The Dissipation of Cyazofamid and Its Main Metabolite CCIM during Wine-Making Process
Abstract
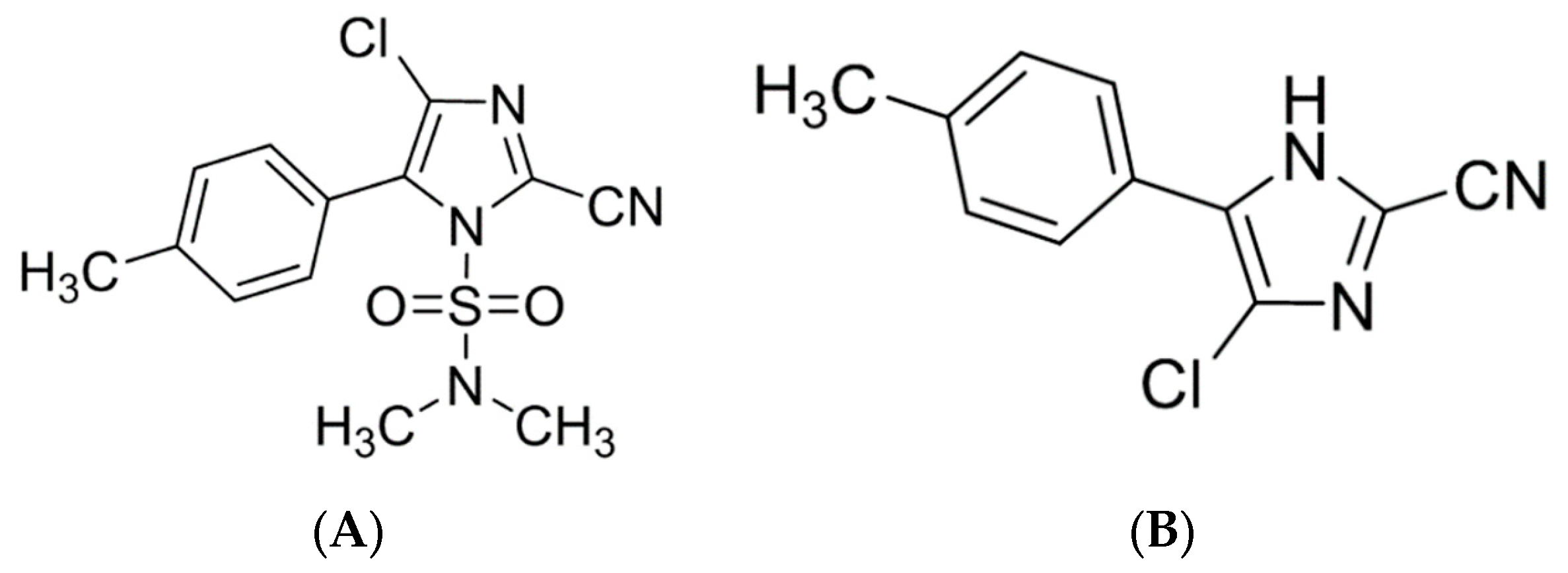
1. Introduction
2. Results and Discussion
2.1. Method Validation
2.2. Effects of Processing
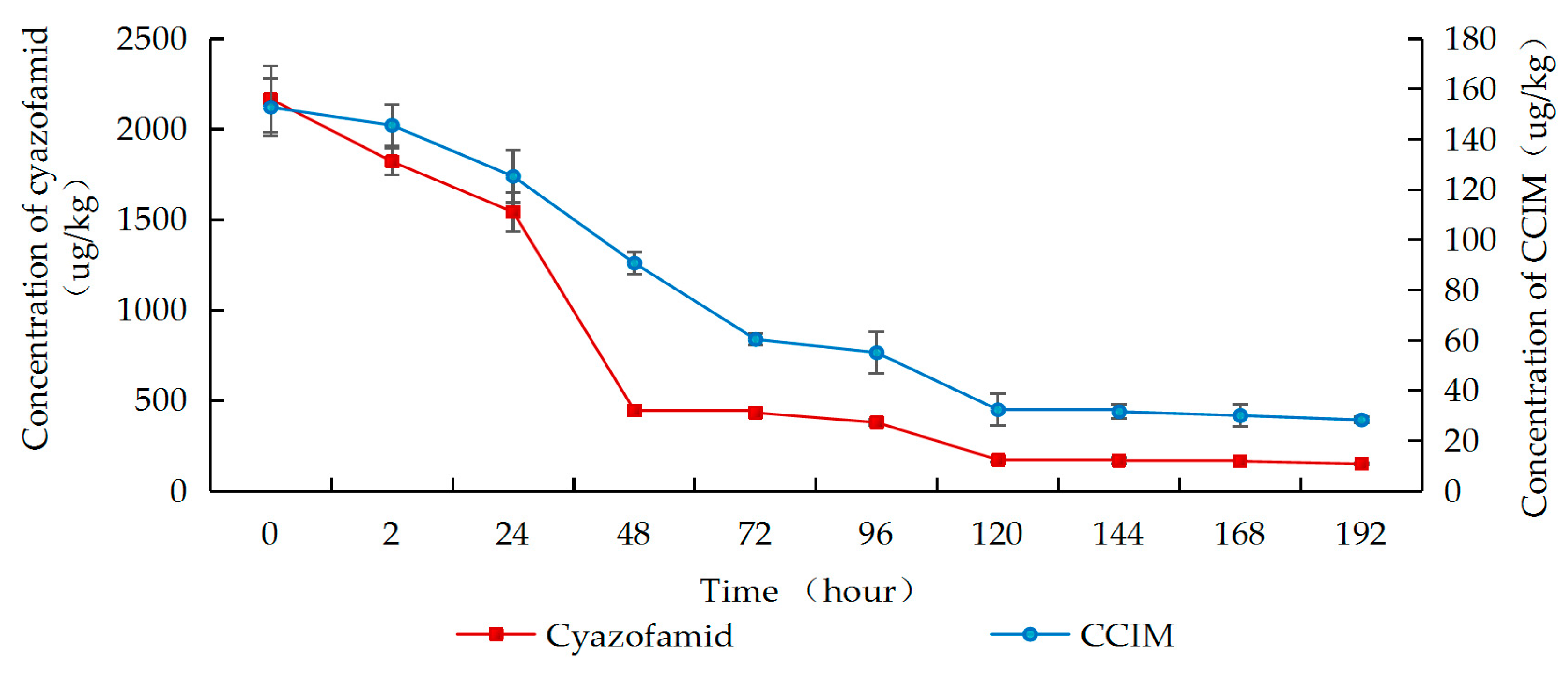
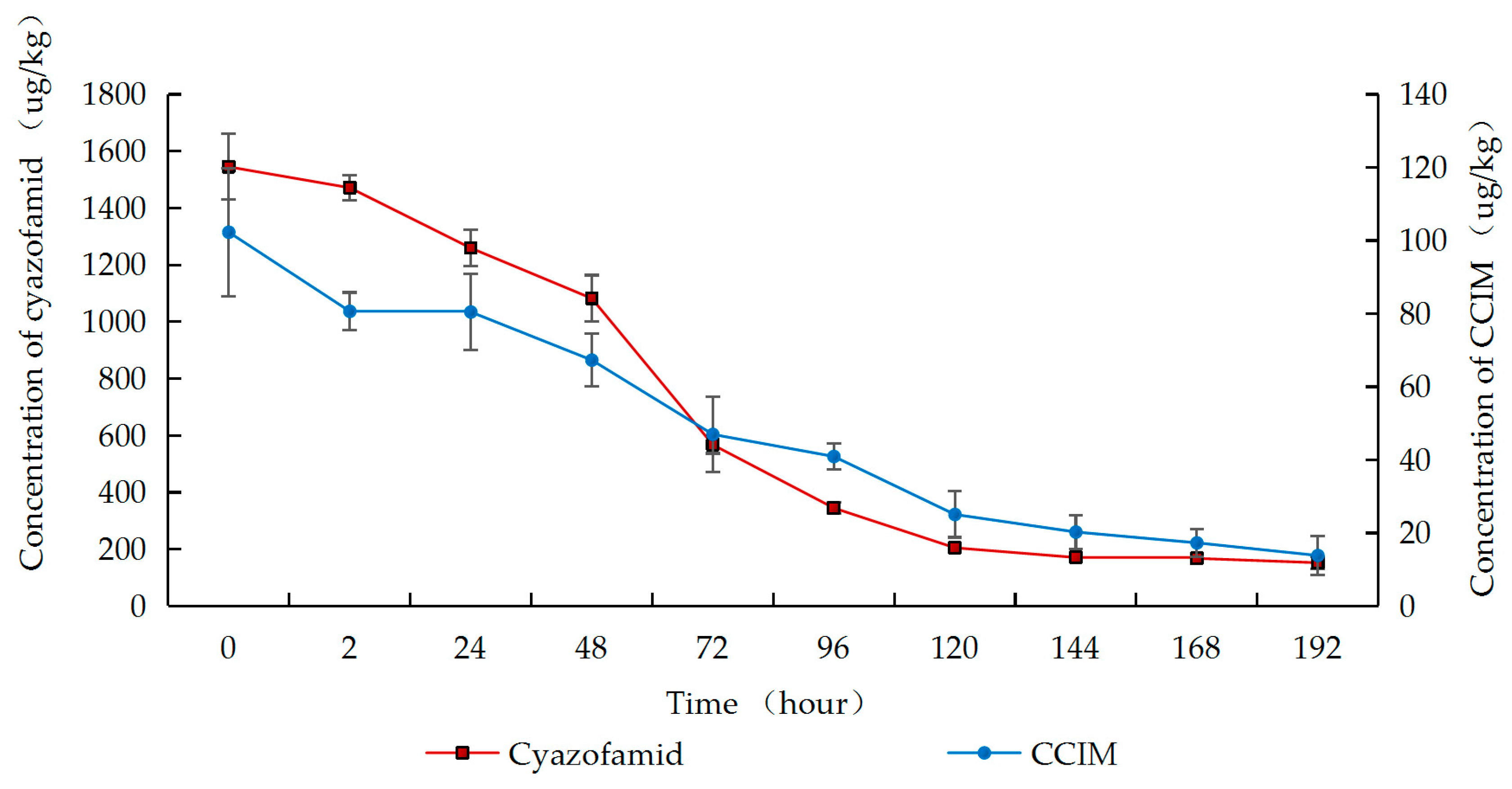
2.3. Degradation of Cyazofamid and Its Metabolite CCIM During Wine-Making Process
2.4. Processing Factors
3. Materials and Methods
3.1. Materials
3.2. Field Experiments
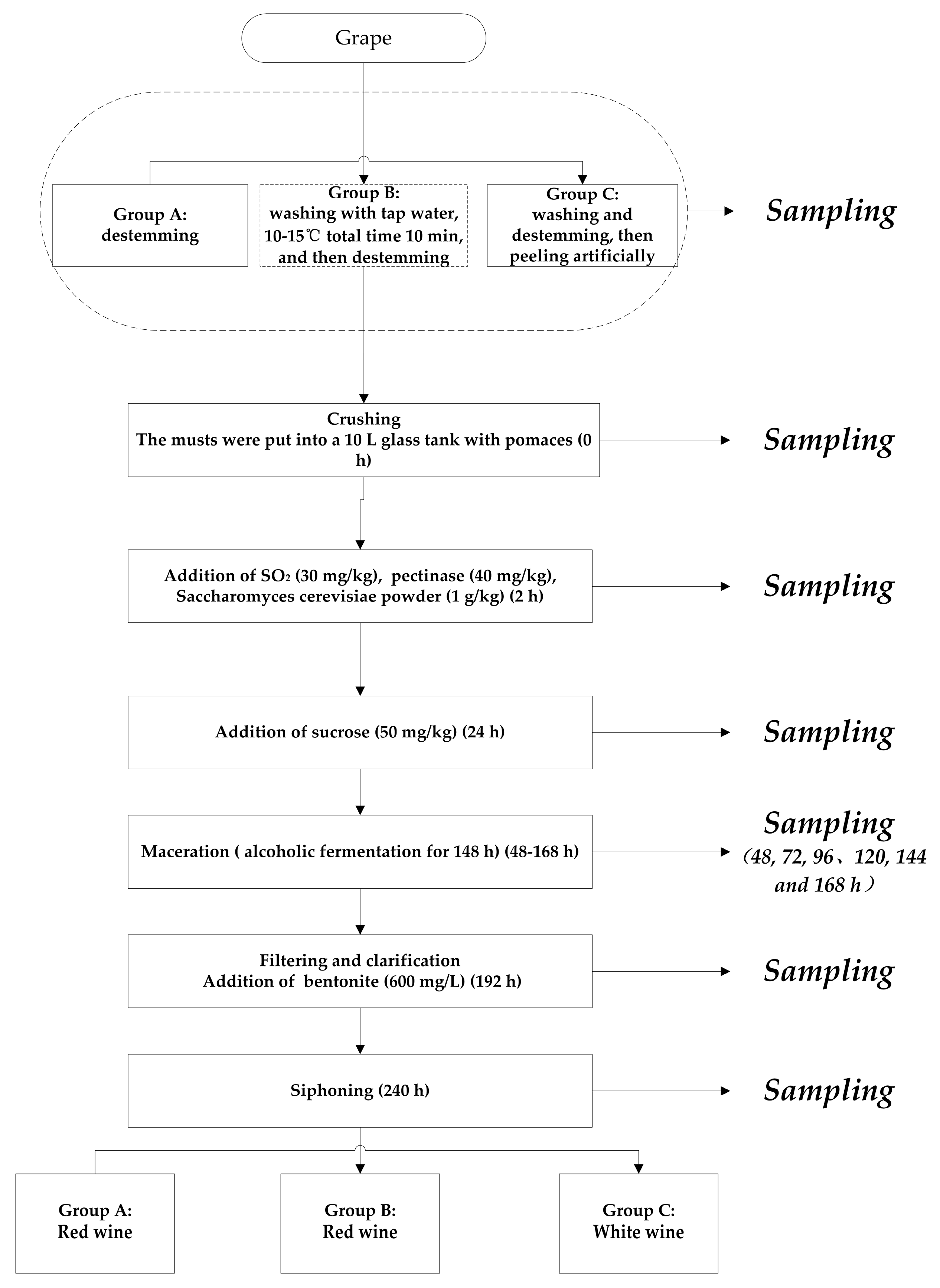
3.3. Winemaking and Sampling
3.4. Extraction and Clean-Up Procedure
3.5. Instrumentation and HPLC-MS/MS Analytical Conditions
3.6. Recovery Assay
3.7. Data Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- García-Lomillo, J.; González-SanJosé, M.L. Applications of wine pomace in the food industry: Approaches and functions. Compr. Rev. Food Sci. Food 2017, 16, 3–22. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; Silva-James, N.K.D.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste. Manag. 