High Density Supercritical Carbon Dioxide for the Extraction of Pesticide Residues in Onion with Multivariate Response Surface Methodology
Abstract
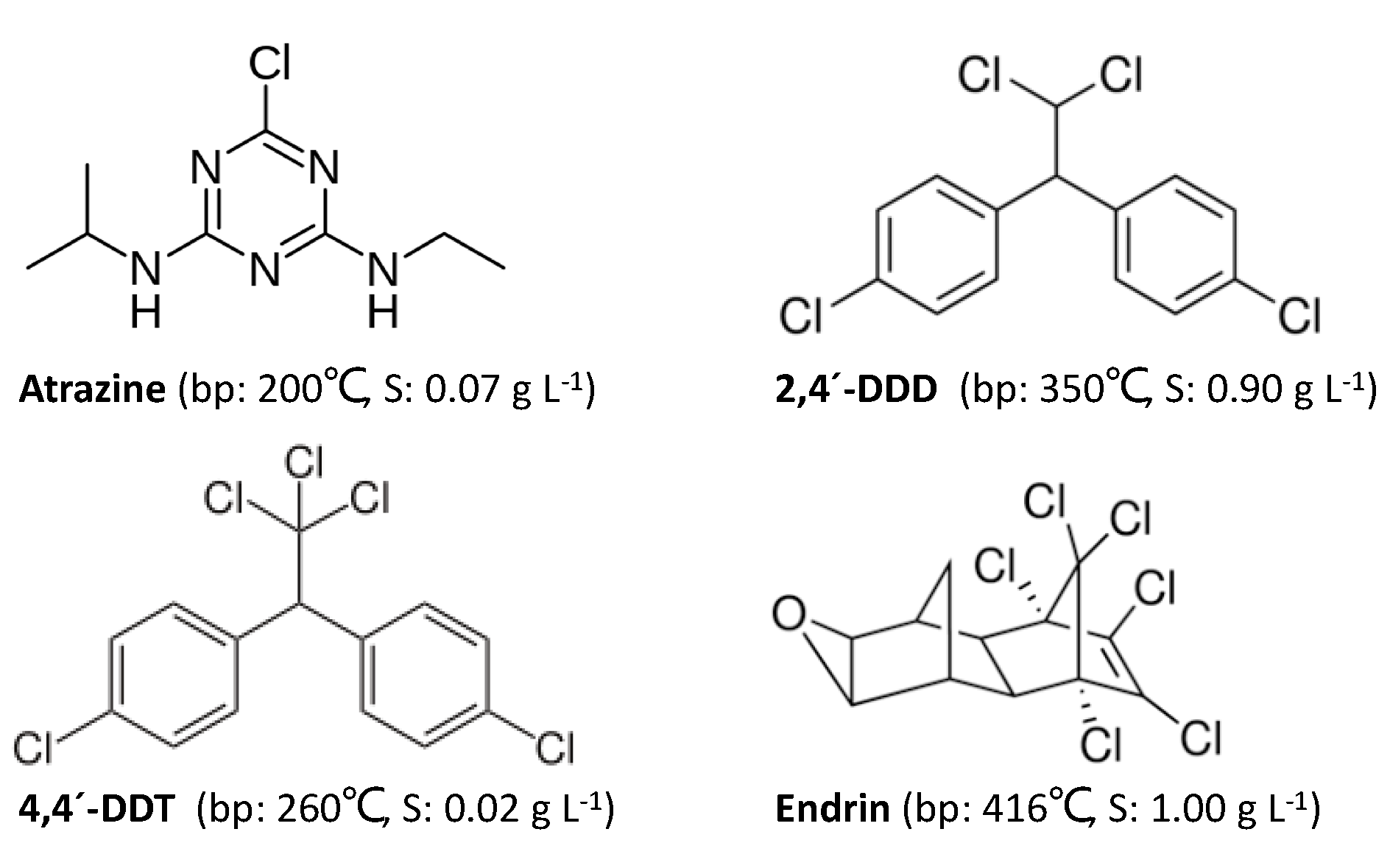
1. Introduction
2. Results and Discussion
2.1. Multivariate Parameter Optimization
2.2. Analytical Performance of the New Extraction Method
2.3. Comparison of the New SFE Method with Other Extraction Methods in the Literature
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Sample Site and Sampling
3.3. Multivariate Experimental Design
3.4. Supercritical Fluid Extraction Procedure
3.5. GC-MS Analysis
3.6. Method Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tolcha, T.; Merdassa, Y.; Megersa, N. Low-density extraction solvent based solvent-terminated dispersive liquid-liquid microextraction for quantitative determination of ionizable pesticides in environmental waters. J. Sep. Sci. 2013, 36, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.T.; Tanabe, S.; Koh, C.H. Distribution of organochlorine pesticides in sediments from Kyeonggi Bay and nearby areas. Environ. Pollut. 2001, 114, 207–2013. [Google Scholar] [CrossRef]
- Zhou, Q.; Pang, L.; Xie, G.; Xiao, J.; Bai, H. Determination of atrazine and simazine in environmental water samples by dispersive liquid-liquid microextraction with high performance liquid chromatography. Anal. Sci. 2009, 25, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Sanagi, M.M.; Abbas, H.H.; Ibrahim, W.A.W.; Aboul-Enein, H.Y. Dispersive liquid-liquid microextraction method based on solidification of floating organic droplet for the determination of triazine herbicides in water and sugarcane samples. Food Chem. 2012, 133, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Barriada-Pereira, M.; Concha-Granña, E.; González-Castro, M.J.; Muniategui-Lorenzo, S.; López-Mahía, P.; Prada-Rodríguez, D.; Fernández-Fernández, E. Microwave-assisted extraction versus Soxhlet extraction in the analysis of 21 organochlorine pesticides in plants. J. Chromatogr. A 2003, 1008, 115–122. [Google Scholar] [CrossRef]
- Beyene, Y.; Ikenaka, Y.; Saengtienchai, A.; Watanabe, K.P.; Nakayama, S.M.M.; Ishizuka, M. Organochlorine pesticides and heavy metals in fish from Lake Awassa, Ethiopia: Insights from stable isotope analysis. Chemosphere 2013, 91, 857–863. [Google Scholar]
- Chang, C.; Wei, S.; Huang, S. Improved solvent collection system for a dispersive liquid-liquid microextraction of organochlorine pesticides from water using low-density organic solvent. J. Sep. Sci. 2011, 34, 837–843. [Google Scholar] [CrossRef]
