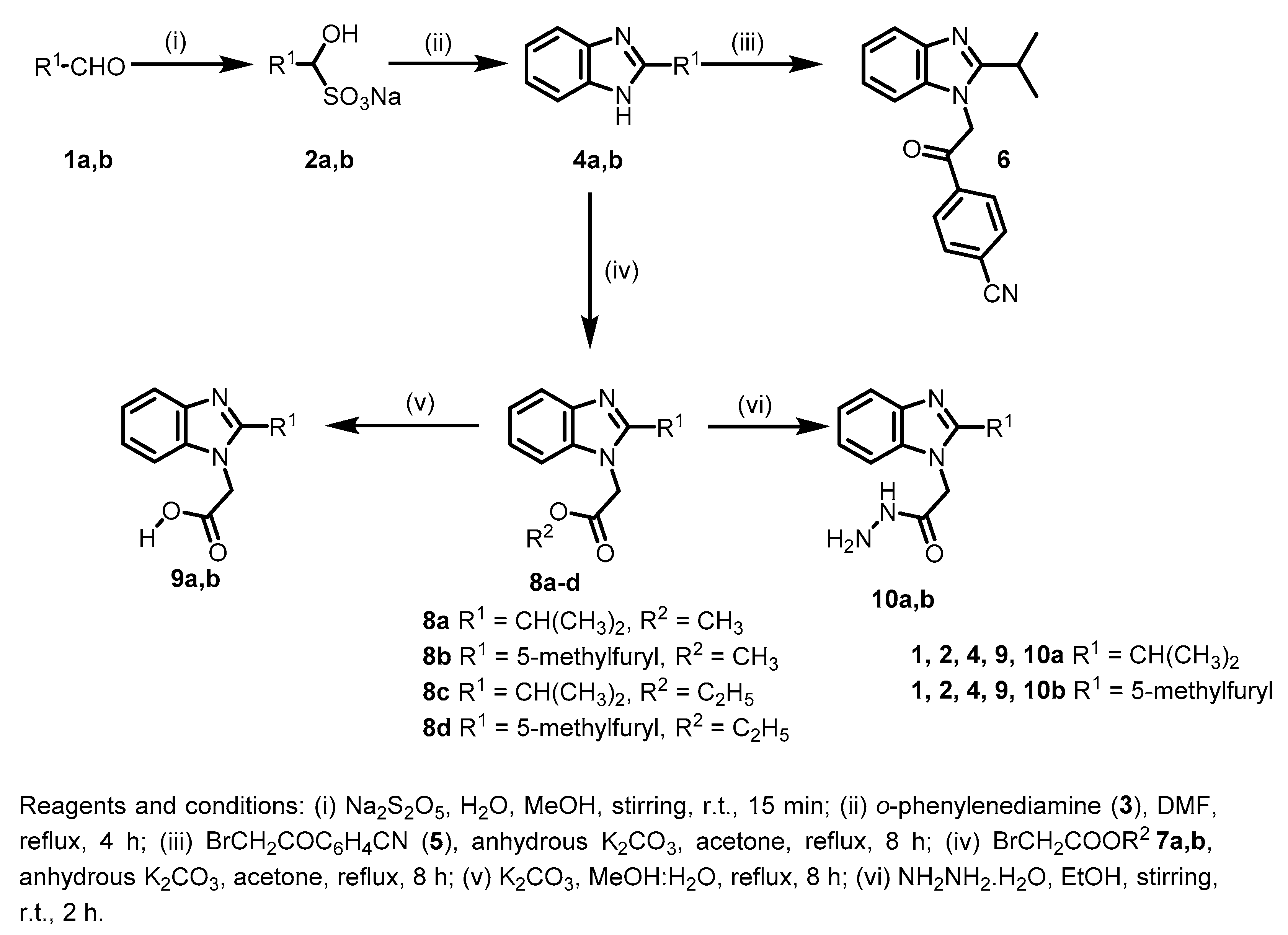
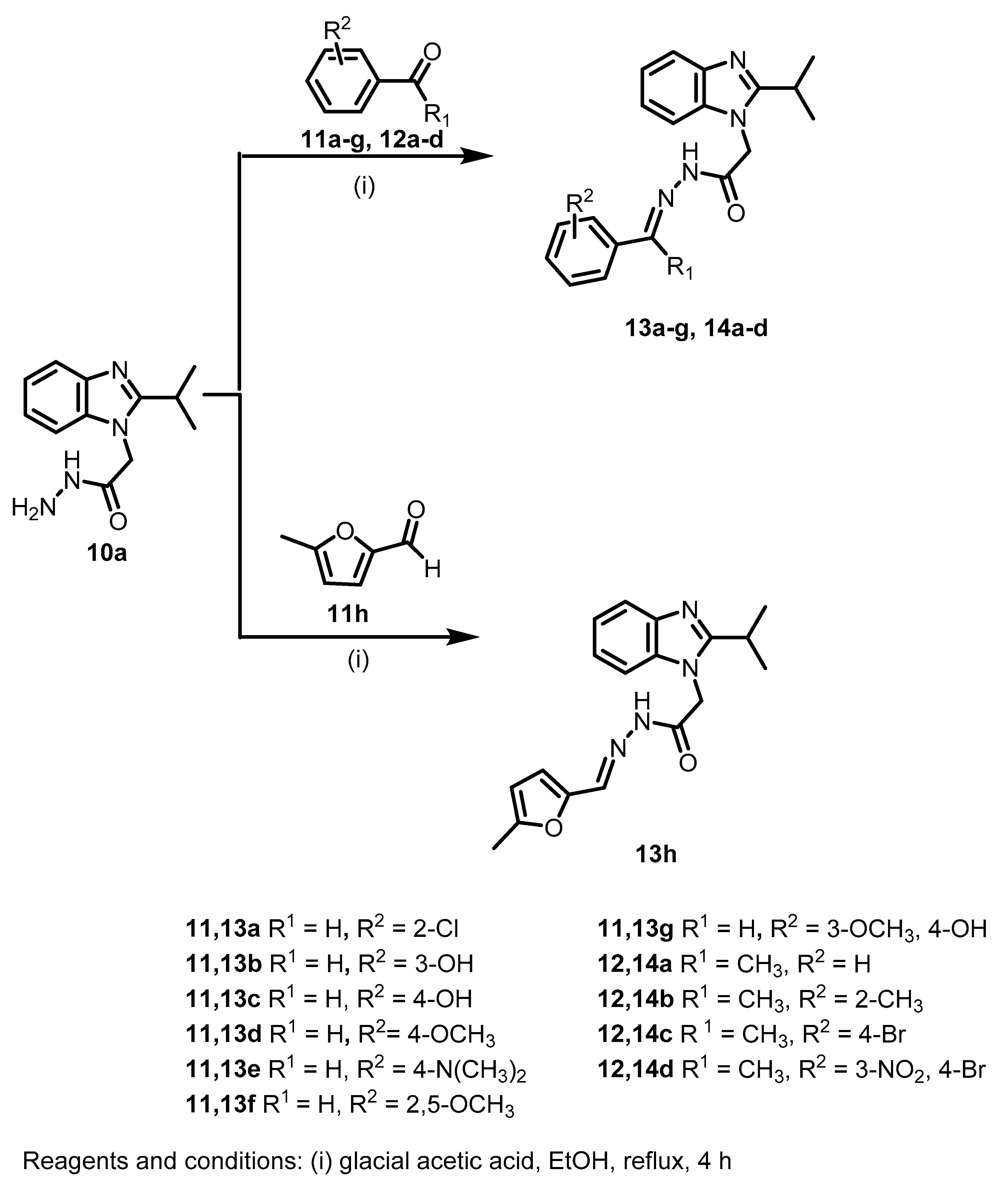
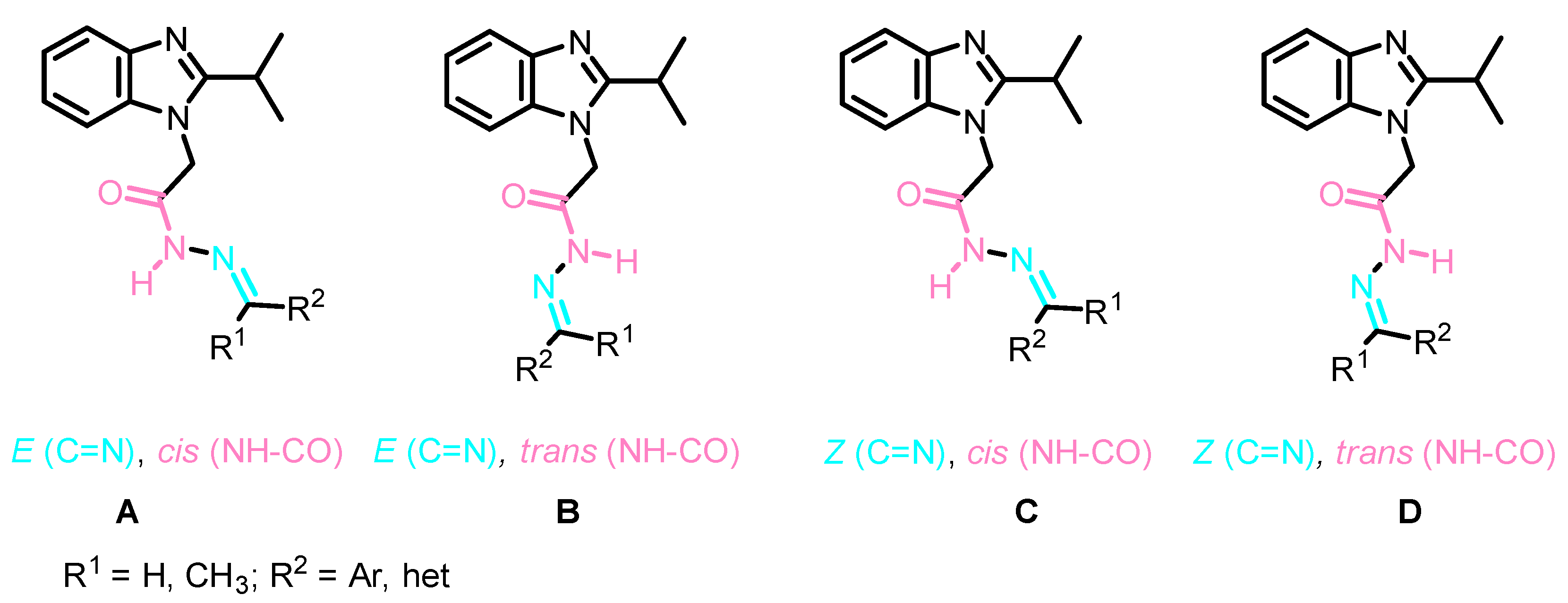
3.1.2. Synthesis and Analytical Data of Starting and Target Benzimidazoles
2-Isopropyl-1H-benzo[d]imidazole (4a)
A saturated solution of Na2S2O5 (2.85 g, 15 mmol in 2 mL water) was added to a solution of isobutyraldehyde (1a) (1.08 g, 15 mmol) in methanol (15 mL), and the mixture was stirred at r.t. for 15 min. The mixture was left in the fridge overnight, and the precipitated bisulfite adducts 2a was filtered and dried. Subsequently, a mixture of the formed adduct 2a (1.76 g, 10 mmol) and 1,2-phenylenediamine (3) (1.08 g, 10 mmol) was refluxed in DMF (15 mL) for 4 h. The reaction mixture was cooled to room temperature and poured onto ice/water (50 mL) to give the crude product 4a (CAS No. 5851-43-4), which was collected by filtration and further purified by recrystallization from methanol to give 4a as a buff powder (1.40 g, 88%); mp: 233–235 °C; 1H-NMR (DMSO-d6, 400 MHz): δH 1.33 (d, 3J = 7.2 Hz, 6H), 3.12 (sep, 3J = 7.2 Hz, 1H), 7.08–7.10 (m, 2H), 7.44–7.45 (m, 2H), 12.10 ppm (s, 1H); Anal. Calcd. for C10H12N2: C, 74.97; H, 7.55; N, 17.48. Found: C, 74.59; H, 7.31; N, 17.25.
2-(5-Methylfuran-2-yl)-1H-benzo[d]imidazole (4b) was synthesized according to the previously reported procedure [
17].
