Effects of Lycium barbarum L. Polysaccharides on Inflammation and Oxidative Stress Markers in a Pressure Overload-Induced Heart Failure Rat Model
Abstract
1. Introduction
2. Results
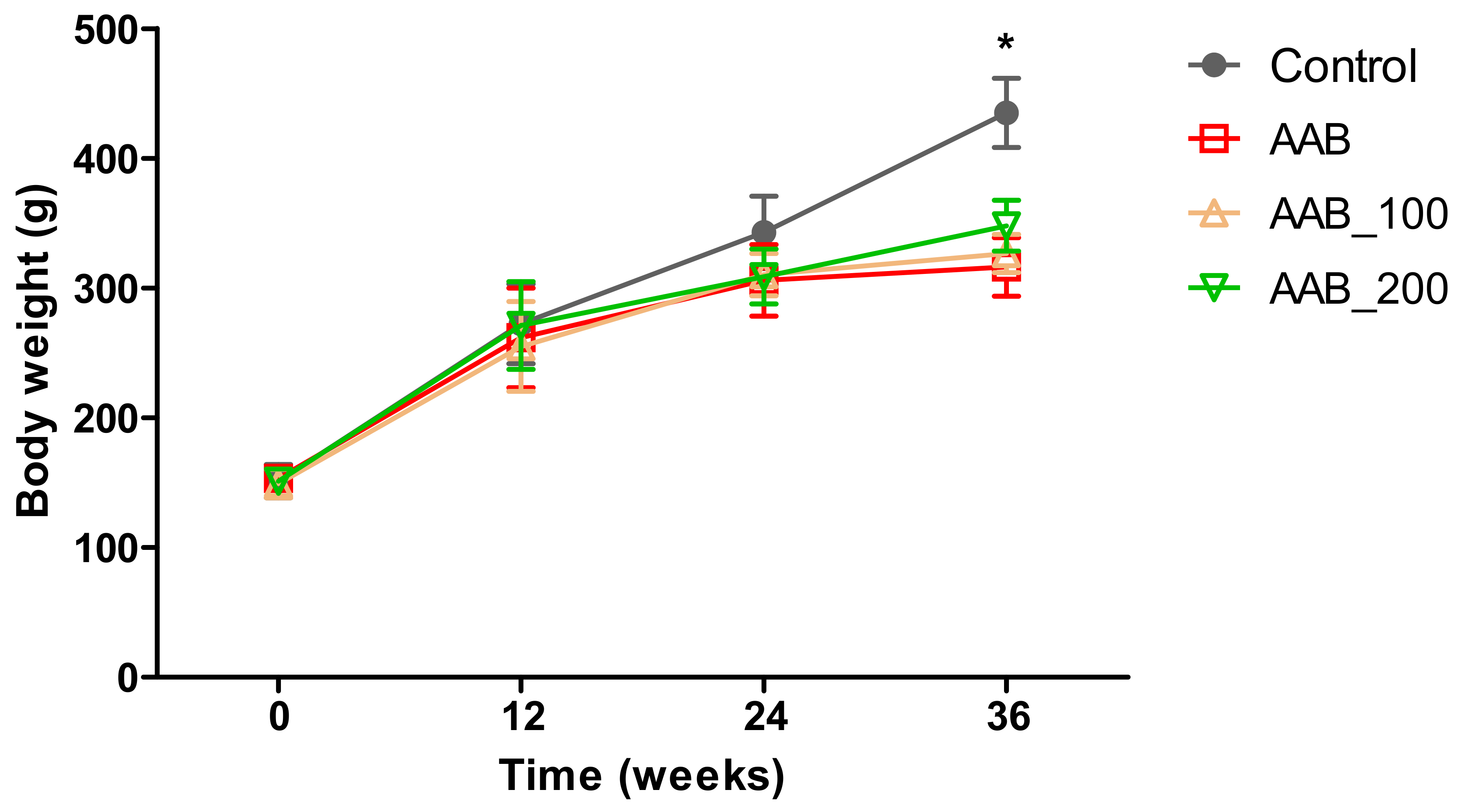
2.1. Body Weight
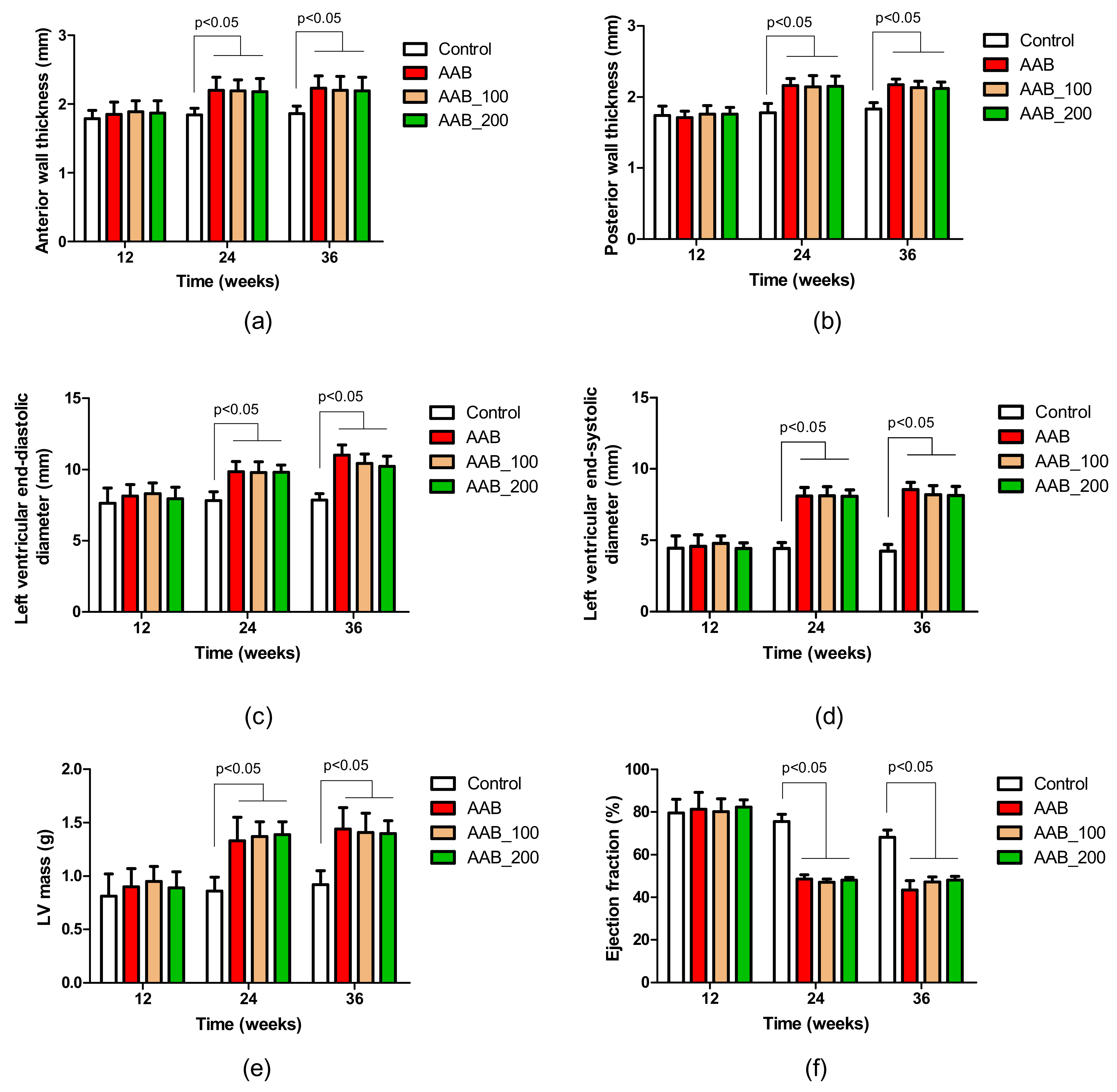
2.2. Effects of LBPs on Echocardiographic Parameters
2.3. Effects of LBPs on Hematology Parameters
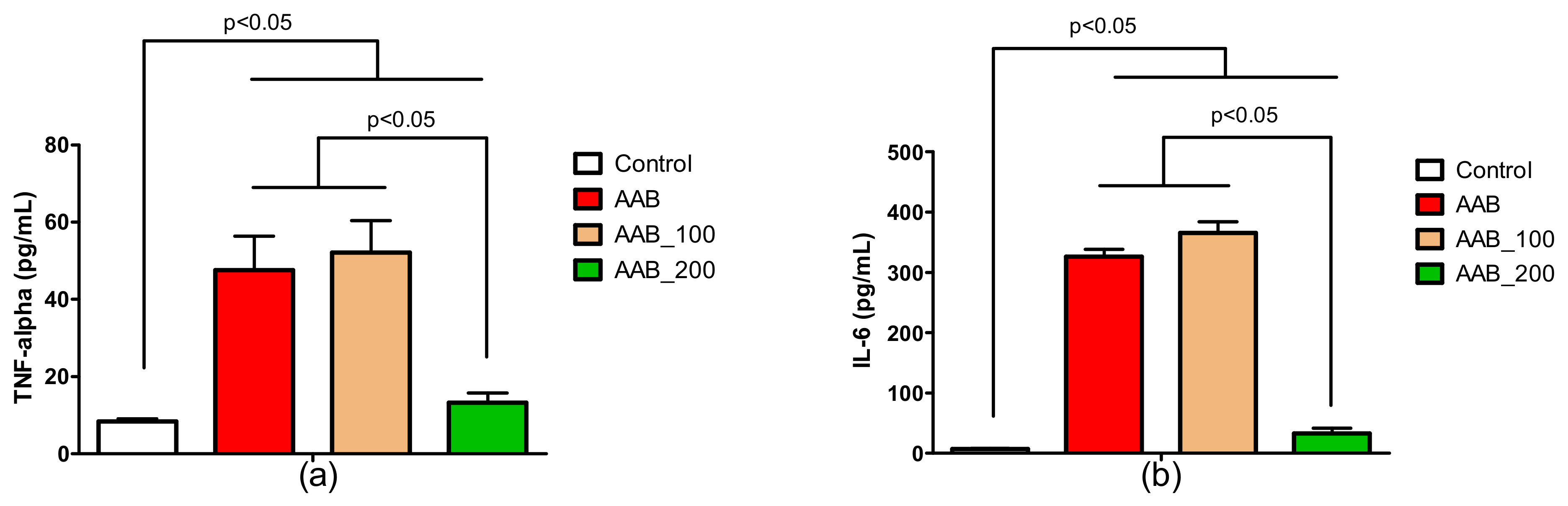
2.4. Effects of LBPs on Plasma Cytokines Levels
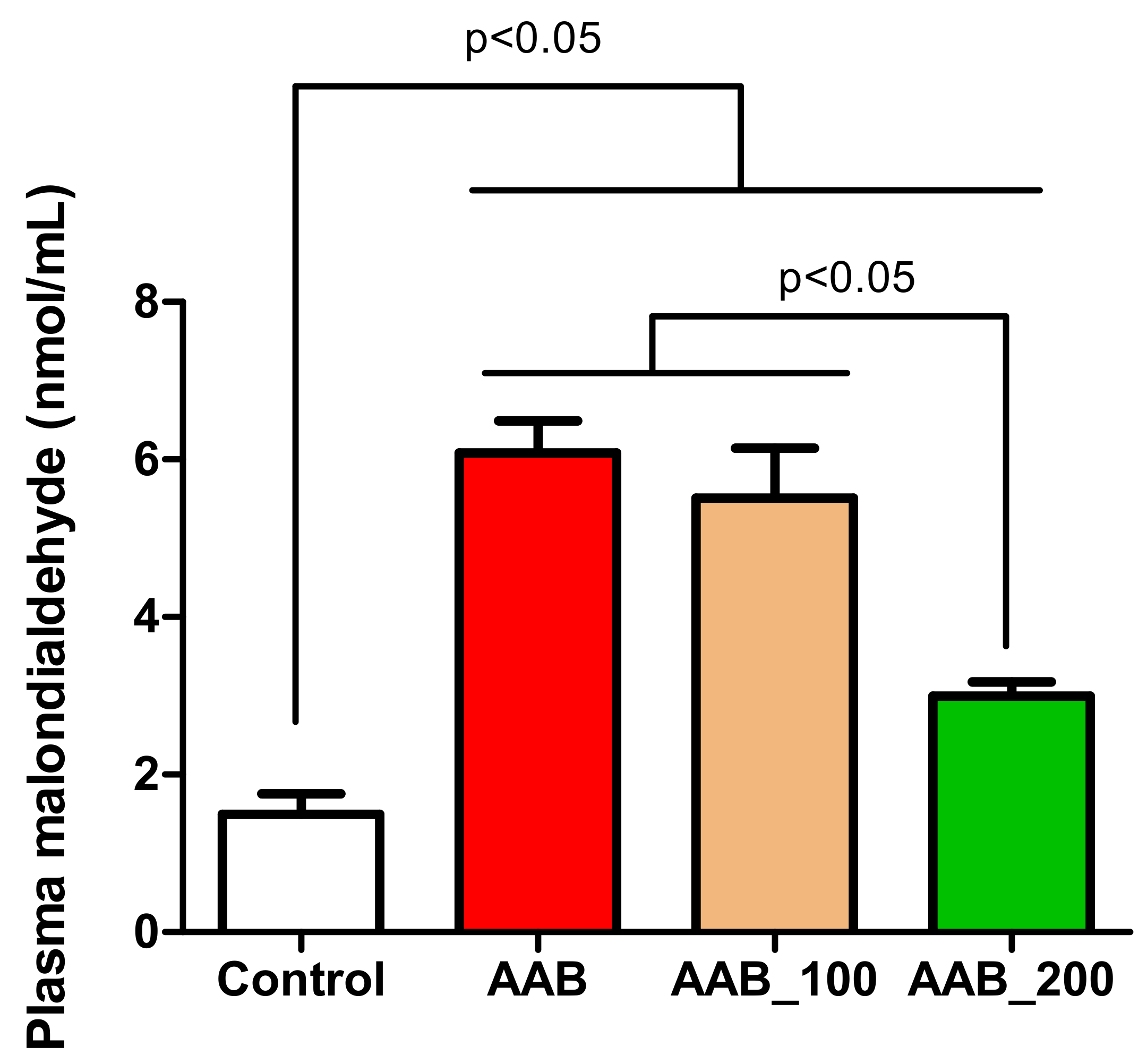
2.5. Effects of LBPs on Plasma Lipid Peroxidation
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Protocol
4.2. Echocardiography Measurements
4.3. Complete Blood Count
4.4. Proinflammatory Plasma Cytokines
4.5. Plasma Lipid Peroxidation
4.6. Data Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, K.F.; Pothineni, N.V.K.; Rutland, J.; Ding, Z.; Mehta, J.L. Immunity, Inflammation, and Oxidative Stress in Heart Failure: Emerging Molecular Targets. Cardiovasc. Drugs Ther. 2017, 31, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Hasenfuss, G. Animal models of human cardiovascular disease, heart failure and hypertrophy. Cardiovasc. Res. 1998, 39, 60–76. [Google Scholar] [CrossRef]
- Pop, C.; Berce, C.; Ghibu, S.; Pop, A.; Kiss, B.; Irimie, A.; Popa, Ș.T.; Cismaru, G.; Loghin, F.; An, M.Ș. Validation and characterisation of a heart failure animal model. Farmacia 2016, 64, 435–443. [Google Scholar]
