Preparation of Aluminosilicate Ferrierite Zeolite Nanosheets with Controllable Thickness in the Presence of a Sole Organic Structure Directing Agent
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Starting Materials
3.2. Synthesis of OSDA
3.3. Synthesis of Aluminosilicate FER Zeolite Nanosheets
3.4. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dusselier, M.; Davis, M.E. Small-Pore Zeolites: Synthesis and Catalysis. Chem. Rev. 2018, 118, 5265–5329. [Google Scholar] [CrossRef]
- Moliner, M.; Martínez, C.; Corma, A. Synthesis Strategies for Preparing Useful Small Pore Zeolites and Zeotypes for Gas Separations and Catalysis. Chem. Mater. 2014, 26, 246–258. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Y.; Yang, W.; Tang, Y.; Xie, Z. Recent Advances of Pore System Construction in Zeolite-Catalyzed Chemical Industry Processes. Chem. Soc. Rev. 2015, 44, 8877–8903. [Google Scholar] [CrossRef]
- Davis, M.E.; Lobo, R.F. Zeolite and Molecular Sieve Synthesis. Chem. Mater. 1992, 4, 756–768. [Google Scholar] [CrossRef]
- Jeon, M.Y.; Kim, D.; Kumar, P.; Lee, P.S.; Rangnekar, N.; Bai, P.; Shete, M.; Elyassi, B.; Lee, H.S.; Narasimharao, K.; et al. Ultra-Selective High-Flux Membranes from Directly Synthesized Zeolite Nanosheets. Nature 2017, 543, 690–694. [Google Scholar] [CrossRef]
- Awala, H.; Gilson, J.-P.; Retoux, R.; Boullay, P.; Goupil, J.-M.; Valtchev, V.; Mintova, S. Template-Free Nanosized Faujasite-Type Zeolites. Nat. Mater. 2015, 14, 447–451. [Google Scholar] [CrossRef]
