EPR Spectroscopy: A Powerful Tool to Analyze Supramolecular Host•Guest Complexes of Stable Radicals with Cucurbiturils
Abstract
:1. Introduction
2. Results
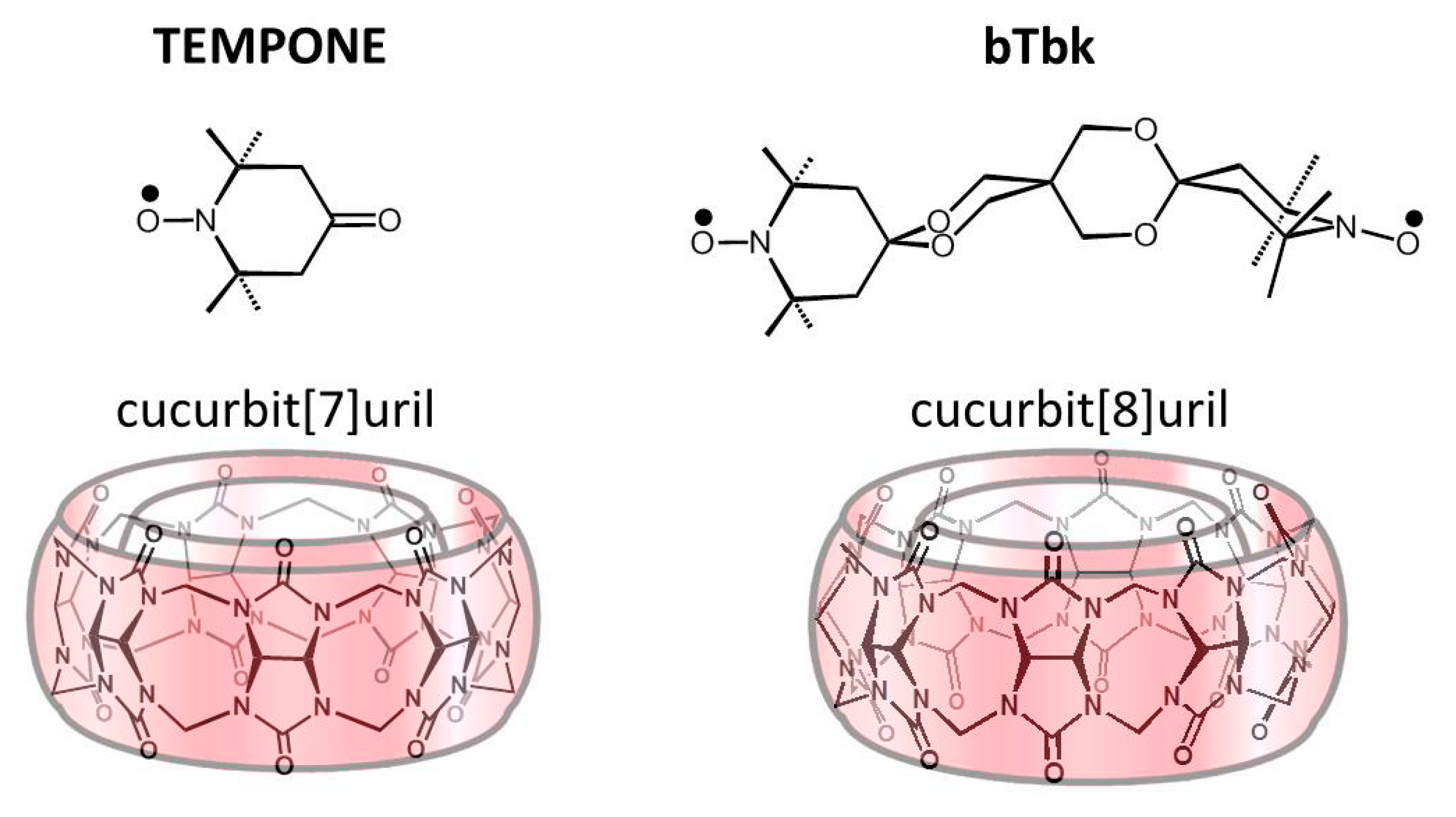
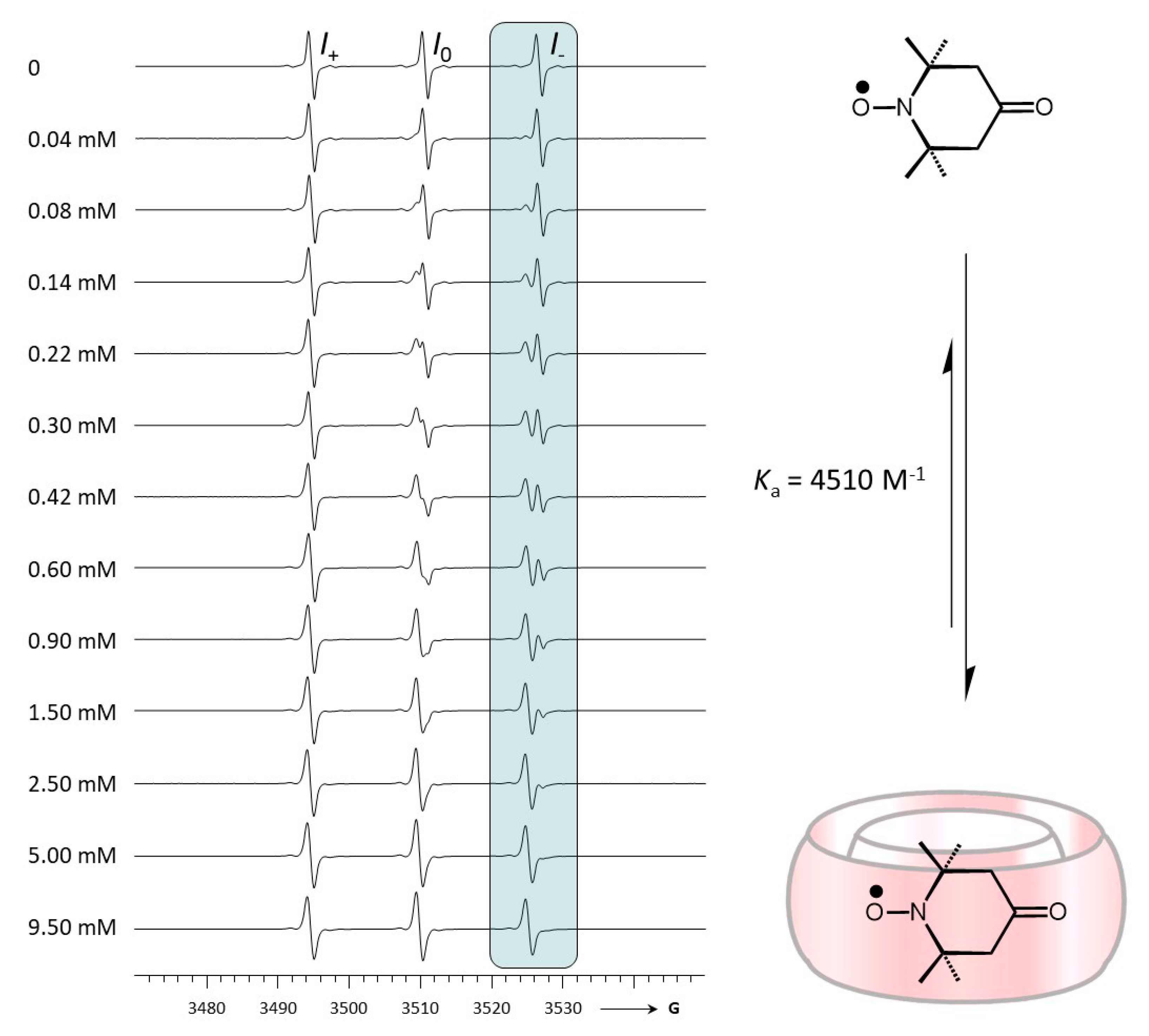
2.1. Complexation between TEMPONE and CB[7]
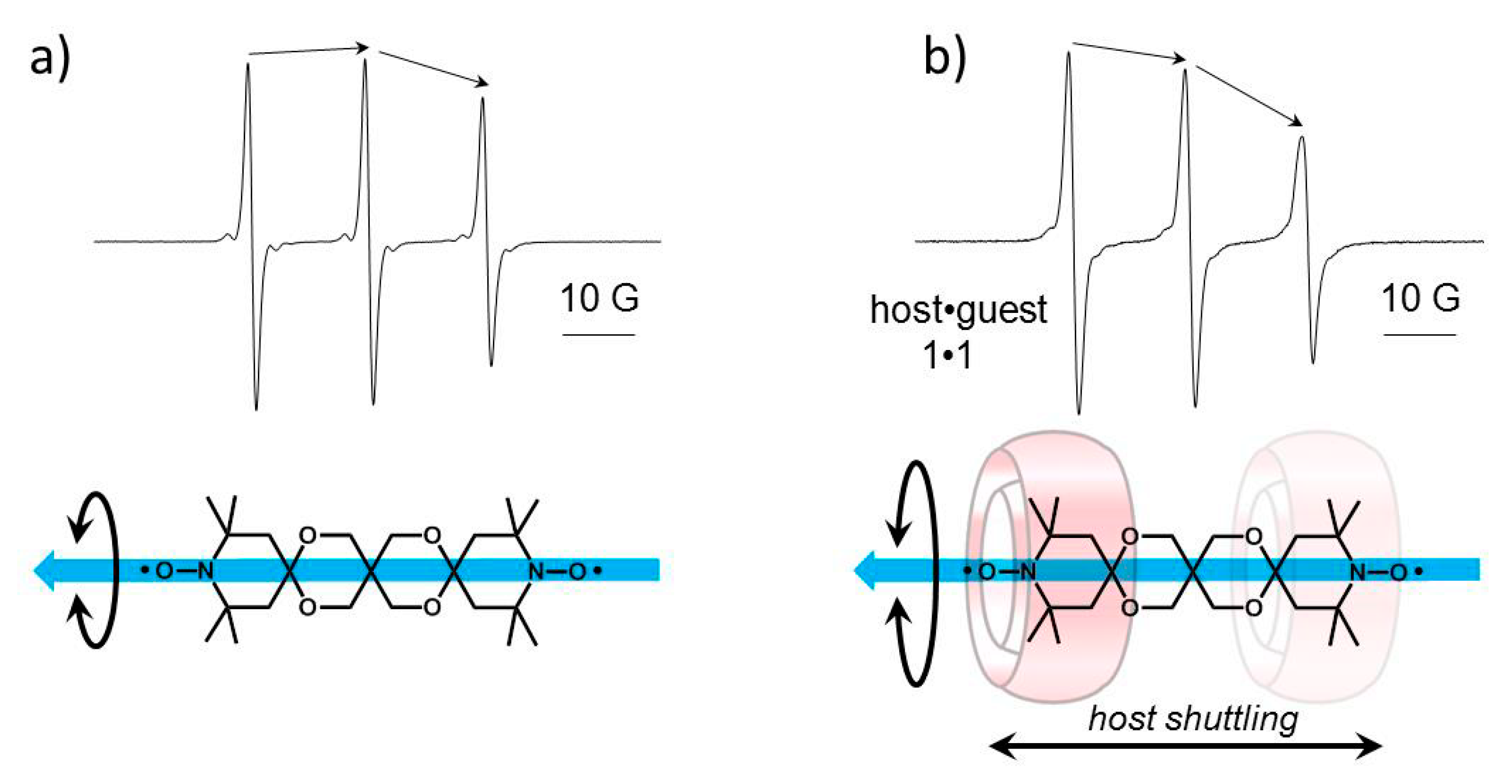
2.2. Complexation between bTbk and CB[8]
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gomberg, M. An instance of trivalent carbon: Triphenylmethyl. J. Am. Chem. Soc. 1900, 22, 757–771. [Google Scholar] [CrossRef] [Green Version]
- Tidwell, T.T. Triarylmethyl and related radicals. In Stable Radicals: Fundamentals and Applied Aspects of Odd-Electron Compounds, 1st ed.; Hicks, R.G., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2010; pp. 1–31. [Google Scholar]
- Keana, J.F. Newer aspects of the synthesis and chemistry of nitroxide spin labels. Chem. Rev. 1978, 78, 37–64. [Google Scholar] [CrossRef]
- Nicolas, J.; Guillaneuf, Y.; Lefay, C.; Bertin, D.; Gigmes, D.; Charleux, B. Nitroxide-mediated polymerization. Prog. Polym. Sci. 2013, 38, 63–235. [Google Scholar] [CrossRef]
- Nakahara, K.; Oyaizu, K.; Nishide, H. Organic radical battery approaching practical use. Chem. Lett. 2011, 40, 222–227. [Google Scholar] [CrossRef]
- Wisser, D.; Karthikeyan, G.; Lund, A.; Casano, G.; Karoui, H.; Yulikov, M.; Menzildjian, G.; Pinon, A.C.; Purea, A.; Engelke, F.; et al. BDPA-Nitroxide Biradicals Tailored for Efficient Dynamic Nuclear Polarization Enhanced Solid-State NMR at Magnetic Fields up to 21.1 T. J. Am. Chem. Soc. 2018, 140, 13340–13349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casano, G.; Karoui, H.; Ouari, O. Polarizing agents: Evolution and outlook in free radical development for DNP. eMagRes 2018, 7, 195–208. [Google Scholar]