4-(2-(2-Isopropyl-1H-benzo[d]imidazol-1-yl)acetyl)benzonitrile (6)
A solution of 4a (0.16 g, 1.5 mmol) and anhydrous K2CO3 (0.21 g, 1.5 mmol) was stirred in dry acetone (20 mL) at room temperature for 30 min. 2-Bromo-4′-cyanoacetophenone (5) (0.34 g, 1.5 mmol) was added, and the reaction mixture was refluxed for 8 h. The reaction mixture was then poured onto ice/water (100 mL) with continuous stirring, and the precipitated product was collected by filtration and recrystallized from ethanol to give analytically pure derivative 6 as a grey powder (0.37 g, 81%); mp 209–211 °C; IR (KBr): ṽ 3095, 2927, 2231, 1706, 1624, 1542, 1506, 1467 cm−1; 1H-NMR (DMSO-d6, 400 MHz) δH 1.31 (d, 3J = 6.8 Hz, 6H), 3.34 (sep, 3J = 6.8 Hz, 1H), 6.24 (s, 2H), 7.27–7.34 (m, 2H), 7.64 (d, 3J = 7.6 Hz, 1H), 7.69 (d, 3J = 7.6 Hz, 1H), 8.13 (d, 3J = 8.0 Hz, 2H), 8.29 ppm (d, 3J = 8.0 Hz, 2H); 13C-NMR (DMSO-d6, 100 MHz) δC 21.17, 25.34, 50.86, 111.48, 116.05, 116.52, 118.09, 123.37, 123.47, 129.23, 132.85, 134.06, 137.28, 160.08, 192.57 ppm; Anal. Calcd. for C19H17N3O: C, 75.23; H, 5.65; N, 13.85. Found: C, 75.55; H, 5.29; N, 13.53.
General procedure I for the synthesis of 8a,c
A solution of 4a and anhydrous K2CO3 was stirred for 30 min in dry acetone (20 mL). Methyl bromoacetate (7a) or ethyl bromoacetate (7b) was added drop-wise, and the mixture was stirred under reflux for 8 h. The reaction mixture was then poured onto ice / water (100 mL) with continuous stirring. The precipitated product was collected by filtration and recrystallized from ethanol to give analytically pure derivatives 8a,c.
Methyl 2-(2-isopropyl-1H-benzo[d]imidazol-1-yl)acetate (8a)
According to the general procedure I, 4a (2.40 g, 15 mmol), anhydrous K2CO3 (2.07 g, 15 mmol) and methyl bromoacetate (7a) (2.30 g, 15 mmol) were reacted in dry acetone (20 mL) to give 8a as a grey powder (2.20 g, 63%); mp 93–95 °C; IR (KBr): ṽ 3042, 2975, 1744, 1613, 1512, 1459 cm−1; 1H-NMR (DMSO-d6, 400 MHz) δH 1.27 (d, 3J = 6.8 Hz, 6H), 3.19 (sep, 3J = 6.8 Hz, 1H), 3.70 (s, 3H), 5.21 (s, 2H), 7.15–7.18 (m, 2H), 7.42–7.44 (m, 1H), 7.55–7.57 (m, 1H); 13C-NMR (DMSO-d6, 100 MHz) δC 21.55, 25.47, 44.08, 52.40, 109.89, 118.50, 121.52, 121.77, 135.35, 142.10, 160.00, 168.96 ppm; Anal. Calcd. for C13H16N2O2: C, 67.22; H, 6.94; N, 12.06. Found: C, 67.45; H, 6.65; N, 11.72.
Methyl 2-(2-(5-methylfuran-2-yl)-1H-benzo[d]imidazol-1-yl)acetate (
8b) was synthesized according to the previously reported procedure [
17].
Ethyl 2-(2-isopropyl-1H-benzo[d]imidazol-1-yl)acetate (8c)
According to the general procedure I, 4a (2.40 g, 15 mmol), anhydrous K2CO3 (2.07 g, 15 mmol) and ethyl bromoacetate (7b) (2.51 g, 15 mmol) were reacted in dry acetone (20 mL) to give 8c as a white powder (2.30 g, 62%); mp 103–105 °C; IR (KBr): ṽ 3068, 2983, 1738, 1618, 1510, 1461 cm−1; 1H-NMR (DMSO-d6, 400 MHz): δH 1.20 (t, 3J = 7.2 Hz, 3H), 1.28 (d, 3J = 6.8 Hz, 6H), 3.18 (sep, 3J = 6.8 Hz, 1H), 4.16 (q, 3J = 7.2 Hz, 2H), 5.19 (s, 2H), 7.15–7.18 (m, 2H), 7.41–7.44 (m, 1H), 7.55–7.57 (m, 1H); 13C-NMR (DMSO-d6, 100 MHz): δC 14.00, 21.53, 25.53, 44.25, 61.33, 109.86, 118.50, 121.50, 121.77, 135.38, 142.11, 160.01, 168.44 ppm; Anal. Calcd. for C14H18N2O2: C, 68.27; H, 7.37; N, 11.37. Found: C, 68.61; H, 6.99; N, 11.05.
2-(2-Isopropyl-1H-benzo[d]imidazol-1-yl)acetic acid (9a)
A solution of 8a (0.47 g, 2 mmol) and K2CO3 (0.28 g, 2 mmol) in methanol:water 10:1 mixture (10 mL) was refluxed for 4 h. Solvent was evaporated under reduced pressure, and the precipitated product was collected, washed and recrystallized from ethanol to give 9a as gray needle crystals (0.40 g, 91%); mp 238–240 °C; IR (KBr) ṽ 3417, 2976, 2939, 1608, 1513, 1468 cm−1; 1H-NMR (DMSO-d6, 400 MHz): δH 1.30 (d, 3J = 6.8 Hz, 6H), 3.23–3.27 (m, 1H), 4.77 (s, 2H), 7.32–7.34 (m, 2H), 7.46–7.48 (m, 1H), 7.60–7.62 ppm (m, 1H); 13C-NMR (DMSO-d6, 100 MHz): δC 21.57, 26.73, 45.31, 110.06, 118.28, 121.34, 121.66, 135.58, 141.87, 160.16, 170.13, 172.35 ppm; Anal. Calcd. for C12H14N2O2: C, 66.04; H, 6.47; N, 12.84. Found: C, 66.32; H, 6.21; N, 12.52.
2-(2-(5-Methylfuran-2-yl)-1H-benzo[d]imidazol-1-yl)acetic acid (
9b) was synthesized according to the previously reported procedure [
17].
2-(2-Isopropyl-1H-benzo[d]imidazol-1-yl)acetohydrazide (10a)
Hydrazine hydrate (0.60 g, 12 mmol) was added drop-wise to a solution of 8a (0.70 g, 3 mmol) in ethanol (15 ml). The reaction mixture was stirred at room temperature for 1 h and then poured onto ice/water (100 mL). The precipitated product was collected by filtration, washed with water and dried to afford 10a as a white powder (0.50 g, 71%); mp: 243–245 °C; IR (KBr) ṽ 3433, 3292, 3163, 3073, 2965, 1646, 1552, 1508 cm−1; 1H-NMR (DMSO-d6, 400 MHz) δH 1.30 (d, 3J = 6.8 Hz, 6H), 3.23 (sep, 3J = 6.8 Hz, 1H), 4.34 (br., 2H), 4.82 (s, 2H), 7.13–7.16 (m, 2H), 7.38–7.40 (m, 1H), 7.53–7.55 (m, 1H), 9.53 ppm (s, 1H); 13C-NMR (DMSO-d6, 100 MHz) δC 21.67, 25.60, 44.17, 109.92, 118.42, 121.33, 121.55, 135.47, 142.09, 160.27, 166.18 ppm; Anal. Calcd. for C12H16N4O: C, 62.05; H, 6.94; N, 24.12. Found: C, 62.35; H, 6.67; N, 23.83.
2-(2-(5-Methylfuran-2-yl)-1H-benzo[d]imidazol-1-yl)acetohydrazide (
10b) was synthesized according to the previously reported procedure [
17].
General procedure II for the synthesis of Schiff bases 13a–h and 14a–d
A mixture of 10a (1 mmol), aldehyde 11a–g or ketone 12a–d (1 mmol) and glacial acetic acid (1 mL) in ethanol (20 mL) was refluxed for 4 h. The reaction mixture was then poured onto ice/water (50 mL) and neutralized with dilute ammonia, and the precipitated product was filtered, dried and further purified by recrystallization from appropriate solvent to give the corresponding analytically pure compound.
(E)-N′-(2-Chlorobenzylidene)-2-(2-isopropyl-1H-benzo[d]imidazol-1-yl)acetohydrazide (13a)
According to the general procedure II, 10a (0.23 g, 1 mmol) was reacted with 2-chlorobenzaldehyde (11a) (0.14 g, 1 mmol) in ethanol in the presence of acetic acid (1 mL). Work-up followed by crystallization from methanol/dichloromethane (1:1) gave 13a as a white powder (0.25 g, 71%); mp 211–213 °C; IR (KBr) ṽ 3428, 3063, 2962, 1679, 1634, 1634 cm−1; 1H-NMR (DMSO-d6, 400 MHz) of major conformer δH 1.29 (d, 3J = 6.8 Hz, 6H), 3.19 (sep, 3J = 6.8 Hz, 1H), 5.51 (s, 2H), 7.13–7.15 (m, 2H), 7.42–7.47 (m, 3H), 7.52–7.57 (m, 2H), 8.15 (dd, 3J = 7.6 Hz, 4J = 1.6 Hz, 1H), 8.47 (s, 1H), 11.95 ppm (s, 1H); 1H-NMR (DMSO-d6, 400 MHz) of minor conformer δH 1.32 (d, 3J = 7.2 Hz, 6H), 3.22 (sep, 3J = 6.8 Hz, 1H), 5.05 (s, 2H), 7.13–7.15 (ov. m, 2H), 7.42–7.47 (ov. m, 3H), 7.52–7.57 (ov. m, 2H), 7.94 (dd, 3J = 7.6 Hz, 4J = 1.2 Hz, 1H), 8.65 (s, 1H), 12.16 (s, 1H); 13C-NMR (DMSO-d6, 100 MHz) of major conformer δC 21.62, 25.58, 43.92, 109.91, 118.33, 121.14, 121.44, 127.28, 127.55, 129.87, 131.25, 131.48, 133.03, 135.78, 140.27, 142.17, 160.37 and 168.64 ppm; 13C-NMR (DMSO-d6, 100 MHz) of minor conformer δC 21.62, 25.58, 44.63, 109.81, 118.45, 121.33, 121.60, 126.90, 127.66, 129.92, 131.13, 131.69, 133.21, 135.54, 142.12, 143.49, 160.21 and 163.69 ppm; Anal. Calcd. for C19H19ClN4O: C, 64.31; H, 5.40; N, 15.79. Found: C, 64.63; H, 5.15; N, 15.53.
(E)-N′-(3-Hydroxybenzylidene)-2-(2-isopropyl-1H-benzo[d]imidazol-1-yl)acetohydrazide (13b)
According to the general procedure II, 10a (0.23 g, 1 mmol) was reacted with 3-hydroxybenzaldehyde (0.12 g, 1 mmol) (11b) in the presence of acetic acid in ethanol. Work-up followed by crystallization from ethanol gave 13b as white crystals (0.23 g, 69%); mp 249–251 °C; 1H-NMR (DMSO-d6, 400 MHz) of major conformer δH 1.30 (d, 3J = 6.8 Hz, 6H), 3.18 (sep, 3J = 6.8 Hz, 1H), 5.47 (s, 2H), 6.85 (dd, 3J = 7.2 Hz, 4J = 1.6 Hz, 1H), 7.13–7.20 (m, 4H), 7.25 (d, 3J = 8.0 Hz, 1H), 7.40-7.42 (m, 1H), 7.55–7.58 (m, 1H), 8.00 (s, 1H), 9.66 (s, 1H), 11.72 ppm (s, 1H); 1H-NMR (DMSO-d6, 400 MHz) of minor conformer δH 1.32 (d, 3J = 6.8 Hz, 6H), 3.23 (ov. sep, 3J = 6.8 Hz, 1H), 5.03 (s, 2H), 6.82 (ov. dd, 3J = 7.2 Hz, 4J = 2.0 Hz, 1H), 7.09 (d, 3J = 7.6 Hz, 1H), 7.13–7.20 (ov. m, 3H), 7.27 (d, 3J = 7.6 Hz, 1H), 7.40–7.42 (ov. m, 1H), 7.55–7.58 (ov. m, 1H), 8.17 (s, 1H), 9.66 (s, 1H), 11.72 ppm (s, 1H); 13C-NMR (DMSO-d6, 125 MHz) of major conformer δC 21.68, 25.78, 43.90, 110.01, 113.17, 117.54, 118.42, 118.65, 121.40, 121.72, 130.01, 135.33, 135.84, 142.14, 144.77, 157.78, 160.56 and 168.44 ppm; 13C-NMR (DMSO-d6, 125 MHz) of minor conformer δC 21.72, 25.76, 44.70, 109.96, 112.86, 117.80, 118.53, 119.06, 121.57, 121.82, 130.02, 135.33, 135.61, 142.11, 147.93, 157.78, 160.45 and 163.60 ppm; Anal. Calcd. for C19H20N4O2: C, 67.84; H, 5.99; N, 16.66. Found: C, 67.61; H, 6.32; N, 16.31.
