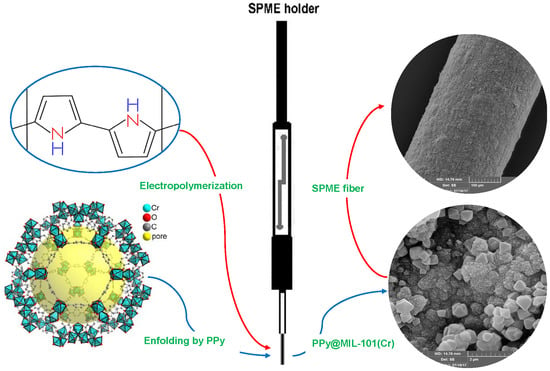
Chromium-Based Polypyrrole/MIL-101 Nanocomposite as an Effective Sorbent for Headspace Microextraction of Methyl tert-Butyl Ether in Soil Samples
Abstract
1. Introduction
2. Results and Discussion
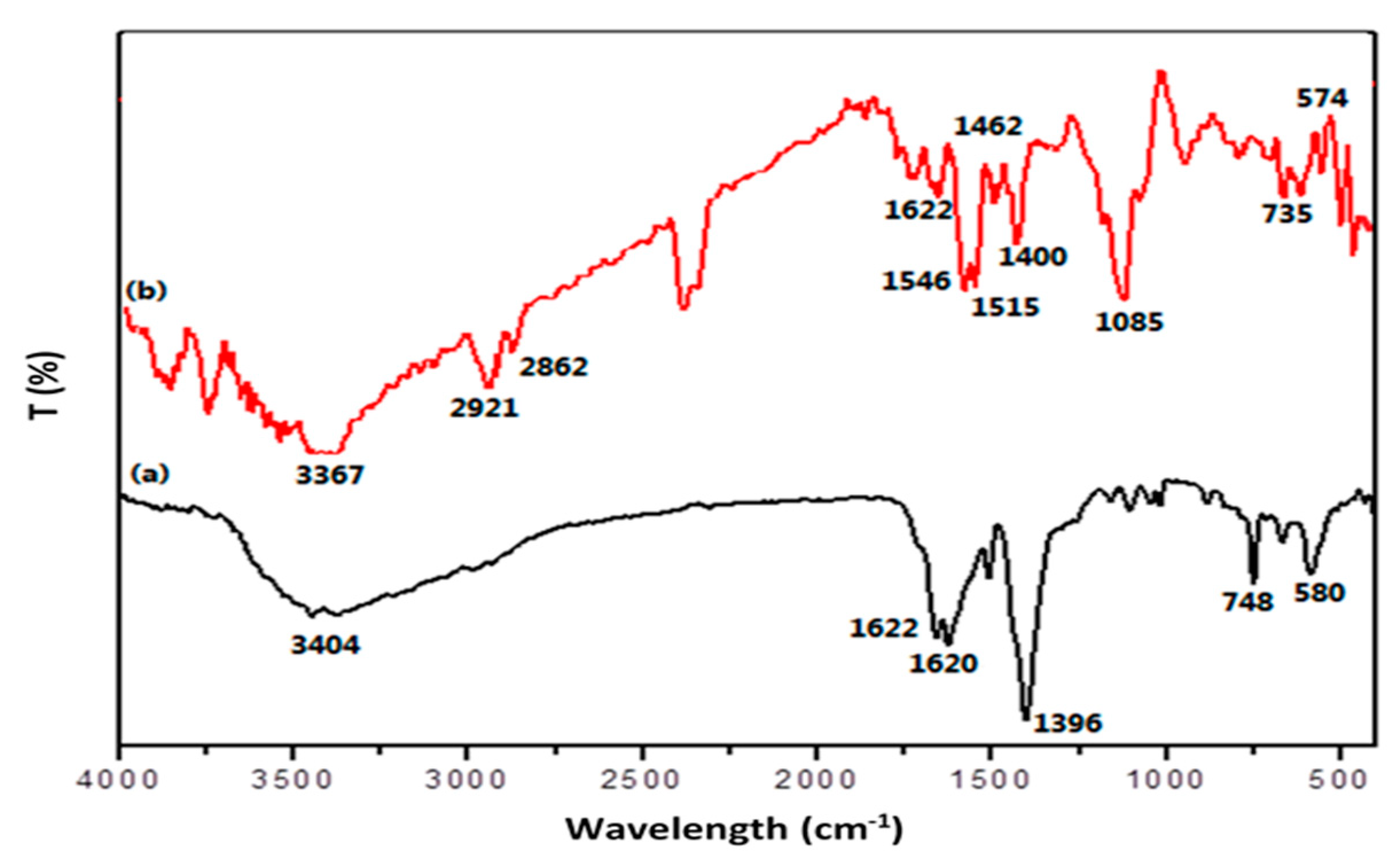
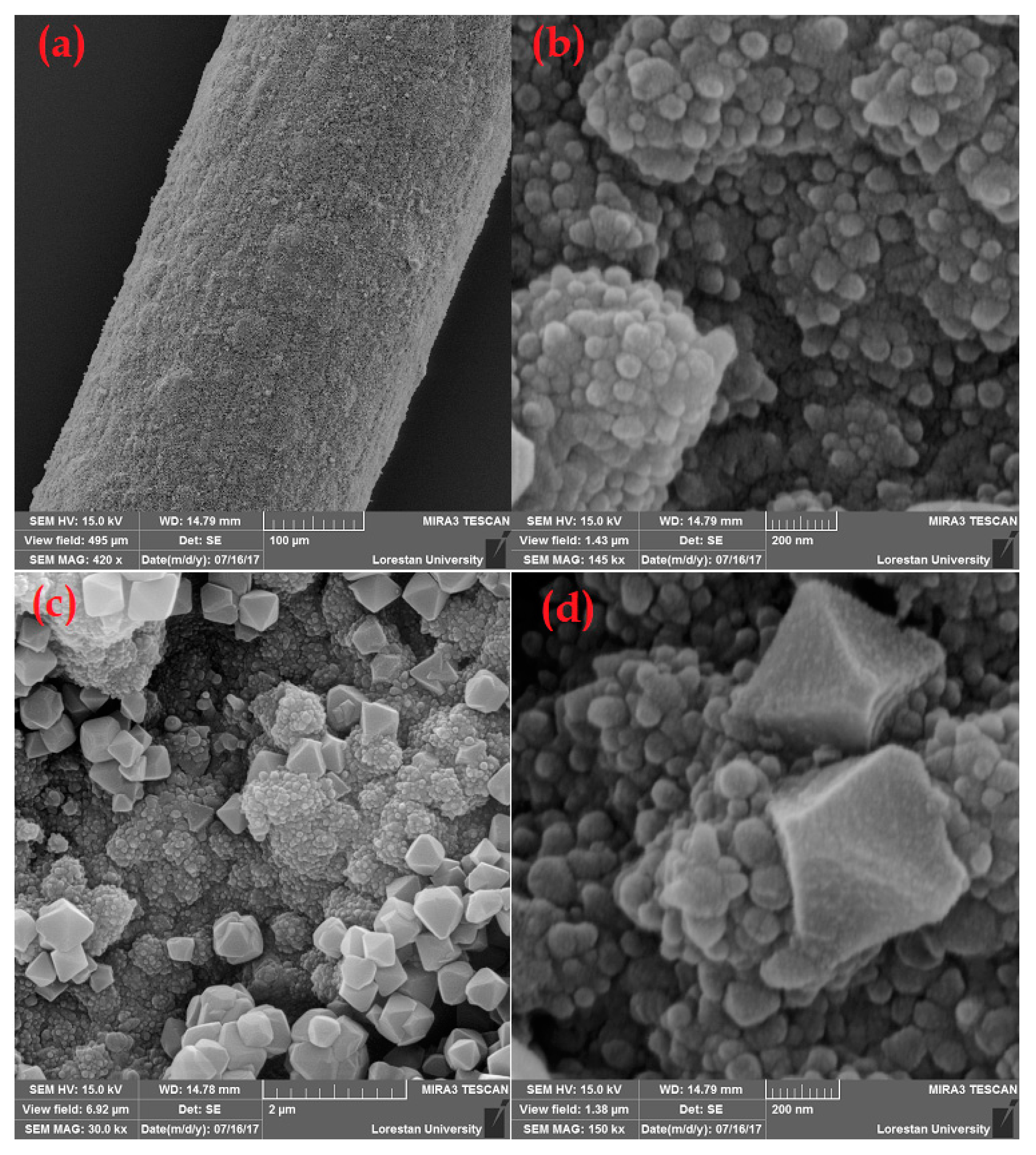
2.1. Characterization of the PPy@MIL-101(Cr) Nanocomposite Sorbent
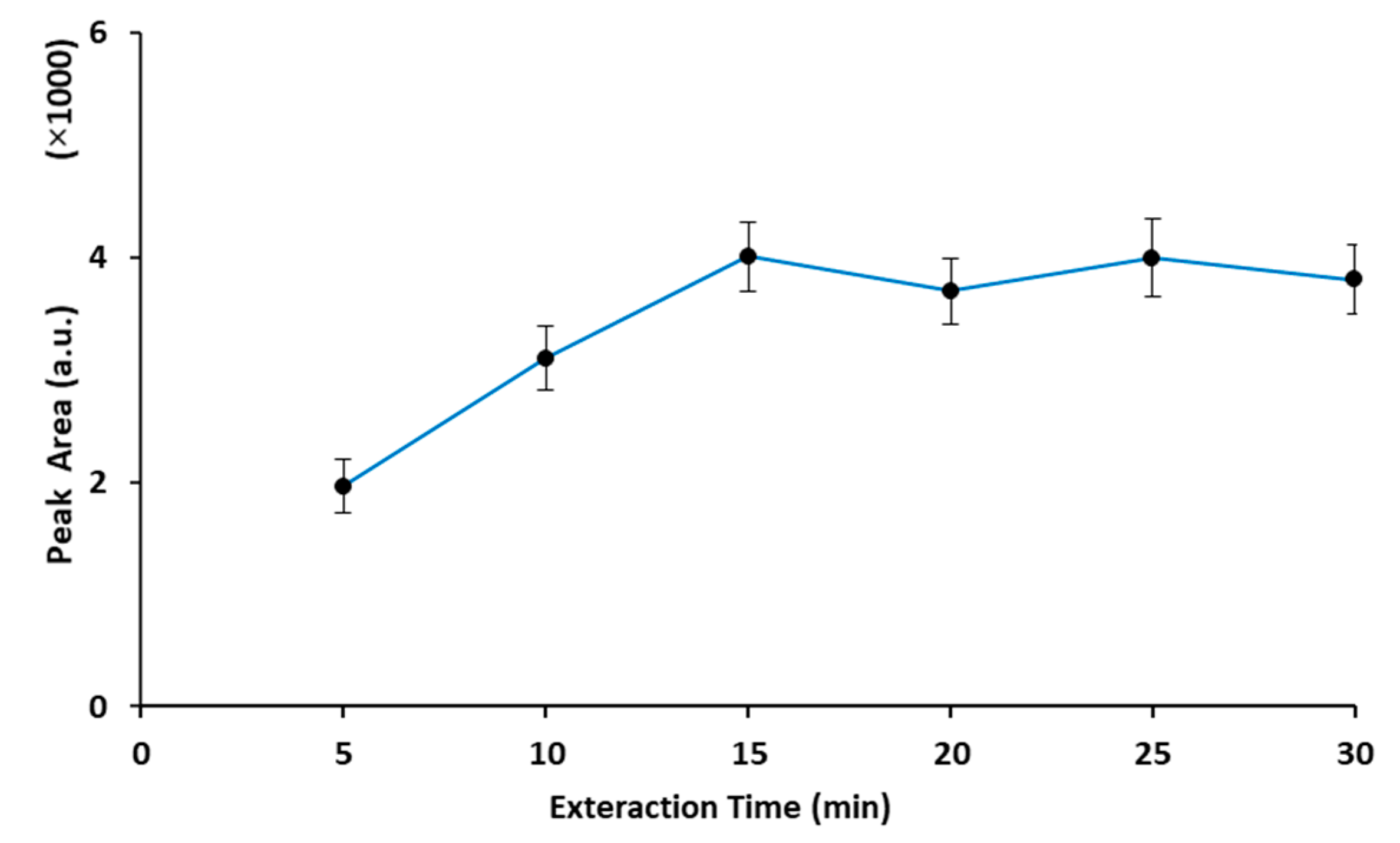
2.2. Optimization of the Extraction Procedure