- Altenbach, C.; López, C.J.; Hideg, K.; Hubbell, W.L. Exploring Structure, Dynamics, and Topology of Nitroxide Spin-Labeled Proteins Using Continuous-Wave Electron Paramagnetic Resonance Spectroscopy. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2015; Volume 564, Part B, pp. 59–100. [Google Scholar] [CrossRef]
- Buck, A.T.; Paletta, J.T.; Khindurangala, S.A.; Beck, C.L.; Winter, A.H. A Noncovalently Reversible Paramagnetic Switch in Water. J. Am. Chem. Soc. 2013, 135, 10594–10597. [Google Scholar] [CrossRef] [Green Version]
- Schulte, B.; Tsotsalas, M.; Becker, M.; Studer, A.; De Cola, L. Dynamic Microcrystal Assembly by Nitroxide Exchange Reactions. Angew. Chem. Int. Ed. 2010, 49, 6881–6884. [Google Scholar] [CrossRef]
- Nakabayashi, K.; Ozaki, Y.; Kawano, M.; Fujita, M. A Self-Assembled Spin Cage. Angew. Chem. Int. Ed. 2008, 47, 2046–2048. [Google Scholar] [CrossRef]
- Lucarini, M. Supramolecular Radical Chemistry. In Encyclopedia of Radicals in Chemistry, Biology and Materials; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 1–20. [Google Scholar] [CrossRef]
- Lucarini, M.; Mezzina, E. EPR investigations of organic non-covalent assemblies with spin labels and spin probes. In Electron Paramagnetic Resonance; RSC publishing: London, UK, 2011; Volume 22, pp. 41–70. [Google Scholar] [CrossRef]
- Bardelang, D.; Hardy, M.; Ouari, O.; Tordo, P. Spin Labels and Spin Probes. In Encyclopedia of Radicals in Chemistry, Biology and Materials; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 1–51. [Google Scholar] [CrossRef]
- Lagona, J.; Mukhopadhyay, P.; Chakrabarti, S.; Isaacs, L. The Cucurbit[n]uril Family. Angew. Chem. Int. Ed. 2005, 44, 4844–4870. [Google Scholar] [CrossRef]