- Boal, B.W.; Schmidt, J.E.; Deimund, M.A.; Deem, M.W.; Henling, L.M.; Brand, S.K.; Zones, S.I.; Davis, M.E. Facile Synthesis and Catalysis of Pure-Silica and Heteroatom LTA. Chem. Mater. 2015, 27, 7774–7779. [Google Scholar] [CrossRef]
- Fickel, D.W.; D’Addio, E.; Lauterbach, J.A.; Lobo, R.F. The Ammonia Selective Catalytic Reduction Activity of Copper-Exchanged Small-Pore Zeolites. Appl. Catal. B: Environ. 2011, 102, 441–448. [Google Scholar] [CrossRef]
- Freyhardt, C.C.; Tsapatsis, M.; Lobo, R.F.; Balkus, K.J.; Davis, M.E. A High-Silica Zeolite with a 14-Tetrahedral-Atom Pore Opening. Nature 1996, 381, 295–298. [Google Scholar] [CrossRef]
- Corma, A.; Fornes, V.; Pergher, S.B.; Maesen, T.L.M.; Buglass, J.G. Delaminated Zeolite Precursors as Selective Acidic Catalysts. Nature 1998, 396, 353–356. [Google Scholar] [CrossRef]
- Park, W.; Yu, D.; Na, K.; Jelfs, K.E.; Slater, B.; Sakamoto, Y.; Ryoo, R. Hierarchically Structure-Directing Effect of Multi-Ammonium Surfactants for the Generation of MFI Zeolite Nanosheets. Chem. Mater. 2011, 23, 5131–5137. [Google Scholar] [CrossRef]
- Wu, Q.; Zhu, L.; Chu, Y.; Liu, X.; Zhang, C.; Zhang, J.; Xu, H.; Xu, J.; Deng, F.; Feng, Z.; et al. Sustainable Synthesis of Pure Silica Zeolites from a Combined Strategy of Zeolite Seeding and Alcohol Filling. Angew. Chem. Int. Ed. 2019, 58, 12138–12142. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, X.; Qi, G.; Guo, Q.; Pan, S.; Meng, X.; Xu, J.; Deng, F.; Fan, F.; Feng, Z.; et al. Sustainable Synthesis of Zeolites without Addition of Both Organotemplates and Solvents. J. Am. Chem. Soc. 2014, 136, 4019–4025. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wu, Q.; Chu, Y.; Jiang, J.; Zhang, L.; Pan, S.; Zhang, C.; Zhu, L.; Deng, F.; Meng, X.; et al. Efficient Synthesis of Aluminosilicate RTH Zeolite with Good Catalytic Performances in NH3-SCR and MTO Reactions. J. Mater. Chem. A 2018, 6, 8705–8711. [Google Scholar] [CrossRef]
- Li, J.; Corma, A.; Yu, J. Synthesis of New Zeolite Structures. Chem. Soc. Rev. 2015, 44, 7112–7127. [Google Scholar] [CrossRef]
- Gu, F.N.; Wei, F.; Yang, J.Y.; Lin, N.; Lin, W.G.; Wang, Y.; Zhu, J.H. New Strategy to Synthesis of Hierarchical Mesoporous Zeolites. Chem. Mater. 2010, 22, 2442–2450. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, Y.; Zhu, L.; Rigutto, M.; van der Made, A.; Yang, C.; Pan, S.; Wang, L.; Zhu, L.; Jin, Y.; et al. Highly Mesoporous Single-Crystalline Zeolite Beta Synthesized Using a Nonsurfactant Cationic Polymer as a Dual-Function Template. J. Am. Chem. Soc. 2014, 136, 2503–2510. [Google Scholar] [CrossRef]
- Grand, J.; Talapaneni, S.N.; Vicente, A.; Fernandez, C.; Dib, E.; Aleksandrov, H.A.; Vayssilov, G.N.; Retoux, R.; Boullay, P.; Gilson, J.-P.; et al. One-Pot Synthesis of Silanol-Free Nanosized MFI Zeolite. Nat. Mater. 2017, 16, 1010–1015. [Google Scholar] [CrossRef]
- Xu, D.; Ma, Y.; Jing, Z.; Han, L.; Singh, B.; Feng, J.; Shen, X.; Cao, F.; Oleynikov, P.; Sun, H.; et al. π–π Interaction of Aromatic Groups in Amphiphilic Molecules Directing for Single-Crystalline Mesostructured Zeolite Nanosheets. Nat. Commun. 2014, 5, 4262. [Google Scholar] [CrossRef]
- Xie, D.; McCusker, L.B.; Baerlocher, C.; Zones, S.I.; Wan, W.; Zou, X. SSZ-52, a Zeolite with an 18-Layer Aluminosilicate Framework Structure Related to That of the DeNOx Catalyst Cu-SSZ-13. J. Am. Chem. Soc. 2013, 135, 10519–10524. [Google Scholar] [CrossRef]