2.2.1. Extraction Time
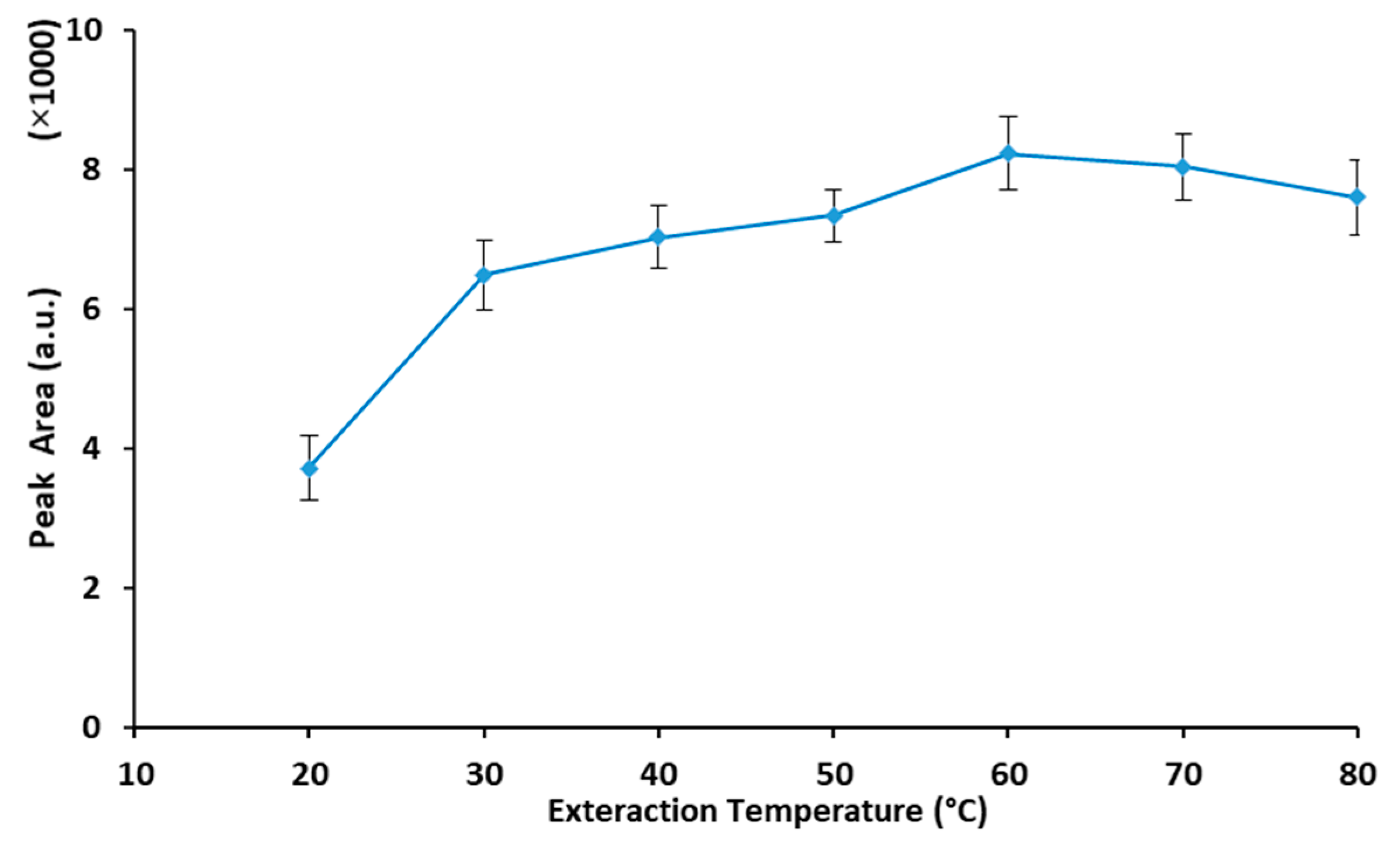
2.2.2. Extraction Temperature
2.2.3. Desorption Condition
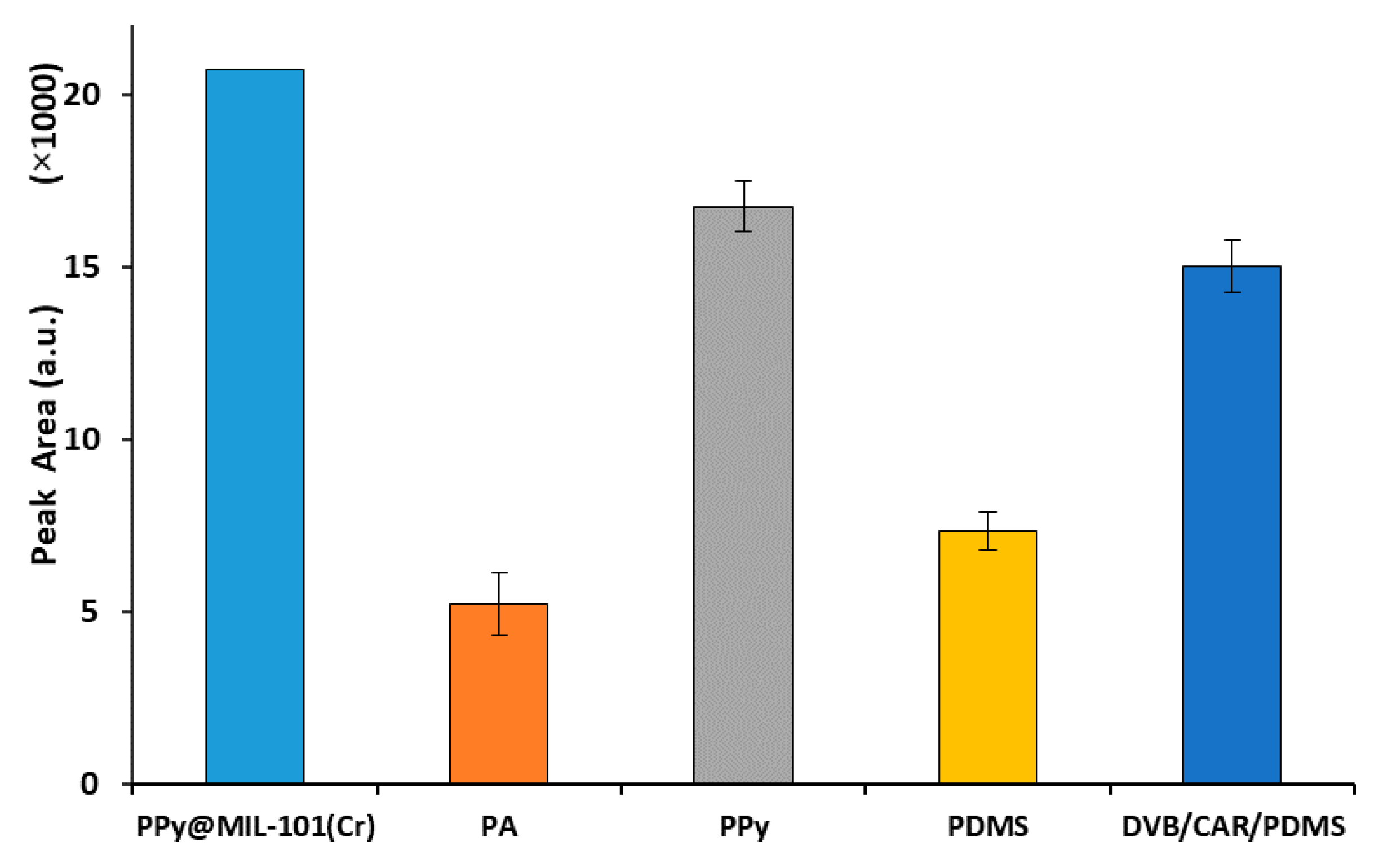
2.3. Comparison of the PPy@MIL-101(Cr)-Coated Fiber with Commercial Fibers
2.4. Analytical Performances
2.5. Determination of MTBE in Contaminated Soil Samples
3. Experimental
3.1. Materials
3.2. Instruments and Conditions
3.3. Preparation of MIL-101(Cr)
3.4. Fabrication of the SPME Fiber Coated by PPy@ MIL-101(Cr)