- Lee, J.W.; Samal, S.; Selvapalam, N.; Kim, H.-J.; Kim, K. Cucurbituril Homologues and Derivatives: New Opportunities in Supramolecular Chemistry. Acc. Chem. Res. 2003, 36, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Barrow, S.J.; Kasera, S.; Rowland, M.J.; del Barrio, J.; Scherman, O.A. Cucurbituril-Based Molecular Recognition. Chem. Rev. 2015, 115, 12320–12406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assaf, K.I.; Nau, W.M. Cucurbiturils: From synthesis to high-affinity binding and catalysis. Chem. Soc. Rev. 2015, 44, 394–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masson, E.; Ling, X.; Joseph, R.; Kyeremeh-Mensah, L.; Lu, X. Cucurbituril chemistry: A tale of supramolecular success. RSC Adv. 2012, 2, 1213–1247. [Google Scholar] [CrossRef]
- Cao, L.; Sekutor, M.; Zavalij, P.Y.; Mlinaric-Majerski, K.; Glaser, R.; Isaacs, L. Cucurbit[7]uril·Guest Pair with an Attomolar Dissociation Constant. Angew. Chem. Int. Ed. 2014, 53, 988–993. [Google Scholar] [CrossRef]
- Rekharsky, M.V.; Mori, T.; Yang, C.; Ko, Y.H.; Selvapalam, N.; Kim, H.; Sobransingh, D.; Kaifer, A.E.; Liu, S.; Isaacs, L.; et al. A synthetic host-guest system achieves avidin-biotin affinity by overcoming enthalpy−entropy compensation. Proc. Natl. Acad. Sci. USA 2007, 104, 20737–20742. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Kim, Y.; Yoon, M.; Lim, S.; Park, S.M.; Seo, G.; Kim, K. Highly Selective Carbon Dioxide Sorption in an Organic Molecular Porous Material. J. Am. Chem. Soc. 2010, 132, 12200–12202. [Google Scholar] [CrossRef]
- Yin, H.; Wang, R. Applications of Cucurbit[n]urils (n = 7 or 8) in Pharmaceutical Sciences and Complexation of Biomolecules. Isr. J. Chem. 2018, 58, 188–198. [Google Scholar] [CrossRef]
- Walker, S.; Oun, R.; McInnes, F.J.; Wheate, N.J. The Potential of Cucurbit[n]urils in Drug Delivery. Isr. J. Chem. 2011, 51, 616–624. [Google Scholar] [CrossRef]
- Ouari, O.; Bardelang, D. Nitroxide Radicals with Cucurbit[n]urils and Other Cavitands. Isr. J. Chem. 2018, 58, 343–356. [Google Scholar] [CrossRef]
