Tannins of Conifer Bark as Nordic Piquancy—Sustainable Preservative and Aroma?
Abstract
:1. Introduction
2. Results and Discussion
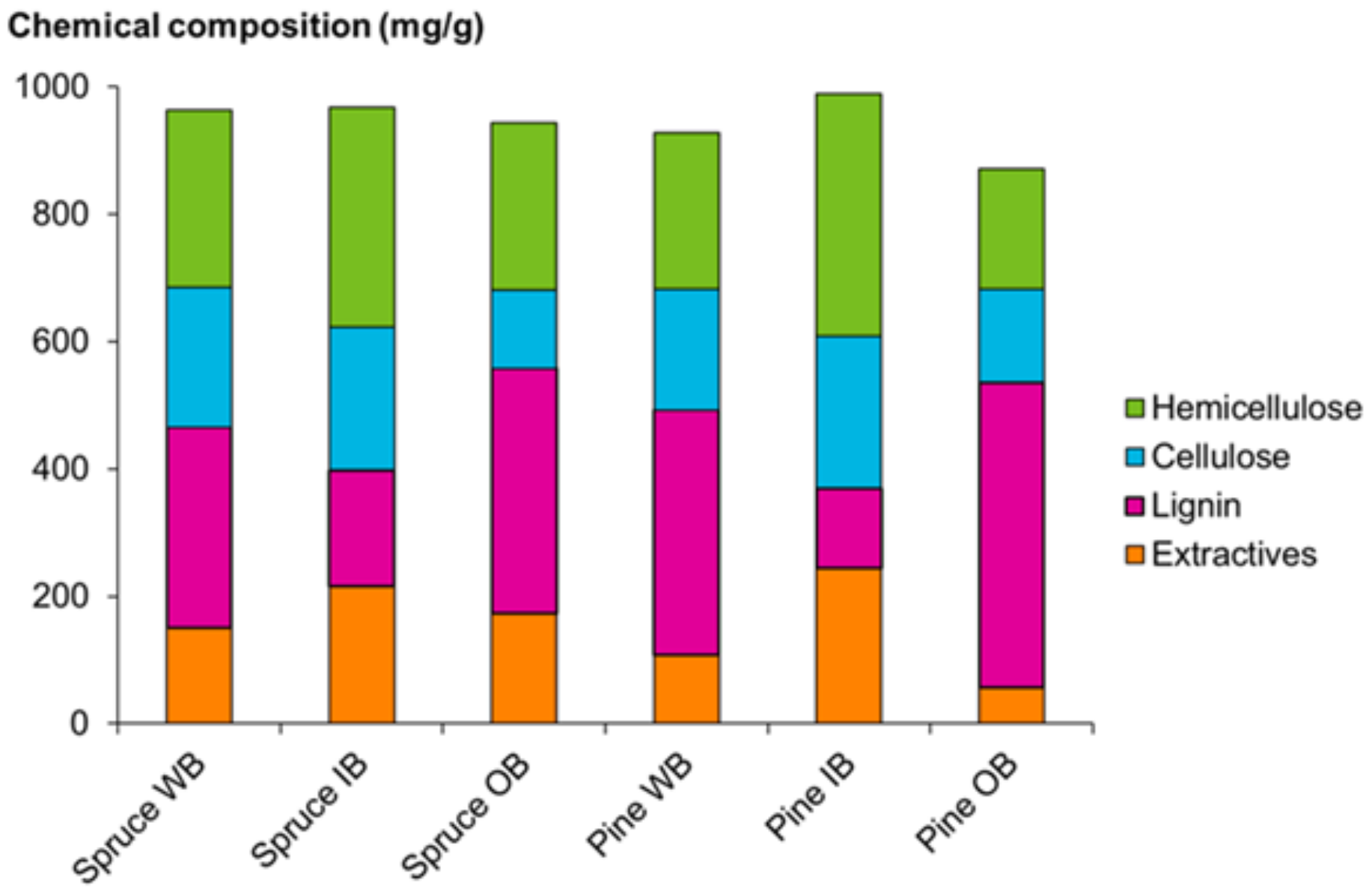
2.1. Composition of Bark Raw Materials
2.2. Yield and Composition of Bark Extracts
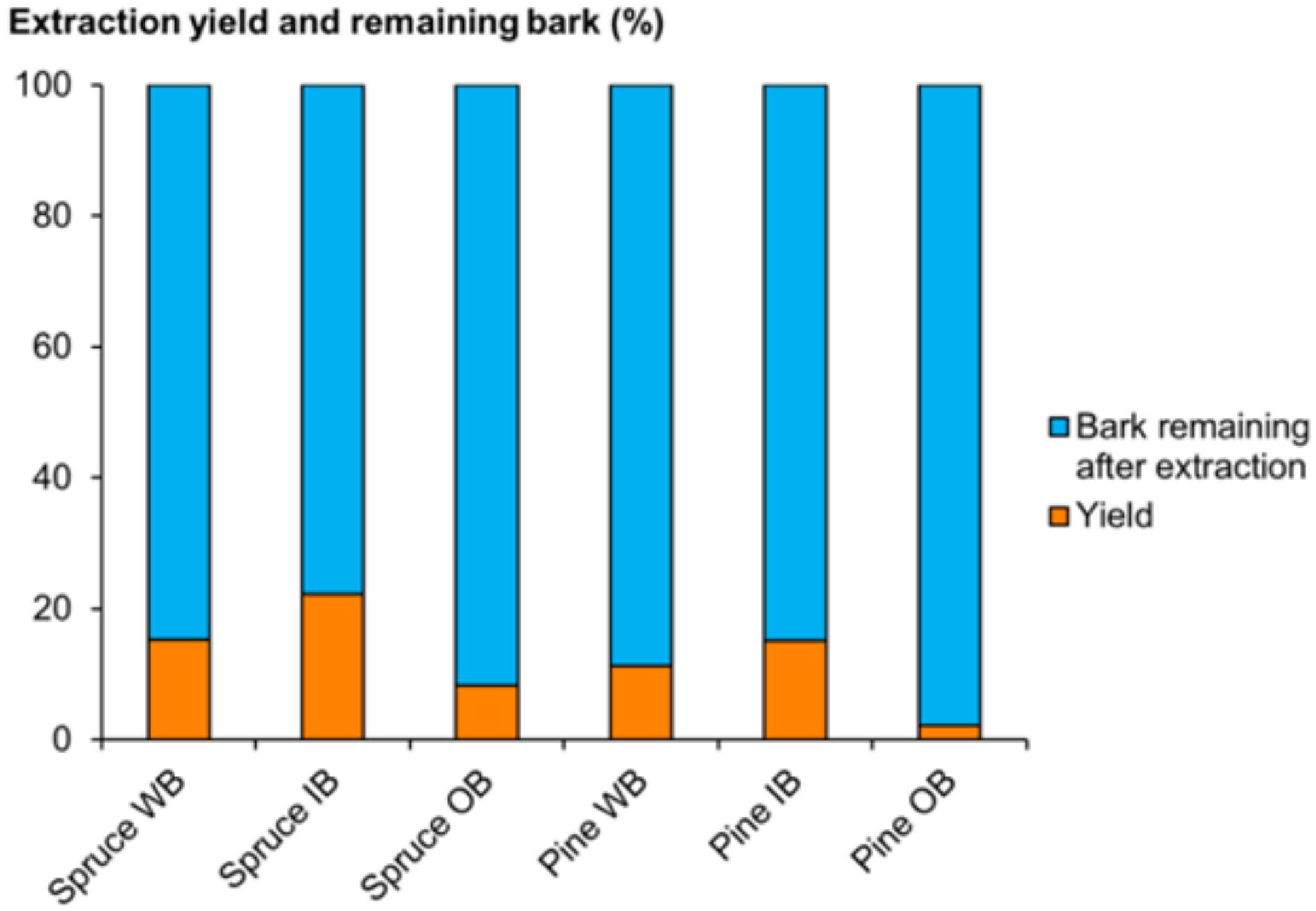
2.2.1. Extraction Yield
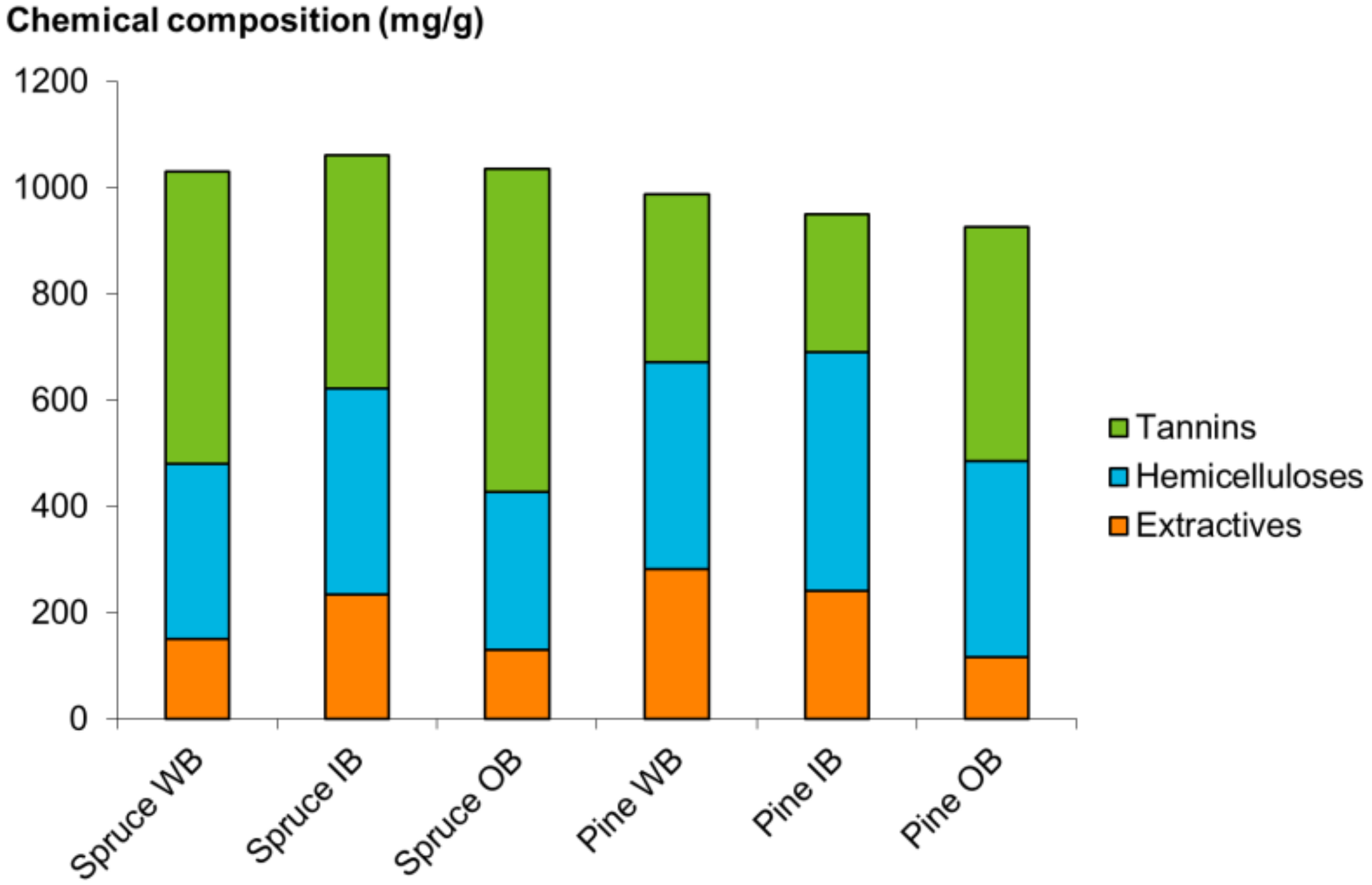
2.2.2. Chemical Composition of Bark Extracts
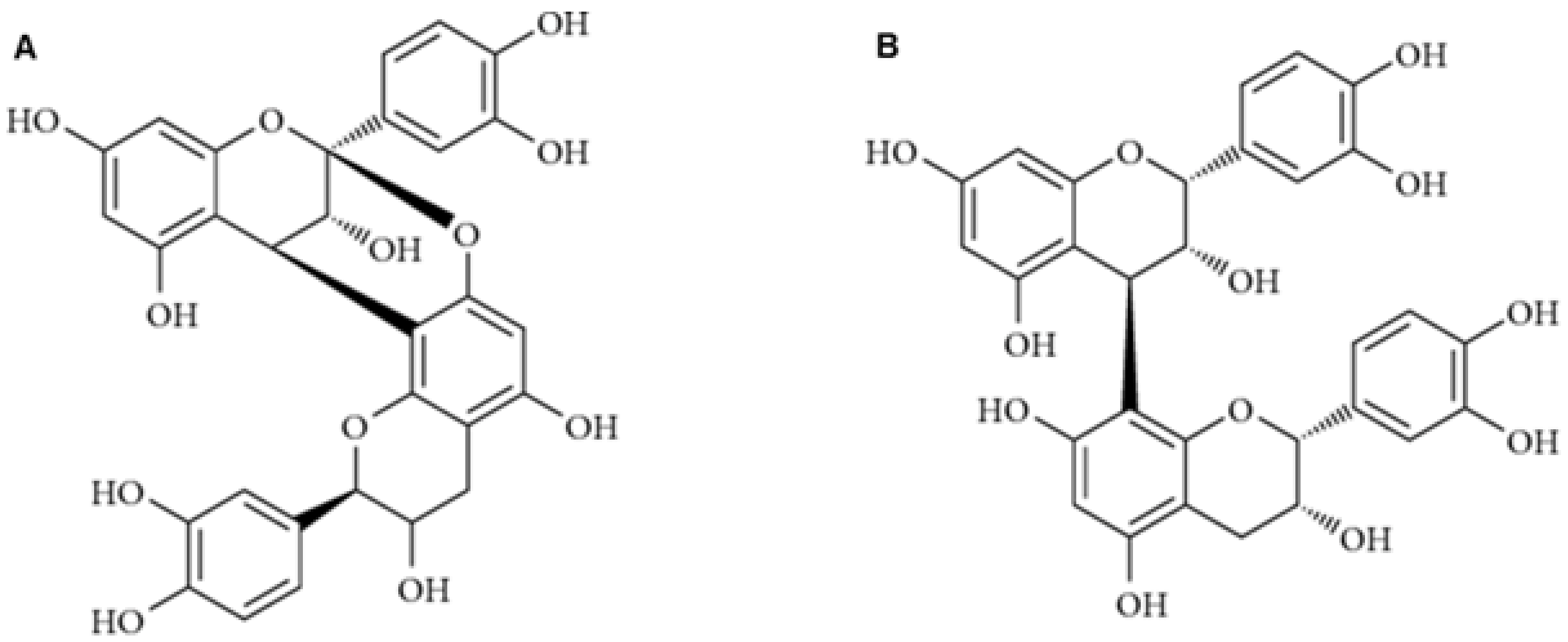
2.2.3. Chemical Composition and Properties of Condensed Tannins
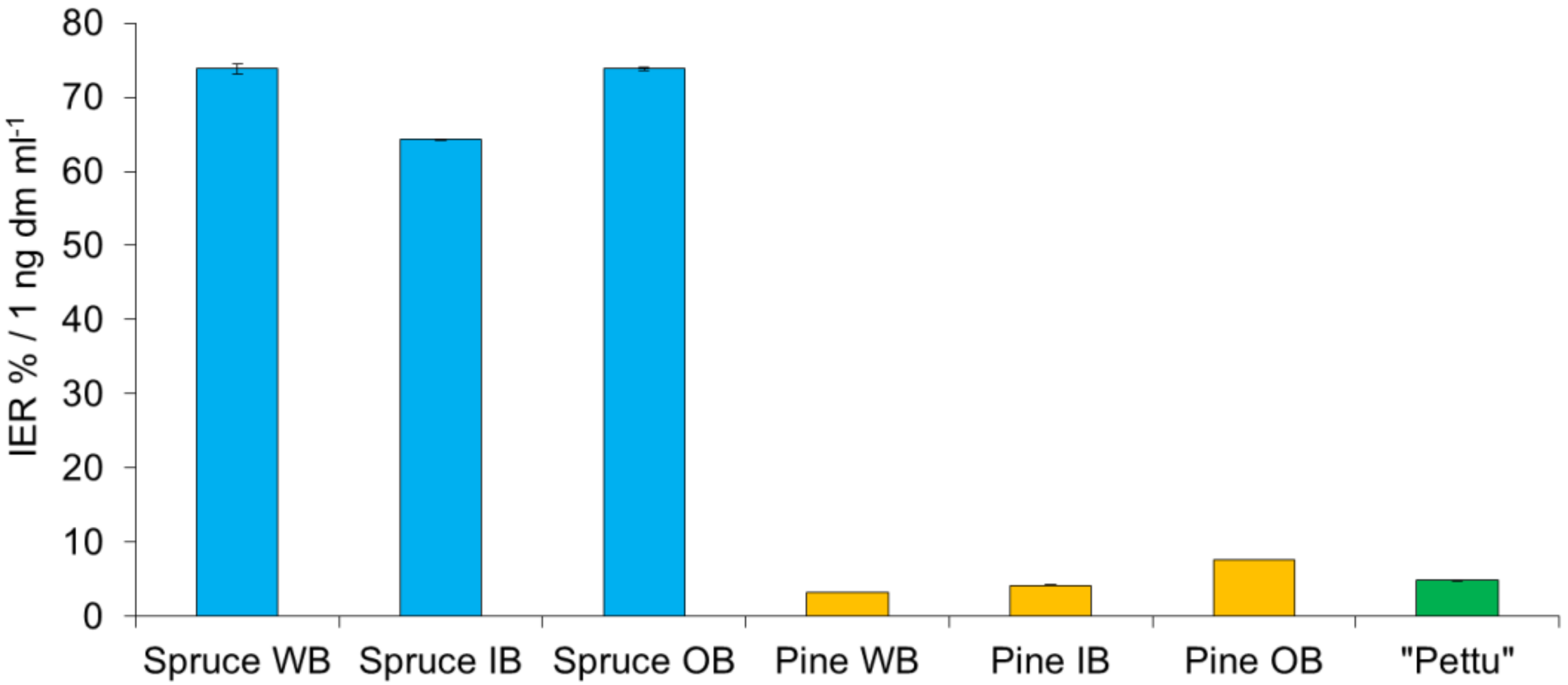
2.3. Inhibition of Lipid Oxidation by Tannin Extracts in a Liposome Model
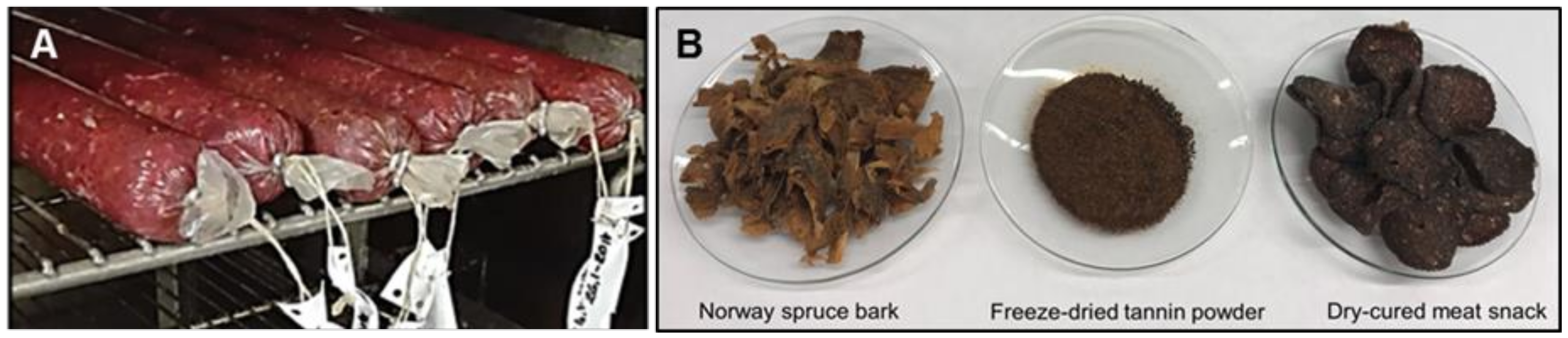
2.4. The Treatment of Meat Snacks
2.5. TBA of the Meat Snacks
2.6. Sensory Assessment of Meat Snacks with Tannin Additions
2.7. Safety of Bark-Derived Flava-3-ols and Proanthocyanidins in Foodstuffs
3. Materials and Methods
3.1. Chemicals
3.2. Bark Materials
3.3. Tannin Extraction
3.4. Chemical Composition of Bark
3.4.1. Lipophilic and Hydrophilic Extractives
3.4.2. Hemicellulose Content
3.4.3. Cellulose Content