- Wang, Y.; You, J.; Ren, R.; Xiao, Y.; Gao, S.; Zhang, H.; Yu, A. Determination of triazines in honey by dispersive liquid-liquid microextraction high-performance liquid chromatography. J. Chromatogr. A 2010, 1217, 4241–4246. [Google Scholar] [CrossRef]
- Daba, D.; Hymete, A.; Bekhit, A.A.; Mohamed, A.M.I.; Bekhit, A.E. Multi residue analysis of pesticides in wheat and khat collected from different regions of Ethiopia. Bull. Environ. Contam. Toxicol. 2011, 86, 336–341. [Google Scholar] [CrossRef]
- Gebremichael, S.; Birhanu, T.; Dejene, A.T. Analysis of organochlorine pesticide residues in human and cow’s milk in the towns of Asendabo, Serbo and Jimma in South-Western Ethiopia. Chemosphere 2013, 90, 1652–1657. [Google Scholar] [CrossRef]
- Cheng, J.; Xiao, J.; Zhou, Y.; Xia, Y.; Guo, F.; Li, J. Dispersive liquid-liquid microextraction based on solidification of floating organic droplet method for the determination of diethofencarb and pyrimethanil in aqueous samples. Microchim. Acta 2011, 172, 51–55. [Google Scholar] [CrossRef]
- Mnif, W.; Hassine, A.I.H.; Bouaziz, A.; Bartegi, A.; Thomas, O.; Roig, B. Effect of endocrine disruptor pesticides. Int. J. Environ. Res. Public Health 2011, 8, 2265–2303. [Google Scholar] [CrossRef] [PubMed]
- Saraji, M.; Boroujeni, M.K. Recent developments in dispersive liquid-liquid microextraction. Anal. Bioanal. Chem. 2014, 406, 2027–2066. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, B.; Teju, E.; Gure, A.; Megersa, N. Ionic-liquid-based dispersive liquid-liquid microextraction combined with high-performance liquid chromatography for the determination of multiclass pesticide residues in water samples. J. Sep. Sci. 2015, 38, 829–835. [Google Scholar] [CrossRef]
- Tobiszewski, M.; Mechlińska, A.; Zygmunt, B.; Namieśnik, J. Green analytical chemistry in sample preparation for determination of trace organic pollutants. Trends Anal. Chem. 2009, 28, 943–951. [Google Scholar] [CrossRef]
- Tsai, W.; Huang, H. Dispersive liquid-liquid microextraction with little solvent consumption combined with gas chromatography-mass spectrometry for the pretreatment of organochlorine pesticides in aqueous samples. J. Chromatogr. A 2009, 1216, 5171–5175. [Google Scholar] [CrossRef]
- Ye, C.; Zhoua, Q.; Wang, X. Improved single drop microextraction for high sensitive analysis. J. Chromatogr. A 2007, 1139, 7–13. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Y.; Liu, D.; Zhou, Z. Determination of organophosphorus pesticides in soil by dispersive liquid-liquid microextraction and gas chromatography. J. Chromatogr. Sci. 2012, 50, 15–20. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, Z.; Wang, X.; Qiu, C. Sample preparation. J. Chromatogr. A 2008, 1184, 191–219. [Google Scholar] [CrossRef]
