Naphthoquinone Derivatives with Anti-Inflammatory Activity from Mangrove-Derived Endophytic Fungus Talaromyces sp. SK-S009
Abstract
1. Introduction
2. Results and Discussion
2.1. Metabolites Isolation
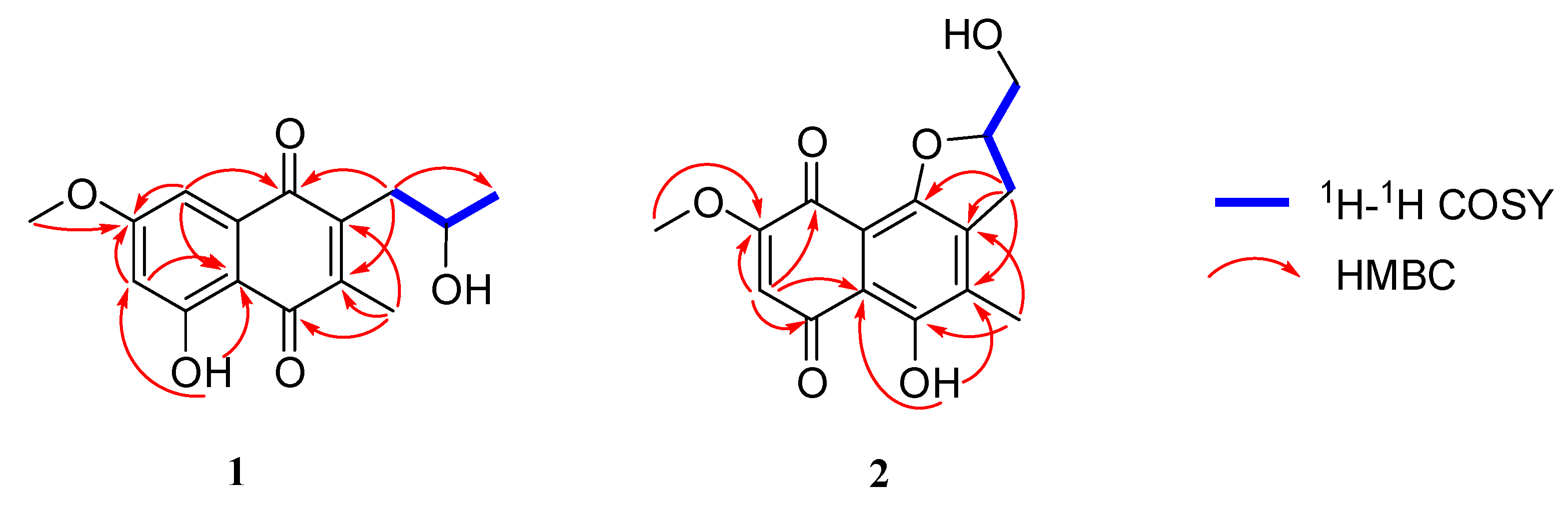
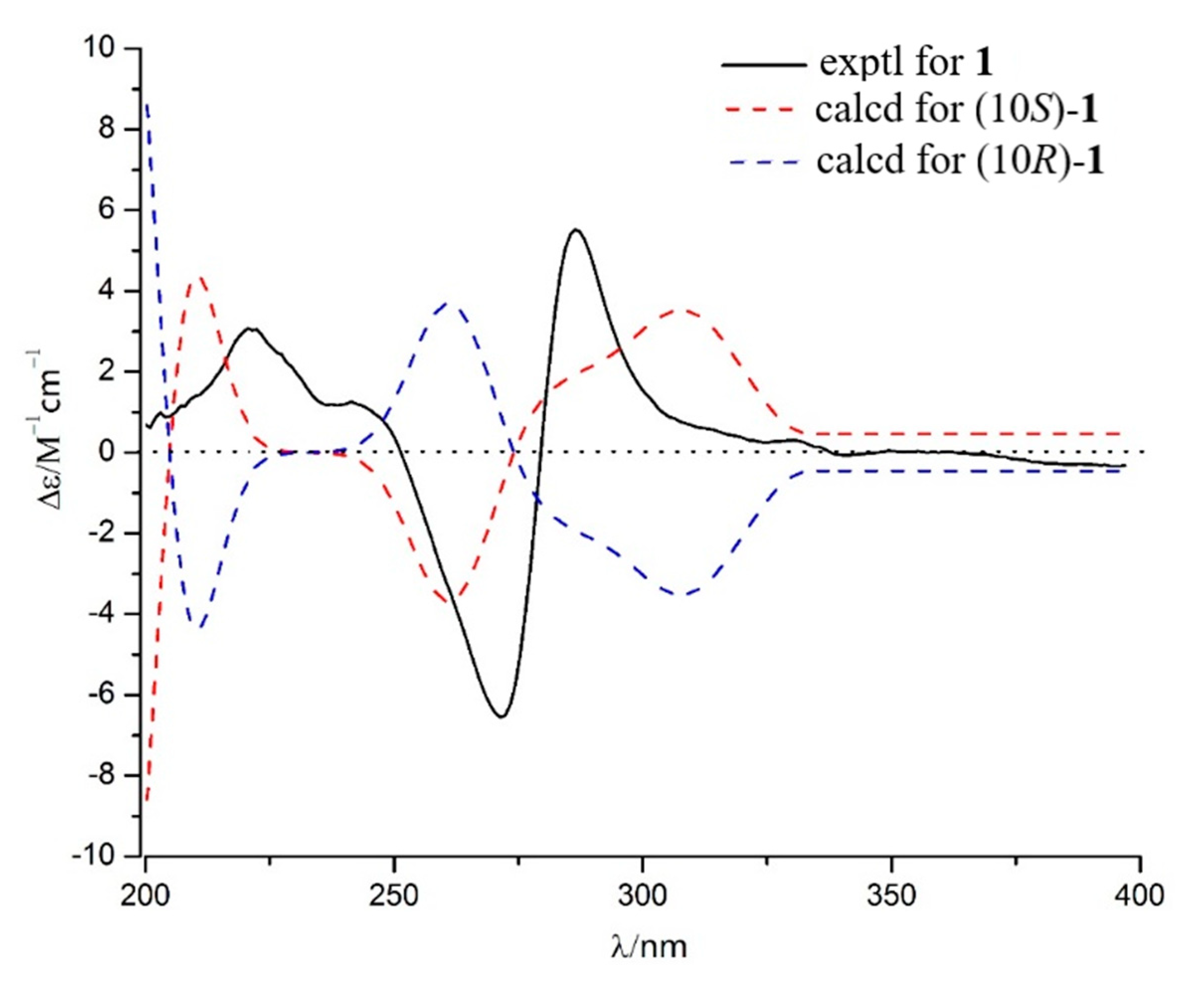
2.2. Structure Identification
2.3. Inhibitory Effects on NO Production
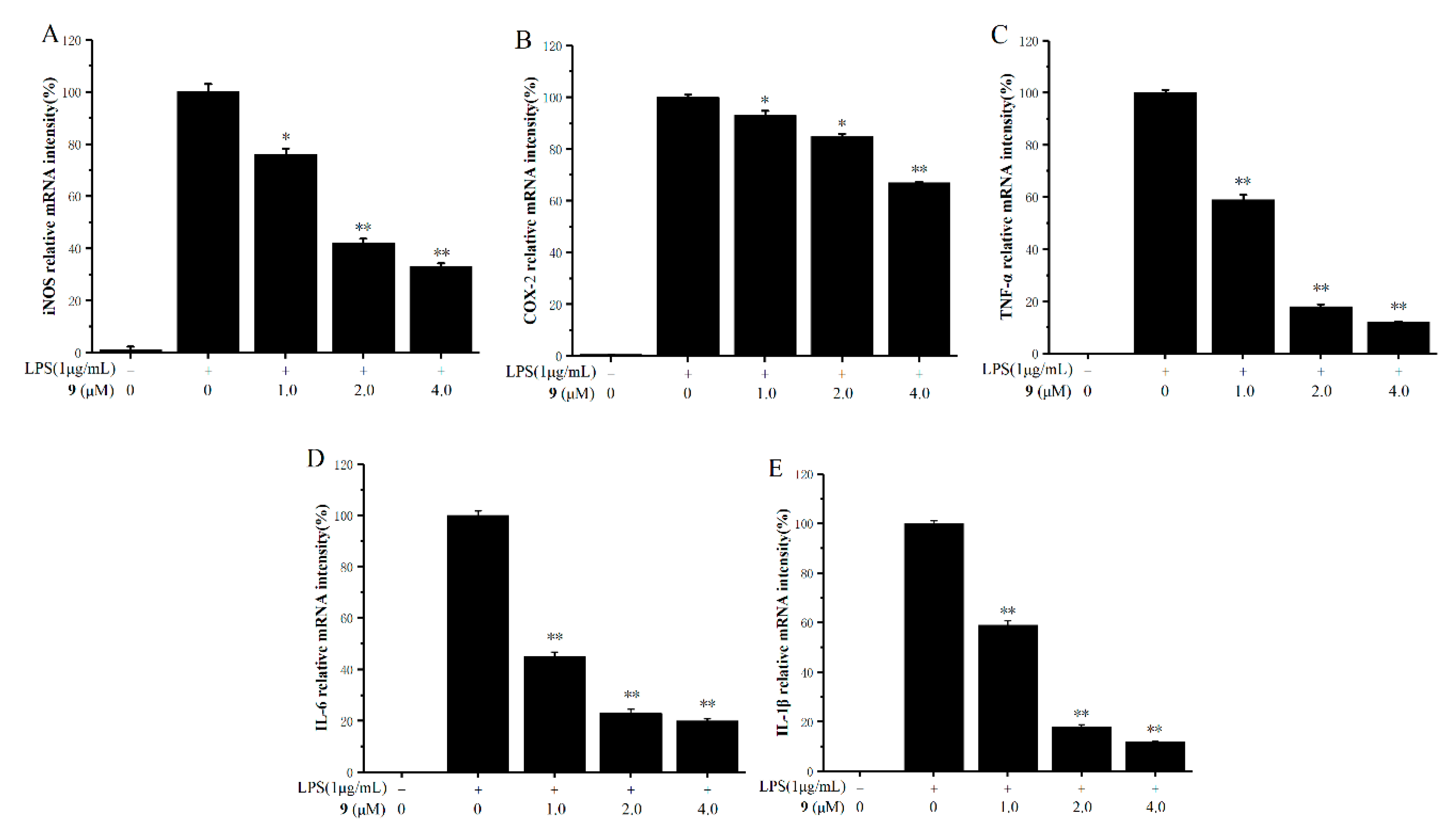
2.4. Inhibitory Effects on the Production of Inducible Nitric Oxide Synthase (iNOS), Cyclooxygenase-2 (COX-2), and Pro-Inflammatory Factors
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Extraction and Isolation
3.3.1. Talanaphthoquinone A (1)
3.3.2. Talanaphthoquinone B (2)
3.4. Measurement of NO Production and Cell Viability
3.5. Real-Time PCR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Heller, R.A.; Schena, M.; Chai, A.; Shalon, D.; Bedilion, T.; Gilmore, J.; Woolley, D.E.; Davis, R.W. Discovery and analysis of inflammatory disease-related genes using cdna microarrays. Proc. Natl. Acad. Sci. USA 1997, 94, 2150–2155. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.S.; Tsai, H.L.; Chau, L.Y. Induction of heme oxygenase-1 expression in murine macrophages is essential for the anti-inflammatory effect of low dose 15-deoxy-delta 12, 14-prostaglandin J2. J. Biol. Chem. 2003, 278, 19325–19330. [Google Scholar] [CrossRef] [PubMed]
- Wiesel, P.L.; Foster, C.; Pellacani, A.; Layne, M.D.; Hsieh, C.M.; Huggins, G.S.; Strauss, P.; Yet, S.F.; Perrella, M.A. Thioredoxin facilitates the induction of heme oxygenase-1 in response to inflammatory mediators. J. Biol. Chem. 2000, 275, 24840–24846. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.S.; Abimael, D.R.; Taglialatelascafati, O. Marine pharmacology in 2012–2013: Marine compounds with Antibacterial, antidiabetic, antifungal, anti-Inflammatory, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous systems, and other miscellaneous mechanisms of action. Mar. Drugs 2017, 15, 2510–2573. [Google Scholar]
- Cui, H.; Liu, Y.; Li, J.; Huang, X.; Yan, T.; Cao, W.; Liu, H.; Long, Y.; She, Z. Diaporindenes A−D: Four unusual 2, 3-dihydro-1H-indene analogues with anti-inflammatory activities from the mangrove endophytic fungus Diaporthe sp. SYSU-HQ3. J. Org. Chem. 2018, 83, 11804–11813. [Google Scholar] [CrossRef]
- Liu, H.; Chen, S.; Liu, W.; Liu, Y.; Huang, X.; She, Z. Polyketides with immunosuppressive activities from mangrove endophytic fungus Penicillium sp. ZJ-SY2. Mar. Drugs 2016, 14, 217–223. [Google Scholar] [CrossRef]
- Rosario, N.; Antonio, T. Bioactive compounds produced by strains of Penicillium and Talaromyces of marine origin. Mar. Drugs 2016, 14, 37–71. [Google Scholar]
- Zhang, L.; Zhang, W.; Liu, J.; Hu, J. C− F bond cleavage by intramolecular SN2 reaction of alkyl fluorides with O-and N-Nucleophiles. J. Org. Chem. 2009, 74, 2850–2853. [Google Scholar] [CrossRef]
- MangasSánchez, J.J.; Busto, E.E.; Gotor-Fernández, V.V.; Gotor, V.V. Straightforward synthesis of enantiopure 2, 3-dihydrobenzofurans by a sequential stereoselective biotransformation and chemical intramolecular cyclization. Org. Lett. 2010, 12, 3498–3501. [Google Scholar] [CrossRef]
