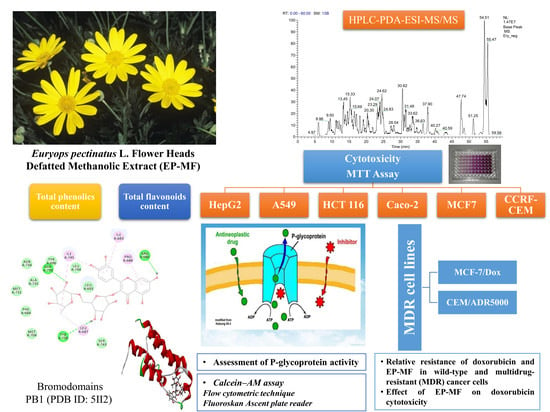
Euryops pectinatus L. Flower Extract Inhibits P-glycoprotein and Reverses Multi-Drug Resistance in Cancer Cells: A Mechanistic Study
Abstract
1. Introduction
2. Results and Discussion
2.1. Phytochemical Analysis
2.1.1. Quantitation of Total Phenolic and Flavonoid Content
2.1.2. Metabolites Profiling of E. pectinatus Flower Heads Methanolic Fraction
Phenolic Acids and Their Derivatives
Flavonol Glycosides
2.2. Cytotoxicity of E. pectinatus Using MTT Assay
2.2.1. IC50 Values and Relative Resistance of Doxorubicin and EP-MF in Wild-type and Multidrug-resistant MCF-7/Dox, CEM/ADR5000 Leukemia Cells
2.2.2. Effects of Combinations of EP-MF and Doxorubicin
2.2.3. Effects of the Extract on P-glycoprotein Activity in CEM/ADR5000 Cells and in Caco-2 Cells
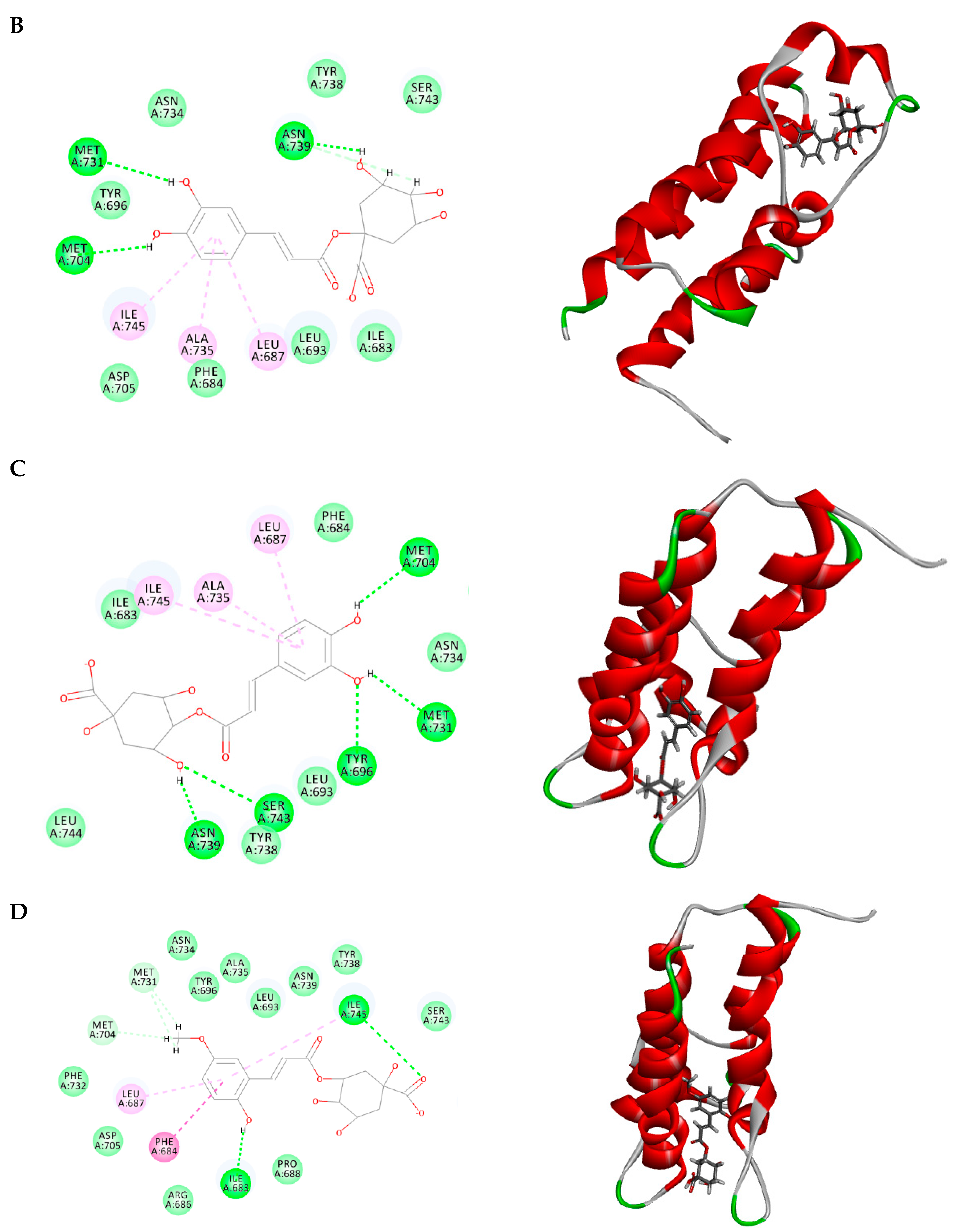
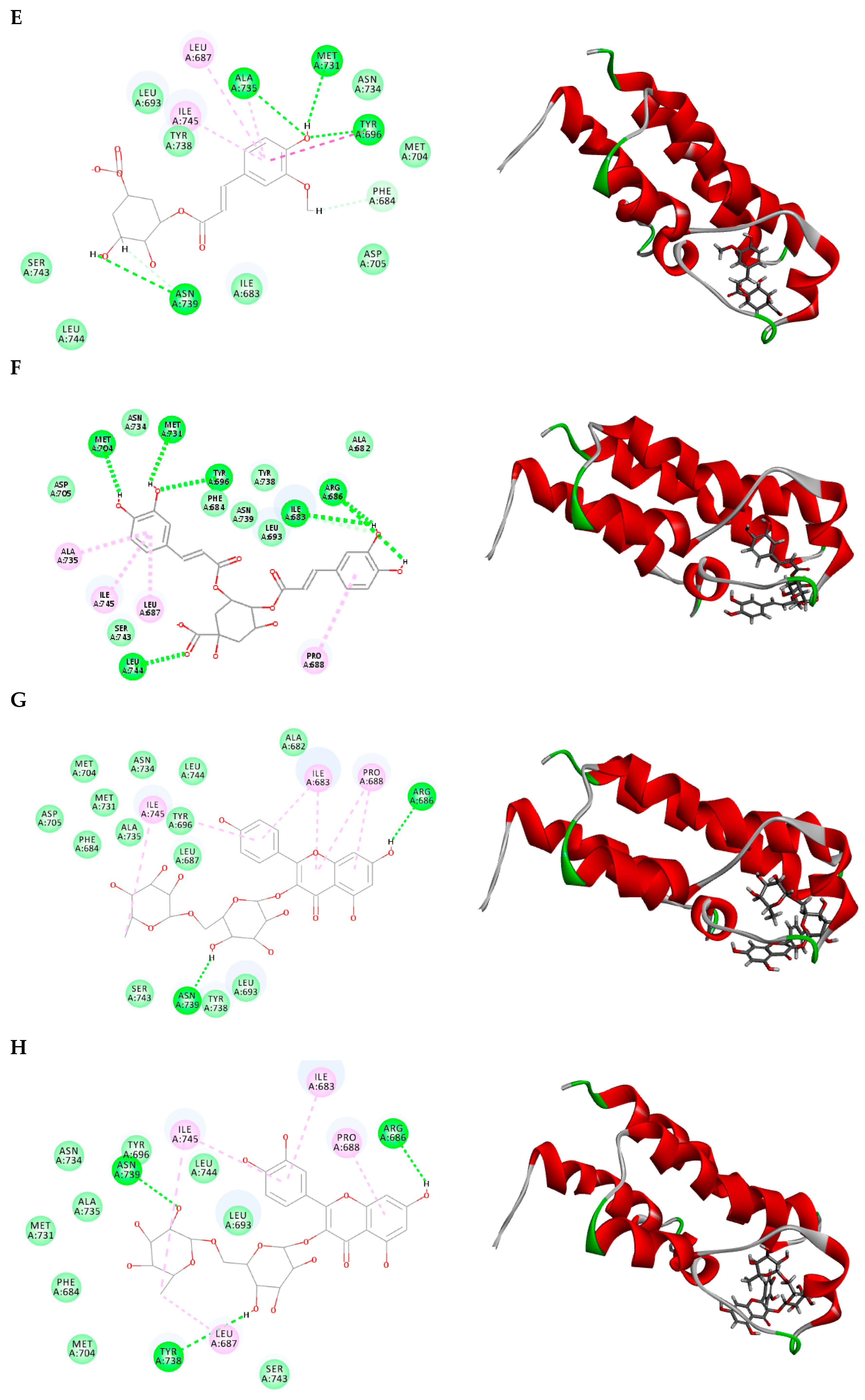
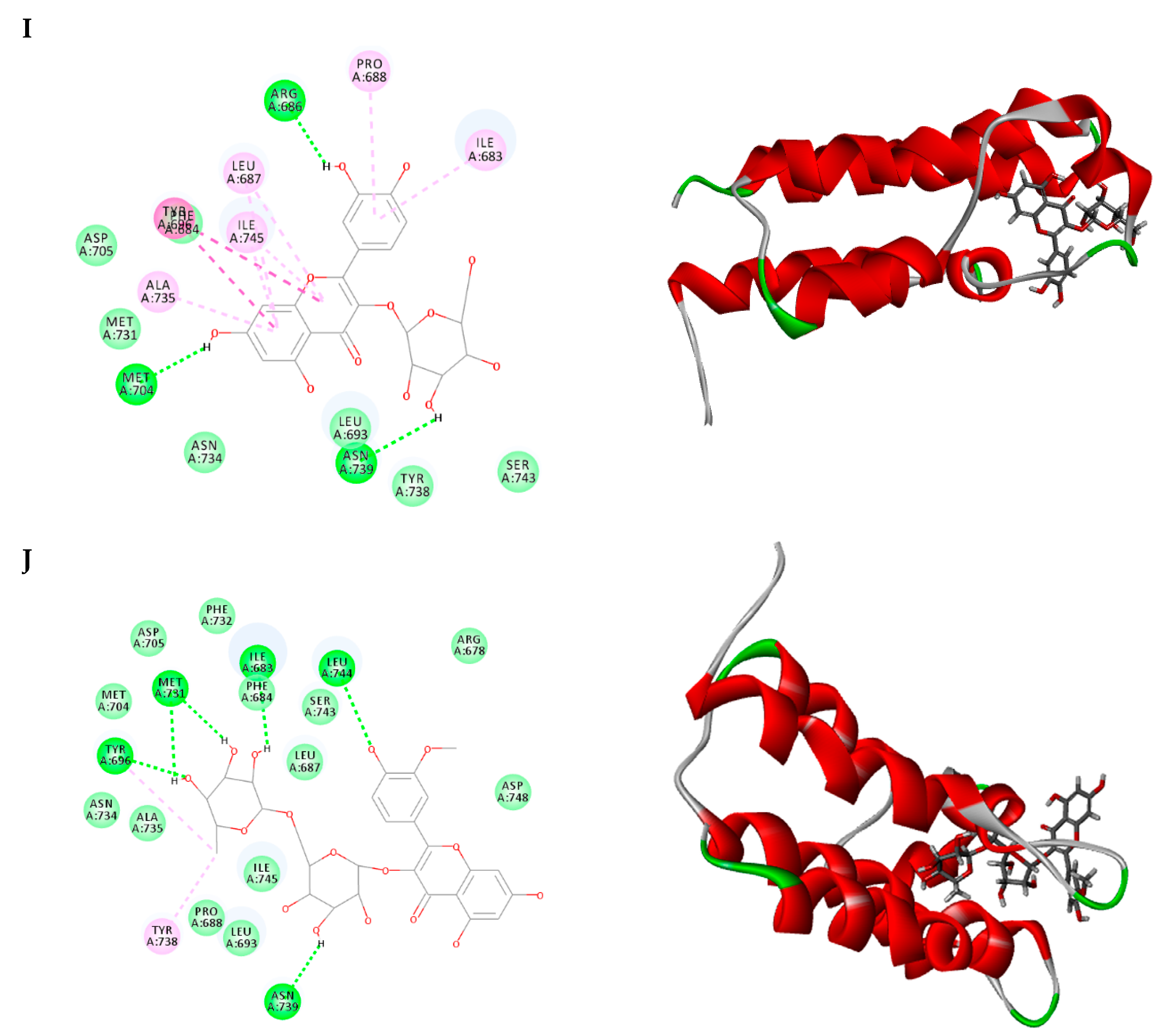
2.3. Molecular Docking
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material
3.3. Preparation of Plant Extract
3.4. Phytochemical Analysis
3.4.1. Quantitation of Total Flavonoids
3.4.2. Quantitation of Total Phenolic Compounds
3.4.3. HPLC-PDA-ESI-MS/MS Analysis
3.5. Biological Activity
3.5.1. Cytotoxicity and Cell Proliferation Assays
Cell Culture
Assessment of Cytotoxicity Using MTT Assay
Assessment of IC50 and Relative Resistance Values of Doxorubicin and E. pectinatus
Effects of E. pectinatus on Doxorubicin Cytotoxicity
3.5.2. Effects of E. pectinatus on P-glycoprotein
Assessment of P-glycoprotein Activity via Calcein–AM Assay Using Flow Cytometric Technique
Calcein–AM Assay Using a Fluoroskan Ascent Plate Reader
3.6. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HPLC-PDA-ESI-MS | High performance liquid chromatography-photodiode array-electrospray ionization-mass spectrometry |
| E. pectinatus | Euryops pectinatus |
| EP-MF | Euryops pectinatus defatted methanol fraction |
| HepG2 | liver hepatocellular carcinoma cell line |