- Merdassa, Y.; Liu, J.; Megersa, N. Development of a one-step microwave-assisted extraction method for simultaneous determination of organophosphorus pesticides and fungicides in soils by gas chromatography-mass spectrometry. Talanta 2013, 114, 227–234. [Google Scholar] [CrossRef]
- Teo, C.C.; Tan, S.N.; Yong, J.W.H.; Hew, C.S.; Ong, E.S. Pressurized hot water extraction (PHWE). J. Chromatogr. A 2010, 1217, 2484–2494. [Google Scholar] [CrossRef]
- Camel, V. Supercritical fluid extraction as a useful method for pesticides determination. Analusis 1998, 26, 99–111. [Google Scholar] [CrossRef]
- Rissato, S.R.; Galhiane, M.S.; Knoll, F.R.N.; Apon, B.M. Supercritical fluid extraction for pesticide multiresidue analysis in honey: Determination by gas chromatography with electron-capture and mass spectrometry detection. J. Chromatogr. A 2004, 1048, 153–159. [Google Scholar] [CrossRef]
- Machado, B.A.S.; Pereira, C.G.; Nunes, S.B.; Padilha, F.F.; Umsza-Guez, M.A. Supercritical fluid extraction using CO2: Main applications and future perspectives. Sep. Sci. Technol. 2013, 48, 2741–2760. [Google Scholar] [CrossRef]
- Turner, C.; Whitehand, L.C.; Nguyen, T.; Mckeon, T. Optimization of a supercritical fluid extraction/reaction methodology for the analysis of castor oil using experimental design. J. Agric. Food Chem. 2004, 52, 26–32. [Google Scholar] [CrossRef]
- Rissato, S.R.; Galhiane, M.S.; de Souza, A.G.; Apon, B.M. Development of a supercritical fluid extraction method for simultaneous determination of organophosphorus, organohalogen, organonitrogen and pyretroids pesticides in fruit and vegetables and its comparison with a conventional method by GC-ECD and GC-MS. J. Braz. Chem. Soc. 2005, 16, 1038–1047. [Google Scholar] [CrossRef]
- Motohashi, N.; Nagashima, H.; Párkányi, C. Supercritical fluid extraction for the analysis of pesticide residues in miscellaneous samples. J. Biochem. Biophys. Methods 2000, 43, 313–328. [Google Scholar] [CrossRef]
- Sharif, K.M.; Rahman, M.M.; Azmir, J.; Mohamed, A.; Jahurul, M.H.A.; Sahena, F.; Zaidul, I.S.M. Experimental design of supercritical fluid extraction. J. Food Eng. 2014, 124, 105–116. [Google Scholar] [CrossRef]
- Couto, R.M.; Fernandes, J.; Gomes da Silva, M.D.R.; Simões, P.C. Supercritical fluid extraction of lipids from spent coffee grounds. J. Supercrit. Fluids 2009, 51, 159–166. [Google Scholar] [CrossRef]
- Belo, Y.N.; Al-Hamimi, S.; Chimuka, L.; Turner, C. Ultrahigh-pressure supercritical fluid extraction and chromatography of Moringa oleifera and Moringa peregrina seed lipids. Anal. Bioanal. Chem. 2019, 411, 3685–3693. [Google Scholar] [CrossRef]
