Insoluble-Bound Polyphenols Released from Guarana Powder: Inhibition of Alpha-Glucosidase and Proanthocyanidin Profile
Abstract
1. Introduction
2. Results and Discussion
2.1. Screening
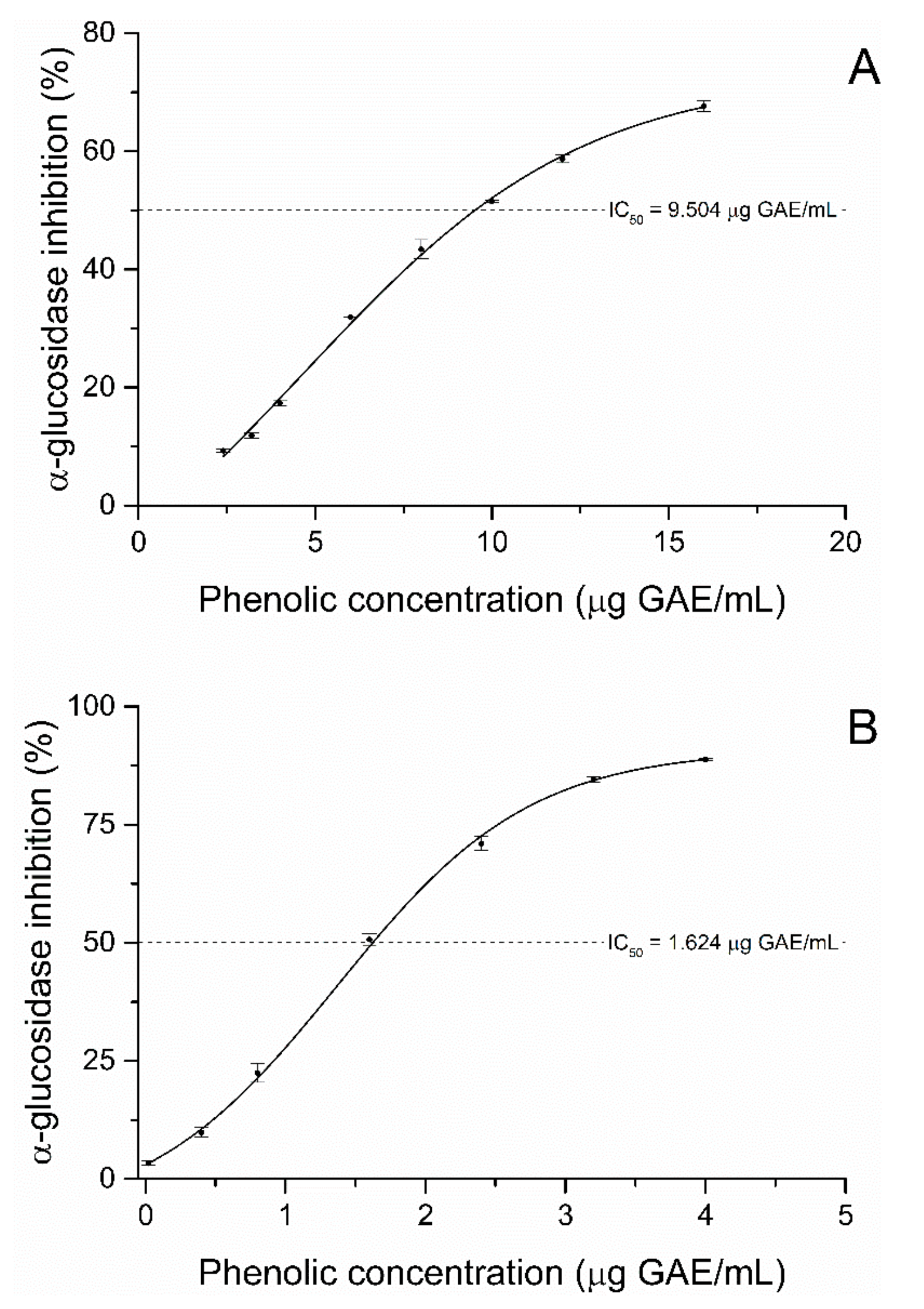
2.2. Alpha-Glucosidase Inhibition Assay
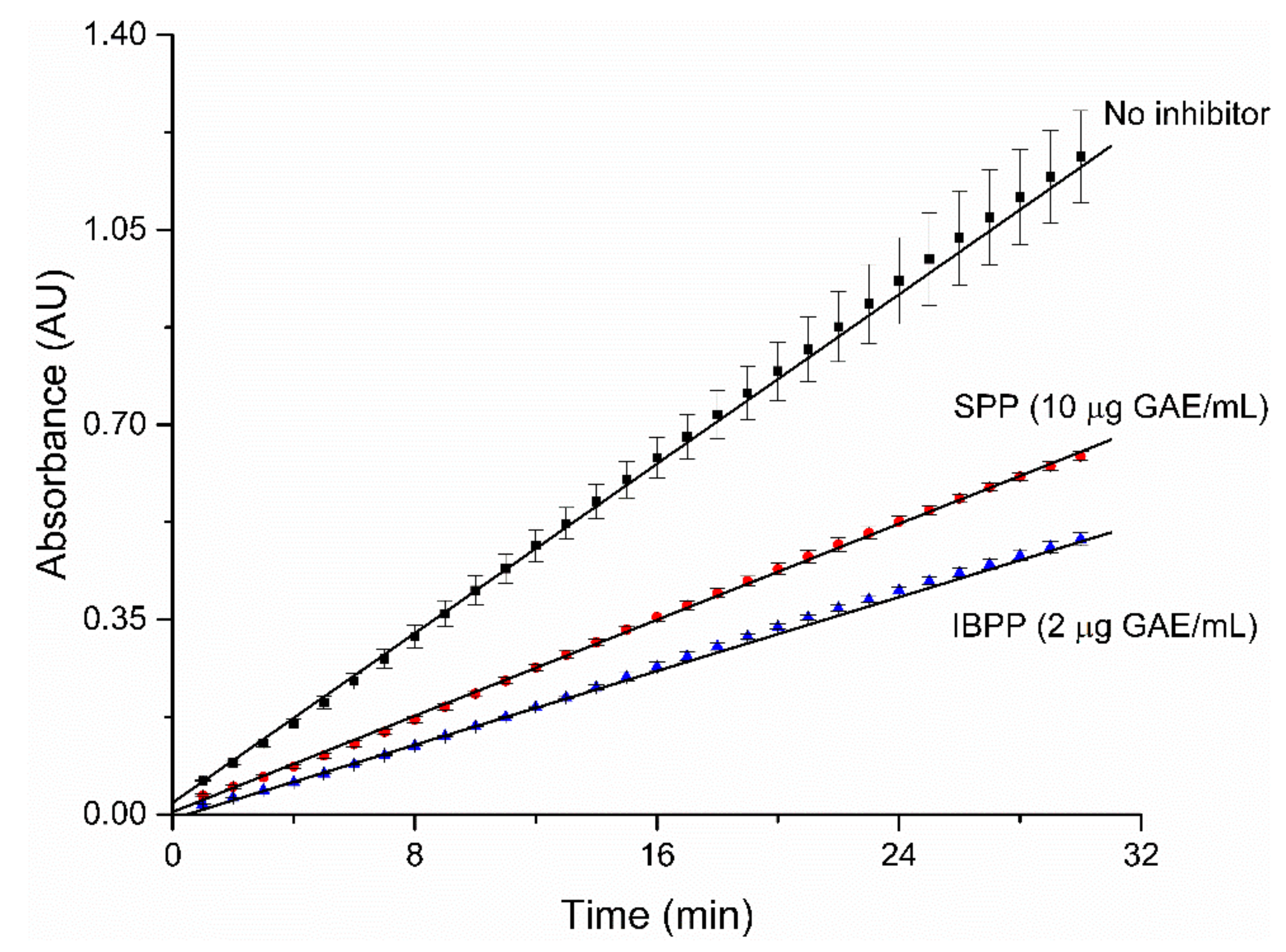
2.3. Mode of Inhibition
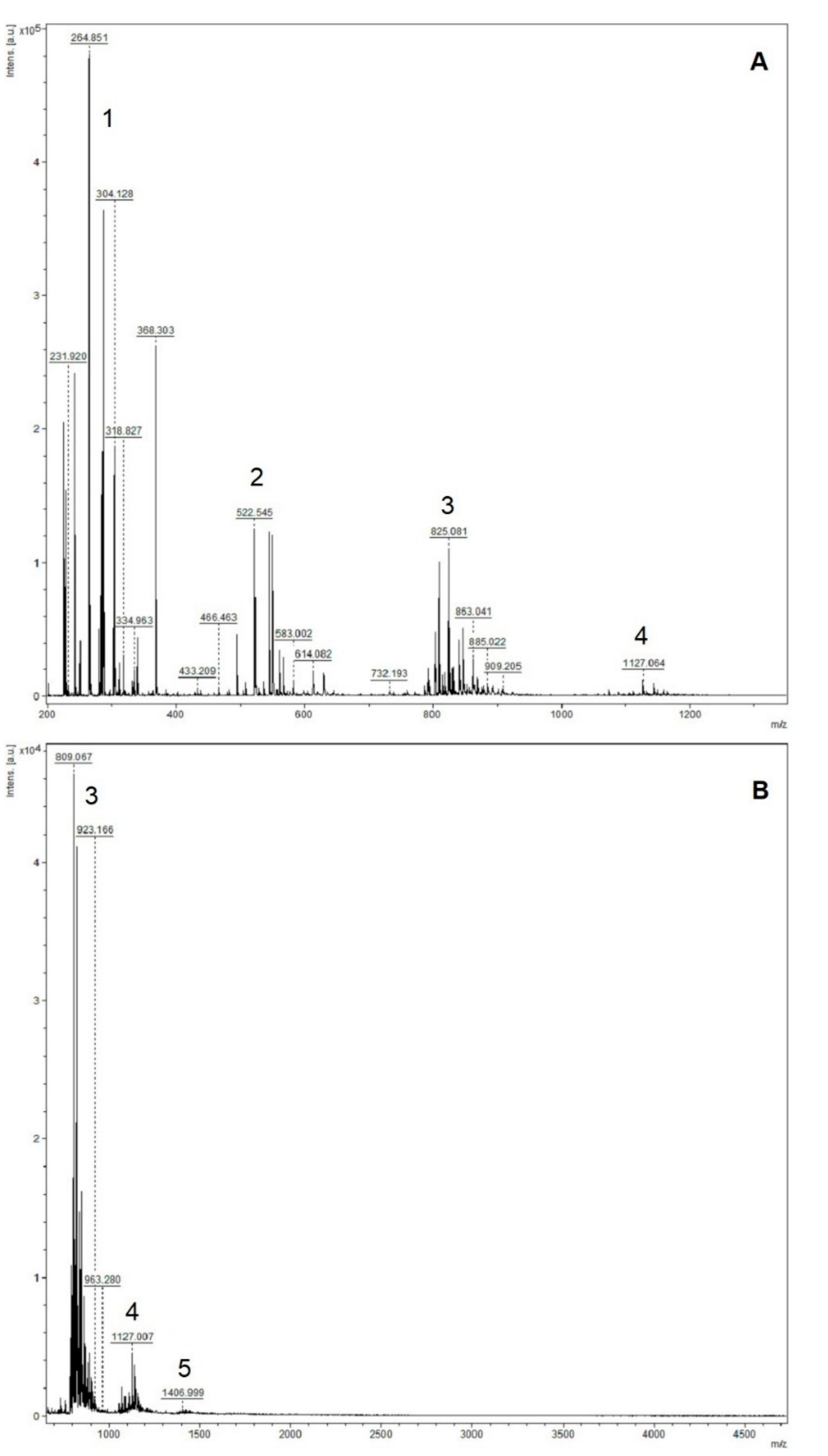
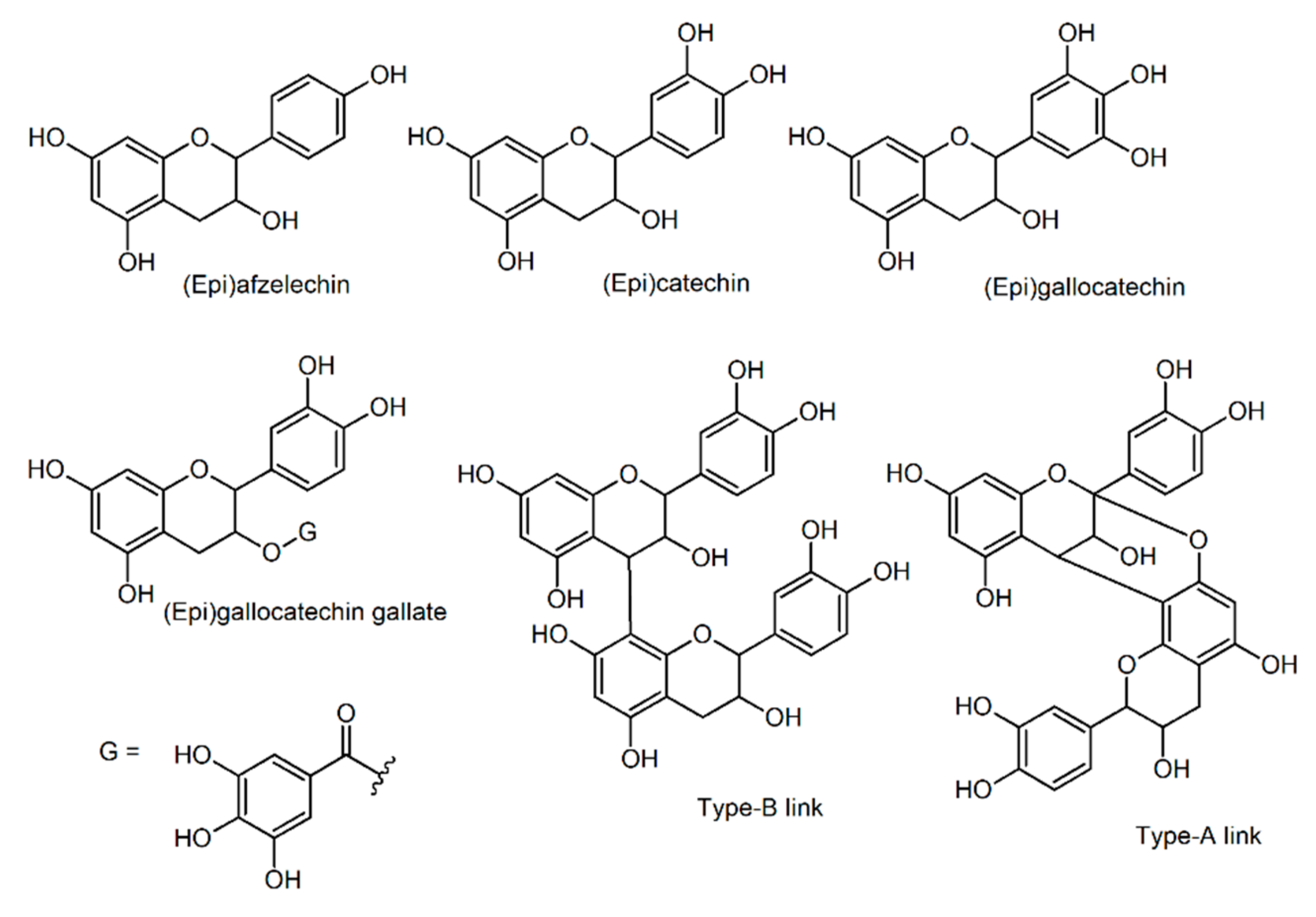
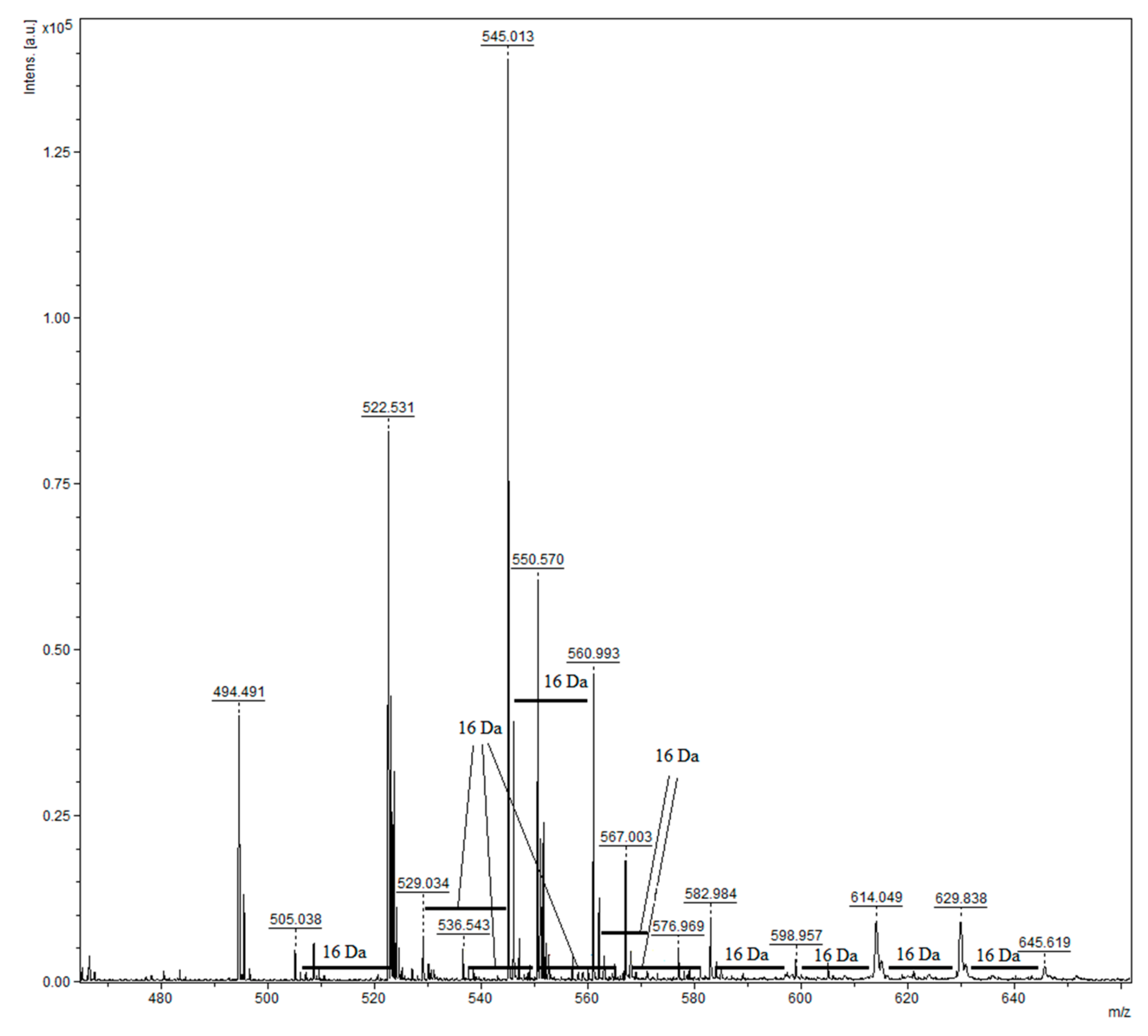
2.4. Insoluble-Bound Phenolic Profile
3. Materials and Methods
3.1. Materials
3.2. Soluble Polyphenol (SPP) Fraction Obtention
3.3. Insoluble-Bound Polyphenol (IBPP) Fraction Obtention
3.4. Total Phenolic Content (TPC)
3.5. Enzymatic Assays
3.6. Mass Spectroscopy Analysis of the IBPP Fraction
3.7. Data Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| SPP | Soluble polyphenol |
| IBPP | Insoluble-bound polyphenol |
| MALDI | Matrix-assisted Laser Desorpsion/Ionization |
| TPC | Total Phenolic Content |
References
- Smith, N.; Atroch, A.L. Guarana’s Journey from regional tonic to aphrodisiac and global energy drink. Evidence-Based Complement. Altern. Med. 2010, 7, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Haskell, C.F.; Kennedy, D.O.; Wesnes, K.A.; Milne, A.L.; Scholey, A.B. A double-blind, placebo-controlled, multi-dose evaluation of the acute behavioural effects of guarana in humans. J. Psychopharmacol. 2007, 21, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Krewer, C.D.; Ribeiro, E.E.; Ribeiro, E.A.M.; Moresco, R.N.; da Rocha, M.; Montagner, G.; Machado, M.M.; Viegas, K.; Brito, E.; da Cruz, I.B.M. Habitual intake of guarana and metabolic morbidities: An epidemiological study of an elderly Amazonian population. Phyther. Res. 2011, 25, 1367–1374. [Google Scholar]
- Lima, N.D.; Teixeira, L.; Gambero, A.; Ribeiro, M.L. Guarana (Paullinia cupana) stimulates mitochondrial biogenesis in mice fed high-fat diet. Nutrients 2018, 10, 12. [Google Scholar]