- Smeets, S.; Xie, D.; McCusker, L.B.; Baerlocher, C.; Zones, S.I.; Thompson, J.A.; Lacheen, H.S.; Huang, H.-M. SSZ-45: A High-Silica Zeolite with Small Pore Openings, Large Cavities, and Unusual Adsorption Properties. Chem. Mater. 2014, 26, 3909–3913. [Google Scholar] [CrossRef]
- Choi, M.; Na, K.; Kim, J.; Sakamoto, Y.; Terasaki, O.; Ryoo, R. Stable Single-Unit-Cell Nanosheets of Zeolite MFI as Active and Long-Lived Catalysts. Nature 2009, 461, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Liu, Y.; Sun, Q.; Dai, Z.; Gies, H.; Wu, Q.; Pan, S.; Bian, C.; Tian, Z.; Meng, X.; et al. Design and Preparation of Efficient Hydroisomerization Catalysts by the Formation of Stable SAPO-11 Molecular Sieve Nanosheets with 10–20 nm Thickness and Partially Blocked Acidic Sites. Chem. Commun. 2017, 53, 4942–4945. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, N.; Xian, H.; Cheng, Q.; Tan, Y.; Tsubaki, N.; Li, X. Facilely Synthesized H-Mordenite Nanosheet Assembly for Carbonylation of Dimethyl Ether. ACS Appl. Mater. Inter. 2015, 7, 8398–8403. [Google Scholar] [CrossRef]
- Margarit, V.J.; Díaz-Rey, M.R.; Navarro, M.T.; Martínez, C.; Corma, A. Direct Synthesis of Nano-Ferrierite along the 10-Ring-Channel Direction Boosts Their Catalytic Behavior. Angew. Chem. 2018, 130, 3517–3521. [Google Scholar] [CrossRef]
- Bonilla, A.; Baudouin, D.; Pérez-Ramírez, J. Desilication of Ferrierite Zeolite for Porosity Generation and Improved Effectiveness in Polyethylene Pyrolysis. J. Catal. 2009, 265, 170–180. [Google Scholar] [CrossRef]