2017, 68, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Grimalt, S.; Dehouck, P. Review of analytical methods for the determination of pesticide residues in grapes. J. Chromatogr. A 2016, 1433, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.L.; Dong, F.S.; Liu, N.; Cheng, Y.P.; Xu, J.; Liu, X.G.; Wu, X.H.; Chen, Z.L.; Zheng, Y.Q. The fate and enantioselective behavior of zoxamide during wine-making process. Food Chem. 2018, 248, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Grazioli, B.; Fumi, M.D.; Silva, A. The role of processing on ochratoxin a content in italian must and wine: A study on naturally contaminated grapes. Int. J. Food Microbiol. 2006, 111, S93–S96. [Google Scholar] [CrossRef]
- Leong, S.L.L.; Hocking, A.D.; Varelis, P.; Giannikopoulos, G.; Scott, E.S. Fate of ochratoxin a during vinification of semillon and shiraz grapes. J. Agric. Food Chem. 2006, 54, 6460–6464. [Google Scholar] [CrossRef]
- Renauld, S.; Lorgeril, M.D. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Covas, M.I.; Gambert, P.; Fitó, M.; dela Torre, R. Wine and oxidative stress: Up-to-date evidence of the effects of moderate wine consumption on oxidative damage in humans. Atherosclerosis 2010, 208, 297–304. [Google Scholar] [CrossRef]
- Holahan, C.J.; Schutte, K.K.; Brennan, P.L.; North, R.J.; Holahan, C.K.; Moos, B.L.; Moos, R.H. Wine consumption and 20-year mortality among late-life moderate drinkers. J. Stud. Alcohol. Drugs 2012, 73, 80–88. [Google Scholar] [CrossRef]
- Gonzálezrodríguez, R.; Canchogrande, B.; Simalgándara, J. Multiresidue determination of 11 new fungicides in grapes and wines by liquid–liquid extraction/clean-up and programmable temperature vaporization injection with analyte protectants/gas chromatography/ion trap mass spectrometry. J. Chromatogr. A 2009, 1216, 6033–6042. [Google Scholar] [CrossRef]
- Pang, N.; Dou, X.; Hu, J. Residue behaviours, dissipation kinetics and dietary risk assessment of pyaclostrobin, cyazofamid and its metabolite in grape. J. Sci. Food Agric. 2019, 99, 6167–6172. [Google Scholar] [CrossRef] [PubMed]
- Mitani, S.; Araki, S.; Yamaguchi, T.; Takii, Y.; Ohshima, T.; Matsuo, N.; Miyoshi, H. The biochemical mode of action of the novel selective fungicide cyazofamid: Specific inhibition of mitochondrial complex III in Phythium spinosum. Pestic. Biochem. Physiol. 2001, 71, 107–115. [Google Scholar] [CrossRef]
- Lozowicka, B. Health risk for children and adults consuming apples with pesticide residue. Sci. Total. Environ. 2015, 502, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, X.L.; Yang, W.C.; Yang, G.F. Comparative kinetics of Qi site inhibitors of cytochrome bc1 complex: Picomolar antimycin and micromolar cyazofamid. Chem. Biol. Drug. Des. 2014, 83, 71–80. [Google Scholar] [CrossRef]
- Singh, N.; Tandon, S. Dissipation kinetics and leaching of cyazofamid fungicide in texturally different agricultural soils. Int. J. Environ. Sci. Technol. 2015, 12, 2475–2484. [Google Scholar] [CrossRef]
- [US EPA] United States Environmental Protection Agency. Pesticide fact sheet: Cyazofamid. 2004. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/registration/fs_PC-085651_01-Sep-04.pdf (accessed on 12 July 2019).