- Yonekura, L.; Martins, C.A.; Sampaio, G.R.; Monteiro, M.P.; Cesar, L.A.M.; Mioto, B.M.; Mori, C.S.; Mendes, T.M.N.; Ribeiro, M.L.; Arcari, D.P.; et al. Bioavailability of catechins from guarana (Paullinia cupana) and its effect on antioxidant enzymes and other oxidative stress markers in healthy human subjects. Food Funct. 2016, 7, 2970–2978. [Google Scholar] [CrossRef] [PubMed]
- Perez-Jimenez, J.; Diaz-Rubio, M.E.; Saura-Calixto, F. Contribution of macromolecular antioxidants to dietary antioxidant capacity: A study in the Spanish mediterranean diet. Plant Foods Hum. Nutr. 2015, 70, 365–370. [Google Scholar] [CrossRef]
- Abeywickrama, G.; Debnath, S.C.; Ambigaipalan, P.; Shahidi, F. Phenolics of selected cranberry cenotypes (Vaccinium macrocarpon Ait.) and their antioxidant efficacy. J. Agric. Food Chem. 2016, 64, 9342–9351. [Google Scholar] [CrossRef]
- Albishi, T.; John, J.A.; Al-Khalifa, A.S.; Shahidi, F. Phenolic content and antioxidant activities of selected potato varieties and their processing by-products. J. Funct. Foods 2013, 5, 590–600. [Google Scholar] [CrossRef]
- John, J.A.; Shahidi, F. Phenolic content, antioxidant and anti-inflammatory activities of seeds and leaves of date palm (Phoenix dactylifera L.). J. Food Bioact. 2019, 5, 120–130. [Google Scholar] [CrossRef]
- Liyana-Pathirana, C.M.; Shahidi, F. Importance of insoluble-bound phenolics to antioxidant properties of wheat. J. Agric. Food Chem. 2006, 54, 1256–1264. [Google Scholar] [CrossRef]
- Kim, K.-H.; Tsao, R.; Yang, R.; Cui, S.W. Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 2006, 95, 466–473. [Google Scholar] [CrossRef]
- Pang, Y.; Ahmed, S.; Xu, Y.; Beta, T.; Zhu, Z.; Shao, Y.; Bao, J. Bound phenolic compounds and antioxidant properties of whole grain and bran of white, red and black rice. Food Chem. 2018, 240, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Kumari, D.; Chandrasekara, A.; Shahidi, F. Bioaccessibility and antioxidant activities of finger millet food phenolics. J. Food Bioact. 2019, 6, 100–109. [Google Scholar] [CrossRef]
