- Cao, S.-Q.; Pan, S.-Y.; Yao, X.-L.; Fu, H.-F. Isolation and Purification of Anthocyanins from Blood Oranges by Column Chromatography. Agric. Sci. China 2010, 9, 207–215. [Google Scholar] [CrossRef]
- Tahir, N.I.; Saari, K.; Abas, F.; Parveez, A.; Tarmizi, H.A.; Ramli, U. Identification of Oil Palm (Elaeis Guineensis) Spear Leaf Metabolites Using Mass Spectrometry and Neutral Loss Analysis. J. Oil Palm Res. 2013, 25, 72–83. [Google Scholar]
- Vargas, L.H.G.; Neto, J.C.R.; de Aquino Ribeiro, J.A.; Ricci-Silva, M.E.; Souza, M.T.; Rodrigues, C.M.; Abdelnur, P.V.; Ribeiro, J.A.D.A.; De Oliveira, A.E. Metabolomics analysis of oil palm (Elaeis guineensis) leaf: Evaluation of sample preparation steps using UHPLC–MS/MS. Metabolomics 2016, 12, 1–12. [Google Scholar] [CrossRef]
- Sonam, K.S.; Guleria, S. Synergistic antioxidant activity of natural product. Anna Pharmacol Pharma. 2017, 2, 1–6. [Google Scholar]
- Alzamora, S.M.; Tapia, M.S.; Chanes, J.W. New strategies for minimally processed foods. The role of multitarget preservation. Food Sci. Technol. Int. 1998, 4, 353–361. [Google Scholar] [CrossRef]
- Xiao, J. Dietary flavonoid aglycones and their glycosides: Which show better biological significance? Crit. Rev. Food Sci. Nutr. 2017, 57, 1874–1905. [Google Scholar] [CrossRef]
- Joubert, E.; Winterton, P.; Britz, T.J.; Ferreira, D. Superoxide anion and α, α-diphenyl-β-picrylhydrazyl radical scavenging capacity of rooibos (Aspalathus linearis) aqueous extracts, crude phenolic fractions, tannin and flavonoids. Food Res. Int. 2004, 37, 133–138. [Google Scholar] [CrossRef]
- Darmanyan, A.P.; Gregory, D.D.; Guo, Y.; Jenks, W.S.; Burel, L.; Eloy, D.; Jardon, P. Quenching of singlet oxygen by oxygen- and sulfur-centered radicals: Evidence for energy transfer to peroxyl radicals in solution. J. Am. Chem. Soc. 1998, 120, 396–403. [Google Scholar] [CrossRef]
- Kancheva, V.D. Phenolic antioxidants—Radical-scavenging and chain-breaking activity: A comparative study. Eur. J. Lipid Sci. Technol. 2009, 111, 1072–1089. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Y.; Huo, T.; Chen, Z.; Liu, Y.; Di, D.; Guo, M.; Zhao, L. Multiple interactions on macroporous adsorption resins modified with ionic liquid. Colloids Surf. A Physicochem. Eng. Asp. 2015, 487, 35–41. [Google Scholar] [CrossRef]