- Frantz, S.; Falcao-Pires, I.; Balligand, J.-L.; Bauersachs, J.; Brutsaert, D.; Ciccarelli, M.; Dawson, D.; de Windt, L.J.; Giacca, M.; Hamdani, N.; et al. The innate immune system in chronic cardiomyopathy: A European Society of Cardiology (ESC) scientific statement from the Working Group on Myocardial Function of the ESC. Eur. J. Heart Fail. 2018, 20, 445–459. [Google Scholar] [CrossRef]
- Tsutsui, H.; Kinugawa, S.; Matsushima, S. Oxidative stress and heart failure. J. Physiol. Heart. Circ. Physiol. 2011, 301, 2181–2190. [Google Scholar] [CrossRef]
- Potterat, O. Goji (Lycium barbarum and L. chinense): Phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Med. 2010, 76, 7–19. [Google Scholar] [CrossRef]
- Hou, Y.-M.; Wang, J.; Zhang, X.-Z. Lycium barbarum polysaccharide exhibits cardioprotection in an experimental model of ischemia-reperfusion damage. Mol. Med. Rep. 2017, 15, 2653–2658. [Google Scholar] [CrossRef]
- Xin, Y.-F.; Wan, L.-L.; Peng, J.-L.; Guo, C. Alleviation of the acute doxorubicin-induced cardiotoxicity by Lycium barbarum polysaccharides through the suppression of oxidative stress. Food Chem. Toxicol. 2011, 49, 259–264. [Google Scholar] [CrossRef]