- Wang, Y.-K.; Zhang, X.; Chen, G.-L.; Yu, J.; Yang, L.-Q.; Gao, Y.-Q. Antioxidant property and their free, soluble conjugate and insoluble-bound phenolic contents in selected beans. J. Funct. Foods 2016, 24, 359–372. [Google Scholar] [CrossRef]
- Alshikh, N.; de Camargo, A.C. Phenolics of selected lentil cultivars: Antioxidant activities and inhibition of low-density lipoprotein and DNA damage. J. Funct. Foods 2015, 18, 1022–1038. [Google Scholar] [CrossRef]
- de Camargo, A.C.; Regitano-d’Arce, M.A.B.; Shahidi, F. Phenolic profile of peanut by-products: Antioxidant potential and inhibition of alpha-glucosidase and lipase activities. J. Am. Oil Chem. Soc. 2017, 94, 959–971. [Google Scholar] [CrossRef]
- Gao, Y.; Ma, S.; Wang, M.; Feng, X.-Y. Characterization of free, conjugated, and bound phenolic acids in seven commonly consumed vegetables. Molecules 2017, 22, 1878. [Google Scholar] [CrossRef]
- de Camargo, A.C.; Schwember, A.R.; Parada, R.; Garcia, S.; Maróstica, M.R.; Franchin, M.; Regitano-d’Arce, M.A.B.; Shahidi, F. Opinion on the hurdles and potential health benefits in value-added use of plant food processing by-products as sources of phenolic compounds. Int. J. Mol. Sci. 2018, 19, 3498. [Google Scholar] [CrossRef]
- de Camargo, A.C.; Favero, B.T.; Morzelle, M.C.; Franchin, M.; Alvarez-Parrilla, E.; de la Rosa, L.A.; Geraldi, M.V.; Maróstica Júnior, M.R.; Shahidi, F.; Schwember, A.R. Is chickpea a potential substitute for Soybean? Phenolic bioactives and potential health benefits. Int. J. Mol. Sci. 2019, 20, 2644. [Google Scholar] [CrossRef]
- Cires, M.J.; Wong, X.; Carrasco-Pozo, C.; Gotteland, M. The gastrointestinal tract as a key target organ for the health-promoting effects of dietary proanthocyanidins. Front Nutr. 2016, 3, 57. [Google Scholar] [CrossRef]
- Wong, X.; Carrasco-Pozo, C.; Escobar, E.; Navarrete, P.; Blachier, F.; Andriamihaja, M.; Lan, A.; Tomé, D.; Cires, M.J.; Pastene, E.; et al. Deleterious fffect of p-cresol on human colonic epithelial cells prevented by proanthocyanidin-containing polyphenol extracts from fruits and proanthocyanidin bacterial metabolites. J. Agric. Food Chem. 2016, 64, 3574–3583. [Google Scholar] [CrossRef] [PubMed]