(E)-N′-(4-Hydroxybenzylidene)-2-(2-isopropyl-1H-benzo[d]imidazol-1-yl)acetohydrazide (13c)
According to the general procedure II, 10a (0.23 g, 1 mmol) was reacted with 4-hydroxybenzaldehyde (11c) (0.12 g, 1 mmol) in the presence of acetic acid in ethanol. Work-up followed by crystallization from DMSO gave 13c as colorless crystals (0.24 g, 72%); mp: 296–298 °C; IR (KBr): ṽ 3437, 3211, 3059, 2977, 1682, 1607, 1577, 1510 cm−1; 1H-NMR (DMSO-d6, 400 MHz) of major conformer: δH 1.29 (d, 3J = 6.8 Hz, 6H), 3.17 (sep, 3J = 6.8 Hz, 1H), 5.44 (s, 2H), 6.83 (d, 3J = 8.8 Hz, 2H), 7.12–7.14 (m, 2H), 7.39–7.42 (m, 1H), 7.55–7.57 (m, 1H), 7.61 (d, 3J = 8.4 Hz, 2H), 7.97 (s, 1H), 9.94 (s, 1H), 11.57 ppm (s, 1H); 1H-NMR (DMSO-d6, 400 MHz) of minor conformer: δH 1.31 (d, 3J = 6.8 Hz, 6H), 3.23 (sep, 3J = 6.8 Hz, 1H), 5.01 (s, 2H), 6.82 (d, 3J = 8.8 Hz, 2H), 7.14–7.16 (ov. m, 2H), 7.38–7.42 (ov. m, 1H), 7.55–7.57 (ov. m, 1H), 7.53 (d, 3J = 8.4 Hz, 2H), 8.15 (s, 1H), 9.94 (ov. s, 1H), 12.15 ppm (s, 1H); 13C-NMR (DMSO-d6, 100 MHz) of major conformer: δC 21.65, 25.72, 44.88, 109.99, 115.76, 118.37, 121.27, 121.60, 125.06, 128.93, 135.85, 142.14, 144.74, 159.46, 160.47, 168.10 ppm; 13C-NMR (DMSO-d6, 100 MHz) of minor conformer: δC 21.69, 25.69, 44.64, 109.91, 115.76, 118.49, 121.45, 121.71, 124.96, 129.07, 135.58, 142.10, 148.05, 159.64, 160.37, 163.16 ppm; Anal. Calcd. for C19H20N4O2: C, 67.84; H, 5.99; N, 16.66. Found: C, 67.53; H, 5.66; N, 16.91.
(E)-2-(2-Isopropyl-1H-benzo[d]imidazol-1-yl)-N′-(4-methoxybenzylidene)acetohydrazide (13d)
According to the general procedure II, 10a (0.23 g, 1 mmol) was reacted with 4-methoxybenzaldehyde (11d) (0.14 g, 1 mmol) in the presence of acetic acid in ethanol. Work-up followed by crystallization from DMSO gave 13d as colorless crystals (0.32 g, 91%); mp: 190–192 °C; IR (KBr): ṽ 3441, 2964, 2928, 1672, 1609, 1570, 1456 cm−1; 1H-NMR (DMSO-d6, 400 MHz) of major conformer δH 1.29 (d, 3J = 6.8 Hz, 6H), 3.18 (d, 3J = 6.8 Hz, 1H), 3.80 (s, 3H), 5.47 (s, 2H), 7.02 (d, 3J = 8.8 Hz, 2H), 7.12–7.14 (m, 2H), 7.39–7.43 (m, 1H), 7.55–7.57 (ov., 1H), 7.73 (d, 3J = 8.8 Hz, 2H), 8.02 (s, 1H), 11.65 ppm (s, 1H); 1H-NMR (DMSO-d6, 400 MHz) of minor conformer δH 1.31 (d, 3J = 6.8 Hz, 6H), 3.23 (d, 3J = 6.8 Hz, 1H), 3.79 (s, 3H), 5.01 (s, 2H), 7.04 (d, 3J = 8.8 Hz, 2H), 7.16–7.17 (m, 2H), 7.39–7.43 (ov. m, 1H), 7.55–7.57 (ov. m, 1H), 7.65 (d, 3J = 8.8 Hz, 2H), 8.21 (s, 1H), 11.78 ppm (s, 1H); Anal. Calcd. for C20H22N4O2: C, 68.55; H, 6.33; N, 15.99. Found: C, 68.21; H, 6.00; N, 16.27.
(E)-N′-(4-(Dimethylamino)benzylidene)-2-(2-isopropyl-1H-benzo[d]imidazol-1-yl)acetohydrazide (13e)
According to the general procedure II, 10a (0.23 g, 1 mmol) was reacted with 4-dimethylaminobenzaldehyde (0.15 g, 1 mmol) (11f) in the presence of acetic acid in ethanol. Work-up followed by crystallization from dioxan/n-hexan (1:1) gave 13e as orange crystals (0.24 g, 67%); mp: 225–227 °C; IR (KBr): ṽ 3441, 3056, 2964, 1672, 1607, 1456 cm−1; 1H-NMR (DMSO-d6, 400 MHz) of major conformer δH 1.29 (d, 3J = 6.8 Hz, 6H), 2.97 (s, 6H), 3.18 (sep, 3J = 6.8 Hz, 1H), 5.43 (s, 2H), 6.75 (d, 3J = 8.8 Hz, 2H), 7.12–7.14 (m, 2H), 7.38–7.41 (m, 1H), 7.50 (d, 3J = 8.8 Hz, 1H), 7.58 (d, 3J = 8.8 Hz, 2H), 7.94 (s, 1H), 11.49 (s, 1H); 1H-NMR (DMSO-d6, 400 MHz) of minor conformer δH 1.31 (d, 3J = 6.8 Hz, 6H), 2.98 (s, 6H), 3.23 (sep, 3J = 6.8 Hz, 1H), 4.98 (s, 2H), 6.76 (d, 3J = 8.8 Hz, 2H), 7.14–7.17 (m, 2H), 7.42-7.43 (m, 1H), 7.52 (d, 3J = 8.8 Hz, 1H), 7.55–7.56 (m, 2H), 8.11 (s, 1H), 11.60 (s, 1H); 13C-NMR (DMSO-d6, 100 MHz) of major conformer δC 21.69, 25.82, 43.92, 110.07, 111.90, 118.43, 121.36, 121.40, 121.74, 128.57, 128.74, 135.88, 142.13, 145.48, 151.67, 160.57, 162.95, 167.89; 13C-NMR (DMSO-d6, 100 MHz) of minor conformer δC 21.74, 25.77, 44.70, 109.99, 111.84, 118.54, 121.18, 121.58, 121.83, 128.57, 129.70, 135.61, 142.10, 148.68, 151.80, 152.16, 160.04, 160.50 ppm; Anal. Calcd. for C21H25N5O: C, 69.40; H, 6.93; N, 19.27. Found: C, 69.71; H, 6.65; N, 19.55.
(E)-N′-(2,4-Dimethoxybenzylidene)-2-(2-isopropyl-1H-benzo[d]imidazol-1-yl)acetohydrazide (13f)
According to the general procedure II, 10a (0.23 g, 1 mmol) was reacted with 2,5-dimethoxybenzaldehyde (11f) (0.17 g, 1 mmol) in the presence of acetic acid in ethanol. Work-up followed by crystallization from dioxan/n-hexan (1:1) gave 13f as orange needle crystals (0.32 g, 85%); mp 202–204 °C; IR (KBr) ṽ 3439, 2929, 2966, 1677, 1637, 1458 cm−1; 1H-NMR (DMSO-d6, 400 MHz) of major conformer δH 1.29 (d, 3J = 6.8 Hz, 6H), 3.19 (sep, 3J = 6.8 Hz, 1H), 3.75 (s, 3H), 3.81 (s, 3H), 5.49 (s, 2H), 7.03 (d, 3J = 2.8 Hz, 1H), 7.05 (s, 1H), 7.12–7.15 (m, 2H), 7.41–7.43 (m, 1H), 7.51 (d, 3J = 2.8 Hz, 1H), 7.55–7.57 (m, 1H), 8.38 (s, 1H), 11.74 (s, 1H); 1H-NMR (DMSO-d6, 400 MHz) of minor conformer 1.32 (d, 3J = 6.8 Hz, 6H), 3.24 (sep, 3J = 6.8 Hz, 1H), 3.70 (s, 3H), 3.81 (ov. s, 3H), 5.01 (s, 2H), 7.00 (d, 3J = 2.8 Hz, 1H), 7.07 (s, 1H), 7.16–7.19 (m, 2H), 7.27 (d, 3J = 2.8 Hz, 1H), 7.41–7.43 (ov. m, 1H), 7.55–7.57 (ov. m, 1H), 8.58 (s, 1H), 11.93 (s, 1H); 13C-NMR (DMSO-d6, 100 MHz) of major conformer δC 21.68, 25.73, 44.00, 55.66, 56.43, 110.07, 110.32, 113.37, 117.29, 118.40, 121.36, 121.66, 122.70, 135.85, 139.96, 142.13, 152.34, 153.39, 160.56, 168.51 ppm; 13C-NMR (DMSO-d6, 100 MHz) of minor conformer δC 21.71, 25.73, 44.73, 55.56, 56.39, 109.26, 109.93, 113.58, 118.14, 118.54, 121.56, 121.82, 122.45, 135.58, 139.96, 143.21, 152.46, 153.35, 160.43, 163.50 ppm; Anal. Calcd. for C21H24N4O3: C, 66.30; H, 6.36; N, 14.73. Found: C, 66.65; H, 6.03; N, 14.51.
(E)-N′-(4-Hydroxy-3-methoxybenzylidene)-2-(2-isopropyl-1H-benzo[d]imidazol-1-yl)acetohydrazide (13g)
According to the general procedure II, 10a (0.23 g, 1 mmol) was reacted with vanillin (11g) (0.15 g, 1 mmol) in the presence of acetic acid in ethanol. Work-up followed by crystallization from dioxan/n-hexan (1:1) gave 13g as white crystals (0.29 g, 81%); mp: 165–167 °C; IR (KBr): ṽ 3446, 3186, 3034, 2970, 1697, 1594, 1512, 1459 cm−1; 1H-NMR (DMSO-d6, 400 MHz) of major conformer δH 1.30 (d, 3J = 6.8 Hz, 6H), 3.19 (sep, 3J = 6.8 Hz, 1H), 3.83 (s, 3H), 5.47 (s, 2H), 6.84 (d, 3J = 8.0 Hz, 1H), 7.12–7.16 (m, 3H), 7.38 (d, 3J = 1.2 Hz, 1H), 7.41–7.43 (m, 1H), 7.55–7.57 (m, 1H), 7.96 (s, 1H), 9.53 (s, 1H), 11.61 ppm (s, 1H); 1H-NMR (DMSO-d6, 400 MHz) of minor conformer δH 1.31 (d, 3J = 8.0 Hz, 6H), 3.23 (sep, 3J = 6.8 Hz, 1H), 3.78 (s, 3H), 5.00 (s, 2H), 6.82 (d, 3J = 8.0 Hz, 1H), 7.09 (dd, 3J = 8.4 Hz, 3J = 1.2 Hz, 1H), 7.12–7.16 (ov. m, 2H), 7.26 (d, 3J = 1.2 Hz, 1H), 7.41–7.43 (ov. m, 1H), 7.55–7.57 (ov. m, 1H), 8.14 (s, 1H), 9.55 (s, 1H), 11.72 ppm (s, 1H); 13C-NMR (DMSO-d6, 100 MHz) of major conformer δC 21.74, 25.87, 44.05, 55.88, 109.87, 110.16, 115.72, 118.49, 121.54, 121.86, 121.88, 125.63, 135.89, 142.13, 145.15, 148.22, 149.11, 160.70, 168.30 ppm; 13C-NMR (DMSO-d6, 100 MHz) of minor conformer δC 21.78, 25.85, 44.73, 55.76, 109.34, 110.05, 115.62, 118.60, 121.72, 121.97, 122.54, 125.51, 135.64, 142.10, 148.25, 148.50, 149.34, 160.60, 163.41 ppm; Anal. Calcd. for C20H22N4O3: C, 65.56; H, 6.05; N, 15.29. Found: C, 65.13; H, 5.79; N, 15.53.