| Caco-2 | colon carcinoma cell line |
| MCF7 | breast cancer cell line |
| A549 | lung cancer cell line |
| HCT 116 | colon cancer cell line |
| CCRF-CEM | acute lymphocytic leukemia cell line |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| MDR | multidrug resistance |
| P-gp | P-glycoprotein |
| ABC | ATP-binding cassette |
| PB1 | human polybromo-1 |
| BRDs | Bromodomains |
References
- Persidis, A. Cancer multidrug resistance. Nat. Biotechnol. 1999, 17, 94. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Hoti, S.L. Development of fourth generation ABC inhibitors from natural products: A novel approach to overcome cancer multidrug resistance. Anti-Cancer Agents Med. Chem. 2015, 15, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Mohana, S.; Ganesan, M.; Agilan, B.; Karthikeyan, R.; Srithar, G.; Beaulah Mary, R.; Ananthakrishnan, D.; Velmurugan, D.; Rajendra Prasad, N.; Ambudkar, S.V. Screening dietary flavonoids for the reversal of P-glycoprotein-mediated multidrug resistance in cancer. Mol. Biosyst. 2016, 12, 2458–2470. [Google Scholar] [CrossRef] [PubMed]
- Elkady, W.M.; Ayoub, I.M. Chemical profiling and antiproliferative effect of essential oils of two Araucaria species cultivated in Egypt. Ind. Crops Prod. 2018, 118, 188–195. [Google Scholar] [CrossRef]
- Jackson, W.P.U. Origins and Meanings of Names of South African Plant Genera; University of Cape Town: Cape Town, South Africa, 1990. [Google Scholar]
- Hafez, E.; Paulis, M.; Ahmed, M.; Fathy, M.N.; Abdel-Lateff, A.; Algandaby, M. Protective and anti-oxidant activity of the Euryops arabicus against paracetamol induced hepatorenal toxicity in rats. J. Toxicol. Clin. 2015, 5. [Google Scholar] [CrossRef]
- Mothana, R.A.; Alsaid, M.S.; Al-Musayeib, N.M. Phytochemical analysis and in vitro antimicrobial and free-radical-scavenging activities of the essential oils from Euryops arabicus and Laggera decurrens. Molecules 2011, 16, 5149–5158. [Google Scholar] [CrossRef] [PubMed]