- Casanova-Marti, A.; Serrano, J.; Portune, K.J.; Sanz, Y.; Blay, M.T.; Terra, X.; Ardevol, A.; Pinent, M. Grape seed proanthocyanidins influence gut microbiota and enteroendocrine secretions in female rats. Food Funct. 2018, 9, 1672–1682. [Google Scholar] [CrossRef] [PubMed]
- Yeo, J.; Shahidi, F. Critical evaluation of changes in the tatio of insoluble bound to soluble phenolics on antioxidant activity of lentils during germination. J. Agric. Food Chem. 2015, 63, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mora, P.; Peñas, E.; Frias, J.; Zieliński, H.; Wiczkowski, W.; Zielińska, D.; Martínez-Villaluenga, C. High-pressure-assisted enzymatic release of peptides and phenolics increases angiotensin converting enzyme I inhibitory and antioxidant activities of pinto bean hydrolysates. J. Agric. Food Chem. 2016, 64, 1730–1740. [Google Scholar] [CrossRef]
- Silva, M.B.R.; Falcão, H.G.; Kurozawa, L.E.; Prudencio, S.H.; Camargo, A.C.; de Shahidi, F.; Ida, E.I. Ultrasound- and hemicellulase-assisted extraction increase β-glucosidase activity, the content of isoflavone aglycones and antioxidant potential of soymilk. J. Food Bioact. 2019, 6. [Google Scholar] [CrossRef][Green Version]
- De Camargo, A.C.; Regitano-D’Arce, M.A.B.; Biasoto, A.C.T.; Shahidi, F. Enzyme-assisted extraction of phenolics from winemaking by-products: Antioxidant potential and inhibition of alpha-glucosidase and lipase activities. Food Chem. 2016, 212, 395–402. [Google Scholar] [CrossRef]
- Bautista-Expósito, S.; Martínez-Villaluenga, C.; Dueñas, M.; Silván, J.M.; Frias, J.; Peñas, E. Combination of pH-controlled fermentation in mild acidic conditions and enzymatic hydrolysis by Savinase to improve metabolic health-promoting properties of lentil. J. Funct. Foods 2018, 48, 9–18. [Google Scholar] [CrossRef]