- Xin, Y.; Zhang, S.; Gu, L.; Liu, S.; Gao, H.; You, Z.; Zhou, G.; Wen, L.; Yu, J.; Xuan, Y. Electrocardiographic and Biochemical Evidence for the Cardioprotective Effect of Antioxidants in Acute Doxorubicin-Induced Cardiotoxicity in the Beagle Dogs. Biol. Pharm. Bull. 2011, 34, 1523–1526. [Google Scholar] [CrossRef]
- Xin, Y.-F.; Zhou, G.-L.; Deng, Z.-Y.; Chen, Y.-X.; Wu, Y.-G.; Xu, P.-S.; Xuan, Y.-X. Protective effect of Lycium barbarum on doxorubicin-induced cardiotoxicity. Phyther. Res. 2007, 21, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xu, Y.; Niu, H.; Tao, T.; Ban, T.; Zheng, L.; Ai, J. Lycium barbarum polysaccharides restore adverse structural remodelling and cardiac contractile dysfunction induced by overexpression of microRNA-1. J. Cell. Mol. Med. 2018, 22, 4830–4839. [Google Scholar] [CrossRef] [PubMed]
- Masci, A.; Carradori, S.; Casadei, M.A.; Paolicelli, P.; Petralito, S.; Ragno, R.; Cesa, S. Lycium barbarum polysaccharides: Extraction, purification, structural characterisation and evidence about hypoglycaemic and hypolipidaemic effects. A review. Food Chem. 2018, 254, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Bui, A.L.; Horwich, T.B.; Fonarow, G.C. Epidemiology and risk profile of heart failure. Nat. Rev. Cardiol. 2011, 8, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Boluyt, M.O.; Converso, K.; Russell, M.W.; Bleske, B.E. Effects of Hawthorn on the Progression of Heart Failure in a Rat Model of Aortic Constriction. Pharmacotherapy 2009, 29, 639–648. [Google Scholar] [CrossRef]