- Nesseem, D.I.; Michel, C.G. Development and characterization of local anti-inflammatory implantation for the controlled release of the hexane extract of the flower-heads of Euryops pectinatus L. (Cass.). Drug Discov. Ther. 2011, 5, 96–106. [Google Scholar] [CrossRef][Green Version]
- Michel, C.G. Essential oil and morphological characters of leaves of Euryops pectinatus (L.) Cass. cultivated in Egypt. Bull. Fac. Pharm. Cairo Univ. 2004, 42, 145–158. [Google Scholar]
- Catarino, M.D.; Silva, A.M.S.; Saraiva, S.C.; Sobral, A.J.F.N.; Cardoso, S.M. Characterization of phenolic constituents and evaluation of antioxidant properties of leaves and stems of Eriocephalus africanus. Arab. J. Chem. 2018, 11, 62–69. [Google Scholar] [CrossRef]
- Simirgiotis, M.; Benites, J.; Areche, C.; Sepúlveda, B. Antioxidant capacities and analysis of phenolic compounds in three endemic Nolana species by HPLC-PDA-ESI-MS. Molecules 2015, 20, 11490–11507. [Google Scholar] [CrossRef]
- Clifford, M.N.; Wu, W.; Kirkpatrick, J.; Kuhnert, N. Profiling the chlorogenic acids and other caffeic acid derivatives of herbal Chrysanthemum by LC-MSn. J. Agric. Food. Chem. 2007, 55, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Inbaraj, B.S.; Chen, B.H. Determination of phenolic acids and flavonoids in Taraxacum formosanum Kitam by liquid chromatography-tandem mass spectrometry coupled with a post-column derivatization technique. Int. J. Mol. Sci. 2012, 13, 260–285. [Google Scholar] [CrossRef] [PubMed]
- Kenny, O.; Smyth, T.J.; Walsh, D.; Kelleher, C.T.; Hewage, C.M.; Brunton, N.P. Investigating the potential of under-utilised plants from the Asteraceae family as a source of natural antimicrobial and antioxidant extracts. Food Chem. 2014, 161, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Engels, C.; Schieber, A.; Gأ¤nzle, M.G. Sinapic acid derivatives in defatted Oriental mustard (Brassica juncea L.) seed meal extracts using UHPLC-DAD-ESI-MSn and identification of compounds with antibacterial activity. Eur. Food Res. Technol. 2012, 234, 535–542. [Google Scholar] [CrossRef]
