The Possible Role of the Nitroso-Sulfide Signaling Pathway in the Vasomotoric Effect of Garlic Juice
Abstract
1. Introduction
2. Results
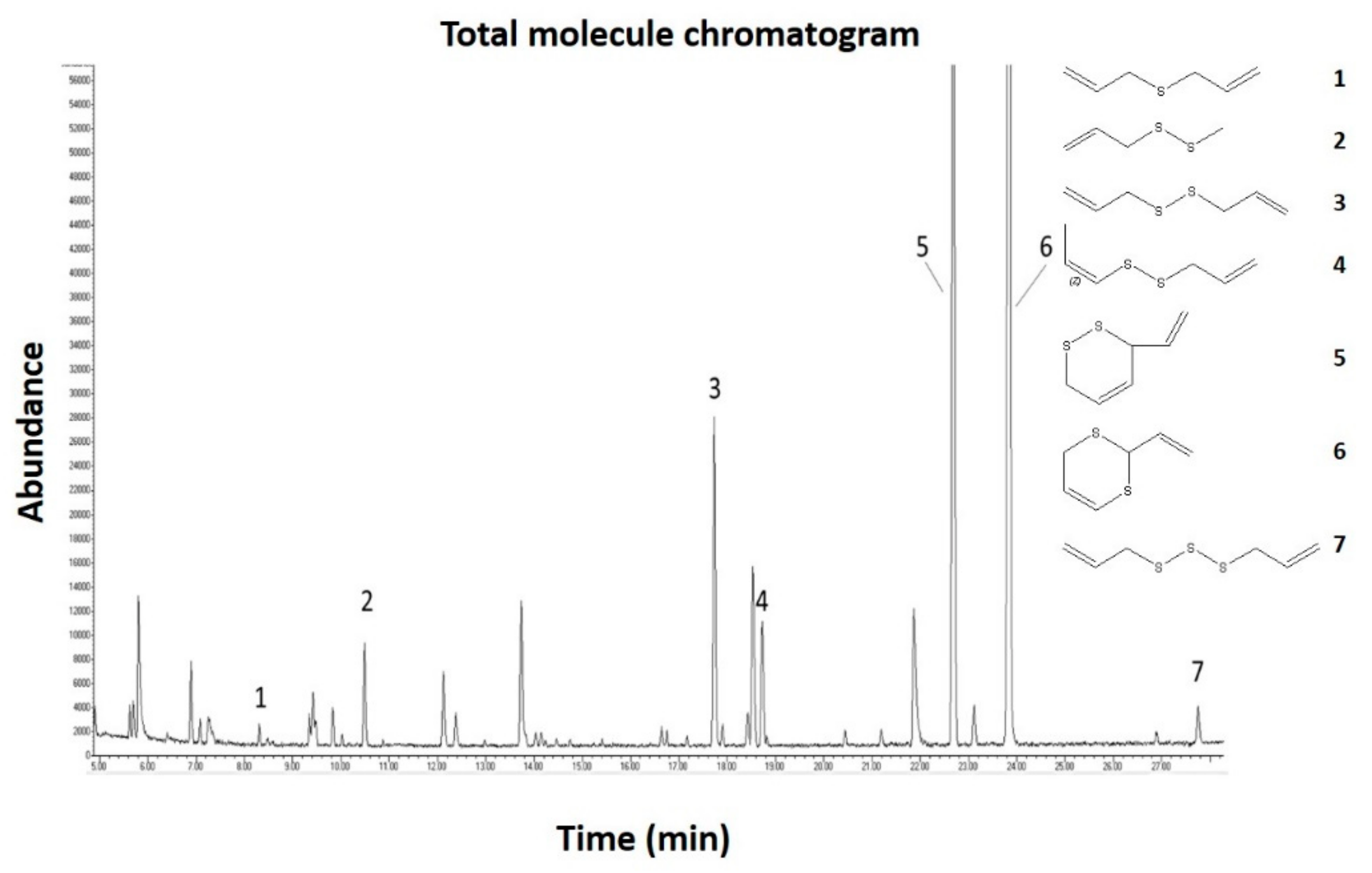
2.1. Gas Chromatography Mass Spectrometric (GC-MS) Analysis of the Garlic Juice
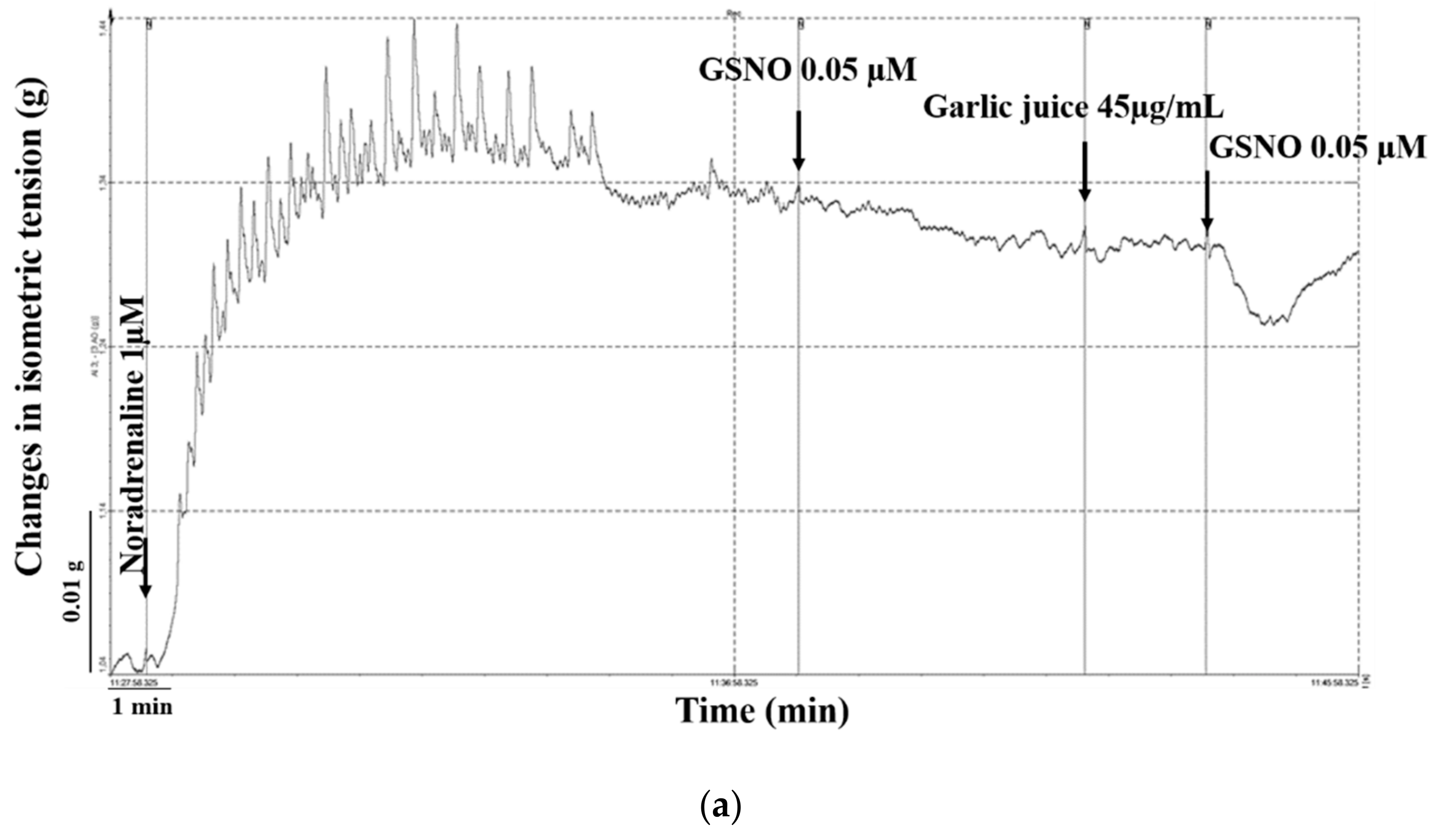
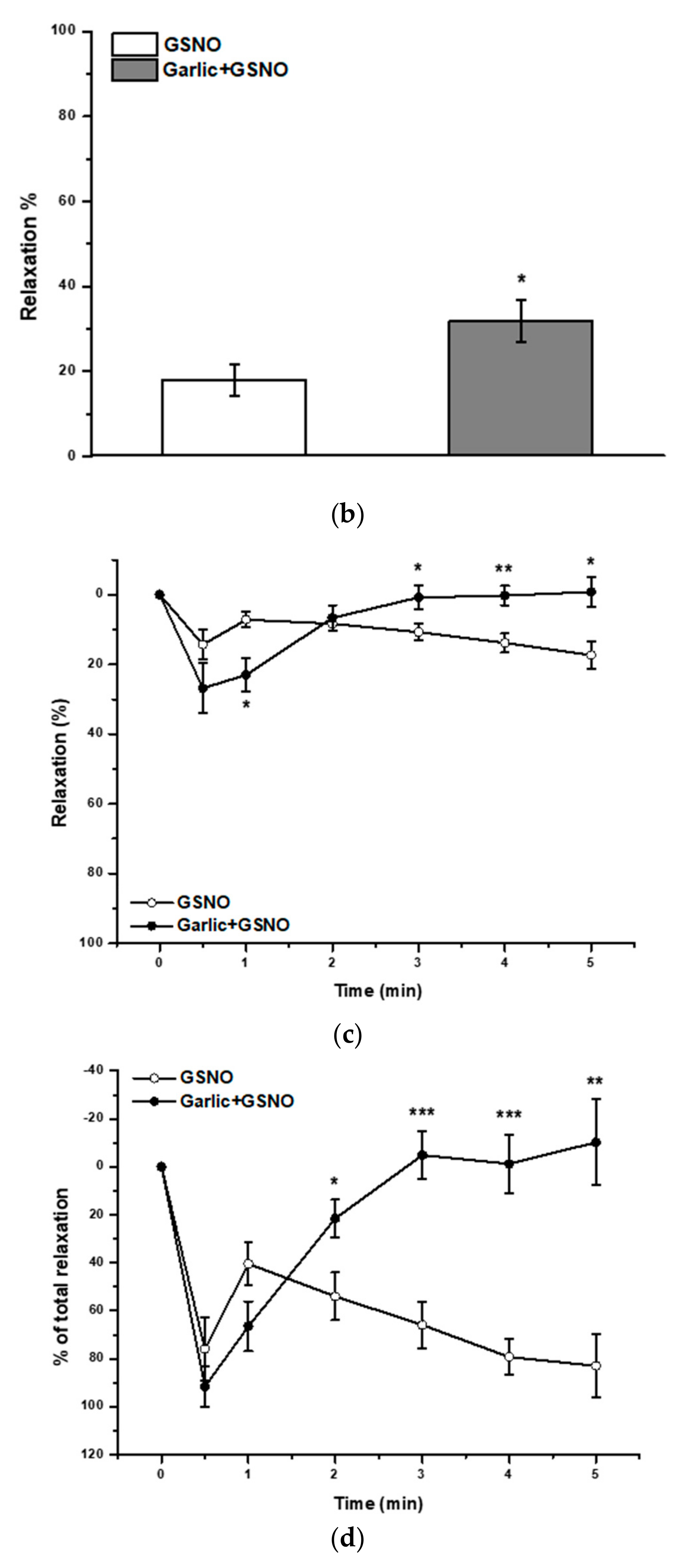
2.2. Effect of Garlic Juice Incubation
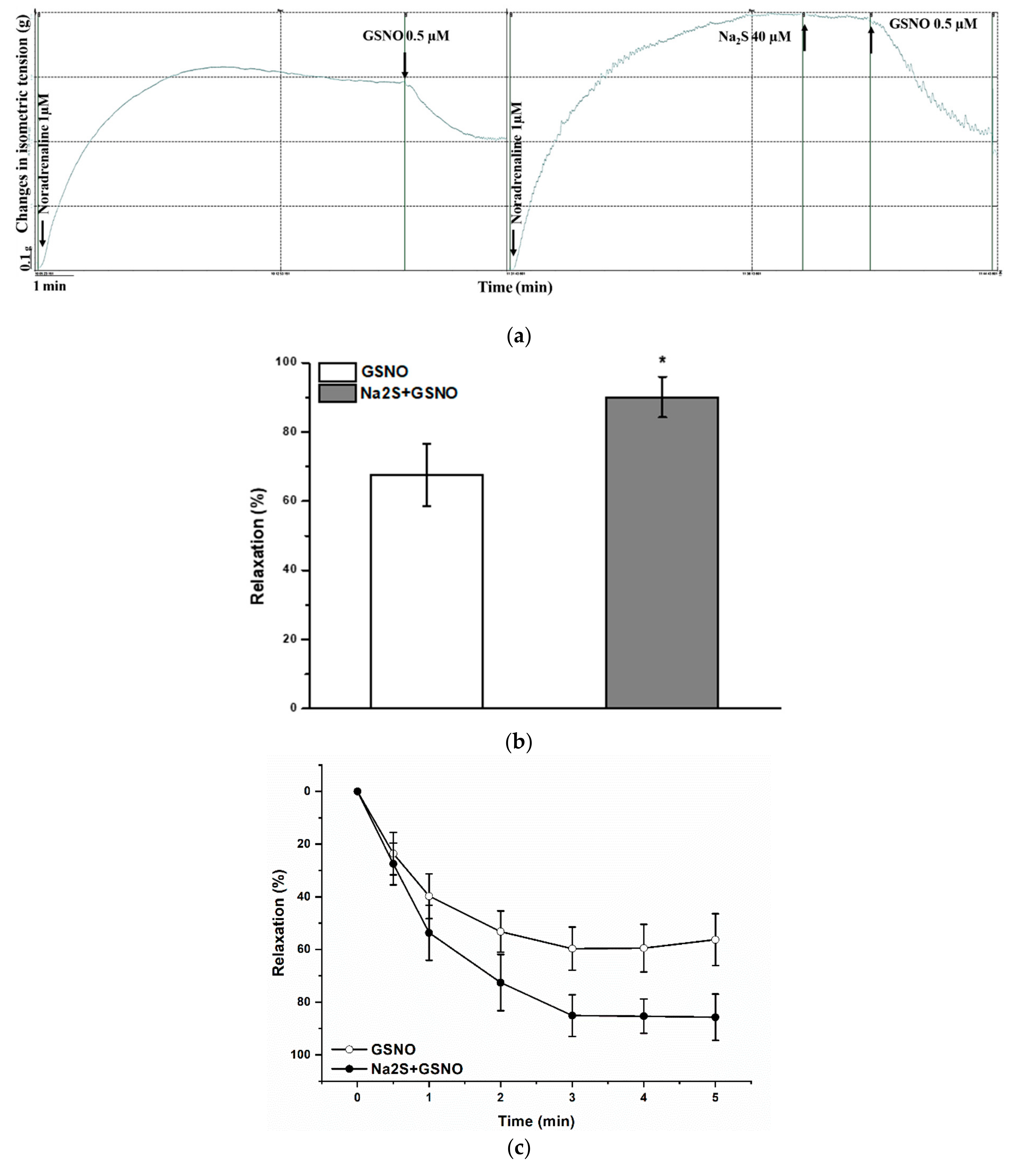
2.3. Effect of H2S-Donor Incubation
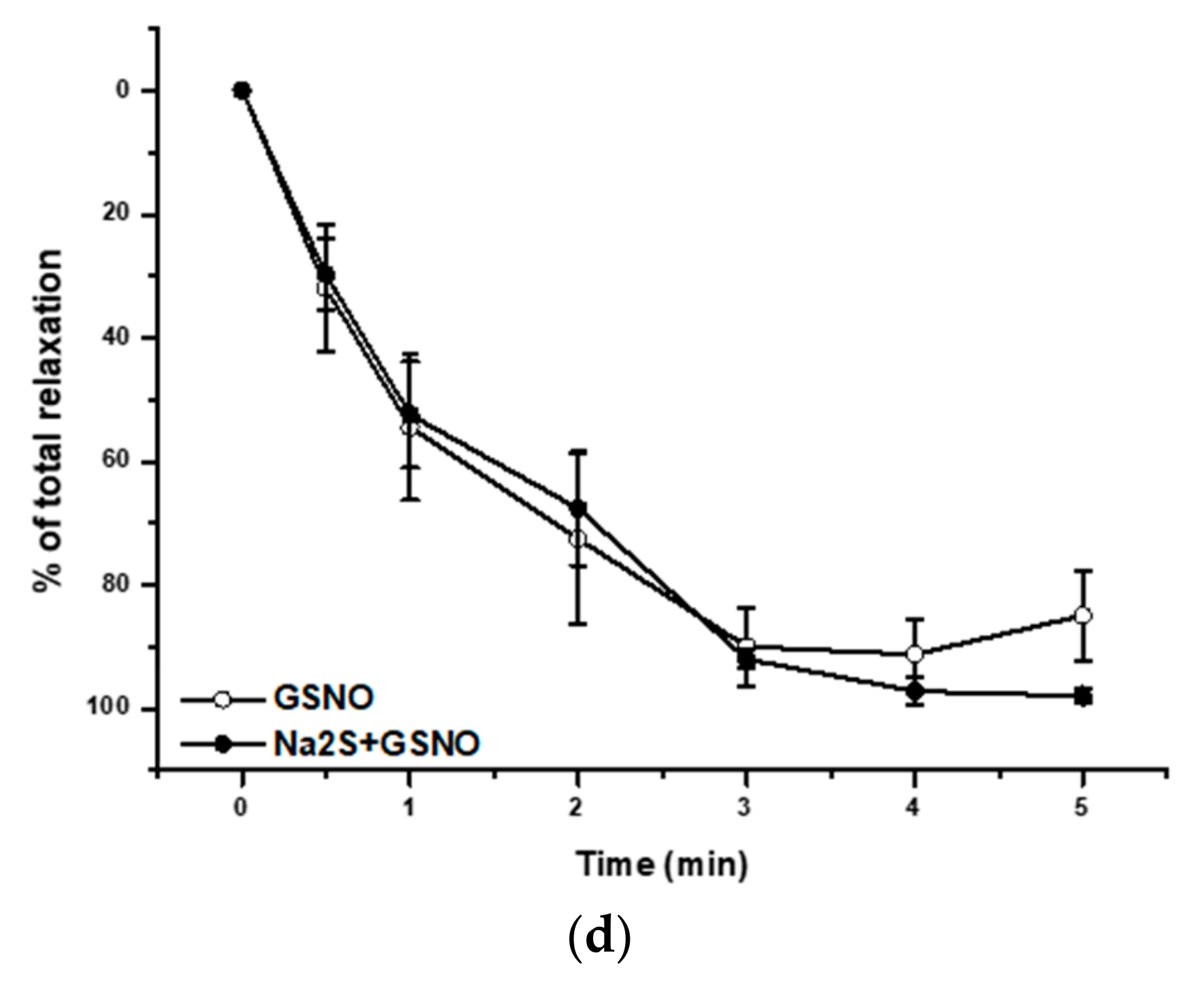
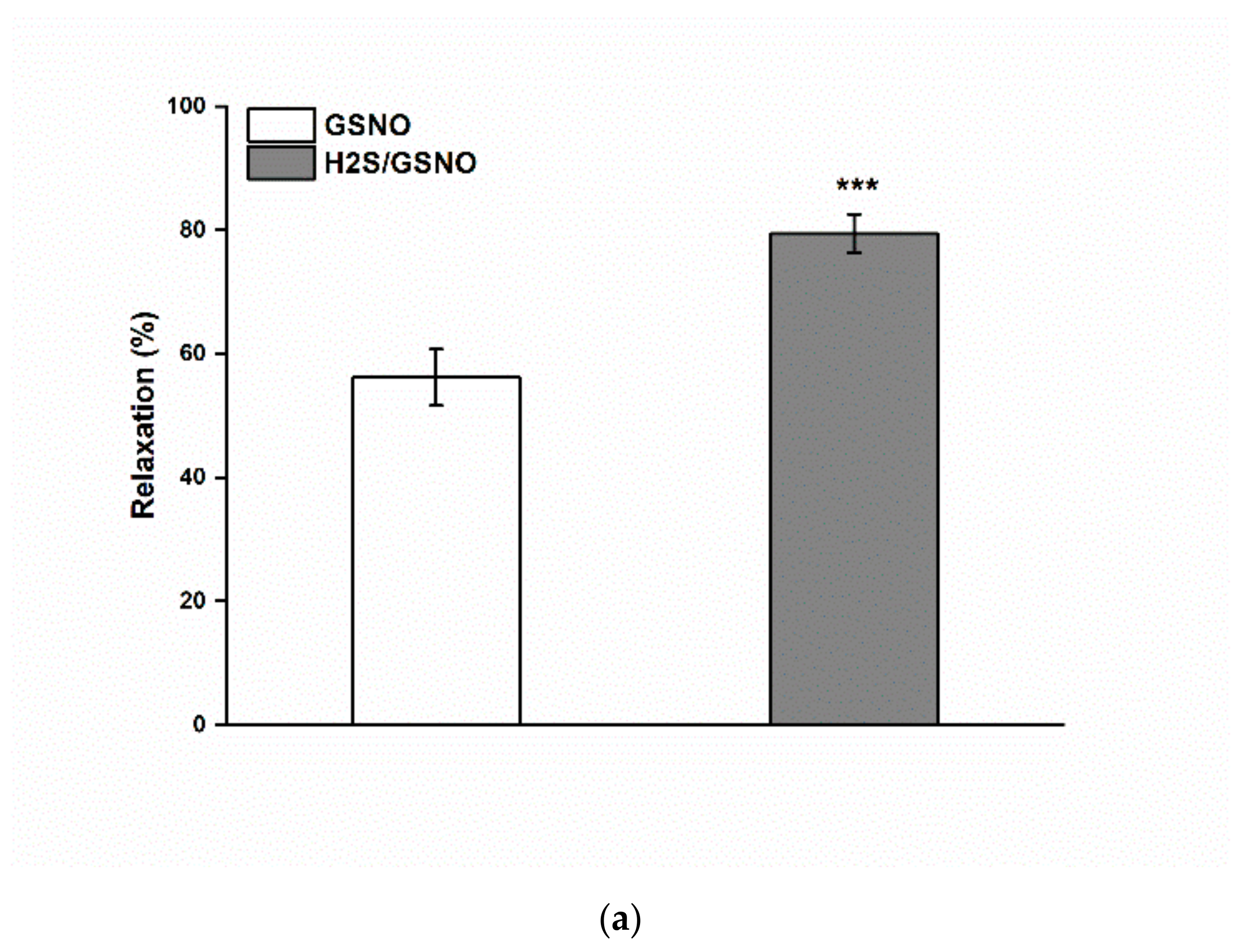
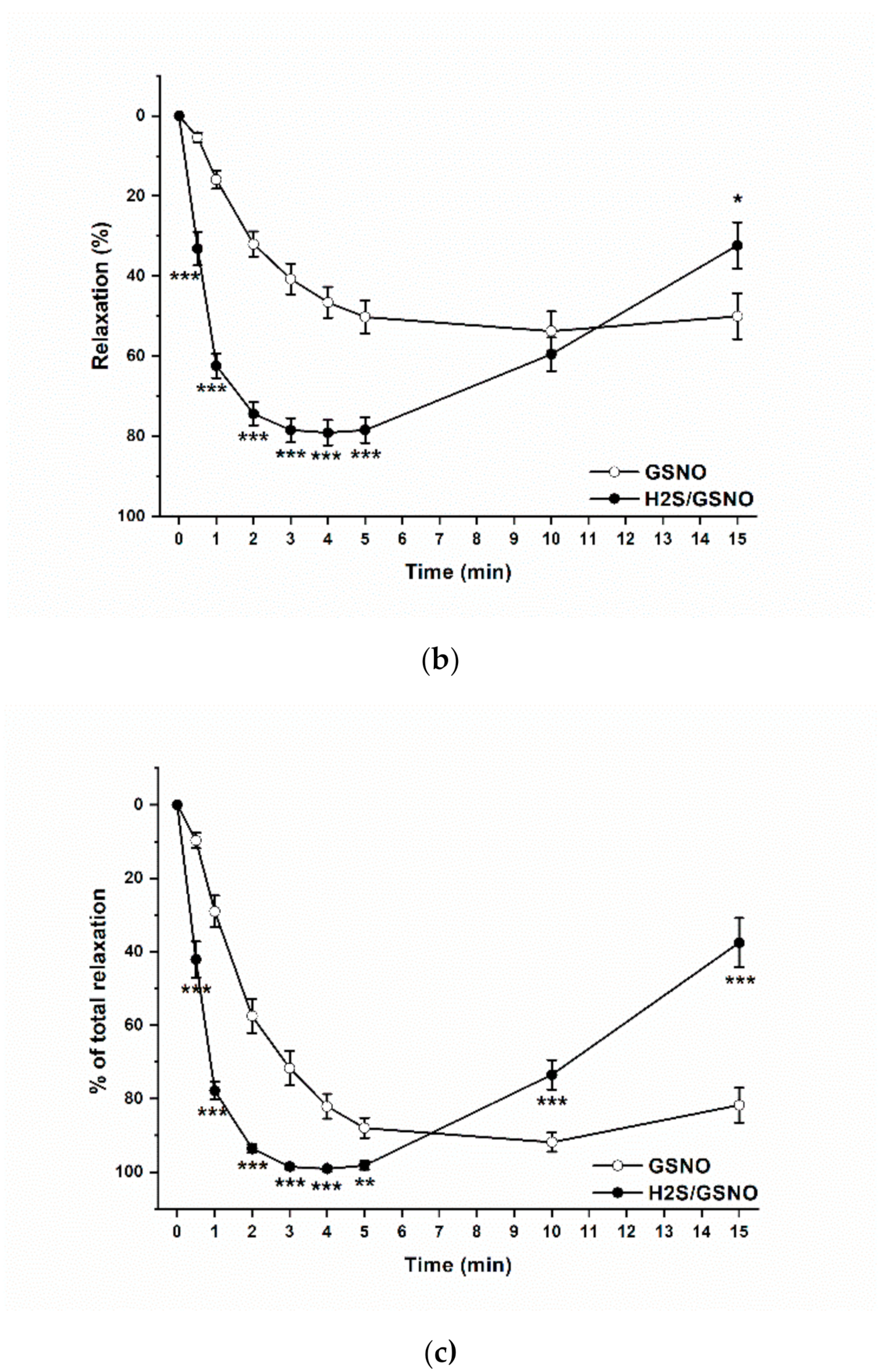
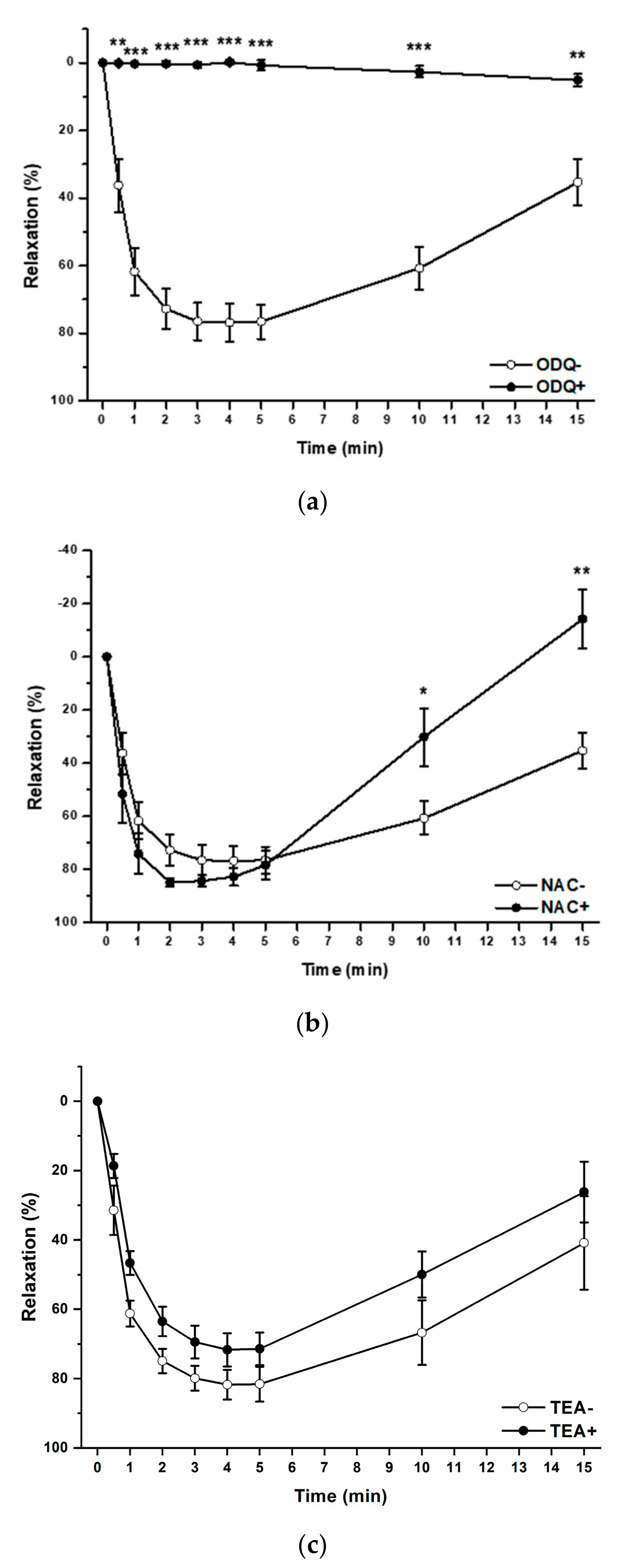
2.4. Effect of the H2S/GSNO Products
3. Discussion
4. Materials and Methods
4.1. Guide for the Use and Care of Laboratory Animals
4.2. Chemicals
4.3. GC-MS Analysis
4.4. Measurement of the Vasoactive Response and Experimental Protocols
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Harauma, A.; Moriguchi, T. Aged garlic extract improves blood pressure in spontaneously hypertensive rats more safely than raw garlic. J. Nutr. 2006, 136, 769S–773S. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Cheng, Z.; Ahmad, H.; Ali, M.; Chen, X.; Wang, M. Garlic, from Remedy to Stimulant: Evaluation of Antifungal Potential Reveals Diversity in Phytoalexin Allicin Content among Garlic Cultivars; Allicin Containing Aqueous Garlic Extracts Trigger Antioxidants in Cucumber. Front. Plant. Sci. 2016, 7, 1235. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Lim, W.C.; Lee, S.J.; Lee, S.H.; Lee, J.H.; Cho, H.Y. Antiobesity Effect of Garlic Extract Fermented by Lactobacillus plantarum BL2 in Diet-Induced Obese Mice. J. Med. Food 2016, 19, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.S. Aged Garlic Extract Modifies Human Immunity. J. Nutr. 2016, 146, 433S–436S. [Google Scholar] [CrossRef]
- Yun, H.M.; Ban, J.O.; Park, K.R.; Lee, C.K.; Jeong, H.S.; Han, S.B.; Hong, J.T. Potential therapeutic effects of functionally active compounds isolated from garlic. Pharmacol. Ther. 2014, 142, 183–195. [Google Scholar] [CrossRef]
- Münchberg, U.; Anwar, A.; Mecklenburg, S.; Jacob, C. Polysulfides as biologically active ingredients of garlic. Org. Biomol. Chem. 2007, 21, 1505–1518. [Google Scholar] [CrossRef]
- Kwak, J.S.; Kim, J.Y.; Paek, J.E.; Lee, Y.J.; Kim, H.R.; Park, D.S.; Kwon, O. Garlic powder intake and cardiovascular risk factors: A meta-analysis of randomized controlled clinical trials. Nutr. Res. Pract. 2014, 8, 644–654. [Google Scholar] [CrossRef]
- Cerella, C.; Dicato, M.; Jacob, C.; Diederich, M. Chemical properties and mechanisms determining the anti-cancer action of garlic-derived organic sulfur compounds. Anticancer Agents. Med. Chem. 2011, 11, 267–271. [Google Scholar] [CrossRef]
- Jacob, C.; Anwar, A. Sulfides in Allium Vegetables. In Chemoprevention of Cancer and DNA Damage by Dietary Factors; Knasmüller, S., DeMarini, D.M., Johnson, I., Gerhäuser, C., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2009; pp. 663–684. [Google Scholar]