- Zhang, W.; Kowal, R.C.; Rusnak, F.; Sikkink, R.A.; Olson, E.N.; Victor, R.G. Failure of Calcineurin Inhibitors to Prevent Pressure-Overload Left Ventricular Hypertrophy in Rats. Circ. Res. 1999, 84, 722–728. [Google Scholar] [CrossRef]
- Norton, G.R.; Woodiwiss, A.J.; Gaasch, W.H.; Mela, T.; Chung, E.S.; Aurigemma, G.P.; Meyer, T.E. Heart failure in pressure overload hypertrophy. The relative roles of ventricular remodeling and myocardial dysfunction. J. Am. Coll. Cardiol. 2002, 39, 664–671. [Google Scholar] [CrossRef]
- Zhang, Y.; Bauersachs, J.; Langer, H.F. Immune mechanisms in heart failure. Eur. J. Heart Fail. 2017, 19, 1379–1389. [Google Scholar] [CrossRef]
- Patten, R.D.; Hall-Porter, M.R. Small Animal Models of Heart Failure. Circ. Hear. Fail. 2009, 2, 138–144. [Google Scholar] [CrossRef]
- Tang, W.-M.; Chan, E.; Kwok, C.-Y.; Lee, Y.-K.; Wu, J.-H.; Wan, C.-W.; Chan, R.Y.-K.; Yu, P.H.-F.; Chan, S.-W. A review of the anticancer and immunomodulatory effects of Lycium barbarum fruit. Inflammopharmacology 2012, 20, 307–314. [Google Scholar] [CrossRef]