- Kimura, Y.; Shimada, A.; Nakajima, H.; Hamasaki, T. Structures of naphthoquinones produced by the fungus, Fusarium sp., and their biological activity toward pollen germination. Agric. Biol. Chem. 1988, 52, 1253–1259. [Google Scholar] [CrossRef]
- Baker, R.A.; Tatum, J.H.; Nemec, S. Antimicrobial activity of naphthoquinones from Fusaria. Mycopathologia 1990, 111, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ding, J.; Ding, K.; Chen, D.; Shan, C.; Mei, G. Phomonaphthalenone A: A novel dihydro-naphthalenone with anti-HIV activity from Phomopsis sp. HCCB04730. Phytochem. Lett. 2013, 6, 257–260. [Google Scholar] [CrossRef]
- Medentsev, A.G.; Akimenko, V.K. Mechanism of phytotoxic action of naphthoquinone pigments of the fungus Fusarium decemcellulare. Phytochemistry 1992, 31, 77–79. [Google Scholar] [CrossRef]
- Xia, X.; Liu, X.; Koo, D.C.; Sun, Z.; Shim, S. Chemical constituents of Fusarium sp. fungus associated with sea cucumbers. Chem. Nat. Com. 2014, 50, 1103–1105. [Google Scholar] [CrossRef]
- Moore, R.E.; Singh, H.; Chang, C.W.J.; Scheuer, P.J. Polyhydroxy naphthoquinones: Preparation and hydrolysis of methoxyl derivatives. Tetrahedron 1967, 23, 3271–3305. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, C.; Zheng, Z. New polyketides isolated from Botryosphaeria australis strain ZJ12-1A. Helv. Chim. Acta 2011, 94, 897–902. [Google Scholar] [CrossRef]
- Poch, G.K.; Gloer, J.B.; Shearer, C.A. New bioactive metabolites from a freshwater isolate of the fungus Kirschsteiniothelia sp. J. Nat. Prod. 1992, 55, 1093–1099. [Google Scholar] [CrossRef]