- Mezzina, E.; Cruciani, F.; Pedulli, G.F.; Lucarini, M. Nitroxide Radicals as Probes for Exploring the Binding Properties of the Cucurbit[7]uril Host. Chem. Eur. J. 2007, 13, 7223–7233. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Captain, B.; Ottaviani, M.F.; Kaifer, A.E. Controlling the Extent of Spin Exchange Coupling in 2,2,6,6-Tetramethylpiperidine-1-oxyl (TEMPO) Biradicals via Molecular Recognition with Cucurbit[n]uril Hosts. Langmuir 2011, 27, 5624–5632. [Google Scholar] [CrossRef] [PubMed]
- Bardelang, D.; Banaszak, K.; Karoui, H.; Rockenbauer, A.; Waite, M.; Udachin, K.; Ripmeester, J.A.; Ratcliffe, C.I.; Ouari, O.; Tordo, P. Probing Cucurbituril Assemblies in Water with TEMPO like Nitroxides: A Trinitroxide Supraradical with Spin−Spin Interactions. J. Am. Chem. Soc. 2009, 131, 5402–5404. [Google Scholar] [CrossRef] [PubMed]
- Mileo, E.; Mezzina, E.; Grepioni, F.; Pedulli, G.F.; Lucarini, M. Preparation and Characterisation of a New Inclusion Compound of Cucurbit[8]uril with a Nitroxide Radical. Chem.-Eur. J. 2009, 15, 7859–7862. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, N.; Porel, M.; Ottaviani, M.F.; Maddipatla, M.V.S.N.; Modelli, A.; Da Silva, J.P.; Bhogala, B.R.; Captain, B.; Jockusch, S.; Turro, N.J.; et al. Self Aggregation of Supramolecules of Nitroxides@Cucurbit[8]uril Revealed by EPR Spectra. Langmuir 2009, 25, 13820–13832. [Google Scholar] [CrossRef]
- Combes, S.; Tran, K.T.; Ayhan, M.M.; Karoui, H.; Rockenbauer, A.; Tonetto, A.; Monnier, V.; Charles, L.; Rosas, R.; Viel, S.; et al. Triangular Regulation of Cucurbit[8]uril 1:1 Complexes. J. Am. Chem. Soc. 2019, 141, 5897–5907. [Google Scholar] [CrossRef]