- Pinar, A.B.; Gómez-Hortigüela, L.; McCusker, L.B.; Pérez-Pariente, J. Controlling the Aluminum Distribution in the Zeolite Ferrierite via the Organic Structure Directing Agent. Chem. Mater. 2013, 25, 3654–3661. [Google Scholar] [CrossRef]
- Itabashi, K.; Kamimura, Y.; Iyoki, K.; Shimojima, A.; Okubo, T. A Working Hypothesis for Broadening Framework Types of Zeolites in Seed-Assisted Synthesis without Organic Structure-Directing Agent. J. Am. Chem. Soc. 2012, 134, 11542–11549. [Google Scholar] [CrossRef]
- Guo, G.; Long, Y.; Sun, Y. Synthesis of FER Type Zeolite with Tetrahydrofuran as the Template. Chem. Commun. 2000, 19, 1893–1894. [Google Scholar] [CrossRef]
- Wuamprakhon, P.; Wattanakit, C.; Warakulwit, C.; Yutthalekha, T.; Wannapakdee, W.; Ittisanronnachai, S.; Limtrakul, J. Direct Synthesis of Hierarchical Ferrierite Nanosheet Assemblies via an Organosilane Template Approach and Determination of Their Catalytic Activity. Micropor. Mesopor. Mater. 2016, 219, 1–9. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, J.; Guo, J.; He, H.; Long, Y. FER Zeolite Crystallized in THF-Na2O-SiO2-Al2O3-H2O Reactant System Containing Catalytic Amount of Organic Additives. Micropor. Mesopor. Mater. 2009, 119, 60–67. [Google Scholar] [CrossRef]
- Seo, G.; Jeong, H.S.; Jang, D.-L.; Cho, D.L.; Hong, S.B. The Role of Carbonaceous Deposits in the Skeletal Isomerization of 1-Butene over Ferrierite Zeolites. Catal. Lett. 1996, 41, 189–194. [Google Scholar] [CrossRef]