- Peng, X.; Chen, B.; Lim, C.C.; Sawyer, D.B. The cardiotoxicology of anthracycline chemotherapeutics: Translating molecular mechanism into preventative medicine. Mol. Interv. 2005, 5, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Home Office. Animals (Scientific Procedures) Act 1986. Code of Practice for the Housing and Care of Animals Used in Scientific Procedures. Available online: http://www.official-documents.gov.uk/document/hc8889/hc01/0107/0107.pdf (accessed on 2 September 2019).
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.; et al. Recommendations for chamber quantification. Eur. J. Echocardiogr. 2006, 7, 79–108. [Google Scholar] [CrossRef] [PubMed]
- Porfire, A.S.; Leucuţa, S.E.; Kiss, B.; Loghin, F.; Pârvu, A.E. Investigation into the Role of Cu/Zn-SOD Delivery System on Its Antioxidant and Antiinflammatory Activity in Rat Model of Peritonitis. Pharmacol. Rep. 2014, 66, 670–676. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| Organ/BW Ratio (mg/g) | Control (n = 10) | AAB (n = 12) | AAB_100 (n = 7) | AAB_200 (n = 9) |
|---|---|---|---|---|
| Heart/BW | 1.59 ± 0.21 | 2.28 ± 0.33 * | 2.27 ± 0.18 * | 2.12 ± 0.18 * |
| Lung/BW | 4.70 ± 0.24 | 5.03 ± 0.63 | 4.87 ± 0.47 | 4.75 ± 0.66 |
| Kidney/BW | 1.96 ± 0.29 | 2.74 ± 0.41 * | 2.96 ± 0.42 * | 2.69 ± 0.35 * |
| Liver/BW | 23.02 ± 1.90 | 23.57 ± 3.00 | 23.55 ± 3.86 | 23.44 ± 2.12 |
| Hematology Parameters | Control (n = 10) | AAB (n = 12) | AAB_100 (n = 7) | AAB_200 (n = 9) |
|---|---|---|---|---|