(E)-2-(2-Isopropyl-1H-benzo[d]imidazol-1-yl)-N′-((5-methylfuran-2-yl)methylene)acetohydrazide (13h)
According to the general procedure II, 10a (0.23 g, 1 mmol) was reacted with 5-methylfurfural (11h) (0.11 g, 1 mmol) in the presence of acetic acid in ethanol. Work-up followed by crystallization from ethanol gave 13h as white crystals (0.24 g, 75%); mp: 131–133 °C; IR (KBr): ṽ 3450, 3100, 2919, 1689, 1618, 1592, 1512, 1452 cm−1; 1H-NMR (DMSO-d6, 400 MHz) of major conformer δH 1.28 (d, 3J = 6.8 Hz, 6H), 2.34 (s, 3H), 3.18 (sep, 3J = 6.8 Hz, 1H), 5.37 (s, 2H), 6.27 (d, 3J = 2.4 Hz, 1H), 6.84 (d, 3J = 3.2 Hz, 1H), 7.12–7.14 (m, 2H), 7.40–7.42 (m, 1H), 7.55–7.58 (m, 1H), 7.88 (s, 1H), 11.65 ppm (s, 1H); 1H-NMR (DMSO-d6, 400 MHz) of minor conformer δH 1.30 (d, 3J = 6.8 Hz, 6H), 2.31 (s, 3H), 3.23 (sep, 3J = 6.8 Hz, 1H), 5.01 (s, 2H), 6.24 (d, 3J = 2.8 Hz, 1H), 6.81 (d, 3J = 3.2 Hz, 1H), 7.16–7.19 (m, 2H), 7.40–7.42 (ov. m, 1H), 7.55–7.58 (ov. m, 1H), 8.05 (s, 1H), 11.65 ppm (s, 1H); 13C-NMR (DMSO-d6, 100 MHz) of major conformer δC 13.61, 21.67, 25.70, 43.89, 108.74, 109.98, 115.86, 118.38, 121.28, 121.61, 134.73, 135.81, 142.18, 147.55, 154.75, 160.49, 168.05 ppm; 13C-NMR (DMSO-d6, 100 MHz) of minor conformer δC 13.51, 21.67, 25.70, 44.65, 108.69, 109.90, 116.03, 118.51, 121.46, 121.72, 135.60, 137.46, 142.13, 147.59, 154.92, 160.34, 163.37 ppm; Anal. Calcd. for C18H20N4O2: C, 66.65; H, 6.21; N, 17.27. Found: C, 66.29; H, 6.57; N, 17.59.
(E)-2-(2-Isopropyl-1H-benzo[d]imidazol-1-yl)-N′-(1-phenylethylidene)acetohydrazide (14a)
According to the general procedure II, 10a (0.23 g, 1 mmol) was reacted with acetophenone (0.12 g, 1 mmol) (12a) in the presence of acetic acid in ethanol. Work-up followed by crystallization from ethanol gave 14a as buff needle crystals (0.28 g, 85%); mp: 176–178 °C; 1H-NMR (DMSO-d6, 400 MHz) of major conformer δH 1.30 (d, 3J = 6.8 Hz, 6H), 2.32 (s, 3H), 3.18 (sep, 3J = 6.8 Hz, 1H), 5.52 (s, 2H), 7.13–7.16 (m, 2H), 7.40–7.46 (m, 4H), 7.56–7.58 (m, 1H), 7.90–7.92 (m, 2H), 11.04 (s, 1H); 1H-NMR (DMSO-d6, 400 MHz) of minor conformer δH 1.33 (d, 3J = 6.8 Hz, 6H), 2.37 (s, 3H), 3.24 (sep, 3J = 6.8 Hz, 1H), 5.18 (s, 2H), 7.13–7.16 (ov. m, 2H), 7.40–7.46 (m, 4H), 7.56–7.58 (m, 1H), 7.76–7.80 (m, 2H), 10.90 (s, 1H); 13C-NMR (DMSO-d6, 100 MHz) of major conformer: δC 13.90, 21.72, 25.80, 44.52, 110.06, 118.44, 121.40, 121.72, 126.52, 128.57, 129.47, 135.89, 138.10, 142.17, 149.23, 160.64, 169.41 ppm; 13C-NMR (DMSO-d6, 100 MHz) of minor conformer: δC 14.48, 21.72, 25.80, 44.59, 110.01, 118.54, 121.56, 121.82, 126.52, 128.52, 129.62, 135.66, 138.04, 142.13, 153.09, 160.53, 164.21 ppm; Anal. Calcd. for C20H22N4O: C, 71.83; H, 6.63; N, 16.75. Found: C, 71.63; H, 6.91; N, 16.49.
(E)-2-(2-Isopropyl-1H-benzo[d]imidazol-1-yl)-N′-(1-(o-tolyl)ethylidene)acetohydrazide (14b)
According to the general procedure II, 10a (0.23 g, 1 mmol) was reacted with 2-methylacetophenone (0.13 g, 1 mmol) (12b) in the presence of acetic acid in ethanol. Work-up followed by crystallization from DMSO gave 14b as colorless crystals (0.27 g, 78%); mp: 238–240 °C; IR (KBr) ṽ 3438, 3073, 2966, 1630, 1525, 1448 cm−1; 1H-NMR (DMSO-d6, 400 MHz) of major conformer δH 1.27 (d, 3J = 6.8 Hz, 6H), 2.26 (s, 3H), 2.43 (s, 3H), 3.12 (sep, 3J = 6.8 Hz, 1H), 5.34 (s, 2H), 7.12–7.14 (m, 2H), 7.24–7.28 (m, 3H), 7.36–7.38 (m, 1H), 7.41–7.43 (m, 1H), 7.54–7.57 (m, 1H), 10.94 ppm (s, 1H); 1H-NMR (DMSO-d6, 400 MHz) of minor conformer δH 1.31 (d, 3J = 6.8 Hz, 6H), 2.30 (s, 3H), 2.41 (s, 3H), 3.23 (sep, 3J = 6.8 Hz, 1H), 5.16 (s, 2H), 7.12–7.14 (ov. m, 2H), 7.24–7.28 (ov. m, 3H), 7.36–7.38 (ov. m, 1H), 7.41–7.43 (ov. m, 1H), 7.54–7.57 (ov. m, 1H), 10.83 ppm (s, 1H); 13C-NMR (DMSO-d6, 100 MHz) of major conformer δC 18.00, 20.57, 21.60, 25.74, 44.33, 109.92, 118.39, 121.26, 121.59, 125.86, 128.36, 128.41, 130.85, 135.34, 135.83, 139.43, 142.14, 151.94, 160.40, 169.16 ppm; 13C-NMR (DMSO-d6, 100 MHz) of minor conformer δC 18.44, 20.10, 21.70, 25.04, 44.47, 109.92, 118.48, 121.43, 121.69, 125.72, 127.99, 128.41, 130.64, 135.13, 135.63, 139.57, 142.14, 156.07, 159.68, 163.99 ppm; Anal. Calcd. for C21H24N4O: C, 72.39; H, 6.94; N, 16.08. Found: C, 72.00; H, 6.71; N, 16.31.
(E)-N′-(1-(4-Bromophenyl)ethylidene)-2-(2-isopropyl-1H-benzo[d]imidazol-1-yl)acetohydrazide (14c)
According to the general procedure II, 10a (0.23 g, 1 mmol) was reacted with 4-bromoacetophenone (12c) (0.20 g, 1 mmol) in the presence of acetic acid in ethanol. Work-up followed by crystallization from methanol gave 14c as white crystals (0.32 g, 78%); mp: 215–217 °C; IR (KBr): ṽ 3435, 2926, 2866, 1664, 1630, 1454 cm−1; 1H-NMR (DMSO-d6, 400 MHz) of major conformer: δH 1.28 (d, 3J = 6.8 Hz, 6H), 2.29 (s, 3H), 3.17 (d, 3J = 6.8 Hz, 1H), 5.51 (s, 2H), 7.12–7.16 (m, 2H), 7.41–7.43 (m, 1H), 7.55–7.57 (m, 1H), 7.62 (d, 3J = 8.4 Hz, 2H), 7.86 (d, 3J = 8.4 Hz, 2H), 11.08 ppm (s, 1H); 1H-NMR (DMSO-d6, 400 MHz) of minor conformer: δH 1.31 (d, 3J = 6.8 Hz, 6H), 2.35 (s, 3H), 3.22 (sep, 3J = 6.8 Hz, 1H), 5.17 (s, 2H), 7.12–7.16 (ov. m, 2H), 7.41–7.43 (ov. m, 1H), 7.55–7.57 (ov. m, 1H), 7.62 (ov. d, 3J = 8.4 Hz, 2H), 7.73 (d, 3J = 8.8 Hz, 2H), 10.93 ppm (s, 1H); 13C-NMR (DMSO-d6, 100 MHz) of major conformer δC 13.63, 21.67, 25.68, 44.44, 109.94, 118.37, 121.22, 121.53, 122.79, 128.49, 131.35, 135.83, 137.23, 142.18, 147.90, 160.48, 169.39 ppm; 13C-NMR (DMSO-d6, 100 MHz) of minor conformer δC 14.17, 21.67, 25.68, 44.51, 109.88, 118.47, 121.38, 121.64, 122.97, 128.60, 131.78, 135.61, 137.16, 142.12, 151.59, 160.34, 164.18 ppm; Anal. Calcd. for C20H21BrN4O: C, 58.12; H, 5.12; N, 13.56. Found: C, 58.43; H, 5.38; N, 13.35.
(E)-N′-(1-(4-Bromo-3-nitrophenyl)ethylidene)-2-(2-isopropyl-1H-benzo[d]imidazol-1-yl)acetohydrazide (14d)
According to the general procedure II, 10a (0.23 g, 1 mmol) was reacted with 4´-bromo-3´-nitroacetophenone (0.24 g, 1 mmol) (12d) in the presence of acetic acid in ethanol. Work-up followed by crystallization from methanol gave 14d as white crystals (0.32 g, 71%); mp: 231–233 °C; IR (KBr): ṽ 3430, 2968, 2929, 1685, 1626, 1531, 1454 cm−1; 1H-NMR (DMSO-d6, 400 MHz) of major conformer δH 1.29 (d, 3J = 6.8 Hz, 6H), 2.33 (s, 3H), 3.17 (sep, 3J = 6.8 Hz, 1H), 5.56 (s, 2H), 7.13–7.16 (m, 2H), 7.41–7.43 (m, 1H), 7.56–7.58 (m, 1H), 7.95 (d, 3J = 8.4 Hz, 1H), 8.09 (dd, 3J = 8.4 Hz, 4J = 1.8 Hz, 1H), 8.47 (d, 3J = 2.0 Hz, 1H), 11.24 ppm (s, 1H); 1H-NMR (DMSO-d6, 400 MHz) of minor conformer δH 1.32 (d, 3J = 6.8 Hz, 6H), 2.40 (s, 3H), 3.17 (ov. sep, 3J = 6.8 Hz, 1H), 5.20 (s, 2H), 7.13–7.16 (ov. m, 2H), 7.41–7.43 (ov. m, 1H), 7.56–7.58 (ov. m, 1H), 7.95 (ov. d, 3J = 8.4 Hz, 1H), 8.09 (dd, 3J = 8.4 Hz, 4J = 1.8 Hz, 1H), 8.32 (s, 1H), 11.10 ppm (s, 1H); 13C-NMR (DMSO-d6, 100 MHz) of major conformer δC 13.53, 21.69, 25.68, 44.57, 110.03, 113.20, 118.38, 121.33, 121.60, 122.57, 131.00, 134.54, 135.80, 138.96, 142.12, 146.05, 150.32, 160.54, 169.69 ppm; 13C-NMR (DMSO-d6, 100 MHz) of minor conformer δC 14.13, 21.58, 25.54, 44.27, 109.93, 113.20, 118.51, 121.48, 121.85, 122.92, 124.77, 132.64, 134.86, 135.36, 137.03, 142.05, 149.67, 160.06, 164.53 ppm; Anal. Calcd. for C20H20BrN5O3: C, 52.41; H, 4.40; N, 15.28. Found: C, 52.110; H, 4.76; N, 15.00.