- Shouk, R.; Abdou, A.; Shetty, K.; Sarkar, D.; Eid, A.H. Mechanisms underlying the antihypertensive effects of garlic bioactives. Nutr. Res. 2014, 34, 106–115. [Google Scholar] [CrossRef]
- Liu, Y.H.; Lu, M.; Hu, L.F.; Wong, P.T.; Webb, D.G.; Bian, J.S. Hydrogen sulphide in the mammalian cardiovascular system. Antioxid. Redox. Signal. 2012, 17, 141–185. [Google Scholar] [CrossRef]
- Cebova, M.; Kosutova, M.; Pechanova, O. Cardiovascular effects of gasotransmitter donors. Physiol. Res. 2016, 65, S291–S307. [Google Scholar] [PubMed]
- Palinkas, Z.; Furtmuller, P.G.; Nagy, A.; Jakopitsch, C.; Pirker, K.F.; Magierowski, M.; Jasnos, K.; Wallace, J.L.; Obinger, C.; Nagy, P. Interactions of hydrogen sulfide with myeloperoxidase. Br. J. Pharmacol. 2015, 172, 1516–1532. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lancaster, J.R. Chemical foundations of hydrogen sulfide biology. Nitric Oxide 2013, 35, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.S.; Cheng, Z.J.; Ellis, A.; Ding, H.; Jiang, Y.; Li, Y.; Hollenberg, M.D.; Triggle, C.R. Nitrosothiol stores in vascular tissue: Modulation by ultraviolet light, acetylcholine and ionomycin. Eur. J. Pharmacol. 2007, 560, 183–192. [Google Scholar] [CrossRef]
- Ondrias, K.; Stasko, A.; Cacanyiova, S.; Sulova, Z.; Krizanova, O.; Kristek, F.; Malekova, L.; Knezl, V.; Breier, A. H(2)S and HS(-) donor NaHS releases nitric oxide from nitrosothiols, metal nitrosyl complex, brain homogenate and murine L1210 leukaemia cells. Pflugers Arch. 2008, 457, 271–279. [Google Scholar] [CrossRef]