- Al-Hamimi, S.; Mayoral, A.A.; Cunico, L.P.; Turner, C. Carbon dioxide expanded ethanol extraction: Solubility and extraction kinetics of α-pinene and cis-verbenol. Anal. Chem. 2016, 88, 4336–4345. [Google Scholar] [CrossRef]
- European Commission. Guidance Ocument on Analytical Quality Control and Method Validation Procedures for Pesticides Residues Analysis in Food and Feed; SANTE/11945/2015; European Commission: Brussels, Belgium, 2015. [Google Scholar]
- Sharif, Z.; Man, Y.B.C.; Hamid, N.S.A.; Keat, C.C. Determination of organochlorine and pyrethroid pesticides in fruit and vegetables using solid phase extraction clean-up cartridges. J. Chromatogr. A 2006, 1127, 254–261. [Google Scholar] [CrossRef]
- Ueno, E.; Oshima, H.; Saito, I.; Matsumoto, H.; Nakazawa, H. Determination of organophosphorus pesticides in onion and Welsh onion by gas chromatography with pulsed flame photometric detector. J. Pestic. Sci. 2003, 28, 422–425. [Google Scholar] [CrossRef][Green Version]
- Zhao, P.; Wang, L.; Luo, J.; Li, J.; Pan, C. Determination of pesticide residues in complex matrices using multi-walled carbon nanotubes as reversed-dispersive solid phase extraction sorbent. J. Sep. Sci. 2012, 35, 153–158. [Google Scholar] [CrossRef]
- Kolberg, D.I.; Prestes, O.D.; Adaime, M.B.; Zanella, R. Development of a fast multiresidue method for the determination of pesticides in dry samples (wheat grains, flour and bran) using QuEChERS based method and GC-MS. Food Chem. 2011, 125, 1436–1442. [Google Scholar] [CrossRef]
- Gure, A.; Lara, F.J.; Garcia-Campana, M.; Megersa, N.; Olmo-Iruela, M. Salting-out assisted liquid-liquid extraction combined with capillary HPLC for the determination of sulfonyl urea herbicides in environmental water and banana juice samples. Talanta 2014, 127, 51–55. [Google Scholar] [CrossRef]
Sample Availability: Sample of the particular onion used in this study is not available. |



| Parameters | N1 | N2 | N3 | N4 | N5 | N6 | N7 | N8 | N9 | N10 | N11 | N12 | N13 | N14 | N15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Density (g mL−1) (Pressure (bar)) | 0.7 (168) | 1.0 (668) | 0.7 (168) | 1.0 (668) | 0.7 (114) | 1.0 (528) | 0.7 (223) | 1.0 (807) | 0.85 (211) | 0.85 (211) | 0.85 (388) | 0.85 (388) | 0.85 (300) | 0.85 (300) | 0.85 (300) |
| Volume (mL) | 10 | 10 | 40 | 40 | 25 | 25 | 25 | 25 | 10 | 40 | 10 | 40 | 25 | 25 | 25 |
| Temperature (°C) | 55 | 55 | 55 | 55 | 40 | 40 | 70 | 70 | 40 | 40 | 70 | 70 | 55 | 55 | 55 |
| Pesticides | N1 | N2 | N3 | N4 | N5 | N6 | N7 | N8 | N9 | N10 | N11 | N12 | N13 | N14 | N15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Atrazine | 71 | 102 | 100 | 117 | 78 | 88 | 38 | 49 | 74 | 122 | 36 | 78 | 102 | 113 | 128 |