3.4.4. Lignin Content
3.5. Chemical Composition of Tannin Extract Powders
3.5.1. Carbohydrates
3.5.2. Extractives
3.5.3. Yield of Condensed Tannins
3.5.4. Chemical Composition of Condensed Tannins
3.6. Lipid oxidation Inhibition Capacity of Tannin Extracts
3.7. Preparation of Meat Snack Samples
3.8. Thiobarbituric Acid Test for Monitoring the Lipid Oxidation of Meat Snacks
3.9. Sensory Evaluation of Meat Snacks with Tannin Addition
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Salminen, J.-P.; Karonen, M. Chemical ecology of tannins and other phenolics: We need a change in approach. Funct. Ecol. 2011, 25, 325–338. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; Scalbert, A. Proanthocyanidins and tannin-like compounds – nature, occurrence, dietary intake and effects on nutrition and health. J. Sci. Food Agric. 2000, 80, 1094–1117. [Google Scholar] [CrossRef]
- Matthews, S.; Mila, I.; Scalbert, A.; Donnelly, D.M.X. Extractable and non-extractable proanthocyanidins in barks. Phytochemistry 1997, 45, 405–410. [Google Scholar] [CrossRef]
- Hellström, J.K.; Mattila, P.H. HPLC determination of extractable and unextractable proanthocyanidins in plant materials. J. Agric. Food Chem. 2008, 56, 7617–7624. [Google Scholar] [CrossRef] [PubMed]
- Riedl, K.M.; Hagerman, A.E. Tannin−protein complexes as radical scavengers and radical sinks. J. Agric. Food Chem. 2001, 49, 4917–4923. [Google Scholar] [CrossRef] [PubMed]
- Schewe, T.; Kühn, H.; Sies, H. Flavonoids of cocoa inhibit recombinant human 5-lipoxygenase. J. Nutr. 2002, 132, 1825–1829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wink, M. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry 2003, 64, 3–19. [Google Scholar] [CrossRef]
- Koleckar, V.; Kubikova, K.; Rehakova, Z.; Kuca, K.; Jun, D.; Jahodar, L.; Opletal, L. Condensed and hydrolysable tannins as antioxidants influencing the health. Mini Rev. Med. Chem. 2008, 8, 436–447. [Google Scholar] [CrossRef]
- De Bruyne, T.; Pieters, L.; Deelstra, H.; Vlietinck, A. Condensed vegetable tannins: Biodiversity in structure and biological activities. Biochem. Syst. Ecol. 1999, 27, 445–459. [Google Scholar] [CrossRef]
- Dixon, R.A.; Xie, D.-Y.; Sharma, S.B. Proanthocyanidins—a final frontier in flavonoid research? New Phytol. 2005, 165, 9–28. [Google Scholar] [CrossRef] [Green Version]
- Scalbert, A. Antimicrobial properties of tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Jansone, Z.; Muizniece, I.; Blumberga, D. Analysis of wood bark use opportunities. Energy Procedia 2017, 128, 268–274. [Google Scholar] [CrossRef]
- Shirmohammadli, Y.; Efhamisisi, D.; Pizzi, A. Tannins as a sustainable raw material for green chemistry: A review. Ind. Crops Prod. 2018, 126, 316–332. [Google Scholar] [CrossRef]
- Metsämuuronen, S.; Sirén, H. Bioactive phenolic compounds, metabolism and properties: A review on valuable chemical compounds in Scots pine and Norway spruce. Phytochem. Rev. 2019, 18, 623–664. [Google Scholar] [CrossRef] [Green Version]
- Official Statistics of Finland (OSF). Natural Resources Institute Finland, Forest industries’ wood consumption. Available online: https://stat.luke.fi/en/wood-consumption (accessed on 8 January 2020).