- Farag, M.A.; Sakna, S.T.; El-fiky, N.M.; Shabana, M.M.; Wessjohann, L.A. Phytochemical, antioxidant and antidiabetic evaluation of eight Bauhinia L. species from Egypt using UHPLC–PDA–qTOF-MS and chemometrics. Phytochemistry 2015, 119, 41–50. [Google Scholar] [CrossRef]
- Farag, M.A.; El-Ahmady, S.H.; Elian, F.S.; Wessjohann, L.A. Metabolomics driven analysis of artichoke leaf and its commercial products via UHPLC–q-TOF-MS and chemometrics. Phytochemistry 2013, 95, 177–187. [Google Scholar] [CrossRef]
- De Nisco, M.; Manfra, M.; Bolognese, A.; Sofo, A.; Scopa, A.; Tenore, G.C.; Pagano, F.; Milite, C.; Russo, M.T. Nutraceutical properties and polyphenolic profile of berry skin and wine of Vitis vinifera L. (cv. Aglianico). Food Chem. 2013, 140, 623–629. [Google Scholar] [CrossRef]
- Djoukeng, J.D.; Arbona, V.; Argamasilla, R.; Gomez-Cadenas, A. Flavonoid profiling in leaves of Citrus genotypes under different environmental situations. J. Agric. Food. Chem. 2008, 56, 11087–11097. [Google Scholar] [CrossRef]
- Lusa, M.G.; Martucci, M.E.; Loeuille, B.F.; Gobbo-Neto, L.; Appezzato-da-Glória, B.; Da Costa, F.B. Characterization and evolution of secondary metabolites in Brazilian Vernonieae (Asteraceae) assessed by LC-MS fingerprinting. J. Linn. Soc. Lond. Bot. 2016, 182, 594–611. [Google Scholar] [CrossRef]
- Bai, J.; Zhao, S.; Fan, X.; Chen, Y.; Zou, X.; Hu, M.; Wang, B.; Jin, J.; Wang, X.; Hu, J.; et al. Inhibitory effects of flavonoids on P-glycoprotein in vitro and in vivo: Food/herb-drug interactions and structure-activity relationships. Toxicol. Appl. Pharmacol. 2019, 369, 49–59. [Google Scholar] [CrossRef]
- Myrianthopoulos, V.; Gaboriaud-Kolar, N.; Tallant, C.; Hall, M.-L.; Grigoriou, S.; Brownlee, P.M.; Fedorov, O.; Rogers, C.; Heidenreich, D.; Wanior, M. Discovery and optimization of a selective ligand for the switch/sucrose nonfermenting-related bromodomains of polybromo protein-1 by the use of virtual screening and hydration analysis. J. Med. Chem. 2016, 59, 8787–8803. [Google Scholar] [CrossRef] [PubMed]