- Qiu, H.; Mallik, A.K.; Takafuji, M.; Liu, X.; Jiang, S.; Ihara, H. A new imidazolium-embedded C 18 stationary phase with enhanced performance in reversed-phase liquid chromatography. Anal. Chim. Acta 2012, 738, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wang, Y.; Yang, L.; Li, X.; Zhou, L.; Li, Y. Novel adsorbent of polymeric complex derived from chaleting resin with Cu(II) and its removal properties for cyanide in aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2014, 455, 136–146. [Google Scholar] [CrossRef]
- Formagio, A.; Volobuff, C.; Santiago, M.; Cardoso, C.; Vieira, M.; Valdevina Pereira, Z. Evaluation of Antioxidant Activity, Total Flavonoids, Tannins and Phenolic Compounds in Psychotria Leaf Extracts. Antioxidants 2014, 3, 745–757. [Google Scholar] [CrossRef]
- Lee, S.Y.; Mediani, A.; Maulidiani, M.; Khatib, A.; Ismail, I.S.; Zawawi, N.; Abas, F. Comparison of partial least squares and random forests for evaluating relationship between phenolics and bioactivities of Neptunia oleracea. J. Sci. Food Agric. 2018, 98, 240–252. [Google Scholar] [CrossRef]
- Abdul-Hamid, N.A.; Mustaffer, N.H.; Maulidiani, M.; Mediani, A.; Ismail, I.S.; Tham, C.L.; Shadid, K.; Abas, F. Quality evaluation of the physical properties, phytochemicals, biological activities and proximate analysis of nine Saudi date palm fruit varieties. J. Saudi Soc. Agric. Sci. 2018, in press. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
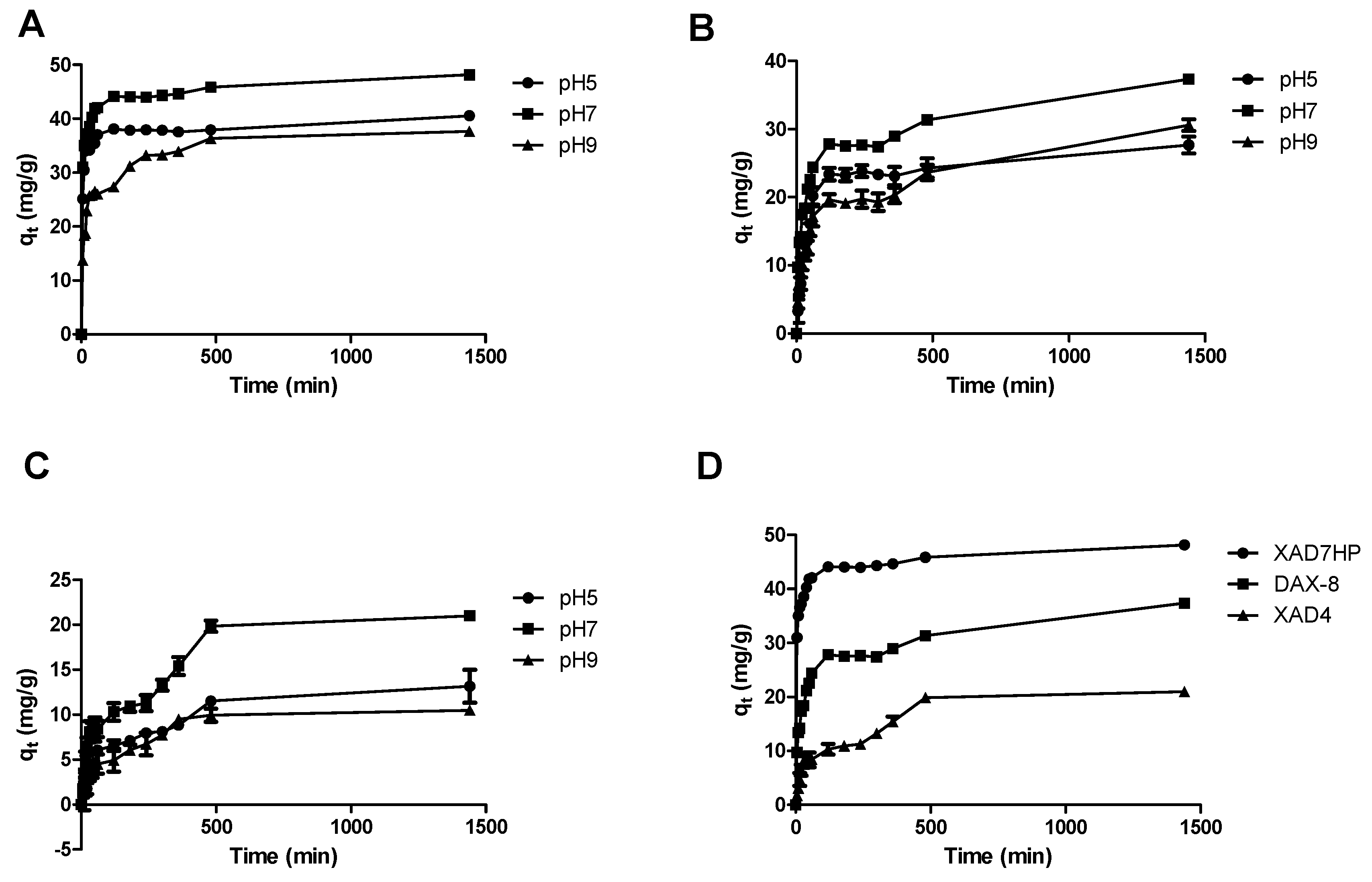
| Resins | pH | qe (exp)(mg/g) | Pseudo-First-Order | Pseudo-Second-Order | Intraparticle Diffusion | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| k1 | qe (cal) (mg/g) | R2 | k2 | qe (cal) (mg/g) | R2 | C | kp | R2 | |||
| XAD7HP | 5 | 40.5667 | 0.0026 | 6.3726 | 0.5460 | 0.0120 | 38.0228 | 0.9999 | 31.7520 | 0.3707 | 0.6049 |
| 7 | 48.1583 | 0.0034 | 10.1381 | 0.7732 | 0.0045 | 45.6621 | 0.9996 | 34.8670 | 0.5890 | 0.7467 | |
| 9 | 37.6979 | 0.0050 | 17.4023 | 0.9444 | 0.0016 | 36.2319 | 0.9956 | 17.2340 | 0.9540 | 0.8673 | |
| DAX-8 | 5 | 27.6583 | 0.0041 | 15.1727 | 0.7054 | 0.0014 | 25.7731 | 0.9969 | 6.4897 | 1.0203 | 0.7685 |
| 7 | 37.3583 | 0.0027 | 19.7707 | 0.7933 | 0.0018 | 30.9598 | 0.9957 | 12.9690 | 0.9407 | 0.8157 | |