- Sulikowski, B.; Janas, J.; Haber, J.; Kubacka, A.; Wloch, E.; Olejniczak, Z. The Synergetic Effect of Cobalt and Indium in Ferrierite Catalysts for Selective Catalytic Reduction of Nitric Oxide with Methane. Chem. Commun. 1998, 24, 2755–2756. [Google Scholar] [CrossRef]
- Guzmán-Vargas, A.; Delahay, G.; Bernard, C. Catalytic Decomposition of N2O and Catalytic Reduction of N2O and N2O + NO by NH3 in the Presence of O2 over Fe-Zeolite. Appl. Catal. B: Environ. 2003, 42, 369–379. [Google Scholar] [CrossRef]
- María, A.A.; Victoriano, B.; César, J.; José, M.M.; Rafael, R.; Francisco, J.R.; Francisco, J. Catalytic Application of Zeolites in the Methanol Conversion to Hydrocarbons. Chem. Lett. 2002, 31, 672–673. [Google Scholar]
- Petersson, M.; Holma, T.; Andersson, B.; Jobson, E.; Palmqvist, A. Lean Hydrocarbon Selective Catalytic Reduction over Dual Pore System Zeolite Mixtures. J. Catal. 2005, 235, 114–127. [Google Scholar] [CrossRef]
- Catizzone, E.; Daele, S.V.; Bianco, M.; Di Michele, A.; Aloise, A.; Migliori, M.; Valtchev, V.; Giordano, G. Catalytic Application of Ferrierite Nanocrystals in Vapour-Phase Dehydration of Methanol to Dimethyl Ether. Appl. Catal. B: Environ. 2019, 243, 273–282. [Google Scholar] [CrossRef]
- Bastiani, R.; Lam, Y.L.; Henriques, C.A.; Teixeira da Silva, V. Application of Ferrierite Zeolite in High-Olefin Catalytic Cracking. Fuel 2013, 107, 680–687. [Google Scholar] [CrossRef]
- Rachwalik, R.; Olejniczak, Z.; Jiao, J.; Huang, J.; Hunger, M.; Sulikowski, B. Isomerization of α-Pinene over Dealuminated Ferrierite-Type Zeolites. J. Catal. 2007, 252, 161–170. [Google Scholar] [CrossRef]