- Regueiro, J.; Olguín, N.; Simal-Gandara, J.; Sunol, C. Toxicity evaluation of new agricultural fungicides in primary cultured cortical neurons. Environ. Res. 2015, 140, 37–44. [Google Scholar] [CrossRef]
- Pesticides Experts Committee. Evaluation Report Cyazofamid. 2004. Available online: http://www.fsc.jp/english/evaluationreports/pesticide/cyazofamid_fullreport (accessed on 13 May 2018).
- [EFSA] European Food Safety Authority. Peer review of the pesticide risk assessment of the active substance cyazofamid. EFSA J. 2016, 14, 4503–4527. [Google Scholar]
- Jin, B.H.; Xie, L.Q.; Guo, Y.F.; Pang, G.F. Multi-residue detection of pesticides in juice and fruit wine: A review of extraction and detection methods. Food Res. Int. 2012, 46, 399–409. [Google Scholar] [CrossRef]
- Čuš, F.; Česnik, H.B.; Bolta, Š.V.; Gregorčič, A. Pesticide residues and microbiological quality of bottled wines. Food Control. 2010, 21, 150–154. [Google Scholar] [CrossRef]
- Barrett, C.B. Measuring food insecurity. Science 2010, 327, 825–828. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Wang, H.; Yong, W.; Zhang, F.; Sun, L.; Yang, M.L.; Wu, Y.N.; Chu, X.G. The effects of washing and cooking on chlorpyrifos and its toxic metabolites in vegetables. Food Control. 2011, 22, 54–58. [Google Scholar] [CrossRef]
- Keikotlhaile, B.M.; Spanoghe, P.; Steurbaut, W. Effects of food processing on pesticide residues in fruits and vegetables: A meta-analysis approach. Food Chem. Toxicol. 2010, 48, 1–6. [Google Scholar] [CrossRef] [PubMed]
- González-Rodríguez, R.M.; Rial-Otero, R.; Cancho-Grande, B.; Gonzalez-Barreiro, C.; Simal-Gándara, J. A review on the fate of pesticides during the processes within the food-production chain. Crit. Rev. Food Sci. Nutr. 2011, 51, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Timme, G.; Walz-Tylla, B. Effects of food preparation and processing on pesticide residues in commodities of plant origin. In Pesticide Residues in Food and Drinking Water: Human Exposure and Risks; Leverkusen (Germany); Hamilton, D., Crossley, S., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2004; pp. 121–148. [Google Scholar]
- Amvrazi, E.G.; Albanis, T.A. Multiclass pesticide determination in olives and their processing factors in olive oil: Comparison of different olive oil extraction systems. J. Agric. Food Chem. 2008, 56, 5700–5709. [Google Scholar] [CrossRef] [PubMed]
- Bonnechère, A.; Hanot, V.; Bragard, C.; Bedoret, T.; Loco, J.V. Effect of household and industrial processing on the levels of pesticide residues and degradation products in melons. Food Addit. Contam. 2012, 29, 1058–1066. [Google Scholar] [CrossRef]
- Yang, Q.X.; Liu, N.; Zhang, S.; Wang, W.J.; Zou, Y.Z.; Gu, Z.M. The dissipation of cyazofamid and its main metabolite CCIM during tomato growth and tomato paste making process. Food Addit. Contam. A 2019, 36, 1327–1336. [Google Scholar] [CrossRef]
- Juan, J.J.; José, L.B.; Jesús, M.; Arias, E.; José, B. Determination of impurities in pesticides and their degradation products formed during the wine-making process by solid-phase extraction and gas chromatography with detection by electron impact mass spectrometry. i. vinclozoline, procymidone and fenitrothion. Rapid Commun Mass Spectrom. 2004, 18, 657–663. [Google Scholar]
- Garau, V.L.; Susana, D.M.A.; Pierluigi, C.; Alberto, A.; Arminda, A.; Paolo, C. Residue-free wines: Fate of some quinone outside inhibitor (QoI) fungicides in the winemaking process. J. Agric. Food Chem. 2009, 57, 2329–2333. [Google Scholar] [CrossRef]