3.5. HS-SPME Procedure
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pankow, J.F.; Thomson, N.R.; Johnson, R.L.; Baehr, A.L.; Zogorski, J.S. The urban atmosphere as a non-point source for the transport of MTBE and other volatile organic compounds (VOCs) to shallow groundwater. Environ. Sci. Technol. 1997, 31, 2821–2828. [Google Scholar] [CrossRef]
- Levchuk, I.; Bhatnagar, A.; Sillanpää, M. Overview of technologies for removal of methyl tert-butyl ether (MTBE) from water. Sci. Total Environ. 2014, 476, 415–433. [Google Scholar] [CrossRef] [PubMed]
- Dodd, D.; Willson, G.; Parkinson, H.; Bermudez, E. Two-year drinking water carcinogenicity study of methyl tertiary-butyl ether (MTBE) in wistar rats. J. Appl. Toxicol. 2013, 33, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Atienza, J.; Aragón, P.; Herrero, M.A.; Puchades, R.; Maquieira, Á. State of the art in the determination of mtbe in natural waters and soils. Crit. Rev. Anal. Chem. 2005, 35, 317–337. [Google Scholar] [CrossRef]
- Schmidt, T.C.; Duong, H.-A.; Berg, M.; Haderlein, S.B. Analysis of fuel oxygenates in the environment. Analyst 2001, 126, 405–413. [Google Scholar] [CrossRef]
- US Environmental Protection Agency. Drinking Water Advisory: Consumer Acceptability Advice and Health Effects Analysis on Methyl Tertiary-Butyl Ether (MtBE); EPA: Washington, DC, USA, 1997; pp. 11–13.