| 9 | 30.5958 | 0.0022 | 19.8004 | 0.7973 | 0.0016 | 22.9885 | 0.9886 | 6.9934 | 0.8093 | 0.8353 | |
| XAD4 | 5 | 13.1521 | 0.0034 | 11.3441 | 0.9153 | 0.0009 | 11.9474 | 0.9573 | 0.2973 | 0.4973 | 0.9422 |
| 7 | 20.9875 | 0.0042 | 17.8553 | 0.8235 | 0.0008 | 18.3824 | 0.9184 | 2.2351 | 0.7137 | 0.9162 | |
| 9 | 10.4937 | 0.0052 | 8.9692 | 0.9312 | 0.0014 | 10.2459 | 0.9400 | 1.0913 | 0.4005 | 0.9677 | |
| Resins | T (K) | Langmuir Equation | Freundlich Equation | ΔH (kJ/mol) | |||||
|---|---|---|---|---|---|---|---|---|---|
| qm | KL | RL | R12 | 1/n | Kf | R22 | |||
| XAD4 | 298 | 4.3497 | 0.4419 | 0.6706 | 0.9401 | 0.6894 | 3.6247 | 0.9351 | −19.96 |
| 308 | 5.0352 | 0.8489 | 0.5146 | 0.8882 | 0.7879 | 3.1212 | 0.9660 | ||
| 318 | 3.6088 | 0.7232 | 0.5544 | 0.8412 | 0.6507 | 2.1153 | 0.9802 | ||
| 328 | 3.0609 | 0.6670 | 0.5743 | 0.7856 | 0.6260 | 1.8461 | 0.9741 | ||
| XAD7HP | 298 | 67.568 | 0.3108 | 0.7433 | 0.8218 | 0.7450 | 80.926 | 0.9967 | −7.49 |
| 308 | 61.350 | 0.3006 | 0.7496 | 0.8561 | 0.7381 | 74.068 | 0.9908 | ||
| 318 | 66.667 | 0.3600 | 0.7143 | 0.8821 | 0.7667 | 76.342 | 0.9943 | ||
| 328 | 86.207 | 0.6466 | 0.5819 | 0.9575 | 0.8429 | 74.790 | 0.9973 | ||
| DAX-8 | 298 | 39.370 | 0.3071 | 0.7456 | 0.8745 | 0.6328 | 37.051 | 0.9960 | −13.41 |
| 308 | 37.594 | 0.3195 | 0.7379 | 0.8269 | 0.5953 | 32.590 | 0.9845 | ||
| 318 | 30.960 | 0.2879 | 0.7576 | 0.9517 | 0.6238 | 29.689 | 0.9970 | ||
| 328 | 27.933 | 0.2961 | 0.7524 | 0.8951 | 0.6045 | 25.578 | 0.9912 | ||
| Peak | tR (min) | λmax, (nm) | [M − H]− (m/z) | Formula | Key MS/MS Fragments (m/z) | Class | Possible Compound | Peak Intensity | |
|---|---|---|---|---|---|---|---|---|---|
| Crude | Fraction | ||||||||
| 1 | 0.65 | 270 | 341.0850 | C12H22O11 | 161.0224, 119.0327, 113.0223, 101.0223, 89.0224 | Sugar | Sucrose | + | − |
| 2 | 0.71 | 268, 374 | 366.9911 | C14H8O18 | 182.9911, 139.0014, 94.0276, 67.0170 | Organic acid | Chelidonic acid dimer | + | − |
| 3 | 0.83 | 258 | 191.0189 | C6H8O7 | 111.0066, 87.0067, 57.0328 | Organic acid | Citric acid | ++ | − |
| 4 | 0.93 | 312 | 315.0697 | C13H16O9 | 152.0092, 109.0195 | Phenolic acid | Dihydroxylbenzoyl-O-hexoside | + | − |