- Silva, C.P.; Soares-Freitas, R.A.M.; Sampaio, G.R.; de Camargo, A.C.; Torres, E.A.F.S. Guarana as a source of bioactive compounds. J. Food Bioact. 2019, 6, 1–5. [Google Scholar] [CrossRef]
- Antonelli-Ushirobira, T.M.; Kaneshima, E.N.; Gabriel, M.; Audi, E.A.; Marques, L.C.; Mello, J.C.P. Acute and subchronic toxicological evaluation of the semipurified extract of seeds of guarand (Paullinia cupana) in rodents. Food Chem. Toxicol. 2010, 48, 1817–1820. [Google Scholar] [CrossRef]
- Dorneles, I.M.P.; Fucks, M.B.; Fontela, P.C.; Frizzo, M.N.; Winkelmann, E.R. Guarana (Paullinia cupana) presents a safe and effective anti-fatigue profile in patients with chronic kidney disease: A randomized, double-blind, three-arm, controlled clinical trial. J. Funct. Foods 2018, 51, 1–7. [Google Scholar] [CrossRef]
- da Silva, G.S.; Canuto, K.M.; Ribeiro, P.R.V.; de Brito, E.S.; Nascimento, M.M.; Zocolo, G.J.; Coutinho, J.P.; de Jesus, R.M. Chemical profiling of guarana seeds (Paullinia cupana) from different geographical origins using UPLC-QTOF-MS combined with chemometrics. Food Res. Int. 2017, 102, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Dias, T.; Silva, M.R.; Damiani, C.; da Silva, F.A. Quantification of catechin and epicatechin in foods by enzymatic-spectrophotometric method with tyrosinase. Food Anal. Methods 2017, 10, 3914–3923. [Google Scholar] [CrossRef]
- Machado, K.N.; de Freitas, A.A.; Cunha, L.H.; Faraco, A.A.G.; de Padua, R.M.; Braga, F.C.; Vianna-Soares, C.D.; Castilho, R.O. A rapid simultaneous determination of methylxanthines and proanthocyanidins in Brazilian guarana (Paullinia cupana Kunth.). Food Chem. 2018, 239, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Varatharajan, V.; Oh, W.Y.; Peng, H. Phenolic compounds in agri-food by-products, their bioavailability and health effects. J. Food Bioact. 2019, 5, 57–119. [Google Scholar] [CrossRef]
- World Health Organization: Diabetes. Available online: https://www.webcitation.org/70RGtswSF (accessed on 25 June 2018).