- Rasi, S.; Kilpeläinen, P.; Rasa, K.; Korpinen, R.; Raitanen, J.-E.; Vainio, M.; Kitunen, V.; Pulkkinen, H.; Jyske, T. Cascade processing of softwood bark with hot water extraction, pyrolysis and anaerobic digestion. Bioresour. Technol. 2019, 292, 121893. [Google Scholar] [CrossRef]
- Fernández, J.; Pérez-Álvarez, J.A.; Fernández-López, J.A. Thiobarbituric acid test for monitoring lipid oxidation in meat. Food Chem. 1997, 59, 345–353. [Google Scholar] [CrossRef]
- Al-Hijazeen, M.; Lee, E.J.; Mendonca, A.; Ahn, D.U. Effects of tannic acid on lipid and protein oxidation, color, and volatiles of raw and cooked chicken breast meat during storage. Antioxidants 2016, 5, 19. [Google Scholar] [CrossRef] [Green Version]
- Burri, S.C.M.; Granheimer, K.; Rémy, M.; Ekholm, A.; Håkansson, Å.; Rumpunen, K.; Tornberg, E. Lipid oxidation inhibition capacity of 11 plant materials and extracts evaluated in highly oxidised cooked meatballs. Foods 2019, 8, 406. [Google Scholar] [CrossRef] [Green Version]
- Burri, S.C.M.; Ekholm, A.; Bleive, U.; Püssa, T.; Jensen, M.; Hellström, J.; Mäkinen, S.; Korpinen, R.; Mattila, P.H.; Radenkovs, V.; et al. Lipid oxidation inhibition capacity of plant extracts and powders in a processed meat model system. Meat Sci. 2020, 162, 108033. [Google Scholar] [CrossRef]
- Kemppainen, K.; Siika-aho, M.; Pattathil, S.; Giovando, S.; Kruus, K. Spruce bark as an industrial source of condensed tannins and non-cellulosic sugars. Ind. Crops Prod. 2014, 52, 158–168. [Google Scholar] [CrossRef]
- Karonen, M.; Loponen, J.; Ossipov, V.; Pihlaja, K. Analysis of procyanidins in pine bark with reversed-phase and normal-phase high-performance liquid chromatography-electrospray ionization mass spectrometry. Anal. Chim. Acta 2004, 522, 105–112. [Google Scholar] [CrossRef]
- Bianchi, S.; Gloess, A.N.; Kroslakova, I.; Mayer, I.; Pichelin, F. Analysis of the structure of condensed tannins in water extracts from bark tissues of Norway spruce (Picea abies [Karst.]) and silver fir (Abies alba [Mill.]) using MALDI-TOF mass spectrometry. Ind. Crops Prod. 2014, 61, 430–437. [Google Scholar] [CrossRef]
- Bianchi, S.; Kroslakova, I.; Janzon, R.; Mayer, I.; Saake, B.; Pichelin, F. Characterization of condensed tannins and carbohydrates in hot water bark extracts of European softwood species. Phytochemistry 2015, 120, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Krogell, J.; Holmbom, B.; Pranovich, A.; Hemming, J.; Willför, S. Extraction and chemical characterization of Norway spruce inner and outer bark. Nord. Pulp Paper Res. J. 2012, 27, 6–17. [Google Scholar] [CrossRef]
- Latva-Mäenpää, H.; Laakso, T.; Sarjala, T.; Wähälä, K.; Saranpää, P. Variation of stilbene glucosides in bark extracts obtained from roots and stumps of Norway spruce (Picea abies [L.] Karst.). Trees 2013, 27, 131–139. [Google Scholar] [CrossRef]
- Latva-Mäenpää, H.; Laakso, T.; Sarjala, T.; Wähälä, K.; Saranpää, P. Root neck of Norway spruce as a source of bioactive lignans and stilbenes. Holzforschung 2014, 68, 1–7. [Google Scholar] [CrossRef]
- Latva-Mäenpää, H. Bioactive and protective polyphenolics from roots and stumps of conifer trees (Norway spruce and Scots pine). Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2017. Available online: http://urn.fi/URN:ISBN:978-951-51-3466-0.