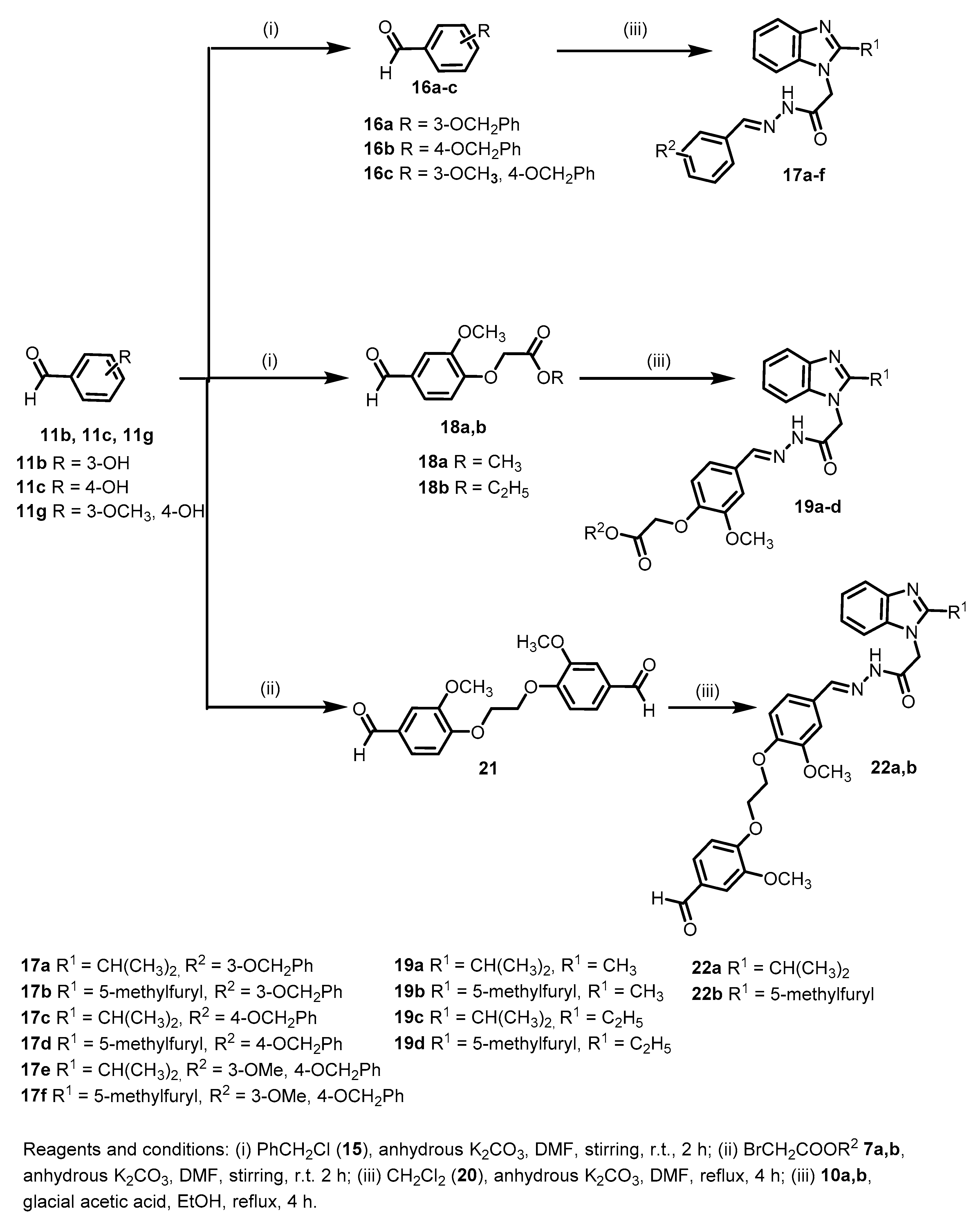
(E)-N′-(3-(Benzyloxy)benzylidene)-2-(2-isopropyl-1H-benzo[d]imidazol-1-yl)acetohydrazide (17a)
According to the general procedure II, 10a (0.23 g, 1 mmol) was reacted with 16a (0.21 g, 1 mmol) in the presence of acetic acid in ethanol. Work-up followed by crystallization from ethanol gave 17a as white crystals (0.32 g, 75%); mp 188–190 °C; 1H-NMR (DMSO-d6, 400 MHz) of major conformer δH 1.29 (d, 3J = 6.8 Hz, 6H), 3.18 (sep, 3J = 6.8 Hz, 1H), 5.16 (s, 2H), 5.48 (s, 2H), 7.10 (d, 3J = 7.6 Hz, 1H), 7.13–7.15 (m, 2H), 7.30–7.38 (m, 5H), 7.40–7.42 (m, 1H), 7.46–7.47 (m, 3H), 7.55–7.57 (m, 1H), 8.04 (s, 1H), 11.79 ppm (s, 1H); 1H-NMR (DMSO-d6, 400 MHz) of minor conformer δH 1.31 (d, 3J = 6.8 Hz, 6H), 3.23 (sep, 3J = 6.8 Hz, 1H), 5.03 (s, 2H), 5.13 (s, 2H), 7.10 (ov. d, 3J = 7.6 Hz, 1H), 7.15–7.17 (m, 2H), 7.30–7.38 (ov. m, 4H), 7.40–7.42 (ov. m, 2H), 7.46–7.47 (ov. m, 3H), 7.55–7.57 (ov. m, 1H), 8.23 (s, 1H, CH), 11.92 ppm (s, 1H); Anal. Calcd. for C26H26N4O2: C, 73.22; H, 6.14; N, 13.14. Found: C, 73.54; H, 6.43; N, 13.37.
(E)-N′-(3-(Benzyloxy)benzylidene)-2-(2-(5-methylfuran-2-yl)-1H-benzo[d]imidazol-1-yl)acetohydrazide (17b)
According to the general procedure II, 10b (0.27 g, 1 mmol) was reacted with 16a (0.21 g, 1 mmol) in the presence of acetic acid in ethanol. Work-up followed by crystallization from ethanol gave 17b as white crystals (0.32 g, 69%); mp 180–182 °C; 1H-NMR (DMSO-d6, 400 MHz) of major conformer δH 2.22 (s, 3H), 5.15 (s, 2H), 5.74 (s, 2H), 6.32 (d, 3J = 2.8 Hz, 1H), 7.02 (d, 3J = 3.6 Hz, 1H), 7.09 (dd, 3J = 8.0 Hz, 4J = 2.4 Hz, 1H), 7.22–7.29 (m, 3H), 7.32–7.35 (m, 3H), 7.37–7.39 (m, 1H), 7.44–7.46 (ov. m, 3H), 7.62–7.64 (m, 2H), 8.07 (s, 1H), 11.80 ppm (s, 1H); 1H-NMR (DMSO-d6, 400 MHz) of minor conformer δH 2.34 (s, 3H), 5.13 (s, 2H), 5.29 (s, 2H), 6.34 (d, 3J = 3.2 Hz, 1H), 7.05 (d, 3J = 3.6 Hz, 1H), 7.09 (ov. dd, 3J = 8.0 Hz, 4J = 2.4 Hz, 1H), 7.22–7.29 (ov. m, 3H), 7.32–7.35 (ov. m, 3H), 7.37–7.39 (ov. m, 2H), 7.44–7.46 (ov. m, 2H), 7.62–7.64 (ov. m, 2H), 8.24 (s, 1H), 11.97 ppm (s, 1H); Anal. Calcd. for C28H24N4O3: C, 72.40; H, 5.21; N, 12.06. Found: C, 72.72; H, 5.44; N, 12.34.
(E)-N′-(4-(Benzyloxy)benzylidene)-2-(2-isopropyl-1H-benzo[d]imidazol-1-yl)acetohydrazide (17c)
According to the general procedure II, 10a (0.23 g, 1 mmol) was reacted with 16b (0.21 g, 1 mmol) in the presence of acetic acid in ethanol. Work-up followed by crystallization from ethanol gave 17c as white crystals (0.32 g, 75%); mp 209–211 °C; 1H-NMR (DMSO-d6, 400 MHz) of major conformer δH 1.29 (d, 3J = 6.8 Hz, 6H), 3.18 (sep, 3J = 6.8 Hz, 1H), 5.16 (s, 2H), 5.47 (s, 2H), 7.10 (d, 3J = 8.4 Hz, 2H), 7.13–7.15 (m, 2H), 7.33–7.35 (m, 1H), 7.38–7.41 (m, 3H), 7.42–7.47 (m, 2H), 7.55–7.58 (m, 1H), 7.73 (d, 3J = 8.4 Hz, 2H), 8.02 (s, 1H), 11.66 ppm (s, 1H); 1H-NMR (DMSO-d6, 400 MHz) of minor conformer δH 1.32 (d, 3J = 7.2 Hz, 6H), 3.23 (sep, 3J = 6.8 Hz, 1H), 5.02 (s, 2H), 5.14 (s, 2H), 7.08 (d, 3J = 8.4 Hz, 2H), 7.15–7.17 (ov. m, 2H), 7.33–7.35 (ov. m, 1H), 7.38–7.41 (ov, m, 2H), 7.42–7.47 (m, 3H), 7.55–7.58 (ov. m, 1H), 7.65 (d, 3J = 8.8 Hz, 2H), 8.21 (s, 1H), 11.79 ppm (s, 1H); 13C-NMR (DMSO-d6, 100 MHz) of major conformer δC 21.60, 25.66, 43.90, 69.40, 110.00, 115.19, 118.28, 121.29, 121.60, 126.80, 127.80, 128.50, 128.74, 135.77, 136.77, 141.95, 144.20, 159.92, 160.40, 168.18 ppm; 13C-NMR (DMSO-d6, 100 MHz) of minor conformer δC 21.65, 25.66, 44.64, 69.40, 109.89, 114.45, 118.43, 121.44, 121.69, 126.72, 127.98, 128.50, 128.86, 135.53, 136.74, 141.99, 147.54, 160.09, 160.30, 163.24 ppm; Anal. Calcd. for C26H26N4O2: C, 73.22; H, 6.14; N, 13.14. Found: C, 73.58; H, 6.47; N, 12.97.
(E)-N′-(4-(Benzyloxy)benzylidene)-2-(2-(5-methylfuran-2-yl)-1H-benzo[d]imidazol-1-yl)acetohydrazide (17d)
According to the general procedure II, 10b (0.27 g, 1 mmol) was reacted with 16b (0.21 g, 1 mmol) in the presence of acetic acid in ethanol. Work-up followed by crystallization from ethanol afforded 17d as white crystals (0.35 g, 76%); mp 201–203 °C; 1H-NMR (DMSO-d6, 400 MHz) of major conformer δH 2.24 (s, 3H), 5.16 (s, 2H), 5.73 (s, 2H), 6.31 (d, 3J = 2.8 Hz, 1H), 7.01 (d, 3J = 3.6 Hz, 1H), 7.09 (d, 3J = 8.8 Hz, 2H), 7.21–7.24 (m, 2H), 7.31–7.35 (m, 1H), 7.39 (t like, 3J = 7.6 Hz, 2H), 7.45–7.47 (m, 2H), 7.59–7.66 (m, 2H), 7.72 (d, 3J = 8.4 Hz, 2H), 8.05 (s, 1H), 11.67 ppm (s, 1H); 1H-NMR (DMSO-d6, 400 MHz) of minor conformer δH 2.34 (s, 3H), 5.14 (s, 2H), 5.26 (s, 2H), 6.34 (d, 3J = 2.8 Hz, 1H), 7.05 (d, 3J = 3.6 Hz, 1H), 7.07–7.10 (m, 2H), 7.21–7.24 (ov. m, 2H), 7.31–7.35 (ov. m, 1H), 7.39 (ov. t like, 3J = 7.6 Hz, 2H), 7.45–7.47 (ov. m, 2H), 7.59–7.66 (ov. m, 4H), 8.21 (s, 1H), 11.83 ppm (s, 1H); Anal. Calcd. for C28H24N4O3: C, 72.40; H, 5.21; N, 12.06. Found: C, 72.62; H, 5.54; N, 12.34.