- Piyanete, C.; Meechai, P.; Nakbanpotecc, W. Antioxidant activities and phenolic contents of extracts from Salvinia molesta and Eichornia crassipes. Res. J. Biol. Sci. 2009, 4, 1113–1117. [Google Scholar]
- Kumazawa, S.; Tanguchi, M.; Suzuki, Y.; Shimura, M.; Kwon, MS.; Nakayama, T. Antioxidant activity of polyphenols in carob pods. J. Agric. Food. Chem. 2002, 50, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Meth. 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Fujisawa, T.; Filppakopoulos, P. Functions of bromodomain-containing proteins and their roles in homeostasis and cancer. Nat. Rev. Mol. Cell Biol. 2017, 246. [Google Scholar] [CrossRef]
- Ayoub, I.M.; Korinek, M.; Hwang, T.-L.; Chen, B.-H.; Chang, F.-R.; El-Shazly, M.; Singab, A.N.B. Probing the antiallergic and anti-inflammatory activity of biflavonoids and dihydroflavonols from Dietes bicolor. J. Nat. Prod. 2018. [Google Scholar] [CrossRef]
Sample Availability: Samples of the plant extract are available from the authors. |
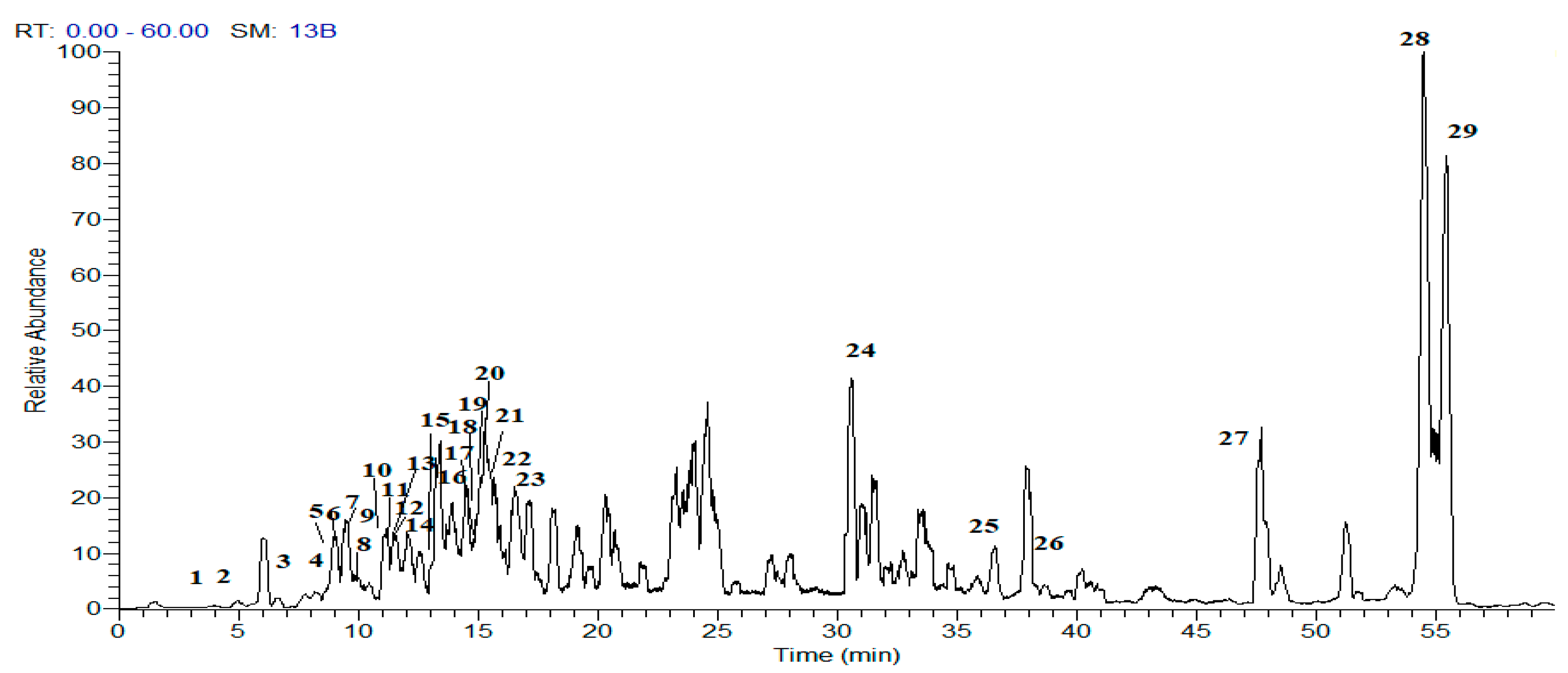

| Compound | Rt (min) | [M − H]− | Fragment Ions | UV–vis (λmax nm) | Class | References | |
|---|---|---|---|---|---|---|---|
| 1 | Malic acid | 4.05 | 133 | 115, 99 | 261 | Organic acid | |
| 2 | Cinnamic acid | 4.15 | 147 | 129, 115 | 261 | Phenolic acid | |
| 3 | Protocatechuic acid | 6.61 | 153 | 109 | 257, 290 | Phenolic acid | [10] |
| 4 | Chlorogenic acid | 8.35 | 353 | 191, 179 | 319, 379 | Phenolic acid | [11] |
| 5 | Caffeic acid-O-hexoside | 8.99 | 341 | 179, 135 | 319 | Phenolic glycoside | [12,13] |
| 6 | Quinic acid | 9.14 | 191 | 173, 127, 111 | 216, 323 | Organic acid | [10] |
| 7 | Caffeoyl quinic acid | 9.48 | 353 | 191, 179 | 324 | Phenolic acid | [17] |