- Roslev, P.; Lentz, T.; Hesselsoe, M. Microbial toxicity of methyl tert-butyl ether (MTBE) determined with fluorescent and luminescent bioassays. Chemosphere 2015, 120, 284–291. [Google Scholar] [CrossRef]
- Church, C.D.; Isabelle, L.M.; Pankow, J.F.; Rose, D.L.; Tratnyek, P.G. Method for determination of methyl tert-butyl ether and its degradation products in water. Environ. Sci. Technol. 1997, 31, 3723–3726. [Google Scholar] [CrossRef]
- Halden, R.U.; Happel, A.M.; Schoen, S.R. Evaluation of standard methods for the analysis of methyl tert-butyl ether and related oxygenates in gasoline-contaminated groundwater. Environ. Sci. Technol. 2001, 35, 1469–1474. [Google Scholar] [CrossRef]
- Cummins, T.M.; Robbins, G.A.; Henebry, B.J.; Goad, C.R.; Gilbert, E.J.; Miller, M.E.; Stuart, J.D. A water extraction, static headspace sampling, gas chromatographic method to determine MTBE in heating oil and diesel fuel. Environ. Sci. Technol. 2001, 35, 1202–1208. [Google Scholar] [CrossRef]
- Yazdi, A.S.; Assadi, H. Determination of trace of methyl tert-butyl ether in water using liquid drop headspace sampling and GC. Chromatographia 2004, 60, 699–702. [Google Scholar] [CrossRef]
- Hu, D.; Yang, J.; Liu, Y.; Zhang, W.; Peng, X.; Wei, Q.; Yuan, J.; Zhu, Z. Health risk assessment for inhalation exposure to methyl tertiary butyl ether at petrol stations in southern China. Int. J. Environ. Res. Public Health 2016, 13, 204. [Google Scholar] [CrossRef]
- Hong, S.; Duttweiler, C.M.; Lemley, A.T. Analysis of methyl tert.-butyl ether and its degradation products by direct aqueous injection onto gas chromatography with mass spectrometry or flame ionization detection systems. J. Chromatogr. A 1999, 857, 205–216. [Google Scholar] [CrossRef]
- Ghiasvand, A.R.; Heidari, N.; Abdolhosseini, S.; Hamdi, A.; Haddad, P.R. Evaluation of a cooling/heating-assisted microextraction instrument using a needle trap device packed with aminosilica/graphene oxide nanocomposites, covalently attached to cotton. Analyst 2018, 143, 2632–2640. [Google Scholar] [CrossRef] [PubMed]
- Jalili, V.; Barkhordari, A.; Ghiasvand, A. A comprehensive look at solid-phase microextraction technique: A review of reviews. Microchem. J. 2020, 152, 104319. [Google Scholar] [CrossRef]
- Hao, L.; Wang, C.; Wu, Q.; Li, Z.; Zang, X.; Wang, Z. Metal-organic framework derived magnetic nanoporous carbon: Novel adsorbent for magnetic solid-phase extraction. Anal. Chem. 2014, 86, 12199–12205. [Google Scholar] [CrossRef]
- Yin, Z.; Wan, S.; Yang, J.; Kurmoo, M.; Zeng, M.-H. Recent advances in post-synthetic modification of metal–organic frameworks: New types and tandem reactions. Coord. Chem. Rev. 2019, 378, 500–512. [Google Scholar] [CrossRef]
- Ahmed, A.; Seth, S.; Purewal, J.; Wong-Foy, A.G.; Veenstra, M.; Matzger, A.J.; Siegel, D.J. Exceptional hydrogen storage achieved by screening nearly half a million metal-organic frameworks. Nat. Commun. 2019, 10, 1568. [Google Scholar] [CrossRef]
- González-Sálamo, J.; Socas-Rodríguez, B.; Hernández-Borges, J.; Rodríguez-Delgado, M.Á. Nanomaterials as sorbents for food sample analysis. Trends Anal. Chem. 2016, 85, 203–220. [Google Scholar] [CrossRef]
- Farha, O.K.; Eryazici, I.; Jeong, N.C.; Hauser, B.G.; Wilmer, C.E.; Sarjeant, A.A.; Snurr, R.Q.; Nguyen, S.T.; Yazaydın, A.Ö.; Hupp, J.T. Metal-organic framework materials with ultrahigh surface areas: Is the sky the limit? J. Am. Chem. Soc. 2012, 134, 15016–15021. [Google Scholar] [CrossRef]