- Sun, R.; Gao, Y.; Shen, K.; Xu, Y.; Wang, C.; Liu, H. Antimicrobial metabolites from the aquatic fungus Delitschia corticola. Phytochem. Lett. 2011, 4, 101–105. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Z.; Liu, H.; Pan, Y.; Li, J.; Liu, L.; She, Z. Dichloroisocoumarins with potential anti-inflammatory activity from the mangrove endophytic fungus Ascomycota sp. CYSK-4. Mar. Drugs 2018, 16, 54. [Google Scholar] [CrossRef]
- Zhao, D.; Shao, C.; Gan, L.; Wang, M.; Wang, C. Chromone derivatives from a sponge-serived strain of the fungus Corynespora cassiicola. J. Nat. Prod. 2015, 78, 286–293. [Google Scholar] [CrossRef]
- Nielsen, K.F.; Smedsgaard, J. Fungal metabolite screening: Database of 474 mycotoxins and fungal metabolites for dereplication by standardised liquid chromatography-UV-mass spectrometry methodology. J. Chrom. A 2003, 1002, 111–136. [Google Scholar] [CrossRef]
- Arsenault, G.P. Fungal metabolites—III: Quinones from fusarium solani D2 purple and structure of (+)-solaniol. Tetrahedron 1968, 24, 4745–4749. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Q.; Xia, G. Polyketides with α-glucosidase inhibitory activity from a mangrove endophytic fungus, Penicillium sp. HN29-3B1. J. Nat. Prod. 2015, 78, 1816–1822. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Lee, J.M.; Jun, S.H.; Lee, S.H.; Kim, N.W.; Lee, J.H.; Ko, N.Y.; Mun, S.H.; Kim, B.K.; Lim, B.O. The anti-inflammatory effects of Pyrolae herba extract through the inhibition of the expression of inducible nitric oxide synthase (iNOS) and NO production. J. Ethnopharmacol. 2007, 112, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Nishiumi, S.; Nishida, M.; Hirai, M.; Azuma, T.; Yoshida, H.; Mizushina, Y.; Yoshida, M. Effects of quinone derivatives, such as 1, 4-naphthoquinone, on DNA polymerase inhibition and anti-inflammatory action. Med. Chem. 2011, 7, 37–44. [Google Scholar] [CrossRef]