(E)-N′-(4-(Benzyloxy)-3-methoxybenzylidene)-2-(2-isopropyl-1H-benzo[d]imidazol-1-yl)acetohydrazide (17e)
According to the general procedure II, 10a (0.23 g, 1 mmol) was reacted with 16c (0.24 g, 1 mmol) in the presence of acetic acid in ethanol. Work-up followed by crystallization from ethanol gave 17e as white crystals (0.32 g, 70%); mp: 180–182 °C; 1H-NMR (DMSO-d6, 400 MHz) of major conformer δH 1.30 (d, 3J = 6.8 Hz, 6H), 3.16–3.25 (m, 1H), 3.83 (s, 3H), 5.14 (s, 2H), 5.49 (s, 2H), 7.10–7.21 (m, 3H), 7.25 (d, 3J = 8.0 Hz, 1H), 7.32–7.46 (m, 7H), 7.55–7.58 (m, 1H), 8.00 (s, 1H), 11.69 ppm (s, 1H); 1H-NMR (DMSO-d6, 400 MHz) of minor conformer δH 1.32 (d, 3J = 7.2 Hz, 6H), 3.16–3.25 (ov. m, 1H), 3.78 (s, 3H), 5.02 (s, 2H), 5.12 (s, 2H), 7.10–7.21 (ov. m, 4H), 7.32–7.46 (ov. m, 7H), 7.55–7.58 (m, 1H), 8.19 (s, 1H), 11.79 ppm (s, 1H); 13C-NMR (DMSO-d6, 100 MHz) of major conformer δC 21.64, 25.70, 43.89, 55.70, 69.94, 109.28, 109.99, 113.25, 118.38, 121.26, 121.35, 121.56, 127.10, 127.93, 128.50, 135.83, 136.82, 142.15, 144.42, 149.43, 149.70, 160.45, 168.29 ppm; 13C-NMR (DMSO-d6, 100 MHz) of minor conformer δC 21.67, 25.70, 44.65, 55.56, 69.94, 108.76, 109.87, 113.10, 118.50, 121.44, 121.69, 121.91, 126.99, 128.01, 128.50, 135.58, 136.78, 142.15, 144.42, 147.87, 149.91, 160.35, 163.33 ppm; Anal. Calcd. for C27H28N4O3: C, 71.03; H, 6.18; N, 12.27. Found: C, 71.36; H, 6.42; N, 11.98.
(E)-N′-(4-(Benzyloxy)-3-methoxybenzylidene)-2-(2-(5-methylfuran-2-yl)-1H-benzo[d]imidazol-1-yl)acetohydrazide (17f)
According to the general procedure II, 10b (0.27 g, 1 mmol) was reacted with 16c (0.24 g, 1 mmol) in the presence of acetic acid in ethanol. Work-up followed by crystallization from ethanol gave 17e as white crystals (0.37 g, 75%); mp 139–141 °C; 1H-NMR (DMSO-d6, 400 MHz) of major conformer δH 2.25 (s, 3H), 3.82 (s, 3H), 5.14 (s, 2H), 5.75 (s, 2H), 6.31 (d, 3J = 2.4 Hz, 1H), 7.01 (d, 3J = 3.2 Hz, 1H), 7.11 (d, 3J = 8.4 Hz, 1H), 7.16–7.26 (m, 3H), 7.32–7.46 (m, 6H), 7.62–7.64 (m, 2H), 8.03 (s, 1H), 11.70 ppm (s, 1H); 1H-NMR (DMSO-d6, 400 MHz) of minor conformer δH 2.35 (s, 3H), 3.78 (s, 3H), 5.16 (s, 2H), 5.27 (s, 2H), 7.06 (d, 3J = 3.2 Hz, 1H), 7.11 (ov. d, 3J = 8.4 Hz, 1H), 7.16–7.26 (ov. m, 3H), 7.32–7.46 (ov. m, 7H), 7.62–7.64 (ov. m, 2H), 8.19 (s, 1H), 11.84 ppm (s, 1H); Anal. Calcd. for C29H26N4O4: C, 70.43; H, 5.30; N, 11.33. Found: C, 70.72; H, 5.04; N, 11.54.
Methyl-(E)-2-(4-((2-(2-(2-isopropyl-1H-benzo[d]imidazol-1-yl)acetyl)hydrazono)methyl)-2-methoxyphenoxy)acetate (19a)
According to the general procedure II, 10a (0.23 g, 1 mmol) was reacted with 18a (0.22 g, 1 mmol) in the presence of acetic acid in ethanol. Work-up followed by crystallization from ethanol gave 19a as colorless crystals (0.28 g, 65%); mp 175–177 °C; 1H-NMR (DMSO-d6, 400 MHz) of major conformer δH 1.30 (d, 3J = 6.8 Hz, 6H), 3.17–3.28 (m, 1H), 3.70 (s, 3H), 3.84 (s, 3H), 4.85 (s, 2H), 5.49 (s, 2H), 6.94 (d, 3J = 8.4 Hz, 1H), 7.13–7.15 (m, 2H), 7.23 (d, 3J = 7.6 Hz, 1H), 7.41–7.43 (m, 1H), 7.45 (s, 1H), 7.55–7.57 (m, 1H), 8.01 (s, 1H), 11.68 ppm (s, 1H); 1H-NMR (DMSO-d6, 400 MHz) of minor conformer δH 1.31 (d, 3J = 8.4 Hz, 6H), 3.17–3.28 (ov. m, 1H), 3.69 (s, 3H), 3.80 (s, 3H), 4.84 (s, 2H), 5.02 (s, 2H), 6.94 (ov. d, 3J = 8.4 Hz, 1H), 7.16–7.19 (m, 2H), 7.23 (d, 3J = 8.4 Hz, 1H), 7.32 (s, 1H), 7.41–7.43 (ov. m, 1H), 7.55–7.57 (ov. m, 1H), 8.19 (s, 1H), 11.82 ppm (s, 1H); 13C-NMR (DMSO-d6, 100 MHz) of major conformer δC 21.64, 25.69, 43.87, 51.90, 55.73, 65.10, 109.53, 109.99, 113.11, 118.38, 121.12, 121.25, 121.55, 127.72, 135.83, 142.15, 144.22, 148.85, 149.19, 160.44, 168.34, 169.08 ppm; 13C-NMR (DMSO-d6, 100 MHz) of minor conformer δC 21.67, 25.69, 44.63, 51.90, 55.61, 65.06, 109.09, 109.86, 113.03, 118.49, 121.42, 121.60, 121.67, 127.62, 135.58, 142.12, 144.22, 147.69, 149.03, 160.34, 163.36, 169.05 ppm; Anal. Calcd. for C23H26N4O5: C, 63.00; H, 5.98; N, 12.78. Found: C, 63.32; H, 5.64; N, 12.54.
Methyl-(E)-2-(2-methoxy-4-((2-(2-(2-(5-methylfuran-2-yl)-1H-benzo[d]imidazol-1-yl)acetyl)hydrazono)methyl)phenoxy)acetate (19b)
According to the general procedure II, 10b (0.27 g, 1 mmol) was reacted with 18a (0.22 g, 1 mmol) in the presence of acetic acid in ethanol. Work-up followed by crystallization from ethanol gave 19b as colorless crystals (0.48 g, 71%); mp 150–152 °C; 1H-NMR (DMSO-d6, 400 MHz) of major conformer δH 2.25 (s, 3H), 3.70 (s, 3H), 3.83 (s, 3H), 4.84 (s, 2H), 5.75 (s, 2H), 6.32 (d, 3J = 2.8 Hz, 1H), 6.94 (d, 3J = 8.4 Hz, 1H), 7.01 (d, 3J = 3.2 Hz, 1H), 7.22–7.25 (m, 3H), 7.43 (s, 1H), 7.62–7.64 (m, 2H), 8.03 (s, 1H), 11.72 ppm (s, 1H); 1H-NMR (DMSO-d6, 400 MHz) of minor conformer δH 2.34 (s, 3H), 3.70 (ov. s, 3H), 3.80 (s, 3H), 4.84 (ov. s, 2H), 5.27 (s, 2H), 6.34 (d, 3J = 2.4 Hz, 1H), 6.93 (d, 3J = 8.0 Hz, 1H), 7.05 (d, 3J = 2.8 Hz, 1H), 7.18 (d, 3J = 8.4 Hz, 1H), 7.22–7.25 (ov. m, 2H), 7.32 (s, 1H), 7.59–7.64 (ov. m, 2H), 8.20 (s, 1H), 11.85 ppm (s, 1H); 13C-NMR (DMSO-d6, 100 MHz) of major conformer δC 13.32, 45.76, 51.94, 55.77, 65.12, 108.48, 109.55, 110.44, 113.20, 113.25, 118.71, 120.99, 122.27, 122.53, 127.79, 136.54, 142.49, 143.66, 144.12, 144.33, 148.86, 149.24, 153.79, 168.66, 169.13 ppm; 13C-NMR (DMSO-d6, 100 MHz) of minor conformer δC 13.46, 48.68, 51.94, 55.67, 65.14, 108.52, 109.14, 110.34, 113.08, 113.51, 118.80, 121.56, 122.41, 127.72, 136.37, 142.46, 143.40, 144.12, 144.29, 147.25, 149.01, 149.22, 154.05, 163.71, 169.10 ppm; Anal. Calcd. for C25H24N4O6: C, 63.02; H, 5.08; N, 11.76. Found: C, 63.22; H, 5.32; N, 12.01.
Ethyl-(E)-2-(4-((2-(2-(2-isopropyl-1H-benzo[d]imidazol-1-yl)acetyl)hydrazono)methyl)-2-methoxyphenoxy)acetate (19c)
According to the general procedure II, 10a (0.23 g, 1 mmol) was reacted with 18b (0.24 g, 1 mmol) in the presence of acetic acid in ethanol. Work-up followed by crystallization from ethanol gave 19c as white crystals (0.36 g, 80%); mp: 93–95 °C; 1H-NMR (DMSO-d6, 400 MHz) of major conformer δH 1.21 (t, 3J = 7.2 Hz, 3H), 1.30 (d, 3J = 7.2 Hz, 6H), 3.19–3.25 (m, 1H), 3.84 (s, 3H), 4.17 (q, 3J = 7.2 Hz, 2H), 4.83 (s, 2H), 5.49 (s, 2H), 6.93 (d, 3J = 8.4 Hz, 1H), 7.14 (dd, 3J = 6.0 Hz, 4J = 2.8 Hz, 2H), 7.23 (dd, 3J = 8.4 Hz, 4J = 2.8 Hz, 1H), 7.42 (dd, 3J = 6.0 Hz, 4J = 3.2 Hz, 1H), 7.45 (d, 4J = 1.2 Hz, 1H), 7.56 (dd, 3J = 6.0 Hz, 4J = 3.2 Hz, 1H), 8.00 ppm (s, 1H); 1H-NMR (DMSO-d6, 400 MHz) of minor conformer δH 1.20 (ov. t, 3J = 6.8 Hz, 3H), 1.31 (d, 3J = 7.2 Hz, 6H), 3.19–3.25 (ov. m, 1H), 3.80 (s, 3H), 4.17 (ov. q, 3J = 7.2 Hz, 2H), 4.81 (s, 2H), 5.03 (s, 2H), 6.92 (d, 3J = 8.4 Hz, 1H), 7.17 (dd, 3J = 8.0 Hz, 4J = 2.8 Hz, 2H), 7.32–7.34 (m, 1H), 7.42 (ov. dd, 3J = 6.0 Hz, 4J = 3.2 Hz, 1H), 7.51 (d, 4J = 1.2 Hz, 1H), 7.56 (ov. dd, 3J = 6.0 Hz, 4J = 3.2 Hz, 1H), 8.20 ppm (s, 1H); 13C-NMR (DMSO-d6, 100 MHz) of major conformer δC 14.10, 21.65, 25.70, 43.89, 55.76, 60.78, 65.22, 109.60, 110.00, 113.17, 118.38, 121.12, 121.27, 121.58, 127.71, 135.83, 142.15, 144.26, 148.90, 149.22, 160.46, 168.35, 168.60 ppm; 13C-NMR (DMSO-d6, 100 MHz) of minor conformer δC 14.10, 21.68, 25.70, 44.63, 55.64, 60.81, 65.18, 109.13, 109.89, 113.09, 118.49, 121.44, 121.69, 127.64, 135.58, 142.12, 147.71, 149.07, 149.16, 149.84, 160.37, 163.40, 168.51, 168.57 ppm; Anal. Calcd. for C24H28N4O5: C, 63.70; H, 6.24; N, 12.38. Found: C, 63.42; H, 5.89; N, 12.14.