| 5 | 0.96 | 288, 310 | 299.0748 | C13H16O8 | 137.0222, 93.0325 | Phenolic acid | Hydroxylbenzoyl-O-hexoside | ++ | − |
| 6 | 1.07 | 286, 324 | 331.0645 | C13H16O10 | 168.0405, 153.0170, 125.0221 | Phenolic acid | Galloyl-O-hexoside | ++ | − |
| 7 | 1.84 | 286, 324 | 329.0528 | C14H18O9 | 167.0326, 153.0092, 123.0440 | Phenolic acid | Vanilloyl-O-hexoside | ++ | − |
| 8 | 2.49 | 278 | 385.0744 | C17H22O10 | 223.0603, 209.0279, 191.0174, 147.0272 | Phenolic acid | Sinapoyl-O-hexoside | ++ | − |
| 9 | 2.57 | 280, 310 | 289.0717 | C15H14O6 | 205.0480, 151.0378, 137.0222, 125.0222, 109.0273 | Catechin | Catechin isomer | + | − |
| 10 | 3.17 | 272, 348 | 609.1411 | C27H30O16 | 519.1104 489.0998, 429.0786, 399.0696, 369.0585 | Flavone | Luteolin-6,8-di-C-hexose | ++ | ++ |
| 11 | 4.26 | 280, 324 | 289.0717 | C15H14O6 | 205.0480, 151.0378, 137.0223, 125.0222, 109.0274 | Catechin | Catechin isomer | + | + |
| 12 | 4.88 | 272, 336 | 593.1464 | C27H30O15 | 503.1155, 473.1051, 383.0739, 353.0638 | Flavone | Apigenin-6,8-di-C-hexose | ++ | +++ |
| 13 | 6.72 | 272, 346 | 609.1411 | C27H30O16 | 489.1001, 429.0789, 399.0679, 369.0604 | Flavone | Luteolin-6,8-di-C-hexose | ++ | + |
| 14 | 7.10 | 272, 334 | 563.1359 | C26H28O14 | 473.1053, 443.0949, 383.0742, 353.0639 | Flavone | Apigenin-6-C-pentose-8-C-hexose | + | ++ |
| 15 | 7.86 | 270, 348 | 447.0896 | C21H20O11 | 357.0588, 339.0480, 327.0483, 297.0379, 285.0381 | Flavone | Isoorientin (Luteolin-6-C-hexose) | +++ | ++++ |
| 16 | 8.52 | 272, 336 | 563.1359 | C26H28O14 | 473.1051, 443.0949, 383.0741, 353.0638 | Flavone | Apigenin-6-C-hexose-8-C-pentose | +++ | ++++ |
| 17 | 9.00 | 270, 350 | 447.0896 | C21H20O11 | 357.0587, 339.0476, 327.0485, 297.0378, 285.0380 | Flavone | Orientin (Luteolin-8-C-hexose) | +++ | +++ |
| 18 | 9.87 | 270, 348 | 593.1464 | C27H30O15 | 473.1049, 429.0792, 369.0590, 357.0589, 327.0485 | Flavone | Luteolin-6-C-hexose- 8-C-deoxyhexose | ++ | +++ |
| 19 | 11.22 | 274, 334 | 563.1359 | C26H28O14 | 503.1168, 473.1056, 443.0950, 383.0743, 353.0639 | Flavone | Apigenin-6-C-pentose-8-C-hexose | + | ++ |
| 20 | 11.60 | 272, 336 | 593.1464 | C27H30O15 | 473.1067, 413.0846, 369.0590, 357.0589, 293.0434 | Flavone | Luteolin-6-C-hexose- 8-C-deoxyhexose | ++ | ++ |