- Fathy, H.M.; Aboushoer, M.I.; Baraka, A.; Abdel-Kader, M.S.; Omar, A.A. A New Naphthoquinone with Anti-inflammatory Activity from an egyptian collection of echiochilon fruticosum. Nat. Prod. Scien. 2009, 15, 22–26. [Google Scholar]
Sample Availability: Not available. |

| Position | 1 | 2 | ||
|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 164.2, C | - | 190.1, C | - |
| 2 | 106.1, C | 6.63, d (2.5) | 109.2, CH | 6.07, s |
| 3 | 165.9, C | - | 161.4, C | - |
| 4 | 107.8, C | 7.17, d (2.5) | 177.6, C | - |
| 4a | 133.7, C | - | 109.1, C | - |
| 5 | 185.2, C | - | 155.0, C | - |
| 6 | 144.6, C | - | 139.0, C | - |
| 7 | 145.7, C | - | 134.2, C | - |
| 8 | 188.6, C | - | 157.3, C | - |
| 8a | 109.7, C | - | 110.3, C | - |
| 9 | 36.8, CH2 | 2.81, s 2.80, d (1.7) | 29.9, CH2 | 3.04, dd (7.1,16.6); 3.20, dd (9.4,17.0) |
| 10 | 67.9, CH | 4.04, dd (11.9, 6.0) | 86.2, CH | 5.15, m |
| 11 | 24.4, CH3 | 1.31, d (6.2) | 64.5, CH2 | 3.76, d (8.6); 4.02, d (12.7) |
| 12 | 12.9, CH3 | 2.21, s | 13.3, CH3 | 2.25, s |
| 13 | 56.1, CH3 | 3.9, s | 56.7, CH3 | 3.88, s |
| 1-OH | - | 12.38, s | - | 13.47, s |
| Compounds | IC50 (μM) | CC50 (μM) a | SI b |
|---|---|---|---|
| 1 | 3.9 ± 0.5 | 30.7 ± 0.5 | 7.9 |
| 2 | 49.7 ± 1.5 | - | |
| 3 | 16.0 ± 0.2 | - | |
| 4 | 22.6 ± 0.5 | - | |
| 5 | 11.2 ± 0.3 | - | |
| 6 | 5.2 ± 0.1 | - | |
| 7 | 14.4 ± 0.6 | 51.4 ± 1.5 | 3.6 |
| 8 | 7.7 ± 0.3 | - | |
| 9 | 1.7 ± 0.2 | 50.3 ± 1.5 | 29.6 |
| 10 | 7.5 ± 0.2 | 15.8 ± 0.4 | 2.1 |
| 11 | 15.5 ± 0.6 | 59.2 ± 1.5 | 3.8 |
| 12 | 5.6 ± 0.3 | 48.4 ± 1.3 | 8.6 |