Ethyl-(E)-2-(2-methoxy-4-((2-(2-(2-(5-methylfuran-2-yl)-1H-benzo[d]imidazol-1-yl)acetyl)hydrazono)methyl)phenoxy)acetate (19d)
According to the general procedure II, 10b (0.27 g, 1 mmol) was reacted with 18b (0.24 g, 1 mmol) in the presence of acetic acid in ethanol. Work-up followed by crystallization from ethanol gave 19d as colorless crystals (0.43 g, 88%); mp 136–138 °C; 1H-NMR (DMSO-d6, 400 MHz) of major conformer δH 1.20 (t, 3J = 6.8 Hz, 3H), 2.25 (s, 3H), 3.83 (s, 3H), 4.16 (q, 3J = 7.2 Hz, 2H), 4.82 (s, 2H), 5.76 (s, 2H), 6.31 (d, 3J = 3.2 Hz, 1H), 6.93 (d, 3J = 8.4 Hz, 1H), 7.01 (d, 3J = 3.2 Hz, 1H), 7.21–7.25 (m, 3H), 7.43 (d, 4J = 1.2 Hz, 1H), 7.62–7.64 (m, 2H), 8.03 (s, 1H), 11.88 ppm (br., 1H); 1H-NMR (DMSO-d6, 400 MHz) of minor conformer δH 1.20 (ov. t, 3J = 6.8 Hz, 3H), 2.34 (s, 3H), 3.79 (s, 3H), 4.16 (ov. q, 3J = 7.2 Hz, 2H), 4.81 (s, 2H), 5.27 (s, 2H), 6.34 (d, 3J = 2.8 Hz, 1H), 6.92 (ov. d, 3J = 8.4 Hz, 1H), 7.05 (d, 3J = 3.2 Hz, 1H), 7.21–7.25 (ov. m, 3H), 7.32 (d, 4J = 1.2 Hz, 1H), 7.62–7.64 (ov. m, 2H), 8.19 (s, 1H), 11.88 ppm (br., 1H); 13C-NMR (DMSO-d6, 100 MHz) of major conformer δC 13.32, 14.11, 45.77, 55.79, 60.81, 65.81, 108.49, 109.61, 110.45, 113.26, 118.72, 120.97, 122.27, 122.53, 127.78, 136.54, 142.50, 143.67, 144.14, 144.34, 148.90, 149.26, 153.79, 168.64, 168.66 ppm; 13C-NMR (DMSO-d6, 100 MHz) of minor conformer δC 13.46, 14.11, 46.36, 55.66, 60.81, 65.23, 108.52, 109.16, 110.35, 113.51, 118.80, 121.55, 122.41, 122.63, 127.74, 136.40, 142.47, 143.41, 143.74, 144.31, 147.26, 149.04, 154.05, 163.73, 168.61 ppm; Anal. Calcd. for C26H26N4O6: C, 63.66; H, 5.34; N, 11.42. Found: C, 63.42; H, 5.59; N, 11.64.
(E)-N′-(4-(2-(4-Formyl-2-methoxyphenoxy)ethoxy)-3-methoxybenzylidene)-2-(2-isopropyl-1H-benzo[d]imidazol-1-yl)acetohydrazide (22a)
According to the general procedure II, 10a (0.23 g, 1 mmol) was reacted with 21 (0.33 g, 1 mmol) in the presence of acetic acid in ethanol. Work-up followed by crystallization from DMSO gave 22a as a white powder (0.26 g, 48%); mp 218–220 °C; 1H-NMR (DMSO-d6, 400 MHz) of major conformer δH 1.29 (d, 3J = 6.8 Hz, 6H), 3.20 (sep, 3J = 6.8 Hz, 1H), 3.82 (s, 3H), 3.83 (s, 3H), 4.38 (s, 2H), 4.47 (s, 2H), 5.49 (s, 2H), 7.12–7.16 (m, 3H), 7.26–7.28 (m, 2H), 7.41–7.45 (m, 3H), 7.55–7.57 (m, 2H), 8.01 (s, 1H), 9.85 (s, 1H), 11.69 ppm (s, 1H); 1H-NMR (DMSO-d6, 400 MHz) of minor conformer δH 1.31 (d, 3J = 6.8 Hz, 6H), 3.20 (ov. sep, 3J = 6.8 Hz, 1H), 3.77 (s, 3H), 3.78 (s, 3H), 4.37 (s, 2H), 4.47 (s, 2H), 5.01 (s, 2H), 7.12–7.16 (m, 3H), 7.26–7.28 (ov. m, 2H), 7.41–7.45 (ov. m, 3H), 7.55–7.57 (ov. m, 2H), 8.20 (s, 1H), 9.85 (ov. s, 1H), 11.80 ppm (s, 1H); 13C-NMR (DMSO-d6, 100 MHz) of major conformer δC 21.68, 25.74, 43.95, 55.62, 55.67, 67.30, 109.24, 109.94, 112.52, 112.87, 118.41, 121.35, 121.53, 121.56, 121.65, 126.04, 127.21, 130.04, 135.84, 142.13, 144.49, 149.20, 149.27, 149.77, 153.23, 160.53, 168.35, 191.62 ppm; 13C-NMR (DMSO-d6, 100 MHz) of minor conformer δC 21.71, 25.74, 44.66, 55.55, 55.62, 67.19, 108.86, 110.06, 112.49, 112.81, 118.53, 121.76, 121.78, 122.03, 126.04, 127.12, 130.00, 135.59, 142.11, 147.91, 149.20, 149.27, 149.95, 153.30, 160.53, 163.41, 191.62 ppm; Anal. Calcd. for C30H32N4O6: C, 66.16; H, 5.92; N, 10.29. Found: C, 66.50; H, 6.22; N, 9.93.
(E)-N′-(4-(2-(4-Formyl-2-methoxyphenoxy)ethoxy)-3-methoxybenzylidene)-2-(2-(5-methylfuran-2-yl)-1H-benzo[d]imidazol-1-yl)acetohydrazide (22b)
According to the general procedure II, 10b (0.27 g, 1 mmol) was reacted with 21 (0.33 g, 1 mmol) in the presence of acetic acid in ethanol. Work-up followed by crystallization from DMSO gave 22b as white crystals (0.48 g, 82%); mp 203–205 °C; 1H-NMR (DMSO-d6, 400 MHz) of major conformer δH 2.25 (s, 3H), 3.81 (s, 3H), 3.82 (s, 3H), 4.37 (s, 2H), 4.47 (s, 2H), 5.76 (s, 2H), 6.32 (d, 3J = 2.8 Hz, 1H), 7.02 (d, 3J = 3.2 Hz, 1H), 7.12 (d, 3J = 8.0 Hz, 1H), 7.22–7.31 (m, 4H), 7.41–7.43 (m, 2H), 7.56 (dd, 3J = 8.0 Hz, 4J = 1.6 Hz 1H), 7.62–7.65 (m, 2H), 8.04 (s, 1H), 9.85 (s, 1H), 11.71 ppm (s, 1H); 1H-NMR (DMSO-d6, 400 MHz) of minor conformer δH 2.35 (s, 3H), 3.80 (ov. s, 3H), 3.83 (s, 3H), 4.37 (s, 2H), 4.47 (s, 2H), 5.27 (s, 2H), 6.34 (d, 3J = 2.8 Hz, 1H), 7.06 (d, 3J = 3.2 Hz, 1H), 7.12 (ov. d, 3J = 8.0 Hz, 1H), 7.22–7.31 (ov. m, 4H), 7.41–7.43 (ov. m, 2H), 7.56 (ov. dd, 3J = 8.0 Hz, 4J = 1.6 Hz, 1H), 7.62–7.65 (ov. m, 2H), 8.20 (s, 1H), 9.85 (ov. s, 1H), 11.85 ppm (s, 1H); Anal. Calcd. for C32H30N4O7: C, 65.97; H, 5.19; N, 9.62. Found: C, 65.63; H, 5.45; N, 9.42.
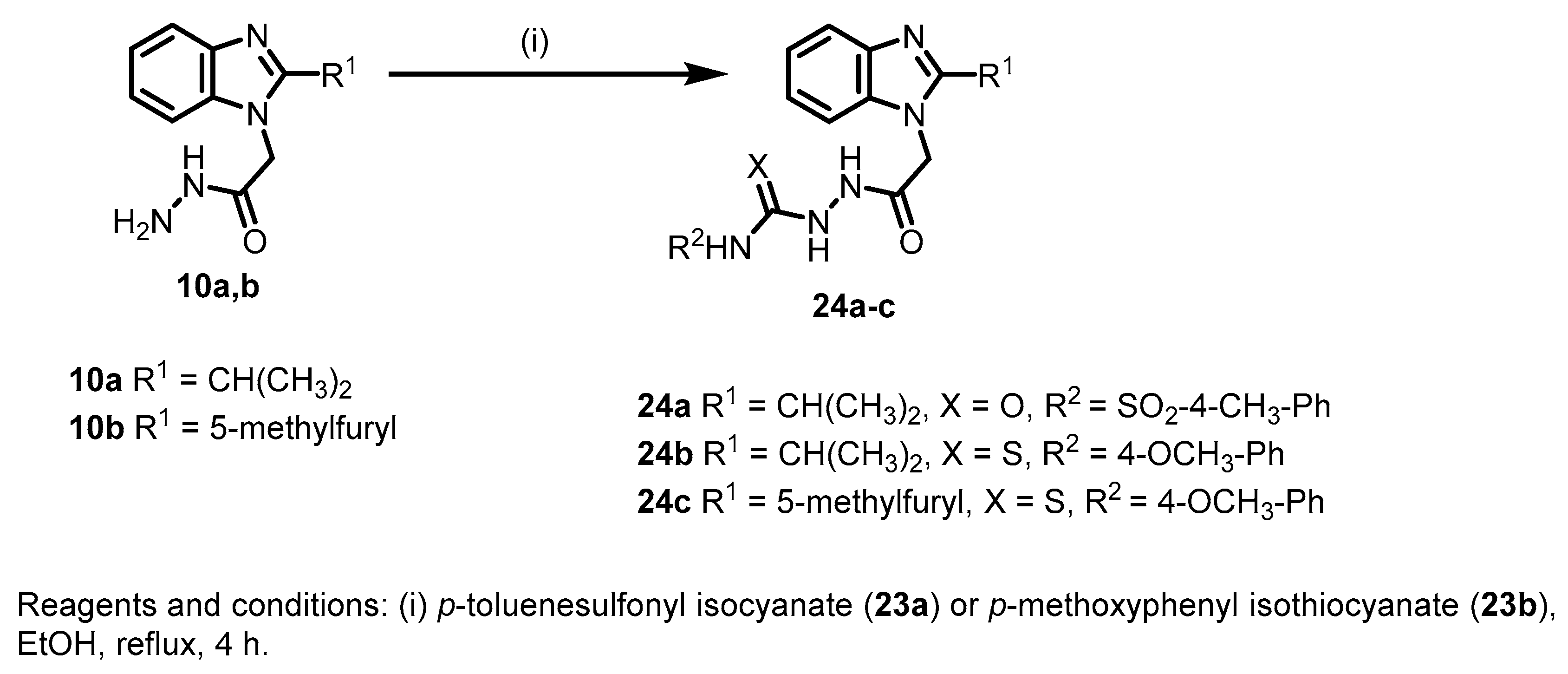
General procedure III for the synthesis of 24a–c
A solution of 10a or 10b (1 mmol), p-toluenesulfonyl isocyanate (23a) or p-methoxyphenyl isothiocyanate (23b) (1 mmol) in ethanol (10 mL) was refluxed for 4 h. The reaction mixture was filtered, dried and further purified by recrystallization from methanol to give the corresponding analytically pure compound.
2-(2-(2-Isopropyl-1H-benzo[d]imidazol-1-yl)acetyl)-N-tosylhydrazine-1-carboxamide (24a)
According to the general procedure III, 10a (0.23 g, 1 mmol) was reacted with p-toluenesulfonyl isocyanate (23a) (0.20 g, 1 mmol) in ethanol to give 24a as white powder (0.28 g, 66%); mp 197–199 °C; 1H-NMR (DMSO-d6, 400 MHz) δH 1.29 (br., 6H), 2.36 (s, 3H), 3.13-3.25 (m, 1H), 4.86 (d, 3J = 25.6 Hz, 2H), 7.14–7.15 (m, 2H), 7.25–7.26 (m, 1H), 7.35–7.38 (m, 2H), 7.54–7.55 (m, 1H), 7.70 (d, 3J = 7.0 Hz, 1H), 7.77 (d, 3J = 7.0 Hz, 1H), 8.60 (br., 1H), 9.54 (br., 1H), 10.25 ppm (br., 1H); Anal. Calcd. for C20H23N5O4S: C, 55.93; H, 5.40; N, 16.31. Found: C, 55.65; H, 5.14; N, 16.62.