- Xie, L.; Liu, S.; Han, Z.; Jiang, R.; Liu, H.; Zhu, F.; Zeng, F.; Su, C.; Ouyang, G. Preparation and characterization of metal-organic framework MIL-101(Cr)-coated solid-phase microextraction fiber. Anal. Chim. Acta 2015, 853, 303–310. [Google Scholar] [CrossRef]
- Ghiasvand, A.R.; Ghaedrahmati, L.; Heidari, N.; Haddad, P.R.; Farhadi, S. Synthesis and characterization of MIL-101(Cr) intercalated by polyaniline composite, doped with silica nanoparticles and its evaluation as an efficient solid-phase extraction sorbent. J. Sep. Sci. 2018, 41, 3910–3917. [Google Scholar] [CrossRef] [PubMed]
- Ghiasvand, A.; Dowlatshah, S.; Nouraei, N.; Heidari, N.; Yazdankhah, F. A solid-phase microextraction platinized stainless steel fiber coated with a multiwalled carbon nanotube-polyaniline nanocomposite film for the extraction of thymol and carvacrol in medicinal plants and honey. J. Chromatogr. A 2015, 1406, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Ghiasvand, A.; Nasirian, A.; Koonani, S.; Nouriasl, K. A platinized stainless steel fiber with in-situ coated polyaniline/polypyrrole/graphene oxide nanocomposite sorbent for headspace solid-phase microextraction of aliphatic aldehydes in rice samples. Biomed. Chromatogr. 2017, 31, e4024. [Google Scholar] [CrossRef] [PubMed]
- Vatani, H.; Yazdi, A.S. Ionic-liquid-mediated poly(dimethylsiloxane)- grafted carbon nanotube fiber prepared by the sol–gel technique for the head space solid-phase microextraction of methyl tert-butyl ether using GC. J. Sep. Sci. 2014, 37, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Mei, Y.; Liu, Y.; Dai, H.; Xia, H.; Zhang, X.; Wu, Y.; Gu, Y.; Peng, X. Simultaneous measurement of methyl tert-butyl ether and tert-butyl alcohol in human serum by headspace solid-phase microextraction gas chromatography–mass spectrometry. Biomed. Chromatogr. 2015, 29, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Tabar-Heydar, K.; Khanalizadeh, G.; Mostaghim, M.; Ahmadi, S.H. A sensitive method for methyl tert-butyl ether analysis in water samples by a new HS-SPME vial and GC. Chromatographia 2009, 69, 1341–1346. [Google Scholar] [CrossRef]
- Nousiainen, M.; Holopainen, S.; Puton, J.; Sillanpää, M. Fast detection of methyl tert-butyl ether from water using solid phase microextraction and ion mobility spectrometry. Talanta 2011, 84, 738–744. [Google Scholar] [CrossRef]
- Achten, C.; Kolb, A.; Püttmann, W. Sensitive method for determination of methyl tert-butyl ether (MTBE) in water by use of headspace-SPME/GC–MS. Fresenius J. Anal. Chem. 2001, 371, 519–525. [Google Scholar] [CrossRef]
- Rastkari, N.; Ahmadkhaniha, R.; Yunesian, M. Single-walled carbon nanotubes as an effective adsorbent in solid-phase microextraction of low level methyl tert-butyl ether, ethyl tert-butyl ether and methyl tert-amyl ether from human urine. J. Chromatogr. B 2009, 877, 1568–1574. [Google Scholar] [CrossRef]
- Bonin, M.A.; Silva, L.K.; Smith, M.M.; Ashley, D.L.; Blount, B.C. Measurement of trihalomethanes and methyl tert-butyl ether in whole blood using gas chromatography with high-resolution mass spectrometry. J. Anal. Toxicol. 2005, 29, 81–89. [Google Scholar] [CrossRef]