- Yong, Q.C.; Hu, L.F.; Wang, S.; Huang, D.; Bian, J.S. Hydrogen sulfide interacts with nitric oxide in the heart: Possible involvement of nitroxyl. Cardiovasc. Res. 2010, 88, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, M.R.; Miljkovic, J.; Nauser, T.; Royzen, M.; Klos, K.; Shubina, T.; Koppenol, W.H.; Lippard, S.J.; Ivanovic-Burmazovic, I. Chemical characterization of the smallest S-nitrosothiol, HSNO; cellular cross-talk of H2S and S-nitrosothiols. J. Am. Chem. Soc. 2012, 134, 12016–12027. [Google Scholar] [CrossRef]
- Eberhardt, M.; Dux, M.; Namer, B.; Miljkovic, J.; Cordasic, N.; Will, C.; Kichko, T.I.; de la Roche, J.; Fischer, M.; Suarez, S.A.; et al. H2S and NO cooperatively regulate vascular tone by activating a neuroendocrine HNO-TRPA1-CGRP signalling pathway. Nat. Commun. 2014, 5, 4381. [Google Scholar] [CrossRef]
- Cortese-Krott, M.M.; Fernandez, B.O.; Santos, J.L.; Mergia, E.; Grman, M.; Nagy, P.; Kelm, M.; Butler, A.; Feelisch, M. Nitrosopersulfide (SSNO(-)) accounts for sustained NO bioactivity of S-nitrosothiols following reaction with sulfide. Redox Biol. 2014, 2, 234–244. [Google Scholar] [CrossRef]
- Berenyiova, A.; Grman, M.; Mijuskovic, A.; Stasko, A.; Misak, A.; Nagy, P.; Ondriasova, E.; Cacanyiova, S.; Brezova, V.; Feelisch, M.; et al. The reaction products of sulfide and S-nitrosoglutathione are potent vasorelaxants. Nitric Oxide 2015, 46, 123–130. [Google Scholar] [CrossRef]