| 21 | 12.44 | 270, 338 | 431.0947 | C21H20O10 | 341.0639, 323.0529, 311.0536, 283.0589 | Flavone | Vitexin (Apigenin-6-C-hexose) | ++ | ++++ |
| 22 | 13.85 | 270, 338 | 431.0947 | C21H20O10 | 341.0638, 323.0536, 311.0536, 283.0588 | Flavone | Isovitexin (Apigenin-8-C-hexose) | ++ | ++++ |
| 23 | 17.19 | 270, 338 | 577.1306 | C27H30O14 | 457.1098, 413.0845, 353.0630, 341.0640, 311.0536, 293.0432 | Flavone | Apigenin-6-C-hexose-8-C-deoxyhexose | ++ | +++ |
| Sample | TFC (mg QCE/g Dried Extract) | Antioxidant Activity (IC50 µg/mL) | |
|---|---|---|---|
| DPPH | NO | ||
| OPL extract | 136.6896 a ± 0.3106 | 15.8767 a ± 2.8610 | 17.8367 a ± 3.3301 |
| Enriched total flavonoid fraction | 487.9729 b ± 0.9748 | 59.4767 b ± 4.4895 | 68.1300 b ± 7.1184 |
| Type | Surface Area (m2/g) | Particle Diameter (mm) | Average Pore Diameter (Å) | Matrix | Polarity |
|---|---|---|---|---|---|
| Amberlite® XAD4 | 750 | 0.250–0.841 | 100 | Styrene–divinylbenzene | Non-polar |
| Amberlite® XAD7HP | 380 | 0.250–0.841 | 300–400 | Acrylic | Moderate polar |
| Supelite™ DAX-8 | 140 | 0.250–0.420 | 225 | Acrylic ester | Moderate polar |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Che Zain, M.S.; Lee, S.Y.; Teo, C.Y.; Shaari, K. Adsorption and Desorption Properties of Total Flavonoids from Oil Palm (Elaeis guineensis Jacq.) Mature Leaf on Macroporous Adsorption Resins. Molecules 2020, 25, 778. https://doi.org/10.3390/molecules25040778
Che Zain MS, Lee SY, Teo CY, Shaari K. Adsorption and Desorption Properties of Total Flavonoids from Oil Palm (Elaeis guineensis Jacq.) Mature Leaf on Macroporous Adsorption Resins. Molecules. 2020; 25(4):778. https://doi.org/10.3390/molecules25040778
Chicago/Turabian StyleChe Zain, Mohamad Shazeli, Soo Yee Lee, Chian Ying Teo, and Khozirah Shaari. 2020. "Adsorption and Desorption Properties of Total Flavonoids from Oil Palm (Elaeis guineensis Jacq.) Mature Leaf on Macroporous Adsorption Resins" Molecules 25, no. 4: 778. https://doi.org/10.3390/molecules25040778
APA StyleChe Zain, M. S., Lee, S. Y., Teo, C. Y., & Shaari, K. (2020). Adsorption and Desorption Properties of Total Flavonoids from Oil Palm (Elaeis guineensis Jacq.) Mature Leaf on Macroporous Adsorption Resins. Molecules, 25(4), 778. https://doi.org/10.3390/molecules25040778