- Jyske, T.; Laakso, T.; Latva-Mäenpää, H.; Tapanila, T.; Saranpää, P. Yield of stilbene glucosides from the bark of young and old Norway spruce stems. Biomass Bioenergy 2014, 71, 216–227. [Google Scholar] [CrossRef]
- Singh, A.P.; Singh, R.; Verma, S.S.; Rai, V.; Kaschula, C.H.; Maiti, P.; Gupta, S.C. Health benefits of resveratrol: Evidence from clinical studies. Med. Res. Rev. 2019, 39, 1851–1891. [Google Scholar] [CrossRef]
- Pietarinen, S.P.; Willför, S.M.; Ahotupa, M.O.; Hemming, J.E.; Holmbom, B.R. Knotwood and bark extracts: Strong antioxidants from waste materials. J. Wood Sci. 2006, 52, 436–444. [Google Scholar] [CrossRef]
- Salem, M.Z.M.; Elansary, H.O.; Elkelish, A.A.; Zeidler, A.; Ali, H.M.; EL-Hefny, M.; Yessoufou, K. In vitro bioactivity and antimicrobial activity of Picea abies and Larix decidua wood and bark extracts. BioResources 2016, 11, 9421–9437. [Google Scholar] [CrossRef] [Green Version]
- Diouf, P.N.; Stevanovic, T.; Cloutier, A. Study on chemical composition, antioxidant and anti-inflammatory activities of hot water extract from Picea mariana bark and its proanthocyanidin-rich fractions. Food Chem. 2009, 113, 897–902. [Google Scholar] [CrossRef]
- Viljanen, K.; Kylli, P.; Kivikari, R.; Heinonen, M. Inhibition of protein and lipid oxidation in liposomes by berry phenolics. J. Agric. Food Chem. 2004, 52, 7419–7424. [Google Scholar] [CrossRef] [PubMed]
- Vuorela, S.; Salminen, H.; Mäkelä, M.; Kivikari, R.; Karonen, M.; Heinonen, M. Effect of plant phenolics on protein and lipid oxidation in cooked pork meat patties. J. Agric. Food Chem. 2005, 53, 8492–8497. [Google Scholar] [CrossRef] [PubMed]
- Vuorela, S.; Kreander, K.; Karonen, M.; Nieminen, R.; Hämäläinen, M.; Galkin, A.; Laitinen, L.; Salminen, J.-P.; Moilanen, E.; Pihlaja, K.; et al. Preclinical evaluation of rapeseed, raspberry, and pine bark phenolics for health related effects. J. Agric. Food Chem. 2005, 53, 5922–5931. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, J.; Pazos, M.; Lois, S.; Medina, I. Contribution of galloylation and polymerization to the antioxidant activity of polyphenols in fish lipid systems. J. Agric. Food Chem. 2010, 58, 7423–7431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Touriño, S.; Selga, A.; Jiménez, A.; Juliá, L.; Lozano, C.; Lizárraga, D.; Cascante, M.; Torres, J.L. Procyanidin fractions from pine (Pinus pinaster) bark: Radical scavenging power in solution, antioxidant activity in emulsion, and antiproliferative effect in melanoma cells. J. Agric. Food Chem. 2005, 53, 4728–4735. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.-P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Rauha, J.-P.; Remes, S.; Heinonen, M.; Hopia, A.; Kähkönen, M.; Kujala, T.; Pihlaja, K.; Vuorela, H.; Vuorela, P. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. Int. J. Food Microbiol. 2000, 56, 3–12. [Google Scholar] [CrossRef]
- Karonen, M.; Hämäläinen, M.; Nieminen, R.; Klika, K.D.; Loponen, J.; Ovcharenko, V.V.; Moilanen, E.; Pihlaja, K. Phenolic extractives from the bark of Pinus sylvestris L. and their effects on inflammatory mediators nitric oxide and prostaglandin E2. J. Agric. Food Chem. 2004, 52, 7532–7540. [Google Scholar] [CrossRef]
- Lamy, E.; Pinheiro, C.; Rodrigues, L.; Capela-Silva, F.; Lopes, O.; Tavares, S.; Gaspar, R. Determinants of tannin-rich food and beverage consumption: Oral perception vs. psychosocial aspects, in Tannins: Biochemistry, Food Sources and Nutritional Properties; Combs, C.A., Ed.; Nova Science Publisher Inc.: Hauppauge, NY, USA, 2016; ISBN 978-1-63484-150-4. [Google Scholar]
- Valsta, L.; Kaartinen, N.; Tapanainen, H.; Männistö, S.; Sääksjärvi, K. (Eds.) Nutrition in Finland – The National FinDiet 2017 Survey. Report 2018_012. 2018. Available online: http://urn.fi/URN:ISBN:978-952-343-238-3 (accessed on 12 December 2019).
- Chira, K.; Schmauch, G.; Saucier, C.; Fabre, S.; Teissedre, P.-L. Grape variety effect on proanthocyanidin composition and sensory perception of skin and seed tannin extracts from Bordeaux wine grapes (Cabernet Sauvignon and Merlot) for two consecutive vintages (2006 and 2007). J. Agric. Food Chem. 2009, 57, 545–553. [Google Scholar] [CrossRef]
- Gonzalo-Diago, A.; Dizy, M.; Fernaández-Zurbano, P. Taste and mouthfeel properties of red wines proanthocyanidins and their relation to the chemical composition. J. Agric. Food Chem. 2013, 61, 8861–8870. [Google Scholar] [CrossRef] [Green Version]
- Vidal, S.; Francis, L.; Guyot, S.; Marnet, N.; Kwiatkowski, M.; Gawel, R.; Cheynier, V.; Waters, E.J. The mouth-feel properties of grape and apple proanthocyanidins in a wine-like medium. J. Sci. Food Agric. 2003, 83, 564–573. [Google Scholar] [CrossRef]
- Chira, K.; Zeng, L.; Le Floch, A.; Péchamat, L.; Jourdes, M.; Teissedre, P.-L. Compositional and sensory characterization of grape proanthocyanidins and oak wood ellagitannin. Tetrahedron 2015, 71, 2999–3006. [Google Scholar] [CrossRef]
- Harrison, R. Practical interventions that influence the sensory attributes of red wines related to the phenolic composition of grapes: A review. Int. J. Food Sci. Tech. 2018, 53, 3–18. [Google Scholar] [CrossRef]
- Peleg, H.; Gacon, K.; Schlich, P.; Noble, A.C. Bitterness and astringency of flavan-3-ol monomers, dimers and trimers. J. Sci. Food Agric. 1999, 79, 1123–1128. [Google Scholar] [CrossRef]
- Hellström, J.K.; Törrönen, A.R.; Mattila, P.H. Proanthocyanidins in common food products of plant origin. J. Agric. Food Chem. 2009, 57, 7899–7906. [Google Scholar] [CrossRef]
- Hu, J.; Webster, D.; Cao, J.; Shao, A. The safety of green tea and green tea extract consumption in adults – Results of a systematic review. Regul. Toxicol. and Pharmacol. 2018, 95, 412–433. [Google Scholar] [CrossRef]
- Makena, P.S.; Chung, K.-T. Effects of various plant polyphenols on bladder carcinogen benzidine-induced mutagenicity. Food Chem. Toxicol. 2007, 45, 1899–1909. [Google Scholar] [CrossRef]
- Isbrucker, R.A.; Bausch, J.; Edwards, J.A.; Wolz, E. Safety studies on epigallocatechin gallate (EGCG) preparations. Part 1: Genotoxicity. Food Chem. Toxicol. 2006, 44, 626–635. [Google Scholar] [CrossRef]
- Isbrucker, R.A.; Edwards, J.A.; Wolz, E.; Davidovich, A.; Bausch, J. Safety studies on epigallocatechin gallate (EGCG) preparations. Part 2: Dermal, acute and short-term toxicity studies. Food Chem. Toxicol. 2006, 44, 636–650. [Google Scholar] [CrossRef]
- Wada, K.; Matsumoto, K. Mutagenic activity of tea flavonoid (−)-epigallocatechin in bacterial and mammalian cells. Genes and Environment 2009, 31, 37–42. [Google Scholar] [CrossRef]
- Yu, C.-L.; Swaminathan, B. Mutagenicity of proanthocyanidins. Food Chem. Toxicol. 1987, 25, 135–139. [Google Scholar] [CrossRef]
- Corcoran, M.P.; McKay, D.L.; Blumberg, J.B. Flavonoid basics: Chemistry, sources, mechanisms of action, and safety. J. Nutr. Gerontol. Geriatr. 2012, 31, 176–189. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Novel Food Catalogue. 2019. Available online: https://ec.europa.eu/food/safety/novel_food/catalogue_en (accessed on 19 December 2019).