- Kimura, H. Physiological Roles of Hydrogen Sulfide and Polysulfides. Handb. Exp. Pharmacol. 2015, 230, 61–81. [Google Scholar] [PubMed]
- Takashima, M.; Kanamori, Y.; Kodera, Y.; Morihara, N.; Tamura, K. Aged garlic extract exerts endothelium-dependent vasorelaxant effect on rat aorta by increasing nitric oxide production. Phytomedicine 2017, 24, 56–61. [Google Scholar] [CrossRef]
- Martins, N.; Petropoulos, S.; Ferreira, I.C. Chemical composition and bioactive compounds of garlic (Allium sativum L.) as affected by pre- and post-harvest conditions: A review. Food. Chem. 2016, 211, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Grman, M.; Nasim, M.J.; Leontiev, R.; Misak, A.; Jakusova, V.; Ondrias, K.; Jacob, C. Inorganic Reactive Sulfur-Nitrogen Species: Intricate Release Mechanisms or Cacophony in Yellow, Blue and Red? Antioxidants 2017, 6, 1–16. [Google Scholar] [CrossRef]
- Ried, K.; Frank, O.R.; Stocks, N.P. Aged garlic extract reduces blood pressure in hypertensives: A dose-response trial. Eur. J. Clin. Nutr. 2013, 67, 64–70. [Google Scholar] [CrossRef]
- Grman, M.; Misak, A.; Cacanyiova, S.; Kristek, F.; Tomaskova, Z.; Bertova, A.; Ondrias, K. The aqueous garlic, onion and leek extracts release nitric oxide from S-nitrosoglutathione and prolong relaxation of aortic rings. Gen. Physiol. Biophys. 2011, 30, 396–402. [Google Scholar] [CrossRef]