| Indomethacin c | 26.3 ± 0.6 |
| Primer | Primer Sequence (5′ to 3′) | |
|---|---|---|
| iNOS | Forward | GTCTTTGACGCTCGGAACTGTAG |
| Reversed | TGAAGTCATGTTTGCCGTCACT | |
| COX-2 | Forward | GATGACTGCCCAACTCCC |
| Reversed | AACCCAGGTCCTCGCTTA | |
| TNF-α | Forward | TGGCTGCTGAAAAGACACATGT |
| Reversed | CCACCAGACGTTCTGCTGTCTAG | |
| IL-1β | Forward | AGTTGACGGACCCCAAAAG |
| Reversed | AGCTGGATGCTCTCATCAGG | |
| IL-6 | Forward | TTCCATCCAGTTGCCTTCTTG |
| Reversed | GGGAGTGGTATCCTCTGTGAAGTC | |
| GADPH | Forward | TGTGTCCGTCGTGGATCTGA |
| Reversed | TTGCTGTTGAAGTCGCAGGAG |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Yan, C.; Li, C.; You, T.; She, Z. Naphthoquinone Derivatives with Anti-Inflammatory Activity from Mangrove-Derived Endophytic Fungus Talaromyces sp. SK-S009. Molecules 2020, 25, 576. https://doi.org/10.3390/molecules25030576
Liu H, Yan C, Li C, You T, She Z. Naphthoquinone Derivatives with Anti-Inflammatory Activity from Mangrove-Derived Endophytic Fungus Talaromyces sp. SK-S009. Molecules. 2020; 25(3):576. https://doi.org/10.3390/molecules25030576
Chicago/Turabian StyleLiu, Hongju, Chong Yan, Changqun Li, Tingting You, and Zhigang She. 2020. "Naphthoquinone Derivatives with Anti-Inflammatory Activity from Mangrove-Derived Endophytic Fungus Talaromyces sp. SK-S009" Molecules 25, no. 3: 576. https://doi.org/10.3390/molecules25030576
APA StyleLiu, H., Yan, C., Li, C., You, T., & She, Z. (2020). Naphthoquinone Derivatives with Anti-Inflammatory Activity from Mangrove-Derived Endophytic Fungus Talaromyces sp. SK-S009. Molecules, 25(3), 576. https://doi.org/10.3390/molecules25030576