| 8 | Caffeoyl quinic acid (isomer) | 9.90 | 353 | 191, 179 | 324 | Phenolic acid | [17] |
| 9 | Caffeoyl quinic acid dimer | 9.57 | 707 | 645, 514, 456, 353 | 290, 322 | Phenolic acid | |
| 10 | Feruloyl quinic acid | 11.04 | 367 | 191, 173 | 295, 322 | Phenolic acid | [11,12] |
| 11 | Caffeic acid | 11.18 | 179 | 135 | 297, 324 | Phenolic acid | [12,13] |
| 12 | Feruloyl quinic acid isomer | 11.47 | 367 | 191, 173 | 295, 322 | Phenolic acid | [11,12] |
| 13 | Sinapic acid | 11.59 | 223 | 205, 179, 163 | 296, 341 | Phenolic acid | [14,15] |
| 14 | Syringetin-3-O-hexoside | 12.15 | 507 | 345 | 289, 325 | Flavonol glycoside | [18] |
| 15 | Dicaffeoyl quinic acid hexoside | 13.09 | 677 | 516 | 289, 324 | Phenolic glycoside | |
| 16 | Caffeoyl malonylhexoside | 13.79 | 427 | 409, 265, 179. 135 | 291, 324 | Phenolic glycoside | |
| 17 | Dicaffeoyl quinic acid | 14.56 | 515 | 515, 353, 191, 179 | 253, 300, 333 | Phenolic acid | [13] |
| 18 | Kaempferol-O-rutinoside | 14.84 | 593 | 431, 285 | 253, 332 | Flavonol glycoside | [16] |
| 19 | Dicaffeoyl quinic acid | 14.94 | 515 | 353, 191, 179 | 253, 300, 333 | Phenolic acid | |
| 20 | Quercetin-3-O-rutinoside (Rutin) | 15.23 | 609 | 343, 301 | 254,348 | Flavonol glycoside | [19] |
| 21 | Quercetin-3-O-hexoside | 15.43 | 463 | 301 | 255, 351 | Flavonol glycoside | [19,20] |
| 22 | Isorhamnetin-O-rutinoside | 16.52 | 623 | 315, 300, 255 | 253, 292, 338 | Flavonol glycoside | [20] |
| 23 | Isorhamnetin-3-O-hexoside | 17.20 | 477 | 315, 314 | 288, 336 | Flavonol glycoside | [16] |
| 24 | Unidentified | 30.66 | 344 | 258, 226 | 282 | Unknown | |
| 25 | Unidentified | 36.57 | 507 | 407, 283, 231, 153 | 279 | Unknown | |
| 26 | Hydroxy-octadecadienoic acid | 38.73 | 295 | 295, 277 | 282 | Fatty acid | [17] |
| 27 | Hydroxyhexadecanoic acid | 47.66 | 271 | 271, 254, 242, 226 | 277 | Fatty acid | [17] |
| 28 | Unidentified | 54.56 | 817 | 577, 559, 538. 443, 317, 285 | 279 | Unknown | |