- Airaksinen, M.M.; Peura, P.; Ala-Fossi-Salokangas, L.; Antere, S.; Lukkarinen, J.; Saikkonen, M.; Stenbäck, F. Toxicity of plant material used as emergency food during famines in Finland. J. Ethnopharmacol. 1986, 18, 273–296. [Google Scholar] [CrossRef]
- Zackrisson, O.; Östlund, L.; Korhonen, O.; Bergman, I. The ancient use of Pinus sylvestris L. (Scots pine) inner bark by Sami people in northern Sweden, related to cultural and ecological factors. Veg. Hist. Archaeobot. 2000, 9, 99–109. [Google Scholar] [CrossRef]
- Mursu, J.; Voutilainen, S.; Nurmi, T.; Helleranta, M.; Rissanen, T.H.; Nurmi, A.; Kaikkonen, J.; Porkkala-Sarataho, E.; Nyyssönen, K.; Virtanen, J.K.; et al. Polyphenol-rich phloem enhances the resistance of total serum lipids to oxidation in men. J. Agric. Food Chem. 2005, 53, 3017–3022. [Google Scholar] [CrossRef]
- Cui, Y.; Xie, H.; Wang, J. Potential biomedical properties of Pinus massoniana bark extract. Phytother. Res. 2005, 19, 34–38. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Feng, J.; Zhang, X.-L.; Cui, Y.-Y. Pine bark extracts: Nutraceutical, pharmacological, and toxicological evaluation. J. Pharmacol. Exp. Ther. 2015, 353, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Oliff, H. Scientific and clinical monograph for Pycnogenol®, 2019 update. The American Botanical Council. Available online: http://abc.herbalgram.org/site/PageServer?pagename=Pycnogenol (accessed on 14 January 2020).
- Francezon, N.; Meda, N.-S.-B.R.; Stevanovic, T. Optimization of bioactive polyphenols extraction from Picea mariana bark. Molecules 2017, 22, 2118. [Google Scholar] [CrossRef] [Green Version]
- Jyske, T.M.; Suuronen, J.-P.; Pranovich, A.V.; Laakso, T.; Watanabe, U.; Kuroda, K.; Abe, H. Seasonal variation in formation, structure, and chemical properties of phloem in Picea abies as studied by novel microtechniques. Planta 2015, 242, 613–629. [Google Scholar] [CrossRef]
- Sundberg, A.; Sundberg, K.; Lillandt, C.; Holmbom, B. Determination of hemicelluloses and pectins in wood and pulp fibres by acid methanolysis and gas chromatography. Nord. Pulp Paper Res. J. 1996, 11, 216–219. [Google Scholar] [CrossRef]
- Schwanninger, M.; Hinterstoisser, B. Klason lignin: Modifications to improve the precision of the standardized determination. Holzforschung 2002, 56, 161–166. [Google Scholar] [CrossRef]
- Mattila, P.H.; Pihlava, J.-M.; Hellström, J.; Nurmi, M.; Eurola, M.; Mäkinen, S.; Jalava, T.; Pihlanto, A. Contents of phytochemicals and antinutritional factors in commercial protein-rich plant products. Food Qual. Saf. 2018, 2, 213–219. [Google Scholar] [CrossRef]
- Mäkinen, S.; Johansson, T.; Vegarud, G.E.; Pihlava, J.M.; Pihlanto, A. Angiotensin I-converting enzyme inhibitory and antioxidant properties of rapeseed hydrolysates. J. Funct. Foods 2012, 4, 575–583. [Google Scholar] [CrossRef]
- Ursini, F.; Maiorino, M.; Valente, M.; Ferri, L.; Gregolin, C. Purification from pig liver of a protein which protects liposomes and biomembranes from peroxidative degradation and exhibits glutathione peroxidase activity on phosphatidylcholine hydroperoxides. Biochim. Biophys. Acta, Lipids Lipid Metab. 1982, 710, 197–211. [Google Scholar] [CrossRef]
- Tirmenstein, M.A.; Pierce, C.A.; Leraas, T.L.; Fariss, M.W. A fluorescence plate reader assay for monitoring the susceptibility of biological samples to lipid peroxidation. Anal. Biochem. 1998, 265, 246–252. [Google Scholar] [CrossRef]
- Draper, H.H.; Hadley, M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990, 186, 421–431. [Google Scholar] [CrossRef]
- Pikul, J.; Leszczynski, D.E.; Kummerow, F.A. Evaluation of three modified TBA methods for measuring lipid oxidation in chicken meat. J. Agric. Food Chem. 1989, 37, 1309–1313. [Google Scholar] [CrossRef]
- Yoshida, H.; Hirooka, N.; Kajimoto, G. Microwave energy effects on quality of some seed oils. J. Food Sci. 1990, 55, 1412–1416. [Google Scholar] [CrossRef]