2-(2-(2-Isopropyl-1H-benzo[d]imidazol-1-yl)acetyl)-N-(4-methoxyphenyl)hydrazine-1-carbothioamide (24b).
According to the general procedure III, 10a (0.23 g, 1 mmol) was reacted with p-methoxyphenyl isothiocyanate (23b) (0.23 g, 1 mmol) in ethanol to give 24b as a white powder (0.30 g, 76%); mp 232–234°C; 1H-NMR (DMSO-d6, 400 MHz) δH 1.31 (d, 3J = 6.8 Hz, 6H), 3.23 (sep, 3J = 6.8 Hz, 1H), 3.75 (s, 3H), 5.00 (s, 2H), 6.92 (d, 3J = 8.8 Hz, 2H), 7.13–7.19 (m, 2H), 7.26 (d, 3J = 8.8 Hz, 2H), 7.42 (d, 3J = 6.8 Hz, 1H), 7.56 (dd, 3J = 8.4 Hz, 4J = 1.6 Hz 1H), 9.62 (s, 2H), 10.45 ppm (s, 1H); Anal. Calcd. for C20H23N5O2S: C, 60.43; H, 5.83; N, 17.62. Found: C, 60.73; H, 5.53; N, 17.42.
N-(4-Methoxyphenyl)-2-(2-(2-(5-methylfuran-2-yl)-1H-benzo[d]imidazol-1-yl)acetyl)hydrazine-1-carbothioamide (24c).
According to the general procedure III, 10b (0.27 g, 1 mmol) was reacted with p-methoxyphenyl isothiocyante (23b) (0.23 g, 1 mmol) in ethanol to give 24c as a white powder (0.35g, 81%); mp: 189–191 °C; 1H-NMR (DMSO-d6, 400 MHz) δH 2.41 (s, 3H), 3.75 (s, 3H), 5.27 (s, 2H), 6.34 (br., 1H), 6.92 (d, 3J = 8.4 Hz, 2H), 7.06 (br., 1H), 7.23–7.27 (m, 4H), 7.53 (d, 3J = 7.6 Hz, 1H), 7.63 (d, 3J = 7.0 Hz, 1H), 9.59 (br., 1H), 9.73 (br., 1H), 10.40 ppm (br., 1H); 13C-NMR (DMSO-d6, 400 MHz) δC 13.70, 45.97, 55.54, 108.64, 110.56, 113.79, 114.10, 118.97, 122.79, 122.92, 131.94, 136.33, 142.49, 143.15, 144.58, 154.86, 157.32 ppm; Anal. Calcd. for C22H21N5O3S: C, 60.68; H, 4.86; N, 16.08. Found: C, 60.34; H, 4.98; N, 16.43.
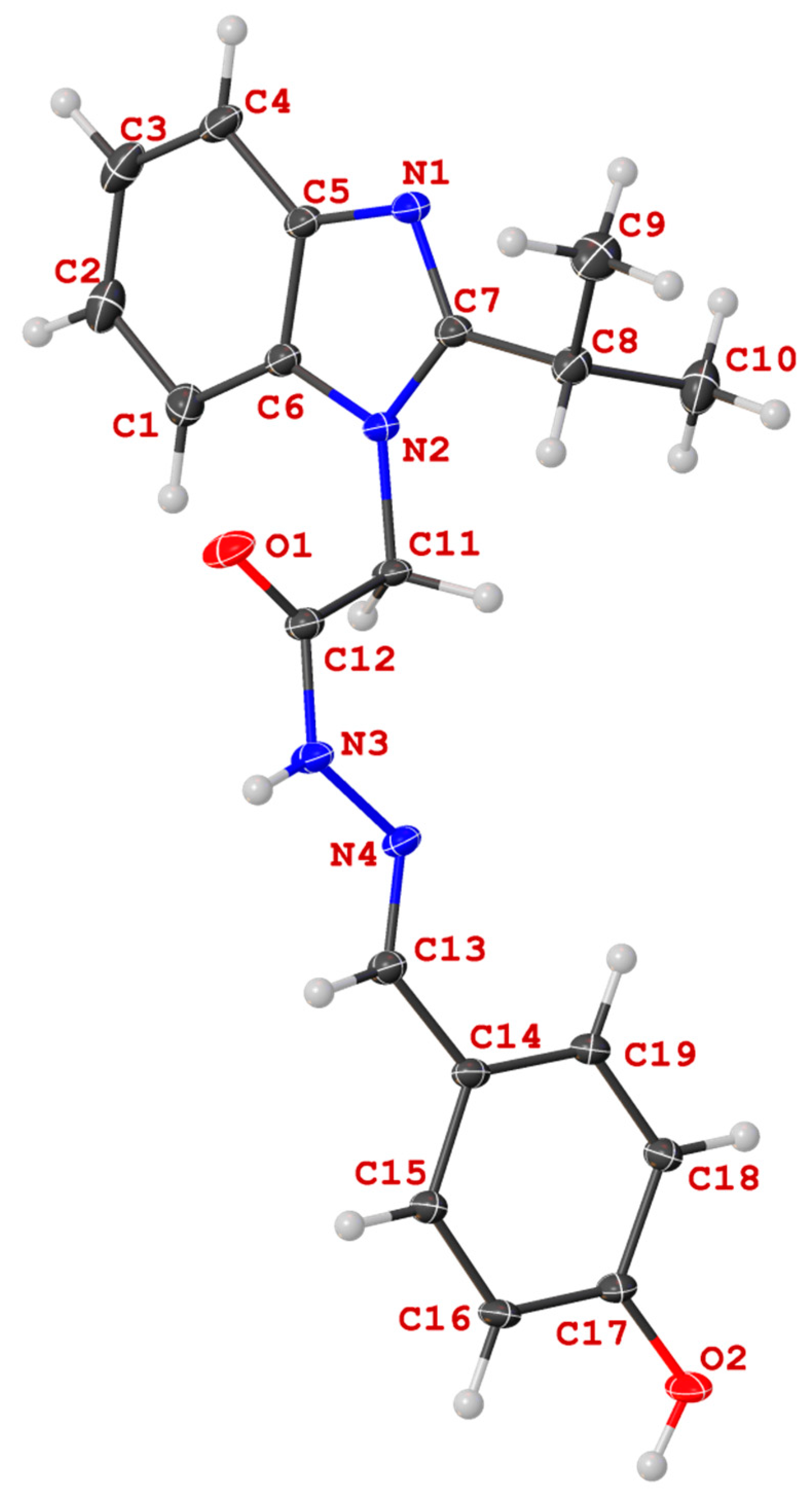
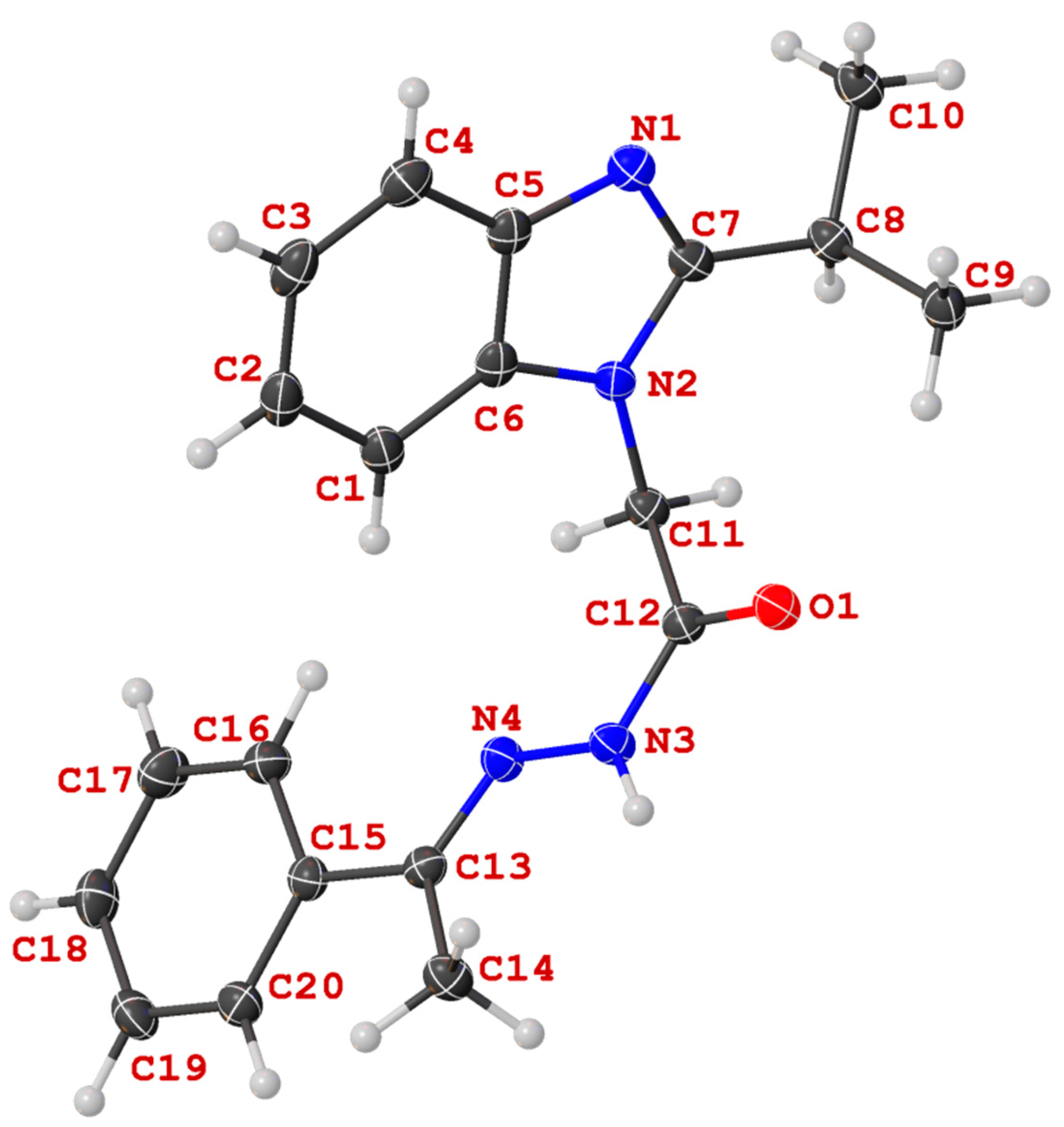
X-ray Crystallography
Crystals were grown following the protocol developed by Hope by dissolving the compound in DMSO and left to slowly crystalize [
53,
54]. Single crystal X-ray diffraction data for all compounds were collected on a Bruker APEX 2 DUO CCD diffractometer by using graphite-monochromated MoK
α (
λ = 0.71073 Å) radiation. Crystals were mounted on a MiTeGen MicroMount and collected at 100(2) K by using an Oxford Cryosystems Cobra low-temperature device. Data were collected by using omega and phi scans and were corrected for Lorentz and polarization effects by using the APEX software suite [
55,
56,
57]. Using Olex2, the structure was solved with the XT structure solution program, using the intrinsic phasing solution method and refined against │F2│ with XL using least-squares minimization [
58,
59]. Hydrogen atoms were generally placed in geometrically calculated positions and refined using a riding model unless otherwise stated. All images were rendered using Olex2 [
58]. Details of data refinements can be found in
Tables S2 and S10 in the Supporting Information. Refinement details for
14a: The hydrogen attached to N3 (H3) was allowed to freely refine. The distance of this bond was fixed using the DFIX restraint at 0.88 (0.01) Å. Refinement details for
13c: The hydrogen attached to N3 (H3) was allowed to freely refine. The distance of this bond was fixed using the DFIX restraint at 0.88 (0.01) Å. The structure contained two solvent DMSO molecules, one of which was disordered over two positions. The disordered DMSO (Part one: S2s; Part two: S3s) were modelled over two positions in a 75:25% occupancy without using any restraint or constraints.
CCDC 1973400 and 1973401 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via
www.ccdc.cam.ac.uk/data_request/cif.