| WBC | 9.01 ± 2.94 | 9.46 ± 1.55 | 9.10 ± 2.82 | 9.18 ± 2.65 |
| LYM | 6.06 ± 1.62 | 6.50 ± 1.44 | 5.89 ± 1.63 | 6.57 ± 2.55 |
| MID | 0.66 ± 0.52 | 0.61 ± 0.35 | 0.59 ± 0.35 | 0.44 ± 0.13 |
| GRA | 2.29 ± 1.04 | 2.34 ± 0.72 | 2.63 ± 1.81 | 2.17 ± 0.81 |
| LY | 69.16 ± 9.35 | 68.09 ± 8.29 | 66.67 ± 12.08 | 70.49 ± 8.72 |
| MI | 6.56 ± 4.44 | 6.43 ± 3.21 | 5.89 ± 2.29 | 5.06 ± 2.10 |
| GR | 24.27 ± 7.06 | 25.46 ± 8.59 | 27.49 ± 11.14 | 24.41 ± 8.74 |
| RBC | 8.50 ± 0.63 | 8.62 ± 0.42 | 8.14 ± 0.57 | 8.14 ± 0.39 |
| HGB | 14.74 ± 0.96 | 14.89 ± 0.52 | 14.70 ± 0.33 | 14.45 ± 0.27 |
| HCT | 42.35 ± 2.37 | 42.80 ± 2.09 | 45.85 ± 4.07 | 45.18 ± 2.20 |
| MCV | 50.11 ± 2.57 | 49.67 ± 1.57 | 56.29 ± 2.06 | 55.67 ± 1.73 |
| MCH | 17.38 ± 0.89 | 17.54 ± 0.66 | 17.59 ± 0.54 | 17.13 ± 0.39 |
| MCHC | 34.77 ± 0.50 | 35.33 ± 0.68 | 31.26 ± 0.83 | 30.83 ± 0.75 |
| RDWc | 15.50 ± 0.74 | 16.61 ± 0.95 | 15.23 ± 1.05 | 15.52 ± 0.87 |
| PLT | 672.60 ± 34.04 | 636.68 ± 195.5 | 673.75 ± 103.35 | 622.17 ± 103.34 |
| PCT | 0.40 ± 0.23 | 0.49 ± 0.16 | 0.53 ± 0.27 | 0.37 ± 0.13 |
| MPV | 6.86 ± 0.46 | 6.96 ± 0.30 | 7.24 ± 0.63 | 7.02 ± 0.34 |
| PDWc | 33.76 ± 2.61 | 33.17 ± 0.93 | 33.70 ± 2.14 | 32.94 ± 0.87 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pop, C.; Berce, C.; Ghibu, S.; Scurtu, I.; Sorițău, O.; Login, C.; Kiss, B.; Ștefan, M.G.; Fizeșan, I.; Silaghi, H.; et