- Misak, A.; Grman, M.; Bacova, Z.; Rezuchova, I.; Hudecova, S.; Ondriasova, E.; Krizanova, O.; Brezova, V.; Chovanec, M.; Ondrias, K. Polysulfides and products of H2S/S-nitrosoglutathione in comparison to H2S, glutathione and antioxidant Trolox are potent scavengers of superoxide anion radical and produce hydroxyl radical by decomposition of H2O2. Nitric Oxide 2018, 76, 136–151. [Google Scholar] [CrossRef]
- Stasko, A.; Brezová, V.; Zalibera, M.; Biskupic, S.; Ondrias, K. Electron transfer: A primary step in the reactions of sodium hydrosulphide, an H2S/HS− donor. Free Rad. Res. 2009, 43, 581–593. [Google Scholar] [CrossRef]
- Whiteman, M.; Armstrong, J.S.; Chu, S.H.; Jia-Ling, S.; Wong, B.S.; Cheng, N.S.; Halliwell, B.; Moore, P.K. The novel neuromodulator hydrogen sulfide: An endogenous peroxynitrite ‘scavenger’? J. Neurochem. 2004, 90, 765–768. [Google Scholar] [CrossRef]
- Whiteman, M.; Cheung, N.S.; Zhu, Y.-Z.; Chu, S.H.; Sian, J.L.; Wong, B.S.; Armstrong, J.S.; Moore, P.K. Hydrogen sulphide: A novel inhibitor of hypochlorous acid-mediated oxidative damage in the brain? Biochem. Biophys. Res. Comm. 2005, 326, 794–798. [Google Scholar] [CrossRef]
- Grman, M.; Misak, A.; Kurakova, L.; Brezova, V.; Cacanyiova, S.; Berenyiova, A.; Balis, P.; Tomasova, L.; Kharma, A.; Domínguez-Álvarez, E.; et al. Products of Sulfide/Selenite Interaction Possess Antioxidant Properties, Scavenge Superoxide-Derived Radicals, React with DNA, and Modulate Blood Pressure and Tension of Isolated Thoracic Aorta. Oxid. Med. Cell. Longev. 2019, 2019, 9847650. [Google Scholar] [CrossRef] [PubMed]