- Casano, G.; Poulhès, F.; Tran, T.K.; Ayhan, M.M.; Karoui, H.; Siri, D.; Gaudel-Siri, A.; Rockenbauer, A.; Jeschke, G.; Bardelang, D.; et al. High binding yet accelerated guest rotation within a cucurbit[7]uril complex. Toward paramagnetic gyroscopes and rolling nanomachines. Nanoscale 2015, 7, 12143–12150. [Google Scholar] [CrossRef] [Green Version]
- Mao, J.; Akhmetzyanov, D.; Ouari, O.; Denysenkov, V.; Corzilius, B.; Plackmeyer, J.; Tordo, P.; Prisner, T.F.; Glaubitz, C. Host−Guest Complexes as Water-Soluble High-Performance DNP Polarizing Agents. J. Am. Chem. Soc. 2013, 135, 19275–19281. [Google Scholar] [CrossRef]
- Matsuki, Y.; Maly, T.; Ouari, O.; Karoui, H.; Le Moigne, F.; Rizzato, E.; Lyubenova, S.; Herzfeld, J.; Prisner, T.; Tordo, P.; et al. Dynamic Nuclear Polarization with a Rigid Biradical. Angew. Chem. Int. Ed. 2009, 48, 4996–5000. [Google Scholar] [CrossRef] [Green Version]
- Rockenbauer, A.; Szabo-Planka, T.; Arkosi, Z.; Korecz, L. A Two-Dimensional (Magnetic Field and Concentration) Electron Paramagnetic Resonance Method for Analysis of Multispecies Complex Equilibrium Systems. Information Content of EPR Spectra. J. Am. Chem. Soc. 2001, 123, 7646–7654. [Google Scholar] [CrossRef]
- Wheate, N.J.; Anil Kumar, P.G.; Torres, A.M.; Aldrich-Wright, J.R.; Price, W.S. Examination of Cucurbit[7]uril and Its Host-Guest Complexes by Diffusion Nuclear Magnetic Resonance. J. Phys. Chem. B 2008, 112, 2311–2314. [Google Scholar] [CrossRef]
- Grant, M.P.; Wheate, N.J.; Aldrich-Wright, J.R. Diffusion Coefficient of Cucurbit[n]urils (n = 6 or 7) at Various Concentrations, Temperatures, and pH. J. Chem. Eng. Data 2009, 54, 323–326. [Google Scholar] [CrossRef]
- Guagnini, F.; Antonik, P.M.; Rennie, M.L.; O’Byrne, P.; Khan, A.R.; Pinalli, R.; Dalcanale, E.; Crowley, P.B. Cucurbit[7]uril-Dimethyllysine Recognition in a Model Protein. Angew. Chem. Int. Ed. 2018, 57, 7126–7130. [Google Scholar] [CrossRef] [PubMed]