| 29 | Unidentified | 55.42 | 815 | 785, 733, 606, 560, 538, 483, 415, 278, 235 | 275 | Unknown | |
| Drug IC50 (μg/mL) | MCF-7 | HepG2 | A549 | Caco-2 | HCT-116 | CCRF-CEM |
|---|---|---|---|---|---|---|
| Doxorubicin | 0.44 | 0.977 | 5.842 | 8.508 | 6.87 | 0.033 |
| 5-Fluorouracil | 1.71 | 4.12 | 10.32 | 20.22 | 18.33 | 1.22 |
| E. pectinatus-MF | 23.32 | 16.15 | 52.12 | 17.04 | 31.55 | 28.76 |
| A.Drug | MCF-7 IC50 (µg/mL) Mean ± SE | MCF-7/Dox IC50 (µg/mL) Mean ± SE | Relative Resistance |
| Doxorubicin | 0.44 ± 0.032 | 16.82 * ± 1.12 | 38.23 |
| E. pectinatus-MF | 23.32 a ± 1.52 | 65.92 a,* ± 3.78 | 2.83 |
| B.Drug | CCRF-CEM IC50µg/mL Mean ± SE | CEM/ADR5000 IC50µg/mL Mean ± SE | Relative Resistance |
| Doxorubicin | 0.033 ± 0.006 | 4.57 * ± 0.41 | 138.48 |
| E. pectinatus-MF | 28.76 a ± 1.86 | 97.42 a,* ± 4.36 | 3.39 |
| MCF-7/Dox IC50 (μg/mL) Mean ± SE | CEM/ADR5000 IC50 (μg/mL) Mean ± SE | Caco-2 cells IC50 (μg/mL) Mean ± SE | |
|---|---|---|---|
| Doxorubicin | 16.82 ± 1.12 | 4.57 ± 0.41 | 8.50 ± 0.65 |
| DOX + E. pectinatus-MF | 7.64 a ± 0.54 | 2.18 a ± 0.22 | 5.28 a ± 0.73 |
| Compound | CEM/ADR5000 Intracellular Calcein Fluorescence (% Control) Mean ± SE | |||
| Concentration | ||||
| 0.5 μg/mL | 2.5 μg/mL | 5 μg/mL | 10 μg/mL | |
| Control | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| Verapamil | 100.54 ± 4.24 | 167.63 a ± 9.41 | 268.24 a ± 11.42 | 324.4 a ± 18.71 |
| E. pectinatus-MF | 102.01 ± 6.82 | 162.54 a ± 10.82 | 284.63 a ± 21.42 | 296.9 a ± 12.05 |
| Compound | Caco-2 Intracellular Calcein fluorescence (% Control) Mean ± SE | |||
| Concentration | ||||
| 0.5 μg/mL | 2.5 μg/mL | 5 μg/mL | 10 μg/mL | |
| Control | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| Verapamil | 103.71 ± 8.67 | 165.63 a ± 13.78 | 220.25 a ± 11.12 | 304.13 a ± 12.55 |
| E. pectinatus-MF | 103.67 ± 7.01 | 173.92 a ± 13.86 | 221.74 a ± 20.17 | 288.22 a ± 16.64 |
| Compound | Binding Energy ΔG (Kcal/mol) | |
|---|---|---|
| Rule-based | pH-based | |
| Luteolin (LU2) | −35.58 | −35.58 |