- Cacanyiova, S.; Berenyiova, A.; Balis, P.; Kristek, F.; Grman, M.; Ondrias, K.; Breza, J.; Breza, J., Jr. Nitroso-sulfide coupled signaling triggers specific vasoactive effects in the intrarenal arteries of patients with arterial hypertension. J. Physiol. Pharmacol. 2017, 68, 527–538. [Google Scholar] [PubMed]
- Bertova, A.; Cacanyiova, S.; Kristek, F.; Krizanova, O.; Ondrias, K.; Tomaskova, Z. The hypothesis of the main role of H2S in coupled sulphide-nitroso signalling pathway. Gen. Physiol. Biophys. 2010, 29, 402–410. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bhuiyan, A.I.; Papajani, V.T.; Paci, M.; Melino, S. Glutathione-garlic sulfur conjugates: Slow hydrogen sulfide releasing agents for therapeutic applications. Molecules 2015, 20, 1731–1750. [Google Scholar] [CrossRef] [PubMed]
- Hess, D.T.; Stamler, J.S. Regulation by S-nitrosylation of protein post-translational modification. J. Biol. Chem. 2012, 287, 4411–4418. [Google Scholar] [CrossRef]
- Wedmann, R.; Zahl, A.; Shubina, T.E.; Durr, M.; Heinemann, F.W.; Bugenhagen, B.E.; Burger, P.; Ivanovic-Burmazovic, I.; Filipovic, M.R. Does perthionitrite (SSNO(−)) account for sustained bioactivity of NO? A (bio)chemical characterization. Inorg. Chem. 2015, 54, 9367–9380. [Google Scholar] [CrossRef]