- Bardelang, D.; Casano, G.; Poulhès, F.; Karoui, H.; Filippini, J.; Rockenbauer, A.; Rosas, R.; Monnier, V.; Siri, D.; Gaudel-Siri, A.; et al. Spin exchange monitoring of the strong positive homotropic allosteric binding of a tetraradical by a synthetic receptor in water. J. Am. Chem. Soc. 2014, 136, 17570–17577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, I.; Jeon, W.S.; Kim, H.-J.; Kim, D.; Kim, H.; Selvapalam, N.; Fujita, N.; Shinkai, S.; Kim, K. Cucurbit[7]uril: A Simple Macrocyclic, pH-Triggered Hydrogelator Exhibiting Guest-Induced Stimuli-Responsive Behavior. Angew. Chem. Int. Ed. 2007, 46, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Freed, J.H.; Fraenkel, G.K. Theory of Linewidths in Electron Spin Resonance Spectra. J. Chem. Phys. 1963, 39, 326–348. [Google Scholar] [CrossRef]
- Freed, J.H. Generalized Cumulant Expansions and Spin-Relaxation Theory. J. Chem. Phys. 1968, 49, 376–391. [Google Scholar] [CrossRef]
- Bardelang, D.; Rockenbauer, A.; Karoui, H.; Finet, J.-P.; Biskupska, I.; Banaszak, K.; Tordo, P. Inclusion complexes of EMPO derivatives with 2,6-di-O-methyl-β-cyclodextrin: Synthesis, NMR and EPR investigations for enhanced superoxide detection. Org. Biomol. Chem. 2006, 4, 2874–2882. [Google Scholar] [CrossRef]
- Bardelang, D.; Rockenbauer, A.; Finet, J.-P.; Karoui, H.; Tordo, P. Inclusion complexes of PBN-type nitrone spin traps and their superoxide spin adducts with cyclodextrin derivatives: Parallel determination of the association constants by NMR titrations and 2D-EPR simulations. J. Phys. Chem. B 2005, 109, 10521–10530. [Google Scholar] [CrossRef]
- Karoui, H.; Rockenbauer, A.; Pietri, S.; Tordo, P. Spin trapping of superoxide in the presence of β-cyclodextrins. Chem. Commun. 2002, 24, 3030–3031. [Google Scholar] [CrossRef]