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- de Camargo, A.C.; Biasoto, A.C.T.; Schwember, A.R.; Granato, D.; Rasera, G.B.; Franchin, M.; Rosalen, P.L.; Alencar, S.M.; Shahidi, F. Should we ban total phenolics and antioxidant screening methods? The link between antioxidant potential and activation of NF-κB using phenolic compounds from grape by-products. Food Chem. 2019, 290, 229–238. [Google Scholar] [CrossRef]
- Jiao, Y.; Wang, Y. The effects of sweeteners and sweetness enhancers on obesity and diabetes: A review. J. Food Bioact. 2018, 4, 107–116. [Google Scholar] [CrossRef][Green Version]
- Lange, K.W.; Hauser, J.; Kaunzinger, I.; Nakamura, Y.; Reissmann, A.; Stollberg, E.; Guo, J.; Li, S. Chronic increase in sugar consumption and visual attention in Wistar rats. J. Food Bioact. 2018, 3, 161–167. [Google Scholar] [CrossRef]
- Shahidi, F.; de Camargo, A.C. Tocopherols and tocotrienols in common and emerging dietary sources: Occurrence, applications, and health benefits. Int. J. Mol. Sci. 2016, 17, 1745. [Google Scholar] [CrossRef]
- Chiasson, J.L.; Josse, R.G.; Gomis, R.; Hanefeld, M.; Karasik, A.; Laakso, M. Acarbose for prevention of type 2 diabetes mellitus: The STOP-NIDDM randomised trial. Lancet 2002, 359, 2072–2077. [Google Scholar] [CrossRef]
- Williamson, G. Possible effects of dietary polyphenols on sugar absorption and digestion. Mol. Nutr. Food Res. 2013, 57, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tsao, R. UF-LC-DAD-MSn for discovering enzyme inhibitors for nutraceuticals and functional foods. J. Food Bioact. 2019, 7, 27–35. [Google Scholar] [CrossRef][Green Version]
- Rohr, G.E.; Meier, B.; Sticher, O. Analysis of procyanidins. Stud. Nat. Prod. Chem. 2000, 21, 497–570. [Google Scholar]
- Ambigaipalan, P.; de Camargo, A.C.; Shahidi, F. Phenolic compounds of pomegranate byproducts (outer skin, mesocarp, divider membrane) and their antioxidant activities. J. Agric. Food Chem. 2016, 64, 6584–6604. [Google Scholar] [CrossRef]
- Basile, A.; Rigano, D.; Contea, B.; Bruno, M.; Rosselli, S.; Sorbo, S. Antibacterial and antifungal activities of acetonic extract from Paullinia cupana Mart. seeds. Nat. Prod. Res. 2013, 27, 2084–2090. [Google Scholar] [CrossRef]
- Matsumura, Y.; Ito, T.; Yano, H.; Kita, E.; Mikasa, K.; Okada, M.; Furutani, A.; Murono, Y.; Shibata, M.; Nishii, Y.; et al. Antioxidant potential in non-extractable fractions of dried persimmon (Diospyros kaki Thunb.). Food Chem. 2016, 202, 99–103. [Google Scholar] [CrossRef]
- Yan, S.S.; Shao, H.J.; Zhou, Z.H.; Wang, Q.; Zhao, L.H.; Yang, X.B. Non-extractable polyphenols of green tea and their antioxidant, anti-alpha-glucosidase capacity, and release during in vitro digestion. J. Funct. Foods 2018, 42, 129–136. [Google Scholar] [CrossRef]
- Domínguez-Rodríguez, G.; Marina, M.L.; Plaza, M. Strategies for the extraction and analysis of non-extractable polyphenols from plants. J. Chromatogr. A 2017, 1514, 1–15. [Google Scholar] [CrossRef]
- Kwon, Y.I.; Son, H.J.; Moon, K.S.; Kim, J.K.; Kim, J.G.; Chun, H.S.; Ahn, S.K.; Hong, C.I. Novel alpha-glucosidase inhibitors, CKD-711 and CKD-711a produced by Streptomyces sp. CK-4416. II. Biological properties. J. Antibiot. (Tokyo) 2002, 55, 462–466. [Google Scholar] [CrossRef]
- Varghese, G.K.; Bose, L.V.; Habtemariam, S. Antidiabetic components of Cassia alata leaves: Identification through α-glucosidase inhibition studies. Pharm. Biol. 2013, 51, 345–349. [Google Scholar] [CrossRef]
- Gao, J.; Xu, P.; Wang, Y.; Wang, Y.; Hochstetter, D. Combined effects of green tea extracts, green tea polyphenols or epigallocatechin gallate with acarbose on inhibition against alpha-amylase and alpha-glucosidase in vitro. Molecules 2013, 18, 11614–11623. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Kong, F. Evaluation of the in vitro alpha-glucosidase inhibitory activity of green tea polyphenols and different tea types. J. Sci. Food Agric. 2016, 96, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Strelow, J.; Dewe, W.; Iversen, P.W.; Brooks, H.B.; Radding, J.A.; Mcgee, J.; Weidner, J. Mechanism of action assays for enzymes. In Assay Guidance Manual; Sittampalan, G., Coussens, N., Brimacombe, K., et al., Eds.; Eli Lilly & Co and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2012. [Google Scholar]