| 2,4´-DDD | 41 | 30 | 59 | 87 | 23 | 49 | 17 | 45 | 37 | 52 | 40 | 17 | 93 | 114 | 110 |
| Endrin | 43 | 19 | 61 | 65 | 11 | 49 | 14 | 47 | 23 | 33 | 31 | 34 | 89 | 107 | 97 |
| 4,4´-DDT | 32 | 59 | 34 | 72 | 52 | 46 | 12 | 33 | 45 | 69 | 10 | 29 | 74 | 82 | 83 |
| Pesticide | Linearity (µg kg−1) | (R2) | Predicted Recovery | Experimental Recovery (%) (%RSD) | Repeatability (%RSD) | Reproducibility (%RSD) | LOD (µg kg−1) |
|---|---|---|---|---|---|---|---|
| Atrazine | 7.8–2000 | 0.999 | 121 | 93 (4.8) | 3.8 | 1.8 | 0.2 |
| 2,4’-DDD | 7.8–2000 | 0.998 | 108 | 93 (3.3) | 0.9 | 3.6 | 0.4 |
| Endrin | 31.2–1000 | 0.998 | 98 | 103 (3.7) | 10.2 | 8.3 | 2.0 |
| 4,4’-DDT | 7.8–2000 | 0.994 | 82 | 80 (3.2) | 4.4 | 2.9 | 0.6 |
| Method | Pesticides | Matrix | Recovery (%) | LODs (µg kg−1) | R2 | Ref. |
|---|---|---|---|---|---|---|
| SFE, GC-MS | Organochlorines and s-triazines | Onion | 80–103 | 0.2–2 | 0.997–0.999 | This study |
| SPE, GC-ECD | Organochlorines and pyrethroid | Fruit, vegetables | 54–104 | 0.3–15 | 0.998–0.999 | [33] |
| QuECheRS, GC-FPD | Organophosphorus | Onion | 61–105 | 2–10 | - | [34] |
| QuEChERS, MWCNTs, GC-MS | Multiclass pesticides | Leek, onion, ginger, garlic | 78–110 | 2–20 | >0.99 | [35] |
| QuEChERS, GC-MS | Organochlorine, organophosphate and pyrethroid | Wheat grains, flour, bran | 70–120 | >2.5 | 0.99–1.0 | [36] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tolcha, T.; Gemechu, T.; Al-Hamimi, S.; Megersa, N.; Turner, C. High Density Supercritical Carbon Dioxide for the Extraction of Pesticide Residues in Onion with Multivariate Response Surface Methodology. Molecules 2020, 25, 1012. https://doi.org/10.3390/molecules25041012
Tolcha T, Gemechu T, Al-Hamimi S, Megersa N, Turner C. High Density Supercritical Carbon Dioxide for the Extraction of Pesticide Residues in Onion with Multivariate Response Surface Methodology. Molecules. 2020; 25(4):1012. https://doi.org/10.3390/molecules25041012
Chicago/Turabian StyleTolcha, Teshome, Tura Gemechu, Said Al-Hamimi, Negussie Megersa, and Charlotta Turner. 2020. "High Density Supercritical Carbon Dioxide for the Extraction of Pesticide Residues in Onion with Multivariate Response Surface Methodology" Molecules 25, no. 4: 1012. https://doi.org/10.3390/molecules25041012
APA StyleTolcha, T., Gemechu, T., Al-Hamimi, S., Megersa, N., & Turner, C. (2020). High Density Supercritical Carbon Dioxide for the Extraction of Pesticide Residues in Onion with Multivariate Response Surface Methodology. Molecules, 25(4), 1012. https://doi.org/10.3390/molecules25041012