- Doulia, D.S.; Anagnos, E.K.; Liapis, K.S.; Klimentzos, D.A. Effect of clarification process on the removal of pesticide residues in red wine and comparison with white wine. J. Environ. Sci. Heal. B 2018, 53, 534–545. [Google Scholar] [CrossRef]
- Tang, F.; Xu, Z.; Gao, M.; Li, L.; Li, H.; Cheng, H.; Zhang, C.; Tian, G. The dissipation of cyazofamid and its main metabolite in soil response oppositely to biochar application. Chemosphere 2018, 218, 26–35. [Google Scholar] [CrossRef]
- Christensen, H.B.; Granby, K.; Rabolle, M. Processing factors and variability of pyrimethanil, fenhexamid and tolylfluanid in strawberries. Food Addit. Contam. 2003, 20, 728–741. [Google Scholar] [CrossRef]
- Liu, N.; Pan, X.L.; Zhang, S.; Ji, M.S.; Zhang, Z.H. Enantioselective behaviour of tetraconazole during strawberry wine-making process. Chirality 2018, 30, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.Q.; Dong, F.S.; Xu, J.; Liu, X.G.; Zhang, C.P.; Li, J. Determination of difenoconazole residue in tomato during home canning by UPLC-MS/MS. Food Control. 2012, 23, 542–546. [Google Scholar] [CrossRef]
- Han, Y.T.; Dong, F.S.; Xu, J.; Liu, X.G.; Li, Y.; Kong, Z.Q. Residue change of pyridaben in apple samples during apple cider processing. Food Control. 2014, 37, 240–244. [Google Scholar] [CrossRef]
- Han, Y.T.; Xu, J.; Dong, F.S.; Li, W.M.; Liu, X.G.; Li, Y. The fate of spirotetramat and its metabolite spirotetramat-enol in apple samples during apple cider processing. Food Control. 2013, 34, 283–290. [Google Scholar] [CrossRef]
- [PPDB] Pesticide Properties DataBase. 2018. Available online: https://sitem.herts.ac.uk/aeru/ppdb/en/atoz.htm (accessed on 13 May 2018).
- Cengiz, M.F.; Certel, M.; Karakas, B.; Gocmen, H. Residue contents of captain and procymidone applied on tomatoes grown in greenhouses and their reduction by duration of a pre-harvest interval and post-harvest culinary applications. Food Chem. 2007, 100, 1611–1619. [Google Scholar] [CrossRef]
- Liu, N.; Dong, F.S.; Liu, X.G.; Xu, J.; Li, Y.B.; Han, Y.T.; Zhu, Y.L. Effect of household canning on the distribution and reduction of thiophanate-methyl and its metabolite carbendazim residues in tomato. Food Control. 2014, 43, 115–120. [Google Scholar] [CrossRef]
- Riederer, M.; Schreiber, L. Waxes: The Transport Barriers of Plant Cuticles; The Oily Press: Dundee, Scotland, 1995; Volume 6, pp. 131–156. [Google Scholar]
- Rawn, D.F.K.; Quade, S.C.; Sun, W.F.; Fouguet, A.; Bélanger, A.; Smith, M. Captan residue reduction in apples as a result of rinsing and peeling. Food Chem. 2008, 109, 790–796. [Google Scholar] [CrossRef]
- Cabras, P.; Angioni, A. Pesticide residues in grapes, wine, and their processing products. J. Agric. Food Chem. 2000, 48, 967–973. [Google Scholar] [CrossRef]
- Horvat, R.; Sanja, P.; Plavša, T.; Lukić, I. Bentonite fining during fermentation reduces the dosage required and exhibits significant side-effects on phenols, free and bound aromas, and sensory quality of white wine. Food Chem. 2019, 285, 305–315. [Google Scholar] [CrossRef]
- Lee, H.; Kim, E.; Shin, Y.; Lee, J.; Hur, H.; Kim, J. Identification and formation pattern of metabolites of cyazofamid by soil fungus Cunninghamella elegans. Appl. Biol. Chem. 2016, 59, 9–14. [Google Scholar] [CrossRef]
- [US EPA] Estimated Drinking Water Concentrations of Cyazofamid and Its Degradates of Concern CCIM, CCIM-AM and CTCA, for Use in Human Health Risk Assessment: Petitions for the Use on Herb, Greenhouse Tomato, Greenhouse Pepper, and Bulb Vegetables. 2015; (PC Code 085651; DP Barcode D426776). Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/registration/fs_PC-085651_01-Sep-04.pdf (accessed on 13 May 2019).