- Nava, M.; Martin-Drumel, M.A.; Lopez, C.A.; Crabtree, K.N.; Womack, C.C.; Nguyen, T.L.; Thorwirth, S.; Cummins, C.C.; Stanton, J.F.; McCarthy, M.C. Spontaneous and Selective Formation of HSNO, a Crucial Intermediate Linking H2S and Nitroso Chemistries. J. Am. Chem. Soc. 2016, 138, 11441–11444. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, J.; Lu, Y.; Wang, R. The vasorelaxant effect of H2S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001, 20, 6008–6016. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berenyiova, A.; Grman, M.; Misak, A.; Golas, S.; Cuchorova, J.; Cacanyiova, S. The Possible Role of the Nitroso-Sulfide Signaling Pathway in the Vasomotoric Effect of Garlic Juice. Molecules 2020, 25, 590. https://doi.org/10.3390/molecules25030590
Berenyiova A, Grman M, Misak A, Golas S, Cuchorova J, Cacanyiova S. The Possible Role of the Nitroso-Sulfide Signaling Pathway in the Vasomotoric Effect of Garlic Juice. Molecules. 2020; 25(3):590. https://doi.org/10.3390/molecules25030590
Chicago/Turabian StyleBerenyiova, Andrea, Marian Grman, Anton Misak, Samuel Golas, Justina Cuchorova, and Sona Cacanyiova. 2020. "The Possible Role of the Nitroso-Sulfide Signaling Pathway in the Vasomotoric Effect of Garlic Juice" Molecules 25, no. 3: 590. https://doi.org/10.3390/molecules25030590
APA StyleBerenyiova, A., Grman, M., Misak, A., Golas, S., Cuchorova, J., & Cacanyiova, S. (2020). The Possible Role of the Nitroso-Sulfide Signaling Pathway in the Vasomotoric Effect of Garlic Juice. Molecules, 25(3), 590. https://doi.org/10.3390/molecules25030590