- Feng, P.; Zhang, G.; Chen, X.; Zang, K.; Li, X.; Xu, L. Specific Zone within 8-Membered Ring Channel as Catalytic Center for Carbonylation of Dimethyl Ether and Methanol over FER Zeolite. Appl. Catal. A: Gen. 2018, 557, 119–124. [Google Scholar] [CrossRef]
- Jo, D.; Hong, S.B.; Camblor, M.A. Monomolecular Skeletal Isomerization of 1-Butene over Selective Zeolite Catalysts. ACS Catal. 2015, 5, 2270–2274. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Y.; Chu, W.; Zhao, D.; Chen, F.; Zhu, X.; Li, X.; Liu, S.; Xie, S.; Xu, L. Synthesis and Catalytic Application of FER Zeolites with Controllable Size. J. Mater. Chem. A 2019, 7, 7573–7580. [Google Scholar] [CrossRef]
- Xu, H.; Chen, W.; Zhang, G.; Wei, P.; Wu, Q.; Zhu, L.; Meng, X.; Li, X.; Fei, J.; Han, S.; et al. Ultrathin Nanosheets of Aluminosilicate FER Zeolites Synthesized in the Presence of a Sole Small Organic Ammonium. J. Mater. Chem. A 2019, 7, 16671–16676. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
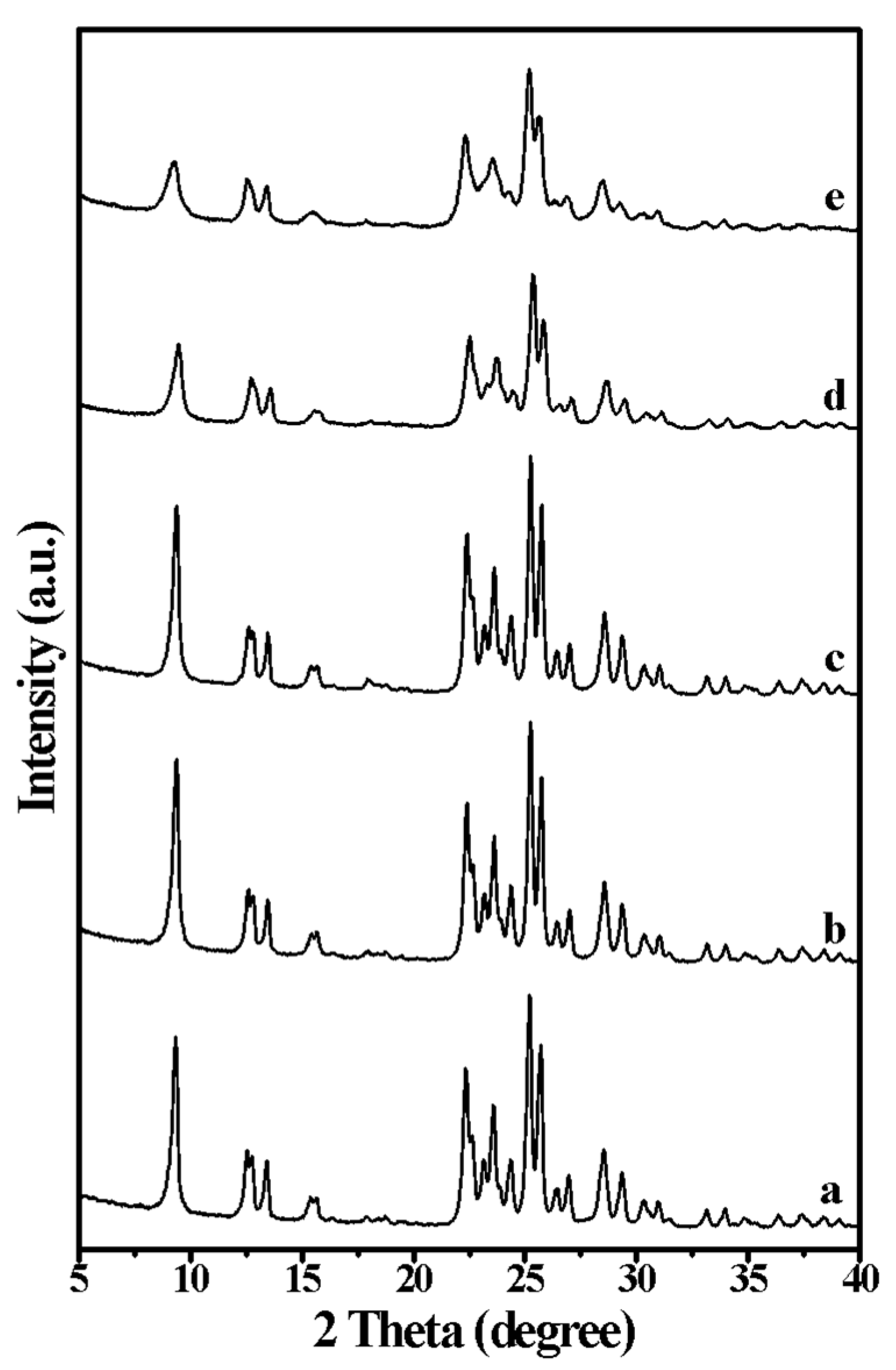
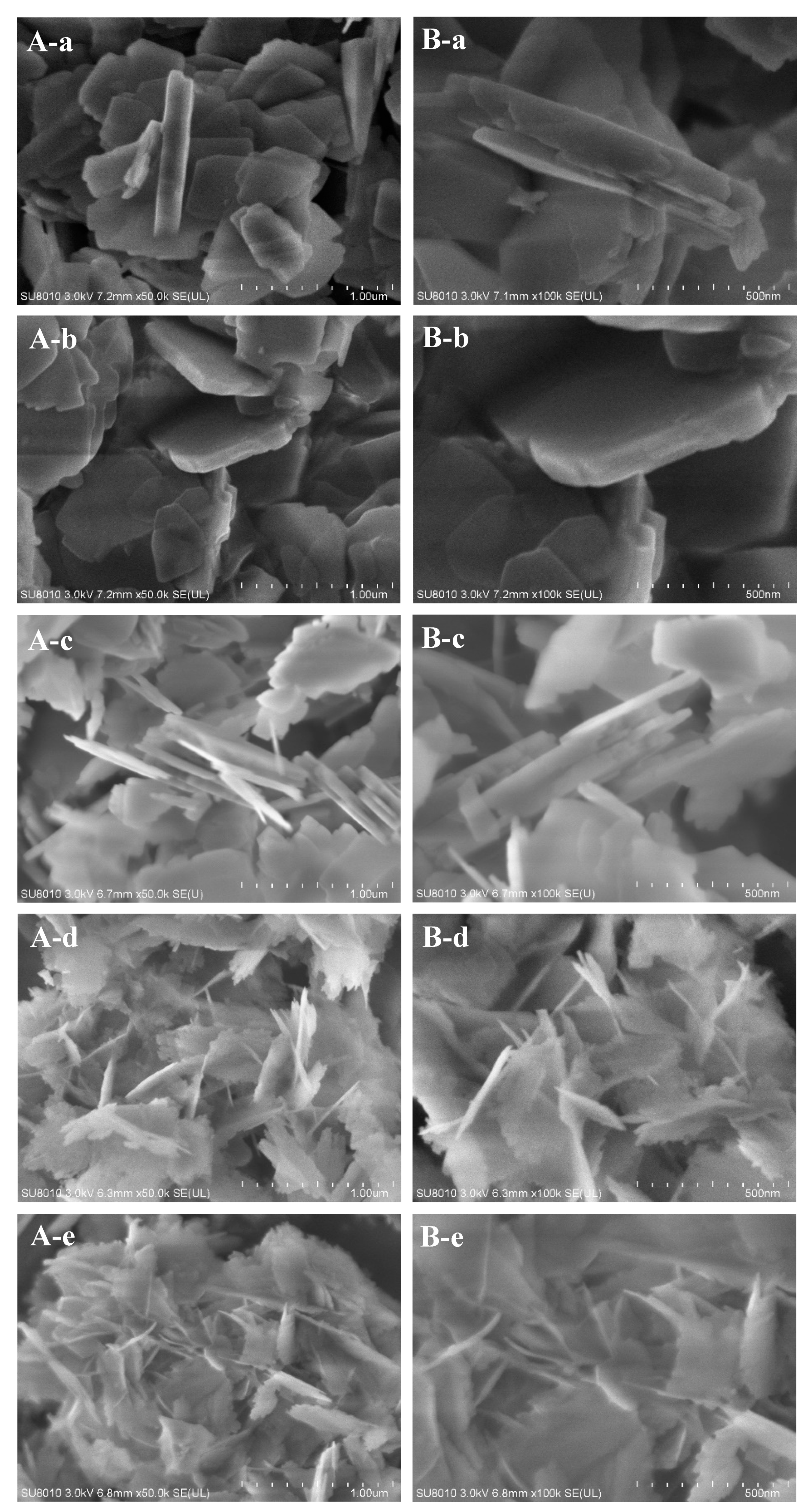
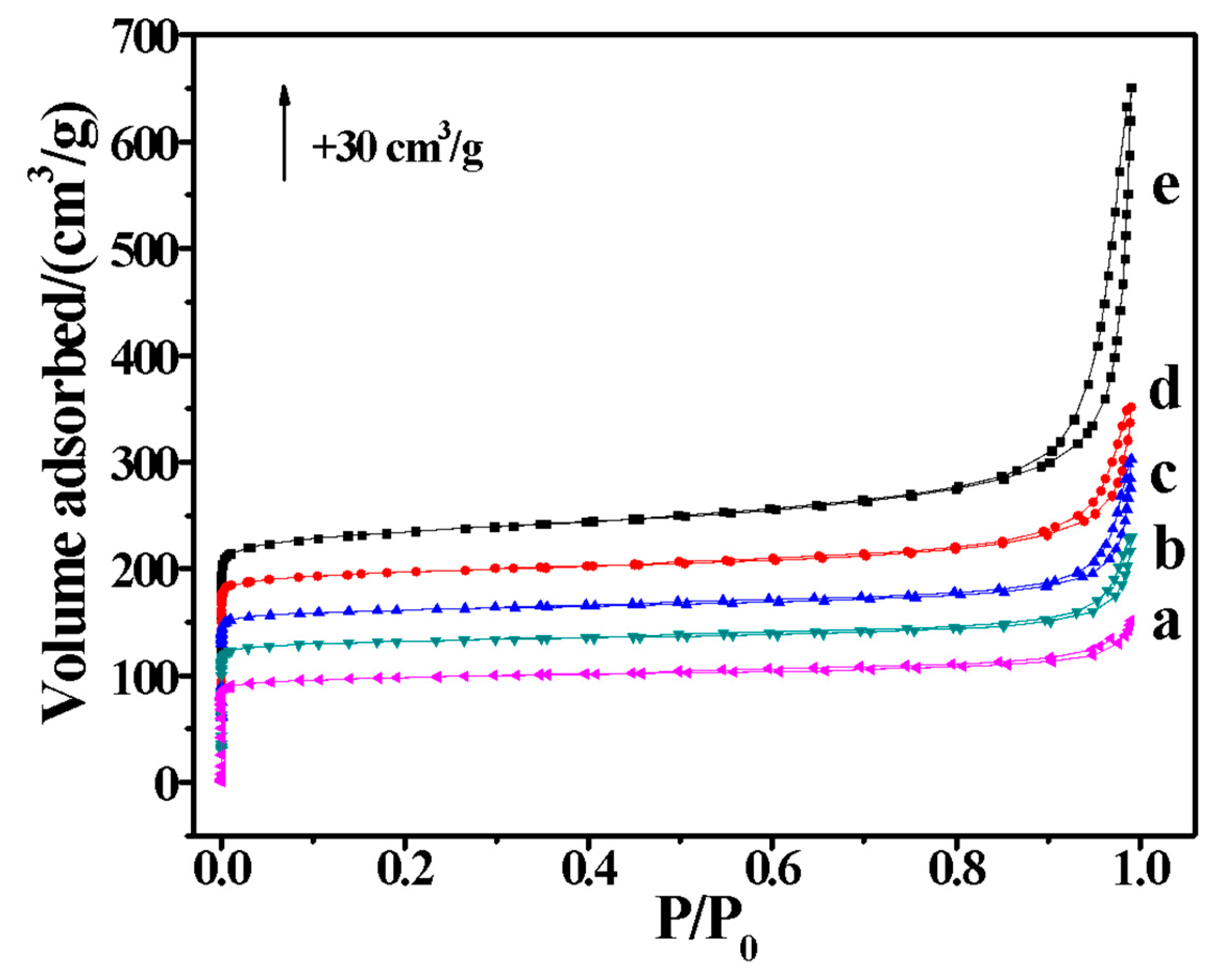
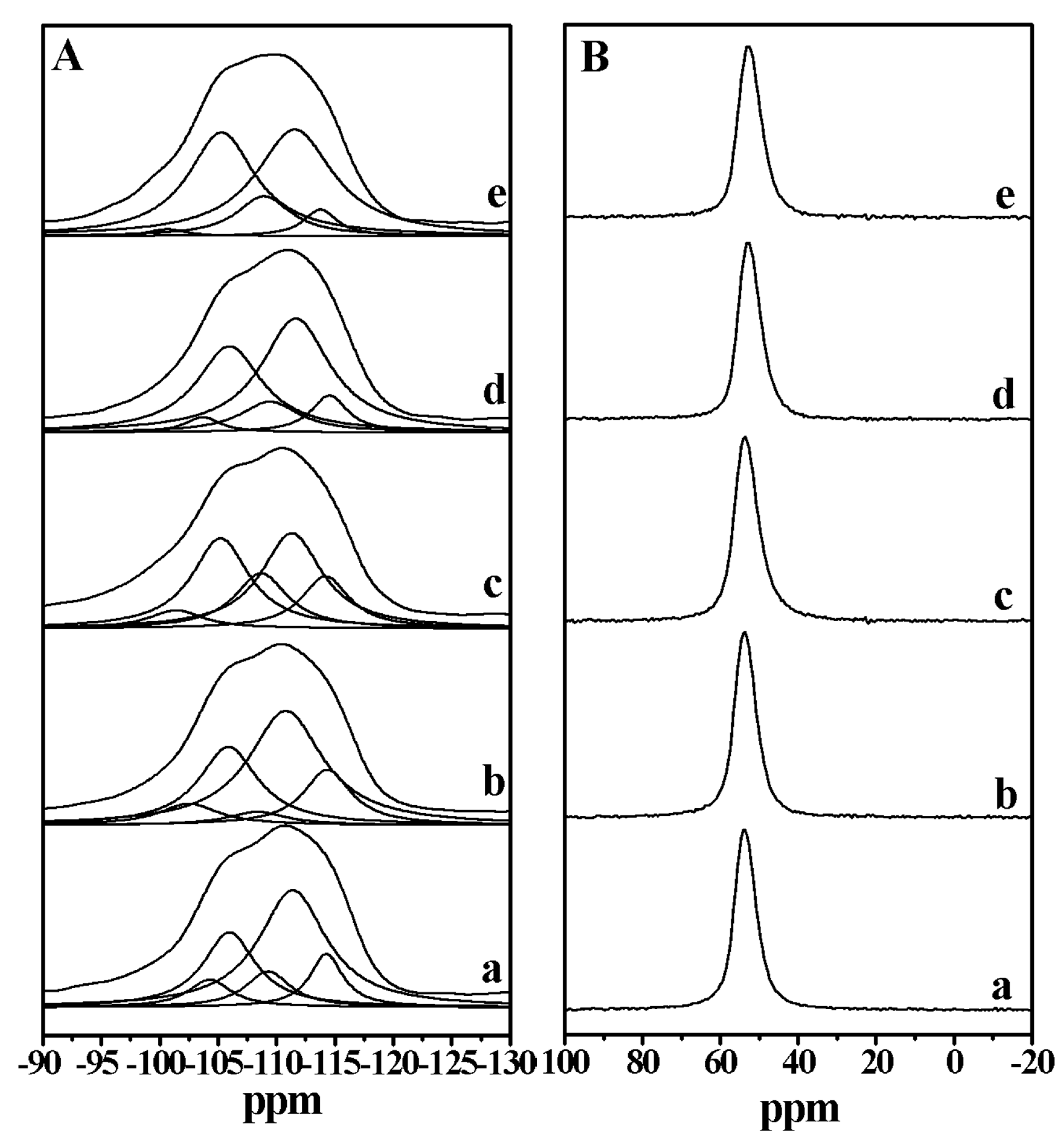
| Run | SiO2/Al2O3 | Na2O/SiO2 | DMP/SiO2 | Seeds/SiO2 | Products | Thickness/nm | SiO2/Al2O3 of the Product |
|---|---|---|---|---|---|---|---|
| 1 | 28.0 | 0.20 | 0 | 0.02 | FER-0 | 100–200 | 17.0 |
| 2 | 28.0 | 0.20 | 0.015 | 0 | FER-0.015 | 50–100 | 16.6 |
| 3 | 28.0 | 0.20 | 0.030 | 0 | FER-0.03 | 30–60 | 16.3 |
| 4 | 28.0 | 0.20 | 0.060 | 0 | FER-0.06 | 10–20 | 16.5 |
| 5 | 28.0 | 0.20 | 0.12 | 0 | FER-0.12 | 6–8 | 16.0 |
| Sample | SBET (m2/g) | Smicro (m2/g) | Sext (m2/g) | Vtot (cm3/g) | Vmicro (cm3/g) | Vmeso (cm3/g) |