- Franchi, P.; Lucarini, M.; Pedulli, G.F.; Sciotto, D. An EPR Investigation of the Kinetics of Inclusion of a Persistent Radical in Water-Soluble Calix[4]arenes. Angew. Chem. Int. Ed. 2000, 39, 263–266. [Google Scholar] [CrossRef]
- Kirilyuk, I.; Polovyanenko, D.; Semenov, S.; Grigorev, I.; Gerasko, O.; Fedin, V.; Bagryanskaya, E. Inclusion Complexes of Nitroxides of Pyrrolidine and Imidazoline Series with Cucurbit[7]uril. J. Phys. Chem. B 2010, 114, 1719–1728. [Google Scholar] [CrossRef]
- Kolman, V.; Khan, M.S.A.; Babinsky, M.; Marek, R.; Sindelar, V. Supramolecular Shuttle Based on Inclusion Complex between Cucurbit[6]uril and Bispyridinium Ethylene. Org. Lett. 2011, 13, 6148–6151. [Google Scholar] [CrossRef]
- Sindelar, V.; Silvi, S.; Kaifer, A.E. Switching a molecular shuttle on and off: Simple, pH-controlled pseudorotaxanes based on cucurbit[7]uril. Chem. Commun. 2006, 2185–2187. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Chen, H.; Ma, X.; Tian, H. A Cucurbit[7]uril Based Molecular Shuttle Encoded by Visible Room-Temperature Phosphorescence. ChemPhysChem 2016, 17, 1934–1938. [Google Scholar] [CrossRef] [PubMed]
- Chernikova, E.Y.; Berdnikova, D.V.; Fedorov, Y.V.; Fedorova, O.A.; Maurel, F.; Jonusauskas, G. Light-induced piston nanoengines: Ultrafast shuttling of a styryl dye inside cucurbit[7]uril. Phys. Chem. Chem. Phys. 2017, 19, 25834–25839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mezzina, E.; Manoni, R.; Romano, F.; Lucarini, M. Spin-Labelling of Host-Guest Assemblies with Nitroxide Radicals. Asian J. Org. Chem. 2015, 4, 296–310. [Google Scholar] [CrossRef]
- Trabolsi, A.; Khashab, N.; Fahrenbach, A.C.; Friedman, D.C.; Colvin, M.T.; Coti, K.K.; Benitez, D.; Tkatchouk, E.; Olsen, J.-C.; Belowich, M.E.; et al. Radically enhanced molecular recognition. Nat. Chem. 2010, 2, 42–49. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are available from the authors. |