| Malic acid (1) | −22.80 | −22.80 |
| Cinnamic acid (2) | −22.16 | −22.16 |
| Protocatechuic acid (3) | −22.52 | −27.03 |
| Chlorogenic acid (4) | −34.63 | −37.35 |
| Quinic acid (6) | −23.99 | −23.99 |
| 1-O-Caffeoyl quinic acid (7 or 8) | −41.14 | −36.80 |
| 4-O-Caffeoyl quinic acid (7 or 8) | −35.94 | −40.48 |
| 3-Feruloyl quinic acid (10 or 12) | −44.29 | −38.26 |
| Caffeic acid (11) | −28.83 | −26.33 |
| 5-Feruloyl quinic acid ((10 or 12) | −44.70 | −37.76 |
| Sinapic acid (13) | −33.58 | −28.39 |
| Dicaffeoyl quinic acid (17 or 19) | −50.49 | −49.04 |
| Kaempferol-O-rutinoside (18) | −43.79 | −53.74 |
| Rutin (20) | −41.21 | −50.12 |
| Quercetin-O-hexoside (21) | −48.53 | −49.53 |
| Isorhamnetin-O-rutinoside (22) | −36.76 | −52.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
M. Elkady, W.; M. Ayoub, I.; Abdel-Mottaleb, Y.; ElShafie, M.F.; Wink, M. Euryops pectinatus L. Flower Extract Inhibits P-glycoprotein and Reverses Multi-Drug Resistance in Cancer Cells: A Mechanistic Study. Molecules 2020, 25, 647. https://doi.org/10.3390/molecules25030647
M. Elkady W, M. Ayoub I, Abdel-Mottaleb Y, ElShafie MF, Wink M. Euryops pectinatus L. Flower Extract Inhibits P-glycoprotein and Reverses Multi-Drug Resistance in Cancer Cells: A Mechanistic Study. Molecules. 2020; 25(3):647. https://doi.org/10.3390/molecules25030647
Chicago/Turabian StyleM. Elkady, Wafaa, Iriny M. Ayoub, Yousra Abdel-Mottaleb, Mohamed F. ElShafie, and Michael Wink. 2020. "Euryops pectinatus L. Flower Extract Inhibits P-glycoprotein and Reverses Multi-Drug Resistance in Cancer Cells: A Mechanistic Study" Molecules 25, no. 3: 647. https://doi.org/10.3390/molecules25030647
APA StyleM. Elkady, W., M. Ayoub, I., Abdel-Mottaleb, Y., ElShafie, M. F., & Wink, M. (2020). Euryops pectinatus L. Flower Extract Inhibits P-glycoprotein and Reverses Multi-Drug Resistance in Cancer Cells: A Mechanistic Study. Molecules, 25(3), 647. https://doi.org/10.3390/molecules25030647