|---|---|---|---|---|---|---|
| H-FER-0 | 326 | 303 | 23 | 0.23 | 0.14 | 0.09 |
| H-FER-0.125 | 355 | 319 | 36 | 0.29 | 0.14 | 0.15 |
| H-FER-0.25 | 367 | 327 | 40 | 0.35 | 0.14 | 0.21 |
| H-FER-0.5 | 377 | 318 | 59 | 0.39 | 0.14 | 0.25 |
| H-FER-1.0 | 391 | 286 | 105 | 0.79 | 0.14 | 0.65 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Yu, Y.; Zhu, L.; Bian, C.; Zhai, H.; Tong, J.; Wu, H.; Shen, C. Preparation of Aluminosilicate Ferrierite Zeolite Nanosheets with Controllable Thickness in the Presence of a Sole Organic Structure Directing Agent. Molecules 2020, 25, 771. https://doi.org/10.3390/molecules25040771
Xu H, Yu Y, Zhu L, Bian C, Zhai H, Tong J, Wu H, Shen C. Preparation of Aluminosilicate Ferrierite Zeolite Nanosheets with Controllable Thickness in the Presence of a Sole Organic Structure Directing Agent. Molecules. 2020; 25(4):771. https://doi.org/10.3390/molecules25040771
Chicago/Turabian StyleXu, Hao, YuXia Yu, LongFeng Zhu, ChaoQun Bian, HangLing Zhai, JianYing Tong, HuiZhen Wu, and Chao Shen. 2020. "Preparation of Aluminosilicate Ferrierite Zeolite Nanosheets with Controllable Thickness in the Presence of a Sole Organic Structure Directing Agent" Molecules 25, no. 4: 771. https://doi.org/10.3390/molecules25040771
APA StyleXu, H., Yu, Y., Zhu, L., Bian, C., Zhai, H., Tong, J., Wu, H., & Shen, C. (2020). Preparation of Aluminosilicate Ferrierite Zeolite Nanosheets with Controllable Thickness in the Presence of a Sole Organic Structure Directing Agent. Molecules, 25(4), 771. https://doi.org/10.3390/molecules25040771