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry, 6th ed.; W. H. Freeman and Company: New York, NY, USA, 2012; ISBN 978-1429234146. [Google Scholar]
- Barrett, A.H.; Farhadi, N.F.; Smith, T.J. Slowing starch digestion and inhibiting digestive enzyme activity using plant flavanols/tannins— A review of efficacy and mechanisms. LWT-Food Sci. Technol. 2018, 87, 394–399. [Google Scholar] [CrossRef]
- Dalonso, N.; de Oliveira Petkowicz, C.L. Guarana powder polysaccharides: Characterisation and evaluation of the antioxidant activity of a pectic fraction. Food Chem. 2012, 134, 1804–1812. [Google Scholar] [CrossRef] [PubMed]
- Renard, C.; Watrelot, A.A.; Le Bourvellec, C. Interactions between polyphenols and polysaccharides: Mechanisms and consequences in food processing and digestion. Trends Food Sci. Technol. 2017, 60, 43–51. [Google Scholar] [CrossRef]
- Laks, P.E.; Hemingway, R.W. Condensed tannins: Base-catalysed reactions of polymeric procyanidins with toluene-α-thiol. Lability of the interflavanoid bond and pyran ring. J. Chem. Soc., Perkin Trans. 1 1987, 2, 465–470. [Google Scholar] [CrossRef]
- White, B.L.; Howard, L.R.; Prior, R.L. Release of bound procyanidins from cranberry pomace by alkaline hydrolysis. J. Agric. Food Chem. 2010, 58, 7572–7579. [Google Scholar] [CrossRef]
- Monagas, M.; Quintanilla-Lopez, J.E.; Gomez-Cordoves, C.; Bartolome, B.; Lebron-Aguilar, R. MALDI-TOF MS analysis of plant proanthocyanidins. J. Pharm. Biomed. Anal. 2010, 51, 358–372. [Google Scholar] [CrossRef]
- Wei, S.D.; Zhou, H.C.; Lin, Y.M.; Liao, M.M.; Chai, W.M. MALDI-TOF MS analysis of condensed tannins with potent antioxidant activity from the leaf, stem bark and root bark of Acacia confusa. Molecules 2010, 15, 4369–4381. [Google Scholar] [CrossRef]
- Chai, W.M.; Wei, M.K.; Wang, R.; Deng, R.G.; Zou, Z.R.; Peng, Y.Y. Avocado proanthocyanidins as a source of tyrosinase inhibitors: Structure characterization, inhibitory activity, and mechanism. J. Agric. Food Chem. 2015, 63, 7381–7387. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Torres, J.L. Analysis of proanthocyanidins in almond blanch water by HPLC-ESI-QqQ-MS/MS and MALDI-TOF/TOF MS. Food Res. Int. 2012, 49, 798–806. [Google Scholar] [CrossRef]
- Perez-Ramirez, I.F.; Reynoso-Camacho, R.; Saura-Calixto, F.; Perez-Jimenez, J.; Pérez-Ramírez, I.F.; Reynoso-Camacho, R.; Saura-Calixto, F.; Pérez-Jiménez, J. Comprehensive characterization of extractable and nonextractable phenolic compounds by high-performance liquid chromatography-electrospray ionization-quadrupole time-of-flight of a grape/pomegranate pomace dietary supplement. J. Agric. Food Chem. 2018, 66, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Ucar, M.B.; Ucar, G.; Pizzi, A.; Gonultas, O. Characterization of Pinus brutia bark tannin by MALDI-TOF MS and 13C NMR. Ind. Crops Prod. 2013, 49, 697–704. [Google Scholar] [CrossRef]
- de Camargo, A.C.; Regitano-d’Arce, M.A.B.; Gallo, C.R.; Shahidi, F. Gamma-irradiation induced changes in microbiological status, phenolic profile and antioxidant activity of peanut skin. J. Funct. Foods 2015, 12, 129–143. [Google Scholar] [CrossRef]
- Ayoub, M.; de Camargo, A.C.; Shahidi, F. Antioxidants and bioactivities of free, esterified and insoluble-bound phenolics from berry seed meals. Food Chem. 2016, 197, 221–232. [Google Scholar] [CrossRef]
- Rahman, M.J.; de Camargo, A.C.; Shahidi, F. Phenolic and polyphenolic profiles of chia seeds and their in vitro biological activities. J. Funct. Foods 2017, 35, 622–634. [Google Scholar] [CrossRef]