- Angioni, A.; Dedola, F.; Garau, V.L.; Schirra, M.; Caboni, P. Fate of iprovalicarb, indoxacarb, and boscalid residues in grapes and wine by GC-ITMS analysis. J. Agric. Food Chem. 2011, 59, 6806–6812. [Google Scholar] [CrossRef] [PubMed]
- [OECD] Organization for Economic Co-Operation and Development. Test No. 508: Mag Pestic Residue in Process Commod, OECD Guidelines for the Testing of Chemicals, Section 5. 2008. Available online: https://www.oecd-ilibrary.org/environment/test-no-508-magnitude-of-the-pesticide-residues-in-processed-commodities_9789264067622-en (accessed on 3 January 2020).
- Romero-Cascales, I.; Jose, I.; Fernández-Fernández, J.M.; Encarna, G. The maceration process during winemaking extraction of anthocyanins from grape skins into wine. Eur. Food Res. Technol. 2005, 221, 163–167. [Google Scholar] [CrossRef]
- Noguerol-Pato, R.; Fernández-Cruz, T.; Sieiro-Sampedro, T.; González-Barreiro, C.; Cancho-Grande, B.; Cilla-García, D.-A.; García-Pastor, M.A.; Martínez-Soria, M.A.-T.; Sanz-Asensio, J.; Simal-Gándara, J. Dissipation of Fungicide Residues during Winemaking and Their Effects on Fermentation and the Volatile Composition of Wines. J. Agric. Food Chem. 2016, 64, 1344–1354. [Google Scholar] [CrossRef] [PubMed]
- Sieiro-Sampedro, T.; Figueiredo-González, M.; González-Barreiro, C.; Simal-Gandara, J.; Cancho-Grande, B.; Rial-Otero, R. Impact of mepanipyrim and tetraconazole in Mencía wines on the biosynthesis of volatile compounds during the winemaking process. Food Chem. 2019, 300, 125–223. [Google Scholar] [CrossRef] [PubMed]
- Sieiro-Sampedro, T.; Pose-Juan, E.; Briz-Cid, N.; Figueiredo-González, M.; Torrado-Agrasar, A.; González-Barreiro, C.; Simal-Gandara, J.; Cancho-Grande, B.; Rial-Otero, R. Mepanipyrim residues on pasteurized red must influence the volatile derived compounds from Saccharomyces cerevisiae metabolism. Food Res. Int. 2019, 126, 108–566. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |

| Compounds | Matrix | Spiked Level/ (ug/kg) | Intra-Day (n = 5) | Inter-Day (n = 15) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | |||||||
| Mean Recoveries/% | RSDr/% | Mean Recoveries/% | RSDr/% | Mean Recoveries/% | RSDr/% | RSDR/% | |||
| Cyazofamid | Raw grape | 5 | 112 l | 5.5 | 108 ijk | 3.1 | 107 j | 0.9 | 5.2 |
| 100 | 108 kl | 3.7 | 103 hj | 2.4 | 105 ij | 1.4 | 3.5 | ||
| 1000 | 98 gh | 4.0 | 100 gh | 5.0 | 101 ghij | 0.7 | 4.2 | ||
| 5000 | 95 efg | 1.2 | 100 gh | 1.1 | 100 ghij | 1.6 | 1.3 | ||
| Wine | 5 | 109 kl | 0.4 | 111 jk | 3.2 | 103 hij | 2.2 | 2.9 | |
| 100 | 109 kl | 2.3 | 103 hi | 0.5 | 104 ij | 0.5 | 3.7 | ||
| 1000 | 100 hi | 4.1 | 100 gh | 6.6 | 94 defg | 4.6 | 4.8 | ||
| 5000 | 99 ghi | 1.0 | 96 efg | 2.4 | 92 cdef | 5.2 | 5.6 | ||
| Pomace | 5 | 105 jk | 5.2 | 113 k | 4.9 | 98 fghi | 6.3 | 3.9 | |
| 100 | 103 ij | 1.1 | 100 gh | 2.4 | 91 bcdef | 2.1 | 1.6 | ||
| 1000 | 86 bc | 2.3 | 106 ij | 3.6 | 84 ab | 5.5 | 4.3 | ||
| 5000 | 90 cd | 3.5 | 92 cde | 4.4 | 83 a | 7.8 | 6.0 | ||