- Halliwell, B.; Chirico, S. Lipid peroxidation: Its mechanism, measurement and significance. Am. J. Clin. Nutr. 1993, 57, 715S–725S. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds (i.e., tannin extracts) are available from the authors. |
| Material | Concentration (g/100 g) | DP 1 | PC 2 (%) | PD 3 (%) | A-type 4 (%) |
|---|---|---|---|---|---|
| Spruce WB | 5.14 ± 0.10 | 4.8 ± 0.1 | 94.8 | 5.2 | 3.3 |
| Spruce IB | 6.75 ± 0.18 | 5.3 ± 0.4 | 94.7 | 5.3 | 3.5 |
| Spruce OB | 4.65 ± 0.02 | 4.4 ± 0.1 | 94.9 | 5.1 | N.D. |
| Pine WB | 14.04 ± 0.30 | 3.4 ± 0.1 | 100 | N.D. | 7.6 |
| Pine IB | 8.79 ± 0.17 | 3.5 ± 0.1 | 100 | N.D. | 8.4 |
| Pine OB | 8.87 ± 0.15 | 3.7 ± 0.1 | 100 | N.D. | 3.2 |
| ”Pettu” | 10.01 ± 0.16 | 4.3 ± 0.1 | 100 | N.D. | 3.6 |
| Score a | Would You Buy the Product? | ||||||
|---|---|---|---|---|---|---|---|
| All evaluators, 16 individuals | |||||||
| Sample b | Outlook | Smell | Taste | Structure | Yes | No | Maybe |
| Extract 1 | 3.48 (0.88) | 3.58 (0.88) | 3.56 (0.82) | 3.33 (0.49) | 5 | 11 | |
| Extract 2 | 3.39 (0.74) | 3.60 (0.66) | 3.65 (0.79) | 3.40 (0.77) | 9 | 7 | |
| Spray extract | 3.38 (0.92 | 3.45 (0.91) | 3.59 (0.76) | 3.45 (0.82) | 6 | 10 | |
| Control | 3.44 (0.72) | 3.52 (0.73) | 3.60 (0.91) | 3.45 (1.03) | 9 | 6 | 1 |
| Men, 8 individuals | |||||||
| Sample | Outlook | Smell | Taste | Structure | Yes | No | Maybe |
| Extract 1 | 3.58 (0.86) | 3.06 (0.94) | 3.69 (0.74) | 3.10 (0.80) | 4 | 4 | |
| Extract 2 | 3.51 (0.86) | 3.07 (0.82) | 3.80 (0.35) | 3.29 (0.46) | 7 | 1 | |
| Spray extract | 3.60 (0.80) | 2.92 (0.96) | 3.77 (0.62) | 3.50 (0.92) | 5 | 3 | |
| Control | 3.70 (0.76) | 3.07 (0.74) | 3.73 (0.58) | 3.42 (1.06) | 6 | 1 | 1 |
| Women, 8 individuals | |||||||
| Sample | Outlook | Smell | Taste | Structure | Yes | No | Maybe |
| Extract 1 | 3.38 (0.93) | 4.10 (1.04) | 3.42 (1.13) | 3.56 (0.64) | 1 | 7 | |
| Extract 2 | 3.27 (0.65) | 4.12 (0.52) | 3.49 (1.07) | 3.49 (1.04) | 2 | 6 | |
| Spray extract | 3.15 (1.07) | 3.98 (0.92) | 3.40 (0.89) | 3.40 (0.74) | 1 | 7 | |
| Control | 3.17 (0.71) | 3.97 (0.74) | 3.47 (1.20) | 3.47 (1.06) | 3 | 5 | |
| Material | Amount (g) | Temperature (°C) | Time (min) | Liquid/Solids (l/kg) | Stirring (rpm) |
|---|---|---|---|---|---|
| Spruce WB 1 | 2 × 200.0 | 90 | 120 | 7.13 | 200 |
| Spruce WB 2 | 3 × 150.0 | 90 | 120 | 10 | 200 |
| Spruce IB 2 | 150.0 | 90 | 120 | 10 | 200 |
| Spruce OB 2 | 100.0 | 90 | 120 | 10 | 200 |
| Pine WB 2 | 150.0 | 90 | 120 | 10 | 200 |
| Pine IB 2 | 150.0 | 90 | 120 | 10 | 200 |
| Pine OB 2 | 150.0 | 90 | 120 | 10 | 200 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raitanen, J.-E.; Järvenpää, E.; Korpinen, R.; Mäkinen, S.; Hellström, J.; Kilpeläinen, P.; Liimatainen, J.; Ora, A.; Tupasela, T.; Jyske, T. Tannins of Conifer Bark as Nordic Piquancy—Sustainable Preservative and Aroma? Molecules 2020, 25, 567. https://doi.org/10.3390/molecules25030567
Raitanen J-E, Järvenpää E, Korpinen R, Mäkinen S, Hellström J, Kilpeläinen P, Liimatainen J, Ora A, Tupasela T, Jyske T. Tannins of Conifer Bark as Nordic Piquancy—Sustainable Preservative and Aroma? Molecules. 2020; 25(3):567. https://doi.org/10.3390/molecules25030567
Chicago/Turabian StyleRaitanen, Jan-Erik, Eila Järvenpää, Risto Korpinen, Sari Mäkinen, Jarkko Hellström, Petri Kilpeläinen, Jaana Liimatainen, Ari Ora, Tuomo Tupasela, and Tuula Jyske. 2020. "Tannins of Conifer Bark as Nordic Piquancy—Sustainable Preservative and Aroma?" Molecules 25, no. 3: 567. https://doi.org/10.3390/molecules25030567
APA StyleRaitanen, J.-E., Järvenpää, E., Korpinen, R., Mäkinen, S., Hellström, J., Kilpeläinen, P., Liimatainen, J., Ora, A., Tupasela, T., & Jyske, T. (2020). Tannins of Conifer Bark as Nordic Piquancy—Sustainable Preservative and Aroma? Molecules, 25(3), 567. https://doi.org/10.3390/molecules25030567