- Dron, J.; Garcia, R.; Millán, E. Optimization of headspace solid-phase microextraction by means of an experimental design for the determination of methyl tert.-butyl ether in water by gas chromatography–flame ionization detection. J. Chromatogr. A 2002, 963, 259–264. [Google Scholar] [CrossRef]
- International Organization for Standardization. 10381-1: Soil Quality-Sampling. Part 1: Guidance on the Design of Sampling Programmes; International Organization for Standardization: Geneva, Switzerland, 2002. [Google Scholar]
- Férey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surblé, S.; Margiolaki, I. A chromium terephthalate-based solid with unusually large pore volumes and surface Area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |

| Method | Fiber | RSD% | LOD | LDR | Sample | Ref. |
|---|---|---|---|---|---|---|
| HS-SPME–GC-FID | IL-mediated PDMS-MWCNTs | 6.5 | 0.007 ng·mL−1 | 0.03–200 ng·mL−1 | Water | [25] |
| HS-SPME–GC–MS | DVB/CAR/PDMS | <10 | 0.03 μg·L−1 | 0.10–40 μg·L−1 | Blood serum | [26] |
| HS-SPME–GC-FID | DVB/CAR/PDMS | 6–8 | 0.02 μg·L−1 | 0.1–400 μg·L−1 | Water | [27] |
| HS-SPME-IMS | PDMS/CAR | 8.3 | 5 mg·L−1 | 10–1390 ng·L−1 | Water | [28] |
| HS-SPME–GC–MS | Carboxen-PDMS | 10–11 | 10 ng·L−1 | 5–250 ng·L−1 | Water | [29] |
| HS-SPME–GC–MS | SWCNTs | <15 | 10 ng·L−1 | 100–5000 ng·L−1 | Urine | [30] |
| HS-SPME–GC–MS | Carboxen-PDMS | 5 | 1.5 ng·L−1 | 0.3–2.4 ng·L−1 | Whole blood | [31] |
| HS-SPME–GC–FID | PDMS/DVB | 6.3 | 0.45 μg·L−1 | 5–500 μg·L−1 | Water | [32] |
| HS-SPME–GC-FID | PPy@MIL-101(Cr) | 8.4 | 0.01 ng·g−1 | 5–40,000 ng·g−1 | Soil | This work |
| Soil Sample | Added (µg ·g−1) | Determined ± SD a (µg·g−1) | Recovery (%) |
|---|---|---|---|
| Kermanshah Oil Refinery Company (sampling from the surface of soil) | 0 | 1.16 ± 0.51 | - |
| 2 | 3.30 ± 0.08 | 107 | |
| Kermanshah Oil Refinery Company (sampling from the depth of soil) | 0 | 1.54 ± 0.25 | - |
| 2 | 3.61 ± 0.17 | 103 | |
| Kabir gas station (sampling from surface soil behind the official building) | 0 | 1.41 ± 0.25 | - |
| 0.5 | 1.98 ± 0.17 | 117 | |
| Besat gas station (sampling from surface soil of gas station area) | 0 | 0.92 ± 0.48 | - |
| 1 | 1.89 ± 0.05 | 96 | |
| Shahrvand gas station (sampling from surface soil of gas station area) | 0 | 2.09 ± 0.34 | - |
| 2 | 4.03 ± 0.09 | 97 | |
| Shahrvand gas station (sampling from firefighting soil bucket) | 0 | 2.72 ± 0.50 | - |
| 2 | 4.48 ± 0.08 | 88 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darabi, J.; Ghiasvand, A. Chromium-Based Polypyrrole/MIL-101 Nanocomposite as an Effective Sorbent for Headspace Microextraction of Methyl tert-Butyl Ether in Soil Samples. Molecules 2020, 25, 644. https://doi.org/10.3390/molecules25030644
Darabi J, Ghiasvand A. Chromium-Based Polypyrrole/MIL-101 Nanocomposite as an Effective Sorbent for Headspace Microextraction of Methyl tert-Butyl Ether in Soil Samples. Molecules. 2020; 25(3):644. https://doi.org/10.3390/molecules25030644
Chicago/Turabian StyleDarabi, Jila, and Alireza Ghiasvand. 2020. "Chromium-Based Polypyrrole/MIL-101 Nanocomposite as an Effective Sorbent for Headspace Microextraction of Methyl tert-Butyl Ether in Soil Samples" Molecules 25, no. 3: 644. https://doi.org/10.3390/molecules25030644
APA StyleDarabi, J., & Ghiasvand, A. (2020). Chromium-Based Polypyrrole/MIL-101 Nanocomposite as an Effective Sorbent for Headspace Microextraction of Methyl tert-Butyl Ether in Soil Samples. Molecules, 25(3), 644. https://doi.org/10.3390/molecules25030644