al. Effects of Lycium barbarum L. Polysaccharides on Inflammation and Oxidative Stress Markers in a Pressure Overload-Induced Heart Failure Rat Model. Molecules 2020, 25, 466. https://doi.org/10.3390/molecules25030466
Pop C, Berce C, Ghibu S, Scurtu I, Sorițău O, Login C, Kiss B, Ștefan MG, Fizeșan I, Silaghi H, et al. Effects of Lycium barbarum L. Polysaccharides on Inflammation and Oxidative Stress Markers in a Pressure Overload-Induced Heart Failure Rat Model. Molecules. 2020; 25(3):466. https://doi.org/10.3390/molecules25030466
Chicago/Turabian StylePop, Cristina, Cristian Berce, Steliana Ghibu, Iuliu Scurtu, Olga Sorițău, Cezar Login, Béla Kiss, Maria Georgia Ștefan, Ionel Fizeșan, Horațiu Silaghi, and et al. 2020. "Effects of Lycium barbarum L. Polysaccharides on Inflammation and Oxidative Stress Markers in a Pressure Overload-Induced Heart Failure Rat Model" Molecules 25, no. 3: 466. https://doi.org/10.3390/molecules25030466
APA StylePop, C., Berce, C., Ghibu, S., Scurtu, I., Sorițău, O., Login, C., Kiss, B., Ștefan, M. G., Fizeșan, I., Silaghi, H., Mocan, A., Crișan, G., Loghin, F., & Mogoșan, C. (2020). Effects of Lycium barbarum L. Polysaccharides on Inflammation and Oxidative Stress Markers in a Pressure Overload-Induced Heart Failure Rat Model. Molecules, 25(3), 466. https://doi.org/10.3390/molecules25030466