| TEMPONE | TEMPONE•CB[7] | |
|---|---|---|
| g | 2.0055 a | 2.0060 a |
| aN/G | 15.99 ± 0.05 b | 15.29 ± 0.05 b |
| aC/G | 6.0 ± 0.5 b | 4.7 ± 0.5 b |
| Wahh/G | 0.82 ± 0.05 a | 0.96 ± 0.06 a |
| I-/I0 | 0.92 a | 0.85 a |
| Ka/M−1 | - | 4510 ± 500 b |
| bTbk | bTbk•CB[8] | |
|---|---|---|
| g | 2.0055 a | 2.0056 a |
| aN/G | 16.59 ± 0.05 b | 16.25 ± 0.05 and 16.59 ± 0.05 b |
| aC/G | 5.8 ±0.5 b | 6.0 ± 0.5 and 5.8 ± 0.5 b |
| Wahh/G | 1.18 ± 0.05 a | 1.44 ± 0.10 a |
| I-/I0 | 0.78 a | 0.67 a |
| Ka 1:1 model/M−1 | - | 36 600 ±5000 M−1, b |
| Ka shuttling model/M−1 | - | 20 900 ± 5000 M−1, b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Karoui, H.; Rockenbauer, A.; Liu, S.; Ouari, O.; Bardelang, D. EPR Spectroscopy: A Powerful Tool to Analyze Supramolecular Host•Guest Complexes of Stable Radicals with Cucurbiturils. Molecules 2020, 25, 776. https://doi.org/10.3390/molecules25040776
Liu F, Karoui H, Rockenbauer A, Liu S, Ouari O, Bardelang D. EPR Spectroscopy: A Powerful Tool to Analyze Supramolecular Host•Guest Complexes of Stable Radicals with Cucurbiturils. Molecules. 2020; 25(4):776. https://doi.org/10.3390/molecules25040776
Chicago/Turabian StyleLiu, Fengbo, Hakim Karoui, Antal Rockenbauer, Simin Liu, Olivier Ouari, and David Bardelang. 2020. "EPR Spectroscopy: A Powerful Tool to Analyze Supramolecular Host•Guest Complexes of Stable Radicals with Cucurbiturils" Molecules 25, no. 4: 776. https://doi.org/10.3390/molecules25040776
APA StyleLiu, F., Karoui, H., Rockenbauer, A., Liu, S., Ouari, O., & Bardelang, D. (2020). EPR Spectroscopy: A Powerful Tool to Analyze Supramolecular Host•Guest Complexes of Stable Radicals with Cucurbiturils. Molecules, 25(4), 776. https://doi.org/10.3390/molecules25040776