- Frazier, R.A.; Deaville, E.R.; Green, R.J.; Stringano, E.; Willoughby, I.; Plant, J.; Mueller-Harvey, I. Interactions of tea tannins and condensed tannins with proteins. J. Pharm. Biomed. Anal. 2010, 51, 490–495. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Lin, G.-M.; Lin, H.-Y.; Chang, S.-T. Characteristics of proanthocyanidins in leaves of Chamaecyparis obtusa var formosana as strong α-glucosidase inhibitors. J. Sci. Food Agric. 2018, 98, 3806–3814. [Google Scholar]
- de Camargo, A.C.; da Silva Lima, R. A perspective on phenolic compounds, their potential health benefits, and international regulations: The revised Brazilian normative on food supplements. J. Food Bioact. 2019, 7, 7–17. [Google Scholar] [CrossRef]
- Brigatto Fontes, L.C.; Ferraz da Silva Torres, A.E.; Yonekura, L. Optimization of the extraction of antioxidants from guarana (Paullinia cupana) and grape (Vitis labrusca var. Izabel) pomace using response surface methodology. African J. Food Sci. Technol. 2014, 5, 53–59. [Google Scholar]
- Gonzales, G.B.; Smagghe, G.; Raes, K.; Van Camp, J. Combined alkaline hydrolysis and ultrasound-assisted extraction for the release of nonextractable phenolics from cauliflower (Brassica oleracea var. botrytis) waste. J. Agric. Food Chem. 2014, 62, 3371–3376. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Shahidi, F.; de Camargo, A.C. JFB–Trends in food bioactives. J. Food Bioact. 2019, 8, 2–5. [Google Scholar] [CrossRef]
Sample Availability: Samples of the guarana powder, SPP fraction and IBPP fraction are available from the authors. |
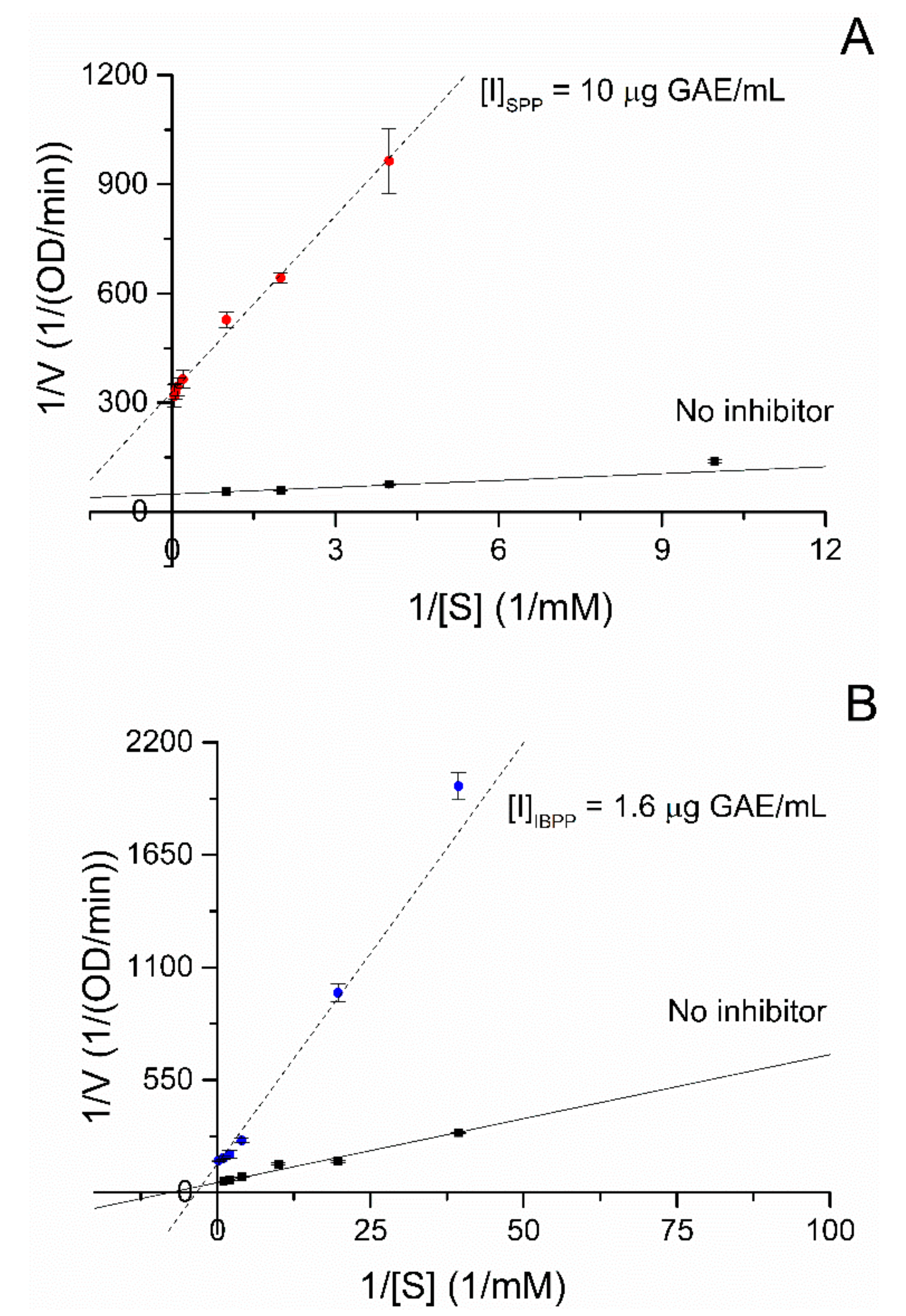
| Km (mM) | Vmax (OD/min) | KI (µg/mL) | K’I (µg/mL) | K’I/KI | Inhibition Mode | |
|---|---|---|---|---|---|---|
| No inhibitor | 0.128 | 0.020 | / | / | / | / |
| SPP | 0.489 | 0.003 | 0.403 | 1.735 | 4.30 | Mixed |
| IBPP | 0.292 | 0.007 | 0.287 | 0.847 | 2.95 | Mixed |
| Calculated [M + H]+ | Observed [M + H]+ | Possible Identity | |
|---|---|---|---|
| Dimers | 544.136 | 545.013 | Type-A afzelechin dimer |
| 560.131 | 560.993 | Type-A afzelechin/catechin dimer | |
| 576.126 | 576.969 | Type-A catechin dimer | |
| Trimers | 814.188 | 815.082 | Type-A afzelechin trimer |
| 830.183 | 831.052 | Type-A (2)afzelechin/catechin trimer | |
| 846.178 | 847.021 | Type-A afzelechin/(2)catechin | |
| 862.173 | 862.995 | Type-A catechin trimer | |
| Tetramers | 1090.288 | 1089.061 | Type-B afzelechin tetramer |
| 1106.283 | 1105.044 | Type-B (3)afzelechin/catechin tetramer | |
| 1122.278 | 1121.009 | Type-B (2)afzelechin/(2)catechin tetramer | |
| 1116.230 | 1117.052 | Type-A (2)afzelechin/(2)catechin tetramer | |
| 1132.225 | 1133.023 | Type-A afzelechin/(3)catechin tetramer | |
| 1148.220 | 1148.998 | Type-A catechin tetramer | |
| 1164.215 | 1164.962 | Type-A (3)catechin/gallocatechin tetramer |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinaffi, A.C.d.C.; Sampaio, G.R.; Soares, M.J.; Shahidi, F.; de Camargo, A.C.; Torres, E.A.F.S. Insoluble-Bound Polyphenols Released from Guarana Powder: Inhibition of Alpha-Glucosidase and Proanthocyanidin Profile. Molecules 2020, 25, 679. https://doi.org/10.3390/molecules25030679
Pinaffi ACdC, Sampaio GR, Soares MJ, Shahidi F, de Camargo AC, Torres EAFS. Insoluble-Bound Polyphenols Released from Guarana Powder: Inhibition of Alpha-Glucosidase and Proanthocyanidin Profile. Molecules. 2020; 25(3):679. https://doi.org/10.3390/molecules25030679
Chicago/Turabian StylePinaffi, Ana Clara da Costa, Geni Rodrigues Sampaio, Maiara Jurema Soares, Fereidoon Shahidi, Adriano Costa de Camargo, and Elizabeth A. F. S. Torres. 2020. "Insoluble-Bound Polyphenols Released from Guarana Powder: Inhibition of Alpha-Glucosidase and Proanthocyanidin Profile" Molecules 25, no. 3: 679. https://doi.org/10.3390/molecules25030679
APA StylePinaffi, A. C. d. C., Sampaio, G. R., Soares, M. J., Shahidi, F., de Camargo, A. C., & Torres, E. A. F. S. (2020). Insoluble-Bound Polyphenols Released from Guarana Powder: Inhibition of Alpha-Glucosidase and Proanthocyanidin Profile. Molecules, 25(3), 679. https://doi.org/10.3390/molecules25030679