| CCIM | Raw grape | 5 | 96 fgh | 1.2 | 95 efg | 0.7 | 89 abcde | 6.4 | 3.1 |
| 100 | 93 def | 0.3 | 91 bcde | 2.1 | 91 bcdef | 5.9 | 1.9 | ||
| 1000 | 89 bcd | 7.7 | 96 efg | 4.1 | 85 abc | 4.9 | 6.7 | ||
| 5000 | 85 b | 2.3 | 88 abcd | 3.7 | 87 abcd | 6.4 | 3.6 | ||
| Wine | 5 | 93 def | 6.2 | 98 fgh | 5.8 | 96 efgh | 3.8 | 2.9 | |
| 100 | 89 bcd | 5.4 | 87 abc | 3.3 | 94 defg | 0.9 | 8.8 | ||
| 1000 | 91 de | 1.9 | 92 cde | 4.1 | 90 abcde | 1.8 | 6.5 | ||
| 5000 | 93 def | 3.3 | 93 def | 2.2 | 88 abcd | 1.1 | 7.2 | ||
| Pomace | 5 | 91 de | 1.7 | 94 ef | 0.9 | 90 abcde | 2.6 | 1.3 | |
| 100 | 86 bc | 2.6 | 98 fgh | 1.2 | 89 abcde | 1.8 | 2.2 | ||
| 1000 | 89 bcd | 2.1 | 86 ab | 0.3 | 85 abc | 2.9 | 0.9 | ||
| 5000 | 80 a | 2.9 | 85 a | 3.5 | 83 a | 3.1 | 3.1 | ||
| Treatments | Compounds | Concentrations (ug/kg) | ||||||
|---|---|---|---|---|---|---|---|---|
| Raw Grape | Washed Grape | Peeled Grape | Grape Skin | Fermentation Wine | Byproduct (Pomace) | Clarification Wine | ||
| Group A | Cyazofamid | 3255.1 b ± 223.6 | - | - | - | 149.7 a ± 3.5 | 3281.6 b ± 60.2 | 95.7 a ± 4.4 |
| CCIM | 236.4 b ± 18.6 | - | - | - | 28.3 a ± 1.1 | 286.1 c ± 2.8 | 18.7 a ± 3.2 | |
| Group B | Cyazofamid | 3289.3 c ± 236.9 | 2073.1 b ± 120.3 | - | - | 152.8 a ± 21.2 | 3267.2 c ± 151.2 | 93.8 a ± 15.3 |
| CCIM | 226.4 d ± 11.0 | 156.2 b ± 12.3 | - | - | 13.9 a ± 5.3 | 196.9 c ± 14.2 | 10.5 a ± 1.3 | |
| Group C | Cyazofamid | 3461.5 b ± 249.1 | - | 173.1 a ± 15.6 | 42396 c ± 500.6 | 18.2 a ± 0.7 | 203.6 a ± 11.4 | 10.9 a ± 1.2 |
| CCIM | 245.6 a ± 11.2 | - | 54.0 a ± 5.9 | 3026.8 b ± 125.6 | <LOQ 1 | 12.7 a ± 0.7 | <LOQ 1 | |
| Treatments | Compounds | PFs of Processing Types | ||||
|---|---|---|---|---|---|---|
| Washing | Peeling | Fermentation | Clarification | Overall Process | ||
| Group A | Cyazofamid | - | - | 0.046 | 0.639 | 0.025 |
| CCIM | - | - | 0.120 | 0.661 | 0.067 | |
| Group B | Cyazofamid | 0.630 | - | 0.074 | 0.614 | 0.024 |
| CCIM | 0.690 | - | 0.089 | 0.755 | 0.039 | |
| Group C | Cyazofamid | - | 0.025 | 0.105 | 0.599 | 0.003 |
| CCIM | - | 0.187 | - | - | - | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Wei, S.; Liu, N.; Gu, Z. The Dissipation of Cyazofamid and Its Main Metabolite CCIM during Wine-Making Process. Molecules 2020, 25, 777. https://doi.org/10.3390/molecules25040777
Yang Q, Wei S, Liu N, Gu Z. The Dissipation of Cyazofamid and Its Main Metabolite CCIM during Wine-Making Process. Molecules. 2020; 25(4):777. https://doi.org/10.3390/molecules25040777
Chicago/Turabian StyleYang, Qingxi, Shiwei Wei, Na Liu, and Zumin Gu. 2020. "The Dissipation of Cyazofamid and Its Main Metabolite CCIM during Wine-Making Process" Molecules 25, no. 4: 777. https://doi.org/10.3390/molecules25040777
APA StyleYang, Q., Wei, S., Liu, N., & Gu, Z. (2020). The Dissipation of Cyazofamid and Its Main Metabolite CCIM during Wine-Making Process. Molecules, 25(4), 777. https://doi.org/10.3390/molecules25040777




